The aim of this study was to assess the possible pharmacological interactions between safinamide and antidepressants, and in particular the appearance of serotonin syndrome with data from real life.
MethodsWe conducted a retrospective observational study of patients with Parkinson's disease from our Movement Disorders Unit, who were under treatment with any antidepressant drug and safinamide. Specifically, symptoms suggestive of serotonin syndrome were screened for. Also, we collected time of simultaneous use, doses of levodopa and other antiparkinsonian drugs.
ResultsClinical records were reviewed for the study period of September 2018 to September 2019. Seventy-eight PD patients who were treated with safinamide of which 25 (32.05%) had a concomitant treatment with an antidepressant drug, being sertraline and escitalopram the most frequent. Mean age was 80 years±8.43 and H&Y stage was 3 [2–4]. Mean dose of levodopa used was 703.75mg±233.15. Median duration of concomitant treatment with safinamide and antidepressant drug was 6 months (IQR 20.5), and over eighteen months in 5 cases. No case of serotonin syndrome was recorded, neither was any of its typical manifestations combined or in isolation.
ConclusionsOur real clinical practice study suggests that concomitant use of safinamide with antidepressant drugs in PD patients seemed to be safe and well tolerated, even in the long term. However, caution is warranted, individualizing treatment regimens and monitoring the potential appearance of adverse effects.
El objetivo de este estudio ha sido evaluar las posibles interacciones farmacológicas entre safinamida y antidepresivos; en particular la aparición del síndrome serotoninérgico mediante datos obtenidos en la vida real.
Material y métodosRealizamos un estudio observacional retrospectivo de pacientes con enfermedad de Parkinson (EP) de nuestra unidad de trastornos del movimiento, que estaban en tratamiento con algún fármaco antidepresivo y safinamida. Específicamente, se examinaron los síntomas sugestivos de síndrome serotoninérgico. Además, se recogieron tiempos de uso simultáneo, dosis de levodopa y otros fármacos antiparkinsonianos concomitantes.
ResultadosSe revisaron las historias clínicas correspondientes al período de estudio de septiembre de 2018 a septiembre de 2019. Setenta y ocho pacientes con EP se encontraban en tratamiento con safinamida, de los cuales 25 (32,05%) se encontraban recibiendo además un fármaco antidepresivo, siendo sertralina y escitalopram los más frecuentes. La edad media fue de 80 años±8,43 y el estadio H&Y fue de 3 [2-4]. La dosis media de levodopa utilizada fue de 703,75mg±233,15. La mediana de duración del tratamiento concomitante con safinamida y un fármaco antidepresivo fue de 6 meses (IQR: 20,5), y más de 18 meses en 5 casos. No se registró ningún caso de síndrome serotoninérgico, ni tampoco ninguno de sus síntomas de forma aislada.
ConclusiónNuestro estudio de práctica clínica real sugiere que el uso concomitante de safinamida con fármacos antidepresivos en pacientes con EP parece ser seguro y bien tolerado, incluso a largo plazo. Sin embargo, es necesaria precaución, individualizando los regímenes de tratamiento, y controlando la posible aparición de efectos adversos.
Depressive disturbances are common in patients with Parkinson's disease (PD) and may influence many other clinical aspects of the disease.1 Frequently, affective disorders predate the onset of motor symptoms, on average, 4–6 years before the diagnosis of PD.2 In several studies, treated or untreated mild depression has been associated with greater disability and it can sometimes precipitate the initiation of dopaminergic therapy. Antidepressants as a drug class have shown utility in depression and anxiety in PD.3,4 Serotonin syndrome is a measure of central nervous system (CNS) hyperexcitability in relation to an excess of serotonin.5 It includes a combination of mental status changes, neuromuscular and autonomic hyperactivity. Symptoms can range from mild to life-threatening. Serotonin syndrome can occur via therapeutic use of serotonergic drugs alone, an intentional overdose of serotonergic drugs, or classically, as a result of a complex drug interaction between two serotonergic drugs that work by different mechanisms.6 MAO-B inhibitors were introduced for the treatment of PD over 40 years ago and remain a popular mainstay of treatment. Monoamine oxidase (MAO) is a mitochondrial enzyme that oxidatively deaminates monoamines and it exists as 2 isoforms, MAO-A and MAO-B. Inhibition of MAO-B, the major isoform in the human brain, prevents the breakdown of extracellular levels of dopamine in the striatum. Inhibition of MAO-B can also be reversible or irreversible.7 Safinamide is a MAO-B inhibitor and glutamate modulator. Unlike other MAO-B inhibitors, the inhibition of safinamide is reversible and more selective compared with that of selegiline and rasagiline, minimizing the risk of hypertensive crises or serotonergic syndrome, and preventing dietary restrictions.8–10 According to the summary of product characteristics, a washout period of five half-lives of the selective serotonin reuptake inhibitors (SSRI) used previously should be considered before safinamide treatment initiation. However, as it has been mentioned above most of the times the patient manifest symptoms of depression and it is not possible to stop antidepressant medication. Then we may consider to start safinamide anyway, and we must always warn the patient about alarm symptoms that indicate he should look for immediate medical help such as vomiting or diarrhea.11,12 Concomitant use of safinamide and fluvoxamine is not recommended and also special care must be taken in those receiving high doses of antidepressants.13 The aim of this study was to assess the possible pharmacological interactions between safinamide and antidepressants, and in particular the appearance of serotonin syndrome.
