The cholinergic system includes neurons located in the basal forebrain and their long axons that reach the cerebral cortex and the hippocampus. This system modulates cognitive function. In Alzheimer's disease (AD) and ageing, cognitive impairment is associated with progressive damage to cholinergic fibres, which leads us to the cholinergic hypothesis for AD.
DevelopmentThe AD produces alterations in the expression and activity of acetyltransferase (ChAT) and acetyl cholinesterase (AChE), enzymes specifically related to cholinergic system function. Both proteins play a role in cholinergic transmission, which is altered in both the cerebral cortex and the hippocampus due to ageing and AD. Dementia disorders are associated with the severe destruction and disorganisation of the cholinergic projections extending to both structures. Specific markers, such as anti-ChAT and anti-AChE antibodies, have been used in light immunohistochemistry and electron microscopy assays to study this system in adult members of certain animal species.
ConclusionsThis paper reviews the main immunomorphological studies of the cerebral cortex and hippocampus in some animal species with particular emphasis on the cholinergic system and its relationship with the AD.
El sistema colinérgico incluye neuronas localizadas en el cerebro basal anterior y sus axones largos proyectan a la corteza cerebral e hipocampo. Este sistema modula la función cognitiva. En la enfermedad de Alzheimer (EA) y en el proceso de envejecimiento la disfunción colinérgica hay una asociación entre el deterioro cognitivo y el daño progresivo de las fibras colinérgicas, lo que conduce al postulado de la hipótesis colinérgica.
DesarrolloEn la EA se producen alteraciones en la expresión y en la actividad de la colina acetiltransferasa (ChAT) y la acetilcolinesterasa (AChE), enzimas específicas relacionadas con la función del SC. Ambas proteínas juegan un papel importante en la transmisión colinérgica mostrando variaciones en la corteza cerebral y en el hipocampo, tanto por el envejecimiento, como por la EA. En ambas estructuras, los desórdenes demenciales están asociados a la destrucción severa y desorganización de las proyecciones colinérgicas que se encuentran afectadas. Para el estudio de este sistema se han usado marcadores específicos como los anticuerpos contra ChAT y AChE que han sido empleados en las técnicas de inmuhistoquímica de luz y microscopia electrónica en algunas especies animales.
ConclusionesEn este trabajo se hace una revisión de los principales estudios inmunomorfológicos de la corteza cerebral e hipocampo de varias especies animales con énfasis en el SC y su relación con la EA.
In mammalian brains, the cholinergic nuclei are located in the basal forebrain (BF), from the medial septum along the diagonal band of Broca to the nucleus basalis of Meynert (NBM) including the substantia innominata. The more rostral neurons located in the medial septum and vertical limb of the diagonal band of Broca innervate the hippocampus (via the hippocampal septum). More caudal neurons, including the NBM and substantia innominata, innervate the cerebral cortex and amygdala. Nuclei in the BF receive reciprocal connections from limbic structures (orbitofrontal, temporal pole, medial region of the temporal lobe, and entorhinal cortex). Brainstem nuclei (the pedunculopontine, tegmental, and lateral dorsal tegmental nuclei) activate the cerebral cortex by means of projections to the thalamus. Both the BF and the brainstem project to the thalamic reticular nucleus; cholinergic nuclei significantly influence limbic and cortical activity both directly and indirectly. Neurotransmission in the cholinergic system (CS) is involved in such processes as memory, learning, sleep, and other functions.1 Problems with