Chronic exposure to low doses of ozone causes oxidative stress and loss of regulation of the inflammatory response, leading to progressive neurodegeneration.
ObjectiveWe studied the effect of chronic exposure to low doses of ozone on IL-17A concentration and expression in neurons, microglia, astrocytes, and T cells in the rat hippocampus.
MethodsWe used 72 Wistar rats, divided into 6 groups (n=12): a control group (no ozone exposure) and 5 groups exposed to ozone (0.25ppm, 4h daily) for 7, 15, 30, 60, and 90 days. We processed 6 rats from each group to quantify IL-17A by ELISA; the remaining 6 were processed for immunohistochemistry (against IL-17A and GFAP, Iba1, NeuN, and CD3).
ResultsThe ELISA study data showed a significant increase in IL-17A concentrations in the 7-, 15-, 30-, and 60-day exposure groups, with regard to the control group (P<.05). Furthermore, they indicate that hippocampal neurons were the cells showing greatest immunoreactivity against IL-17A between 60 and 90 days of exposure to ozone; we also observed an increase in activated astrocytes in the 30- and 60-day exposure groups.
ConclusionExposure to ozone in rats induces an increase in IL-17A expression, mainly in hippocampal neurons, accompanied by hippocampal astrocyte activation during chronic neurodegeneration, similar to that observed in Alzheimer disease in humans.
La exposición crónica a bajas dosis de ozono causa un estado de estrés oxidativo y pérdida de la regulación de la respuesta inflamatoria, lo cual lleva a un proceso de neurodegeneración progresiva.
ObjetivoEstudiar el efecto de la exposición crónica a bajas dosis de ozono sobre la concentración de IL-17A y su expresión en neuronas, microglía, astrocitos y células T en hipocampo de ratas.
MétodosSe utilizaron 72 ratas Wistar, divididas en 6 grupos (n=12): control (sin ozono) y expuestos a ozono (0,25ppm, 4h diarias) durante 7, 15, 30, 60 y 90 días, respectivamente. Seis sujetos de cada grupo fueron procesados para cuantificar IL-17A por ELISA y los 6 restantes para inmunohistoquímica (frente a IL-17A y GFAP, Iba1, NeuN o CD3).
ResultadosLos datos obtenidos por el ELISA mostraron un incremento significativo en las concentraciones de IL-17A en los grupos de 7, 15, 30 y 60 días de exposición, comparados con el control (p<0,05). Los resultados muestran que las neuronas del hipocampo son las células con una mayor inmunorreactividad frente a IL-17A entre los 60 y 90 días de exposición a ozono; además, se observó un aumento de astrocitos activados en los grupos de 30 y 60 días de exposición.
ConclusiónLa exposición a ozono induce un incremento en la expresión de la IL-17A, principalmente en las neuronas hipocampales, acompañado de una activación de astrocitos en el hipocampo de ratas durante el proceso de neurodegeneración progresiva, similar a lo que ocurre en la enfermedad de Alzheimer en humanos.
Air pollution is an important risk factor for several chronic health problems in heavily populated cities.1 Ozone is one of the most significant air pollutants due to its abundance and toxicity. It is formed in the troposphere, through oxidation of volatile organic compounds and carbon monoxide in the presence of nitrogen oxides and sunlight.2 Chronic inhalation of low doses of ozone is associated with the development of chronic/degenerative diseases; it has also been shown to play an important role in such neurodegenerative diseases as Parkinson's disease and Alzheimer disease.3,4 It is well demonstrated that chronic exposure to low doses of ozone causes a state of oxidative stress and irreversible progressive neurodegeneration in animal models.5 This process is accompanied by the loss of regulation of the inflammatory response.3,5,6
Both chronic oxidative stress and the loss of regulation of the inflammatory response are critical factors for the development and progression of the neurodegenerative process.7 In the event of acute central nervous system (CNS) damage, circulating leukocytes are recruited to the CNS and participate in the resolution of the innate inflammatory response.8 However, chronic damage and non-resolution of this response may lead to neuroinflammation associated with neuronal death.8 Both oxidative stress and the loss of regulation of the inflammatory response induce abnormal production of proinflammatory cytokines, chemokines, growth factors, and reactive oxygen species, as well as microglial, astrocyte, and immune cell activation, creating a vicious circle that prevents the self-limitation of the response.6,9
Interleukin 17A (IL-17A) is strongly associated with the progression and development of several neurodegenerative diseases.6,10 IL-17A induces disruption of the tight junctions of the blood-brain barrier; furthermore, it acts as a proinflammatory mediator, inducing the expression of other cytokines (IL-6, transforming growth factor beta, and tumour necrosis factor) and chemokines (IL-8) in some cell populations.11 Th17 cells have classically been identified as the main IL-17A producing cells; however, it has been reported that IL-17A is also expressed by a great variety of cell populations, such as γδ T cells and natural cytotoxic cells (natural killer cells), in addition to glial cells.11
Recently, our working group reported a short-term, systemic Th17/IL-17A inflammatory effect in a mouse model of Alzheimer disease; this effect was observed during the early stages of exposure to low doses of ozone, when the damage caused by this gas is reversible. However, the Th17/IL-17A response notably decreased as the exposure to ozone continued to progress and the neurodegenerative process became irreversible; in these conditions, immunoreactivity to IL-17A was located in the hippocampus.6 This study shows that the oxidative stress caused by ozone exposure first induces a systemic inflammatory response and then a hippocampal inflammatory response, manifesting during the irreversible progressive neurodegenerative process. Despite the above mentioned studies, it remains unclear which is the main IL-17A–expressing cell population in the hippocampus.
