Virtual Reality (VR) uses computer technology to create a simulated environment. VR is a growing technology with promising extensive applications in different areas such as Medicine, entertainment, sports, gaming, and simulation. However, information about VR side effects is still limited. We aimed to identify the most frequent physical side effects caused by VR therapeutic applications.
MethodologyAll available full-text articles evaluating VR as a therapeutic intervention and side effects using the Simulator Sickness Questionnaire (SSQ) between 2016 and 2021 were consulted across 4 electronic (Entrez Pubmed, Scopus, Science Direct, and Wiley databases). The methodological quality was assessed using the PEDro scale.
ResultsTen out of 55 reviewed articles (18%) met inclusion/exclusion criteria, including a sample of 416 patients, mean age of 24.54 (15–52.6)years old. According to the PEDro scale, two articles (20%) were considered good or excellent. Side effects were reported more frequently with head-mounted displays compared to desktop systems, especially disorientation, followed by nausea and oculomotor disturbances.
ConclusionsAlthough VR might have positive effects as a therapeutic tool, VR can also cause side events. As in any other therapeutic intervention, it is important to understand the effectiveness and safety before planning a VR intervention using a well-designed scientific methodology.
La Realidad Virtual (RV) proporciona un entorno simulado mediante el uso de tecnologías informáticas. Aunque la RV es una tecnología en expansión con aplicaciones prometedoras en diferentes áreas de la medicina, ocio, deportes, juegos y simulaciones, todavía existe poca información sobre su seguridad. El objetivo de este estudio es identificar los efectos secundarios más frecuentes provocados por las aplicaciones terapéuticas de la RV.
MetodologíaSe consultaron todos los artículos de texto completo disponibles que evaluaron la RV como intervención terapéutica y sus efectos secundarios utilizando el Simulator Sickness Questionnaire (SSQ), entre 2016 y 2021, en 4 bases electrónicas: Entrez Pubmed, Scopus, Science Direct y bases de datos Wiley. La calidad metodológica se evaluó usando la escala PEDro.
ResultadosDiez de los 55 artículos revisados (18%) cumplieron con los criterios de inclusión/exclusión, incluyendo una muestra total de 416 pacientes, edad media de 24,54 años (15–52,6 años). Dos artículos (20%) fueron considerados buenos o excelentes. Los efectos secundarios asociados a la RV más frecuentes fueron desorientación, náauseas y trastornos oculomotores, especialmente cuando se usaban con «head-mounted displays» comparados con las pantallas del ordenador/TV.
ConclusionesAunque la RV se usa cada vez más en diferentes intervenciones terapéuticas, la RV puede producir efectos secundarios. Para planificar una intervención terapéutica a través de RV, es importante conocer su seguridad y eficacia a través de estudios con una metodología científica adecuada.
The term Virtual Reality (VR) was first used in 1987 by Jaron Lanier, a computer scientist founder of the American company “Virtual Programming Laboratory Research”.1,2 VR allows the user to interact in a 3D virtual world using a screen that provides simulated vision, hearing, and touch.3 VR uses a virtual setting characterized by a changing scenery, which can be visualized by using helmets or Head Mounted Display (HMD), VR glasses, and other haptic devices.4,5 This technology allows the subject to interact and train with a series of lifelike scenes and objects, experiencing a multisensorial dynamic experience, prompting the feeling of being within a real-world inside a virtual one.5–7
The essential elements of VR are simulation, interaction, and immersion. Moreover, three types of VR are differentiated according to the degree of immersion and the stimulation of the sensory systems: fully immersive, semi-immersive, and non-immersive.6 The application of VR allows repeating functional and goal-oriented tasks,4 and can be used within various fields, such as (1) clinical psychology, focusing on the treatment of post-traumatic stress disorders,8 and cognitive rehabilitation,9 (2) neurological rehabilitation treating memory and attention disorders and facilitating spatial-temporal abilities and motor rehabilitation,10 and (3) leisure, entertainment and education.11,12
Though there is enough evidence related to the benefits and advantages rooted in VR usage,12 few authors have observed that prolonged and continuous exposure to VR could cause disturbances named “simulator sickness”, characterized by dizziness, vomiting, and sleepiness.5 In 1989, Baltzley et al.13 conducted a study analyzing the duration of the “simulator sickness” after using a flight simulation. They found that 45% of the subjects suffered side effects, and 25% felt these side effects one hour after finishing the test.13 In 1995, Kay Stanney used the term “cybersickness” to describe the side effects of using VR, including headaches, nausea, postural pains, disorientation, and instability.14 Other terms such as “technopathy” have been used for non-physical side effects of technology, including abusive use of the Internet and social networks.