neurotransmission elicit changes in these functions and this may be one of the causes of senile dementia or Alzheimer disease (AD). Biosynthesis of acetylcholine (C7H16NO2) or ester of ascetic acid and choline takes place in the cytoplasm of the soma and in presynaptic terminals by means of the activity of the choline acetyltransferase enzyme (ChAT). In the synaptic cleft, it is broken down into acetate and choline by the acetylcholinesterase enzyme (AChE) for reuptake by the presynaptic neuron.2,3 Both ChAT and AChE are proteins that function as specific markers of physiological activity by cholinergic neurons. Additionally, both play an important role in the homeostasis of neuronal acetylcholine.4
Cholinergic metabolism dysfunction has been reported in AD,3,5,6 as well as neuronal changes in the acetylcholine innervation, synthesis, breakdown, and reuptake. In humans, the decrease in cholinergic cells reflects a pathological change in the basal forebrain.7–11 Although this is not counted as one of the initial events in AD or the ageing process,12 it does imply cortical denervation related to the elimination of extrinsic projections to the cerebral cortex.6 This situation manifests as cognitive, intellectual, and social dysfunction according to the AD progression pattern and with the following symptoms: agitation, psychosis, depression, apathy, anxiety, sleep disorders, and appetite disorders.12–15 These patients exhibit decreased production of ChAT and AChE in the cerebral cortex and hippocampus, as well as impairment in axonal transport of those enzymes, due to degeneration of cholinergic neurons in the basal forebrain (NBM).16 In quantitative studies, brain tissue in patients with AD displayed a 55% decrease in reactive cholinergic fibres compared to healthy brain tissue.17 Studies of cholinergic innervation of the cerebral cortex and hippocampus and the specific importance of neuronal circuits in those structures have been carried out using a variety of mammal species and a specific combination of antibodies for cholinergic markers such as ChAT and AChE. This has demonstrated that cholinergic innervation is directly related to memory consolidation processes.5,6,18–20
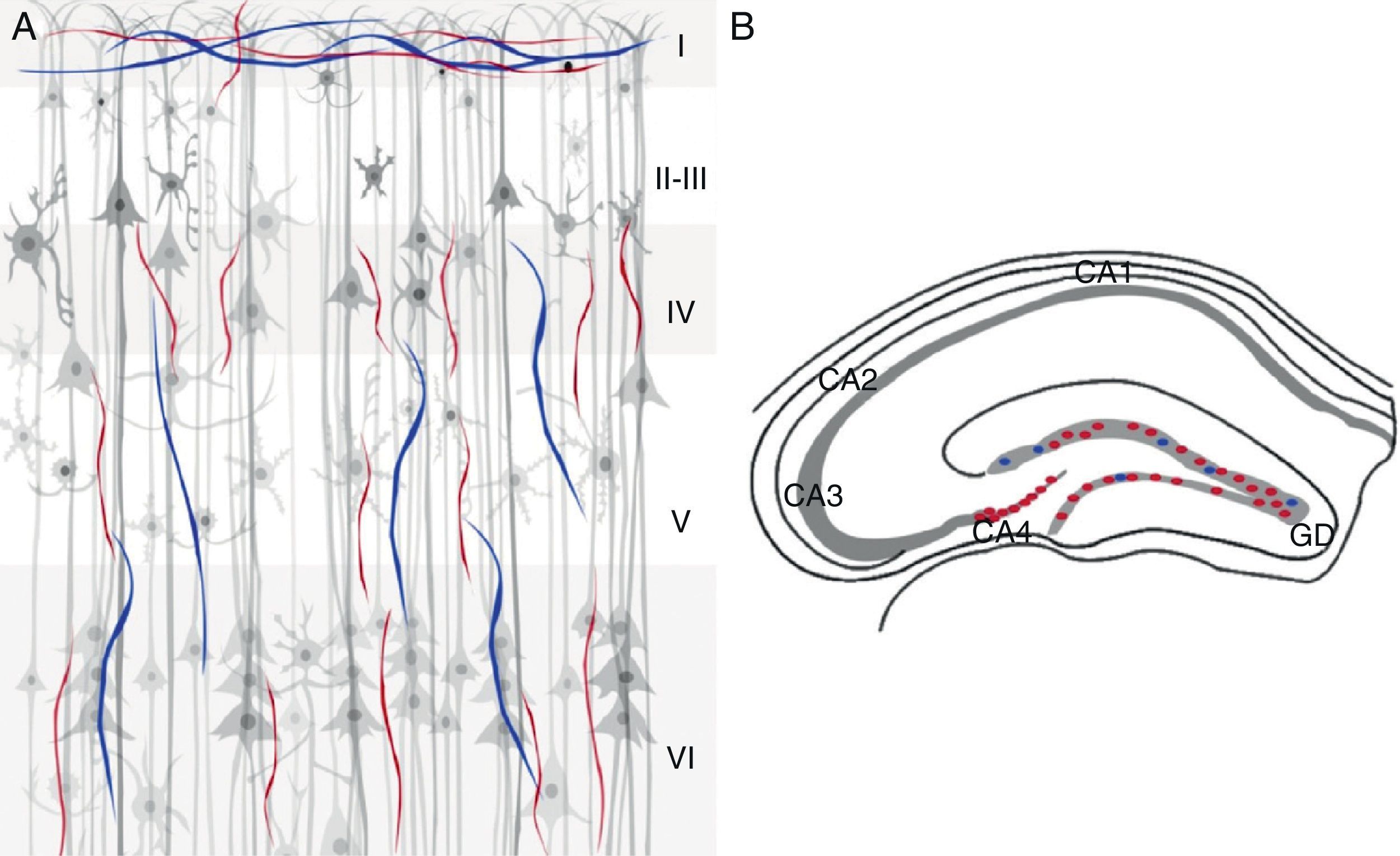
Cholinergic markers in the human brainThe human cerebral cortex possesses a complex and extensive network of cholinergic axons21–23 that innervate or originate in the cholinergic neurons located in the cell nuclei in the NBM (nucleus basalis of Maynert). Both types are immunoreactive to ChAT and AChE enzymes22,24–26 which absorb stain and present at higher densities in cortical layers III and V.17 Most of the neurons that are positive for AChE are pyramidal and located in cortical layer V.17 Immunohistochemical studies performed with the ChAT antibody show a high density of positive fibres and thin cholinergic projections throughout the cerebral cortex.17 In general, fibres that are immunoreactive to ChAT have a thickened appearance like a string of beads with dotted ends.27 All cortical areas contain a combination of cholinergic axons that are oriented horizontally, vertically, and transversally to the cortical surface. Horizontal fibres are located in layer I, and to a lesser extent, in layer II. Vertically oriented fibres are located in most layers. Superficial layers (I–III) display dense cholinergic innervations.22