Materials and methodsWe conducted a retrospective observational study of patients with Parkinson's disease from our Movement Disorders Unit, who were under treatment with any antidepressant drug and safinamide. PD was defined according to UK PD Brain Bank criteria.14 Baseline characteristics of patients were collected (age, sex, Hoehn Yahr stage (H&Y)), as well as safinamide dose, antidepressant and other concomitant opioid-type medications, and side effects related to treatment. Specifically, symptoms suggestive of serotonin syndrome (high body temperature, agitation, increased reflexes, tremor, sweating, dilated pupils, diarrhea) were screened for. Also, we collected time of simultaneous use, doses of levodopa and other antiparkinsonian drugs. Descriptive statistics have been used to summarize and organize the study data. Quantitative variables were described by mean±standard deviation if normally distributed, or by median (first quartile; third quartile) if not normally distributed.
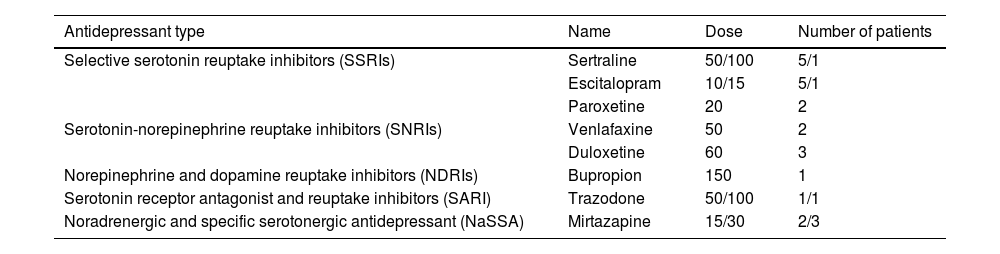
ResultsClinical records were reviewed for the study period of September 2018 to September 2019. Seventy-eight PD patients who were treated with safinamide of which 25 (32.05%) had a concomitant treatment with an antidepressant drug, being sertraline and escitalopram the most frequent [Table 1]. Eight patients were on safinamide 50mg and 17 on safinamide 100mg. Mean age was 80 years±8.43 and H&Y stage was 3.2–4 Mean dose of levodopa used was 703.75mg±233.15. Eight patients were also under treatment with dopaminergic agonists, 4 with opicapone and 6 patients were simultaneously taking opioid medications. Median duration of concomitant treatment with safinamide and antidepressant drug was 6 months (IQR 20.5), and over eighteen months in 5 cases. No case of serotonin syndrome was recorded, neither was any of its typical manifestations combined or in isolation. As for adverse events leading to discontinuation of safinamide, it was registered only in one case, who discontinued the drug due to worsening hallucinations. Nine (12%) patients reported improvement in non-motor symptoms such as sleep (4), pain (2), mood (1), or urinary symptoms (2).