In order to measure the severity of the side effects associated with VR, we can use validated rating scales such as the Pensacola Motion Sickness Questionnaire (MSQ),15 the Simulator Sickness Questionnaire (SSQ),16 and the Virtual Reality Symptom Questionnaire (VRSQ).17 The SSQ was created in 1993, and it is the most frequently used rating scale.18 The SSQ consists of 16 symptoms such as general malaise, fatigue, headache, eyestrain, difficulty focusing, increased salivation, sweating, nausea, difficulty concentrating, headedness, blurry vision, vertigo, upset stomach, open and closed-eyes dizziness, belches, grouped in three domains, including the Nausea (SSQ-N), Oculomotor Symptoms (SSQ-O), and Disorientation (SSQ-D) domains. Each side effect severity is measured using the Likert scale from 0 to 3 (0=absent, 1=minor, 2=mild, 3=serious), and total scores are obtained using a conversion formula (Table 1). The SSQ Total scoring ranges from 0 to 235.62, where the highest scores indicate the most severe disturbances, and for each domain, a score between 5 and 10 is considered as minimal symptoms, between 10 and 15 significant symptoms, between 15 and 20 worrisome symptoms, and more than 20 severe symptoms.16–19
A Simulator sickness questionnaire.
| SSQ symptom | Weights for symptoms | ||
|---|---|---|---|
| N | O | D | |
| General discomfort | 1 | 1 | |
| Fatigue | 1 | ||
| Headache | 1 | ||
| Eyestrain | 1 | ||
| Difficulty focusing | 1 | 1 | |
| Increased salivation | 1 | ||
| Sweating | 1 | ||
| Nausea | 1 | 1 | |
| Difficulty concentrating | 1 | 1 | |
| Fullness of head | 1 | ||
| Blurred vision | 1 | 1 | |
| Dizzy (eyes open) | 1 | ||
| Dizzy (eyes closed) | 1 | ||
| Vertigo | 1 | ||
| Stomach awareness | 1 | ||
| Burping | 1 | ||
| Totala | [1] | [2] | [3] |
| Score | |||
| N=[1]×9.54 | |||
| O=[2]×7.58 | |||
| D=[3]×13.92 | |||
| TS=[1]+[2]+[3]×3.74 | |||
Interestingly, despite the increasing usage of VR, little is known about the safety of this therapeutic tool. Therefore, we conducted a systematic literature review focused on identifying the most frequent physical side effects caused by VR therapeutic applications. This review did not include non-physical side effects such as addictions, anxiety, or social isolation related to VR.
MethodsDesign: This systematic literature review was conducted following the PRISMA guidelines.20
Search strategy: We carried out a systematic review of the Entrez Pubmed, Scopus, Science Direct, and Wiley databases. We performed the search using a combination of MeSH terms and keywords: “virtual reality” OR “head-mounted display”) AND (“cybersickness” OR “motion sickness,” “simulator sickness” OR “visually induced motion sickness,” OR “visual-vestibular” OR “nausea,” located in the title, abstract, or keywords.
Selection criteria: We included clinical trials in Medicine written in English or Spanish using VR as a therapeutic intervention with the HMD, assessing the severity of side events with the SSQ between January 2016 and September 2021. We excluded other applications such as healthcare providers’ training, single-case studies, systematic reviews, or meta-analyses.