The human hippocampus shows immunoreactivity for ChAT and AChE in fimbrial fibres in the subcortical white matter of the parahippocampal gyrus adjacent to the alveolar tract. The fibres directed toward the CA1 region and the parahippocampal gyrus are slender and distributed in a radial pattern.27 Immunoreactivity for ChAT is more intense in the stratum radiatum. The marking pattern in the pyramidal cell layer is lightly dotted; slender fibres oriented in all directions project thick axons toward the cells emanating from the parahippocampal gyrus. Low-intensity immunoreactivity is seen in the CA1 layer, as well as in the stratum oriens and the stratum lacunosum-moleculare.27 Immunoreactive fibres that are positive for AChE are slender in both the stratum oriens and the mossy fibres of the dentate gyrus28 and dense in the stratum pyramidale in the CA2 and CA3 areas. ChAT- and AChE-immunoreactive fibres are located in 2 layers in the dentate gyrus: a band of granule cells and another composed of transversal, densely marked fibres.27 The molecular layer contains ChAT-reactive fibres distributed with a uniform density and oriented parallel to the granule cell layer. ChAT-immunoreactive fibres in the molecular layer are oriented along the rostral-caudal axis, and marking at this level is faint.27 AChE immunoreactivity is denser in the granular cell layer, appearing as a thin band adjacent to that layer. The pyramidal cell layer exhibits moderate innervation in the subicular complex.27,28 The polymorphic and molecular layers show a homogeneous and random distribution of immunoreactivity (Fig. 1).28
ChAT (red) and AChE (blue) cholinergic markers in the human cerebral cortex (A) and hippocampus (B). The 6 cellular layers (I–VI) are indicated in (A); (B) shows the 3 pyramidal cell areas in CA1, CA2, and CA3. Strata are indicated: str. oriens (O), str. pyramidale (P), str. radiatum (R), and str. moleculare (M). The image also shows the dentate gyrus (GD), the source of the mossy fibres (MF) that synapse with the stratum lucidum (CA3), fimbria (Fi), and subiculum (Sub).
ChAT-immunoreactive innervation in the primate cerebral cortex is distributed heterogeneously with fibres and terminals from the NBM.29 In motor regions, AChE has a characteristic pattern in layers V and VI; here, fibres are arranged in a radial pattern and reactive cell bodies are found in layer VI.30
No ChAT-positive neurons can be observed in the hippocampal formation of some primate species because marking is limited to fibres and varicose axons and these fibres are categorised according to their distribution and morphological characteristics (Table 1).
Cholinergic fibres in primates.
| Type I | Fibres are thick and extend in a straight line; they have few varicose axons and numerous ChAT-positive fibres |
| Type II | Diffuse type I fibres with numerous varicose axons |
| Type III | Slender fibres with numerous varicose axons and branching extensions |
ChAT: choline acetyltransferase.
ChAT immunoreactivity is found in CA3 extending to the dentate gyrus. The density of fibres decreases in CA2,31 as does the density of ChAT-labeled fibres in the subiculum and above the CA1 in the pyramidal cell region extending to the molecular layer. Labelling is continuous with fibres located in the stratum lacunosum-moleculare; most immunoreactive fibres are horizontal, type II fibres. Labelling of AChE-positive fibres is decreased in CA1 and the subiculum.31 The dentate gyrus displays orientation-dependent variation in the density of ChAT-positive fibres. In the molecular layer of the antero-dorsal region, fibre distribution is diffuse; however, in more caudal regions and the medial segment adjacent to the fimbria, intensity increases, decreasing in the lateral areas.32 Regarding markers for the AChE enzyme in the dentate gyrus, there was a decrease in fibres in all layers compared to ChAT (Fig. 2).32
ChAT (red) and AChE (blue) cholinergic markers in the primate cerebral cortex (A) and hippocampus (B). The image indicates the 6 cellular layers of the cerebral cortex (I–VI) and the 3 subdivisions of the hippocampus (CA1, CA2, and CA3) in addition to the dentate gyrus (GD), the fimbria (Fi) and the subiculum (Sub).
