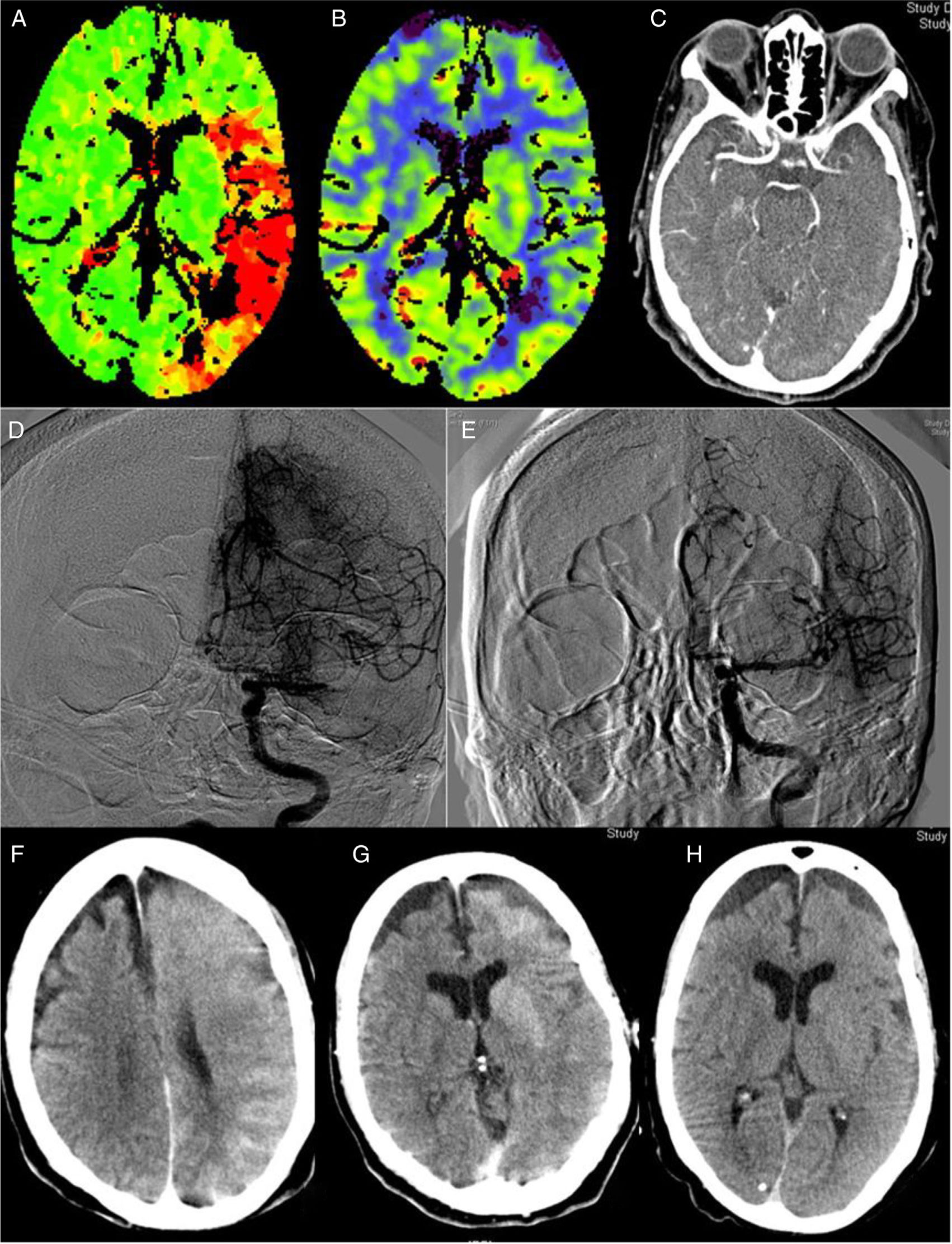
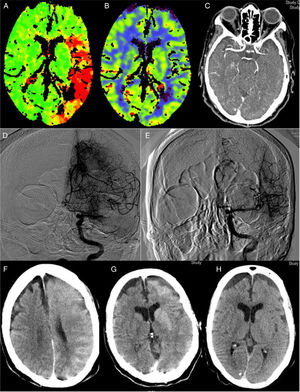
The implementation of acute stroke protocols1 is leading to more extensive use of neurointerventional techniques. Patients with increasingly complex conditions are eligible for this type of treatment, giving rise to new clinical scenarios. We present a case of encephalopathy with onset following successful mechanical thrombectomy associated with imaging evidence of blood–brain barrier (BBB) disruption, which fully resolved both clinically and radiologically. Our patient was an 82-year-old woman with history of hypertension, diabetes, dyslipidaemia, anticoagulation therapy with acenocoumarol for atrial fibrillation, and stage 3B-4 chronic kidney disease due to diabetic nephropathy. The patient consulted due to impaired language production and right hemiparesis. At the time of arrival at hospital, she presented arterial pressure of 150/92, National Institute of Health Stroke Scale (NIHSS) of 17, International Normalised Ratio (INR) of 1.89, and the previously known kidney failure. A computed tomography (CT) scan (Fig. 1A–C) revealed an extensive ischaemic penumbra in the territory of the left middle cerebral artery (MCA), with no signs of established ischaemia with M1 occlusion. The patient underwent primary thrombectomy as fibrinolysis was contraindicated (INR>1.7); complete recanalisation (TICI grade 3) was achieved after one pass of a Penumbra Ace® distal aspiration catheter (Fig. 1D–E). Her condition did not improve, with arterial pressure increasing to 226/100. A brain CT scan performed 2hours after recanalisation (Fig. 1F–G) showed contrast extravasation in the left hemisphere, which led us to a diagnosis of encephalopathy due to BBB rupture secondary to hypertensive crisis and contrast toxicity (iohexol at 350mg/mL). She subsequently developed secondarily generalised focal seizures in the left hemisphere, which did not resolve after administration of 10mg of diazepam and 750mg of intravenous phenytoin; she was therefore administered analgesia/sedation and levetiracetam. A brain CT scan performed at 24hours (Fig. 1H) revealed disappearance of the contrast and signs of oedema in the left hemisphere, with no established infarction. Several electroencephalograms ruled out epileptic activity and therefore phenytoin was suspended. The patient was extubated without incident. A brain CT scan performed 8 days after onset showed no evidence of stroke. The patient was discharged without symptoms (NIHSS: 0) with apixaban adjusted to her renal function prescribed for secondary prevention; levetiracetam was suspended. A brain magnetic resonance imaging study showed no residual ischaemic lesion. At 3 months, she presented a score of 0 on both the NIHSS and the modified Rankin Scale. Multiple reported cases of encephalopathy due to disruption of the BBB may be included on the spectrum of posterior reversible encephalopathy2 and hyperperfusion syndrome.3 Although they share some characteristics, our case presents peculiarities, especially