Review process: Two of the following contributors (LS, EC) independently screened titles and abstracts to determine the eligibility of identified trial reports. Full texts were obtained if there was a need. As required, the study eligibility was resolved by discussion and adjudicated by the lead author (LS).
Quality assessment: We used the PEDro scale to evaluate the methodological quality and risk of bias.20 We obtained the PEDro rating score using the standard classification, ranging from 0 to 3 coded as poor or fair, indicating lack of methodological quality; 4 to 5, acceptable methodology; 6 to 8 good methodology; and 9 to 10 excellent methodology. In other instances, controversial opinions about study eligibility were resolved by discussion and adjudicated by the lead author (LS) as required.20
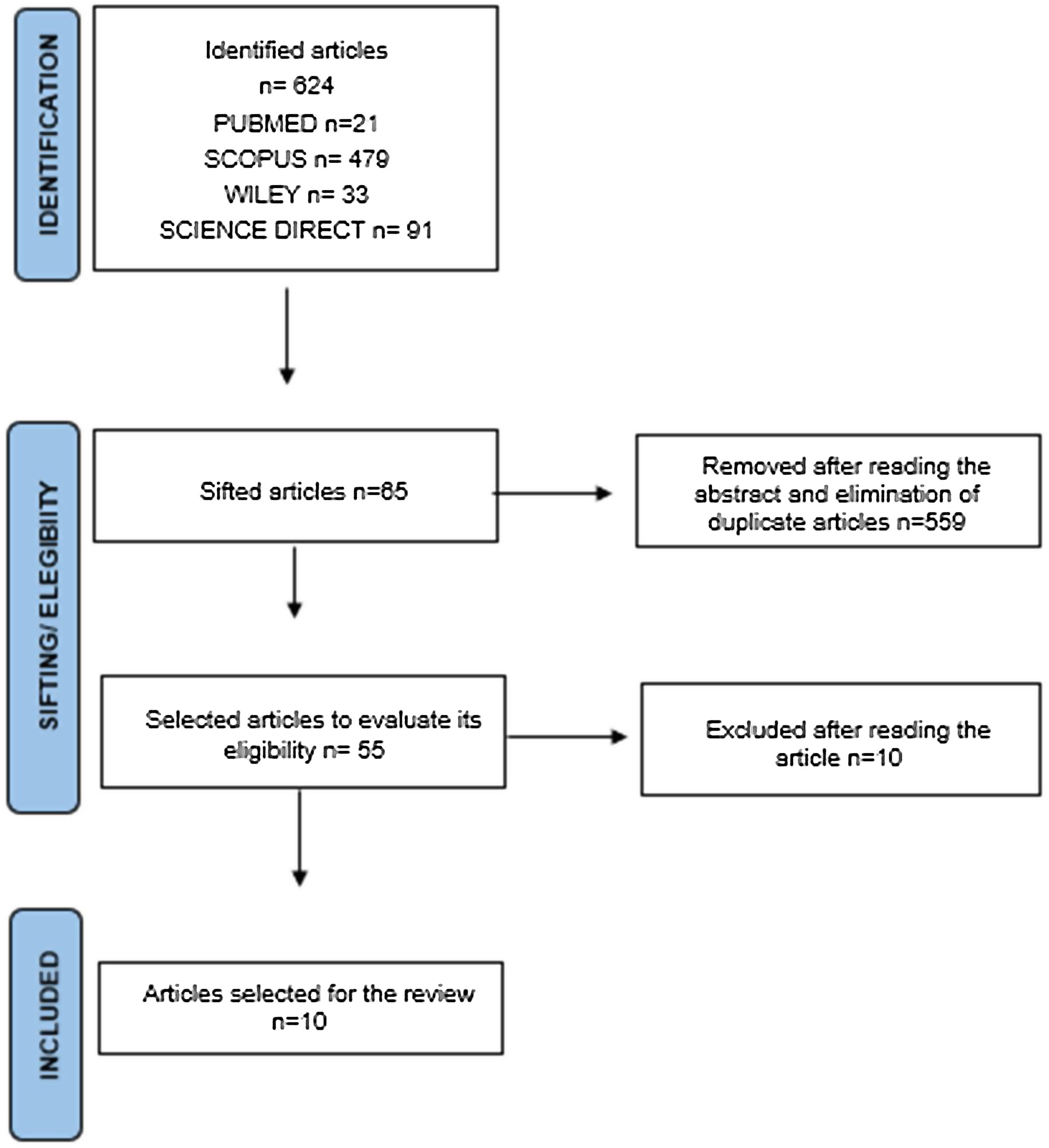
ResultsIn the first analysis stage, 624 articles were identified (Pubmed n=21, Scopus n=479, Wiley n=33, Science Direct n=91). Fig. 1 shows the flow diagram with the selection criteria and data extraction. During the second analysis stage, we read the full text of 55 articles, reducing the inclusion to 10 articles, based on the inclusion/exclusion criteria (Table 2). Two articles (20%) were considered good or excellent, and 8 (80%) were considered poor or fair.