Histochemical techniques show innervation from the NBM in cortical areas given that the NBM is the seat of magnocellular neurons that are the main source of cholinergic innervation.33
ChAT-immunoreactive fibres are observed in layers III and IV of the cerebral cortex, with faint labelling in layer II.2,34 Spindle-shaped cell bodies are also found in these layers.2 This represents only a tiny fraction of the cortical neurons found with less intense labelling than in neurons located in the NBM. Many of these ChAT-positive neurons exhibit a bipolar dendritic pattern; some are stellate, with vertically oriented dendrites extending through cortical layers.34 In the primary visual cortex, area 17 presented dense fibres in layer I with lower fibre density in layers IV and V for both ChAT- and AChE-positive neurons.35
The hippocampus showed a finely granular distribution of ChAT-positive neurons in the stratum oriens, with immunoreactivity being more concentrated in the CA2 and CA3 regions. Granular immunoreactivity in the stratum oriens continued, with ChAT-positive fibres in the alveus and fimbria. The dentate gyrus displayed ChAT-positive granules and fibres distributed dorsally and ventrally, as well as superficially along the molecular layer. The stratum lacunosum-moleculare contains few fibres testing positive for this marker, but they are distributed throughout the CA1 and the transition to the subiculum and toward the subcortical white matter.36 The rat hippocampus receives afferent fibres from the AChE, and they take part in CS activation.37,38 Reports indicate that AChE-positive neurons in the rat brain have similar shapes to ChAT-positive neurons. Researchers have also observed some AChE-positive neurons indicating that the marker is not specific to cholinergic neurons in the cerebral cortex in this species. Furthermore, the immunoreactivity of both markers (ChAT and AChE) is not intense enough to enable observation of double labelling (Fig. 3).39
Cholinergic markers in the mouse brainIn the visual, somatosensory, and frontal regions of the cerebral cortex, ChAT- and AChE-positive fibres are arranged in a trilaminar pattern. Three horizontal bands that are immunoreactive to both markers can be identified in layers I, IV, VI, and to a lesser extent in layer V.40 A narrow band is observed in layers I and II, with wider bands found in layers IV, V, and VI. Additionally, layer VI shows dense accumulation of fibres that are immunoreactive for ChAT.40 Cholinergic fibres in the visual cortex can be identified as a narrow band that crosses layer I parallel to the pial surface. In the somatosensory cortex, most cells that are positive for either ChAT or AChE contain fibres in layer IV. Cholinergic fibres exhibit a higher density in all regions of the frontal cortex, especially in layer I.40 The hippocampal region contains different bands of cholinergic fibres. The pyramidal cell layer is ChAT-immunoreactive, principally in CA4. A narrow band of these fibres can be observed throughout the layer of granular cells in the dentate gyrus (Fig. 4).40
ChAT (red) and AChE (blue) cholinergic markers in the mouse cerebral cortex (A) and hippocampus (B). (A) Region of the cerebral cortex in which the 6 cellular layers can be observed. (B) Region of the hippocampus showing subdivisions (CA1, CA2, CA3, and CA4) and the dentate gyrus (GD).
The development of cholinergic innervation in the cerebral cortex and hippocampus has been studied in a model of AD in which transgenic mice (APPSw,Ind41) overexpress β-amyloid. The study observed ChAT immunoreactivity, indicating that denervation was selective for the CS in this animal model.42 In the triple transgenic model of Alzheimer disease (3×Tg-AD), cholinotropic alterations are age- and sex-dependent and they are more pronounced in the hippocampus, where β-amyloid peptides are first developed. On the other hand, decreased ChAT expression in the same mouse model (3×Tg-AD) may be explained by increased expression of the REST/NRSF transcription factor associated with degenerative neuron disorders observed in AD.43
ConclusionThis review describes the two main CS markers, ChAT and AChE, and their specific distributions in the cerebral cortex and hippocampus of humans, primates, and murines (rats and mice). The cholinergic hypothesis of Alzheimer disease posits that dysfunction of the CS gives rise to cognitive impairment. For that reason, it is important to understand the distribution of CS markers in the areas of the brain most affected by the disease (cerebral cortex and hippocampus). In addition, animal models provide a better approximation of the changes in the CS and their relationship with AD development. This aids in designing pharmacological strategies aimed at preventing or treating the disease.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Orta-Salazar E, Cuellar-Lemus CA, Díaz-Cintra S, Feria-Velasco AI. Marcaje colinérgico en la corteza cerebral y el hipocampo en algunas especies animales y su relación con la enfermedad de Alzheimer. Neurología. 2014;29:497‐503.