Antidepressant medications, dose and number of patients who were on treatment.
| Antidepressant type | Name | Dose | Number of patients |
|---|---|---|---|
| Selective serotonin reuptake inhibitors (SSRIs) | Sertraline | 50/100 | 5/1 |
| Escitalopram | 10/15 | 5/1 | |
| Paroxetine | 20 | 2 | |
| Serotonin-norepinephrine reuptake inhibitors (SNRIs) | Venlafaxine | 50 | 2 |
| Duloxetine | 60 | 3 | |
| Norepinephrine and dopamine reuptake inhibitors (NDRIs) | Bupropion | 150 | 1 |
| Serotonin receptor antagonist and reuptake inhibitors (SARI) | Trazodone | 50/100 | 1/1 |
| Noradrenergic and specific serotonergic antidepressant (NaSSA) | Mirtazapine | 15/30 | 2/3 |
Treatment of motor and non-motor symptoms of PD is complex, and commonly leads to regimens of combination therapy with possibility of interactions. This is especially important when we combine antidepressant and pain treatments with MAOIs, due to the potential risk of serotonin syndrome, especially if combined with other drugs that are frequently used in patients with PD, such as opioids. Data suggest that safinamide may have a lower risk of serotonin syndrome.15–18 Only real clinical experience can corroborate these data, since polypharmacy tends to be avoided for safety in clinical trials. In our study we have found frequent concomitant use of antidepressants and safinamide, with very favorable safety data. No serotonin syndrome was recorded, either minor manifestations, not even in the opioid treatment subgroup. Adherence to safinamide was very good, with only one case of suspension of treatment due to collateral effects (hallucinations), probably due to its dopaminergic properties and to the combination with other drugs with similar mechanism. Although the retrospective registry does not allow evaluating efficacy, in terms of non-motor symptoms control, in a subgroup of patients the use of safinamide was beneficial for the control of several non-motor symptoms, as has been suggested in other real-life clinical studies.19 Pain occurs two or three times more frequently in patients with PD than in individuals of similar age without PD. Prevalence of pain in PD may vary from 34% to 83%, depending on methodological evaluations. It can be considered as a non-motor symptom but also related to concomitant osteoarticular pathology, so is very frequent that patients with Parkinson disease have opioid medications among their treatments.18,19 While SSRIs are the most commonly reported drug associated with serotonin syndrome, physicians should be aware of tramadol as a potential single agent cause for serotonin syndrome.20 The concurrent use of SSRIs with tramadol has been shown to induce serotonin syndrome through synergistic serotonergic action, along with the inhibition of CYP2D6, resulting in higher levels of tramadol enantiomer associated with serotonergic activity.21–24 We found a small group of patients that were taking together antidepressants, tramadol and safinamide [Fig. 1]. Any of them had symptoms suggestive of serotonergic hyperactivity, but we must monitor closely for the possible occurrence of adverse effects. We are aware that this observational study has several limitations due to the small number of patients included and its retrospective design.
ConclusionOur real clinical practice study suggests that concomitant use of safinamide with antidepressant drugs in PD patients seemed to be safe and well tolerated, even in the long term. However, caution is warranted, individualizing treatment regimens and monitoring the potential appearance of adverse effects.
Ethical standardThe study was approved by the local Ethics Committee.
FundingThere are no financial disclosures. Full other financial disclosures of all authors for the previous 12 months. P. Pérez Torre: Hospital Ramón y Cajal, Madrid. IRYCIS, Madrid. V. Mañanes: Hospital Ramón y Cajal, Madrid. IRYCIS, Madrid. E. Monreal: Hospital Ramón y Cajal, Madrid. I Parees: Hospital Ramón y Cajal, Madrid Spain. IRYCIS, Madrid. S. Fanjul: Hospital Ramón y Cajal, Madrid. A Alonso: Hospital Ramon y Cajal, Madrid. J. Martinez: Hospital Ramon y Cajal, Madrid. IRYCIS, Madrid. J. López-Sendón: Hospital Ramón y Cajal. IRYCIS, Madrid, Spain.
Authors’ contributionP. Pérez Torre – research project: conception, organization, execution; statistical analysis: design, execution; manuscript: writing of the first draft.
E. Monreal – research project: organization; statistical analysis: review and critique; manuscript: review and critique.
V. Mañanes – research project: organization; statistical analysis: review and critique, Manuscript: review and critique.
I Parees – research project: organization; statistical analysis: review and critique; manuscript: review and critique.
S. Fanjul – research project: organization; statistical analysis: review and critique; manuscript: review and critique.
A Alonso – research project: organization; statistical analysis: review and critique; manuscript: review and critique.
JC Martínez – research project: organization, execution; statistical analysis: review and critique; manuscript: review and critique.
JL López-Sendón – research project: organization; statistical analysis: review and critique; manuscript: review and critique.
Conflict of interestThere are no conflicts of interest.