Studies main characteristics.
| Reference | Year | Sample | Health condition | Type of design | Objectives and equipments | AgeMean (SD) | Exposing time (min) | SSQ-NMean (SD) | SSQ-OMean (SD) | SSQ-DMean (SD) | Total SSQMean (SD) | PEDro scale score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Guna et al.25 | 2019 | 15 (13M, 2W) | Healthy subjects | Open-label, with post-test assessment only. | To examine the effects of VR technology and VR video content type on VR sickness and on autonomic nervous system of the user. Equipment: head mounted display vs. desktop screens. | 27.2 (NA) | NA | 7 | ||||
| Device 1 | 26.08 (22.36) | 31.33 (23.25) | 55.68 (48.22) | 40.64 (29.20) | ||||||||
| Device 2 | 33.07 (30.99) | 34.87 (35.06) | 53.82 (55.64) | 44.63 (42.63) | ||||||||
| Device 3 | 38.80 (36.67) | 29.31 (28.63) | 51.04 (61.73) | 43.38 (43.33) | ||||||||
| Device 4 | 28.62 (28.62) | 34.36 (29.75) | 47.33 (49.29) | 40.89 (36.32) | ||||||||
| Device 5 | 5.088 (10.11) | 5.559 (10.11) | 4.640 (11.36) | 5.984 (10.27) | ||||||||
| Winter et al.23 | 2021 | 36 (10M, 26W) | Healthy subjects | Experimental design, with control group, pretest and posttest. | To evaluate an immersive VR application for supervised gait rehabilitation of patients with multiple sclerosis or stroke, to test its feasibility and acceptance and to compare its effects to those of a semi-immersive application and to a conventional treadmill training. Equipment: head mounted display vs. desktop screens. | 22 (NA) | 7.5 | 15.37 (11.47) | 12.84 (19.58) | 17.40 (26.07) | 17.04 (18.46) | 3 |
| 14 (6M, 8W) | Patients with multiple sclerosis/stroke | Experimental design, with control group, pretest and posttest. | 52.6 (NA) | 7.5 | 10.90 (12.88) | 11.37 (11.80) | 10.94 (14.63) | 12.82 (11.98) | ||||
| Maloca et al.22 | 2021 | 146 (60M, 84W) | Healthy subjects (children between 12 and 18 years old) | Open-label, with post-test assessment only | To assess children and young people's responses to an ophthalmological VR experience, with a focus on whether it can facilitate understanding, increases interest in science generally and is well-tolerated in this young population. Equipment: head mounted display. | 15 (NA) | 5–15 | 19.36 (NA) | 27.98 (NA) | 34.22 (NA) | 30.43 (30.20) | 3 |
| Yildirim16 | 2019 | 45 (22M, 23W) | Healthy subjects | Randomized clinical trial. | To examine the prevalence of cybersickness during a natural VR interaction, as opposed to a provocative environment designed to induce intense levels of cybersickness. Equipment: head mounted display vs. desktop screens. | 20.6 (2.26) | 10 | 66.78 (2.83) | 55.08 (8.83) | 77.95 (13.16) | 74.30 (7.26) | 9 |
| 55.33 (13.53) | 36.38 (9.13) | 57.54 (15.71) | 55.1 (12.70) | |||||||||
| Jaeseok Heo and Gilwon Yoon30 | 2020 | 17 | Healthy subjects | Open-label, with post-test assessment only. | To quantitatively measure changes in the human brain during VR gaming using EEG and identify the correlations with discomfort symptoms observed after using VR. Equipment: head mounted display. | 26.4 (NA) | NA | 81.10 (54.50) | 69.50 (44.80) | 133.4 (95.2) | 102.4 (2.10) | 2 |