its manifestation after thrombectomy with complete recanalisation. Thrombectomy may have been the cause, as it produces endothelial damage,4 which would trigger disruption of the BBB and subsequent contrast extravasation, which is exacerbated in patients with kidney disease. This type of damage would involve the effect of reperfusion and the toxic effect of contrast, in some patients (especially those with kidney failure). This would occur in previously hypoperfused areas where arterial pressure alterations would play a fundamental role, as in the case of hyperperfusion syndrome after carotid revascularisation.3 Some studies postulate that contrast extravasation following neurointervention may be related with an increase in intracranial bleeding.5,6 A case of hyperperfusion after thrombectomy with poor progression has also been described.7 Reported cases of hyperperfusion after rtPA are more numerous.8 Some cases have been reported of contrast-induced encephalopathy9 after such angiographic procedures as cardiac catheterisation,10 characterised by disruption of the BBB with contrast extravasation to the extravascular space; this is generally reversible within 72 to 96hours. We have not found similar reports to our case in the literature; our most striking finding is the complete recovery observed, with no residual ischaemia despite the patient’s age and the initial clinical severity. This complication, despite being infrequent, should be considered in interventional procedures, especially in those patients at greater risk of BBB damage: risk factors include older age, haemodynamic instability, and kidney disease (especially diabetic nephropathy). It is important to consider this entity in cases of clinical deterioration following neurointervention, once haemorrhagic complications have been ruled out. The measures to be taken include controlling blood pressure, eliminating associated factors, and adding antiepileptics when necessary; these may be suspended in the medium term if there is no structural lesion.2 Clinical and radiological recovery may be expected within 72−96hours.
Perfusion CT (A and B): increased time to peak (A) in an extensive area of the left MCA territory, with no established ischaemia on the cerebral blood volume map (B). CT angiography (C): occlusion at the left proximal M1 segment. Angiography: occlusion at the left proximal M1 segment (D) with complete recanalisation after one pass of a Penumbra Ace® distal aspiration catheter (E). CT scan after thrombectomy (F and G): generalised contrast extravasation in the left hemisphere. CT scan at 24hours (H): signs of oedema in the left hemisphere with disappearance of the accumulated contrast and no signs of established ischaemia.
We would like to thank Francisco Gilo Arrojo, Rafael Manzanares, Antonio Barbosa, José Luis Caniego, and Eduardo Bárcena.
Please cite this article as: Aguirre C, et al. Encefalopatía por contraste secundaria a posible da˜no endotelial tras trom-bectomía mecánica exitosa. Neurología. 2020;35:336–338.