| Risi and Palmisano26 | 2019 | 20 (15M, 5W) | Healthy subjects | Experimental design, with control group, pre-test and post-test. | To examine whether it is possible to: (1) identify people who are more susceptible to this cybersickness; and (2) find general ways to reduce its occurrence and severity. Equipment: head mounted display. | 26.5 (6.9) | 10 | 29.6 (36.6) | 18.9 (30.7) | 35.3 (50.9) | 30.36 (NA) | 3 |
| Shuchisnigd-Deb et al.24 | 2017 | 21 (10M, 11W) | Healthy subjects | Experimental design, with control group, pre-test and post-test. | To confirm that pedestrians will be able to perceive the virtual world as a realistic environment and that they will behave in the simulated world in a way that relatively matches their behavior in the real world. Equipment: head mounted display. | Men 27.84women 29.95 | 35 with 2 breaks (at 15 and 25min) | 8.63 (11.65) | 15.16 (16.95) | 11.93 (19.33) | 14.07 (16.03) | 3 |
| Teixeira and Palmisano27 | 2020 | 38 (25M, 13W) | Healthy subjects | Experimental design, with control group, pre-test and post-test. | To examine the effects of dynamic field-of-view (FOV) restriction on the cybersickness generated by ecological HMD-based gameplay. Equipment: head mounted display. | 22.55 (2.95) | Three periods of 10min each with a 10-min break between each other. | 14.70 (19.24) | 13.92 (19.21) | 16.30 (27.18) | 16.98 (NA) | 3 |
| Grasinni et al.22 | 2021 | 54 (21M, 33W) | Healthy subjects | To update and extend the current literature on the individual factors associated with simulator sickness (SS) and the sense of presence. Equipment: head mounted display. | 23.91 (3.31) | 3 | 60.77 (36.09) | 41.83 (32.15) | 105.43 (68.49) | 72.79 (44.44) | 4 | |
| Clifton et al.28 | 2019 | 25 (13M, 12W) | Healthy subjects | Experimental design with control group pre-test and post-test. | To compare the presence, cybersickness and perceptions of selfmotion (or “vection”) induced when using two common methods of virtual locomotion: steering locomotion and teleporting. Equipment: head mounted display. | 23.92 (5.25) | 16 | 21.66 (NA) | 27.44 (NA) | 35.77 (NA) | 31.63 (NA) | 3 |
NA=not available.
This review included a sample of 416 subjects, 181 (44%) men, 217 (52%) women, and 18 (4%) with no sex specified, with an average age of 24.54 years old ranging from 15 to 52.6years old. Nine articles (90%) included healthy subjects, primarily adults, and one article (10%) included a sample suffering from multiple sclerosis and stroke. In seven articles (70%), VR was used with HMD, and in three articles (30%) combined with desktop screens (Table 2).
Regarding the severity of side events, we found the highest scores in the SSQ-D domain, followed by the SSQ-N and the SSQ-O domains. Within the SSQ symptoms, the most frequent disturbances were concentration difficulties, nausea, headedness, blurry vision, dizziness, and vertigo (Table 2).
DiscussionIn this systematic review of the literature, we found that the quality of the information about side effects related to VR was limited. Most of the safety information was obtained from healthy adult populations. The most frequent adverse events after a VR exposition were disorientation, followed by nausea and oculomotor disturbances.15–22 Although the etiopathogenic mechanisms of these side effects are still unknown, it is hypothesized that VR could cause a conflict between sensory and spatial integration, as the subject receives movement signals as existing, but the vestibular system oppositely indicates there is no movement taking place.16
In terms of age, most of the studies have been carried out on young adults with an average age between 20 and 30years old, except for the studies by Winter et al.23 with an average of 52.6years old, and Maloca et al.22 with an average age of 15years old. Interestingly, whereas young populations reported more frequently fatigue, headedness, and eye strain after using VR,22 older subjects,23 reported fewer side effects in the SSQ-O, and SSQ-D domains, except in the SSQ-N dimension.24,25
Regarding the association between VR types of equipment and the frequency of side effects, Yildirim et al.15 compared the side effects after using two VR headsets against a desktop computer screen. These authors found the use of these two VR headsets was associated with more side effects compared to the desktop computer screen (mean total SSQ scores VR headsets of 74.30 vs. 55.1, respectively), mainly from the SSQ-D and N domains, compared to those using a desktop computer screen (mean total SSQ score of 22.94). Instead, Guna et al.25 analyzed the side effects of VR technology and VR video content type on VR sickness and the participant's autonomic nervous system (ANS), such as skin conductance and temperature, respiratory frequency, and heart rate. They found that VR significantly affected the skin conductance level, and VR sickness effects were significantly reported less often with a TV display type than compared to other VR HMDs.25
The frequency and duration of the adverse events related to VR are still controversial. Whereas Risi et al.,26 Joel Teixeira et al.,27 Clifton et al.28 reported that after the exposition to VR, disorientation became more frequently followed by nausea and oculomotor side events, Deb et al.24 found the oculomotor side effects were the most frequent ones. These differences between the different studies could be explained by factors including the inclusion of different populations with different demographic characteristics and underlying clinical conditions, the use of different devices, VR contents, exposition time, and characteristics of the demanded task.16,26,29,30 For instance, Guna et al.25 compared cybersickness right after visualizing a VR neutral content, such as being on a beach, which generates neutral or pleasing emotions, with the one after an action content, such as being on a roller coaster, which generates exciting emotions. These authors found higher severity, especially disorientation after facing action emotions, compared to using neutral or pleasing VR contents.25 In terms of the impact of VR exposition and the likelihood of causing side effects, whereas some studies found a positive correlation of the duration of the VR exposition with side events severity,29–31 other studies found an adaptation process after repetitive VR increasing exposition time.29–31. When the VR exposition time was under 10min,21,22 disorientation was higher than those participants having a VR exposition time equal to or over 10min.15,26–28
Although this systematic literature review has an important value in drawing the profile of VR side effects mainly in healthy, adult subjects, we are aware of the limitations, including selection bias, and lack of detailed information about the side events’ severity and duration. The results of this systematic review cannot be extrapolated to other populations, such as patients with different medical conditions, subjects of extreme ages, or different RV exposures.
ConclusionVR is a growing technology with promising extensive applications in different areas such as Medicine, entertainment, sports, gaming, and simulation. Although VR has positive effects as a therapeutic tool, VR can also cause side events. As in any other therapeutic intervention, it is important to understand the VR effectiveness and safety before planning a VR intervention. We need further randomized, longitudinal studies using VR as a new and promising therapeutic tool in Medicine.
FundingThe author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was co-financed by the Erasmus+ program of the European Union through the 2018-1-ES01-KA201-050659 project. Accepting funding from the European Commission for the preparation of this publication does not imply the European Commission's acceptance of its contents, which is the authors’ sole responsibility. Therefore, the European Commission is not responsible for the use that may be made of the information disclosed here.
Conflicts of interestNone declared.
We want to thank the research team of the European FORDYSVAR Erasmus+ project as well as the associations and institutions involved for their work.