The aim of this study was to measure the increase in IL-17A concentration and to determine the immunoreactivity of this cytokine in different hippocampal cell populations in rats chronically exposed to low doses of ozone.
MethodsAnimalsWe used 72 male Wistar rats from the vivarium at the medical school at Universidad Nacional Autónoma de México, weighing 250–300g; animals were housed in individual acrylic cages with free access to food and water (NutriCubo, Purina, USA). Animals were kept in a vivarium at controlled temperature and humidity conditions. They were cared for and handled in accordance with the National Institute of Health Guidelines for Animal Treatment and the official Mexican guidelines (NOM-062-SSA-2-2002). The study was approved by the research ethics committee of the School of Medicine at Universidad Nacional Autónoma de México.
General procedureRats were randomly assigned to one of 6 experimental groups (n=12 per group). The control group was exposed to an ozone-free air flow for 4hours daily for 30 days, and groups 2, 3, 4, 5, and 6 were exposed to ozone (0.25ppm) for 4hours daily for 7, 15, 30, 60, and 90 days, respectively.6
Ozone exposureRats were placed inside a chamber with a diffuser connected to an ozone generator with variable output (5L/s). The concentration of ozone produced by the generator was proportional to air intensity and flow. We used a PCI Ozone and Control System monitor (West Coldwell, USA) to measure ozone concentration inside the chamber. To expose the control group to ozone-free air, we used the same chamber, connected to an ozone-free air flow.
After completing the period of air or ozone exposure, all 12 animals from each group were deeply anaesthetised with pentobarbital sodium (50mg/kg), and 6 were perfused with 4% paraformaldehyde. Brains were removed and placed in the same fixative solution at 4°C for 24hours. Conventional histological methods were subsequently used to obtain paraffin-embedded tissue. Brains were cut into 5-μm sagittal slices, and the hippocampal sections were used in the immunofluorescence study. The remaining 6 rats from each group were used to obtain supernatant fluids; hippocampal tissue was cryopreserved at −70°C and then used for IL-17A quantification by ELISA.6
Processing of hippocampal tissue for interleukin 17A quantificationPreviously cryopreserved hippocampal samples were thawed on ice. Samples were then homogenised mechanically in an extraction buffer (50mM, phosphate-buffered saline [PBS] NaCl 0.15M; pH 7.4) and a mixture of protease inhibitors. The homogenate was centrifuged for 20minutes at 11 000 rpm and at a temperature of 4°C; the supernatant was then removed. Total protein concentration of all supernatants was measured with a commercial BCA kit (Thermo Scientific; Rockford, IL, USA).
Quantitative analysis of hippocampal interleukin IL-17A levelsWe used equivalent amounts of proteins to quantify IL-17A by ELISA with the commercial Rat IL-17A ELISA MAX™ Deluxe kit (Biolegend; San Diego, CA, USA). We analysed 6 samples per group, and included a duplicate for each sample. Standard curves were prepared with premixed standards included in the kit. The absorbance of each sample was automatically obtained with an Epoch spectrophotometer (BioTek; Winooski, VT, USA) at a wavelength of 450nm.
Double immunohistochemical study for interleukin IL-17A in neurons, microglia, astrocytes, and T cells in the hippocampusAll double-labelling immunohistochemical studies of hippocampal sections were performed using a rabbit polyclonal anti-IL-17A antibody and various monoclonal mouse antibodies (against Iba1, GFAP, NeuN, or CD3) to identify different cell populations. Prior to this analysis, hippocampal tissue specimens were deparaffinised with xylene and rehydrated. Sections were then washed with a PBS solution (50mM PBS, 0.15M NaCl; pH 7.4) and incubated for 30minutes in 2% bovine serum albumin (fatty acid free, fraction V from MP Biomedical, LLC; Darmstadt, Germany). Samples were impregnated with 0.2% Triton™ X-100 in PBS for 10minutes, and subsequently incubated overnight at 4°C with rabbit polyclonal anti-IL-17A antibody (eBioscience; USA).6 Samples were then washed 3 times with 0.2% Triton™ X-100 in PBS for 10minutes, and the second antibody was added: anti-Iba1 (GeneTex; USA) to identify microglial cells, anti-GFAP (GeneTex; USA) to identify astrocytes, anti-NeuN (GeneTex; USA) to identify mature neurons, or anti-CD3 (BD Biosciences; USA) to identify T cells; the same procedure was then repeated. After washing the slides, samples were incubated with Alexa Fluor® 594 goat anti-rabbit IgG antibody (Life Technologies; USA), labelled with red fluorescence, to observe immunoreactivity to IL-17A. To distinguish the second antibody, which identifies cell populations, we used the Alexa Fluor® 488 goat anti-mouse IgG secondary antibody (Life Technologies; USA), which was labelled with green fluorescence.
Samples were placed on glass slides with Vectashield mounting medium (Vector Laboratories; USA). Representative brain sections from each group were examined with an Olympus BX41 microscope (USA) and photographed with an Evolution VF camera (Media Cybernetics; USA). Fluorescence channels were merged using the ImageJ software for Mac (NIH; USA).
Statistical analysisStatistical analysis was performed using R 3.3.2 for Mac. The Shapiro–Wilk test was used to test for normality.
Having confirmed the normality of the data from the different groups, we used the parametric ANOVA test to identify inter-group differences and the t test for post-hoc analysis. Data were expressed as mean and standard error of the mean. Statistical significance was set at P<.05.
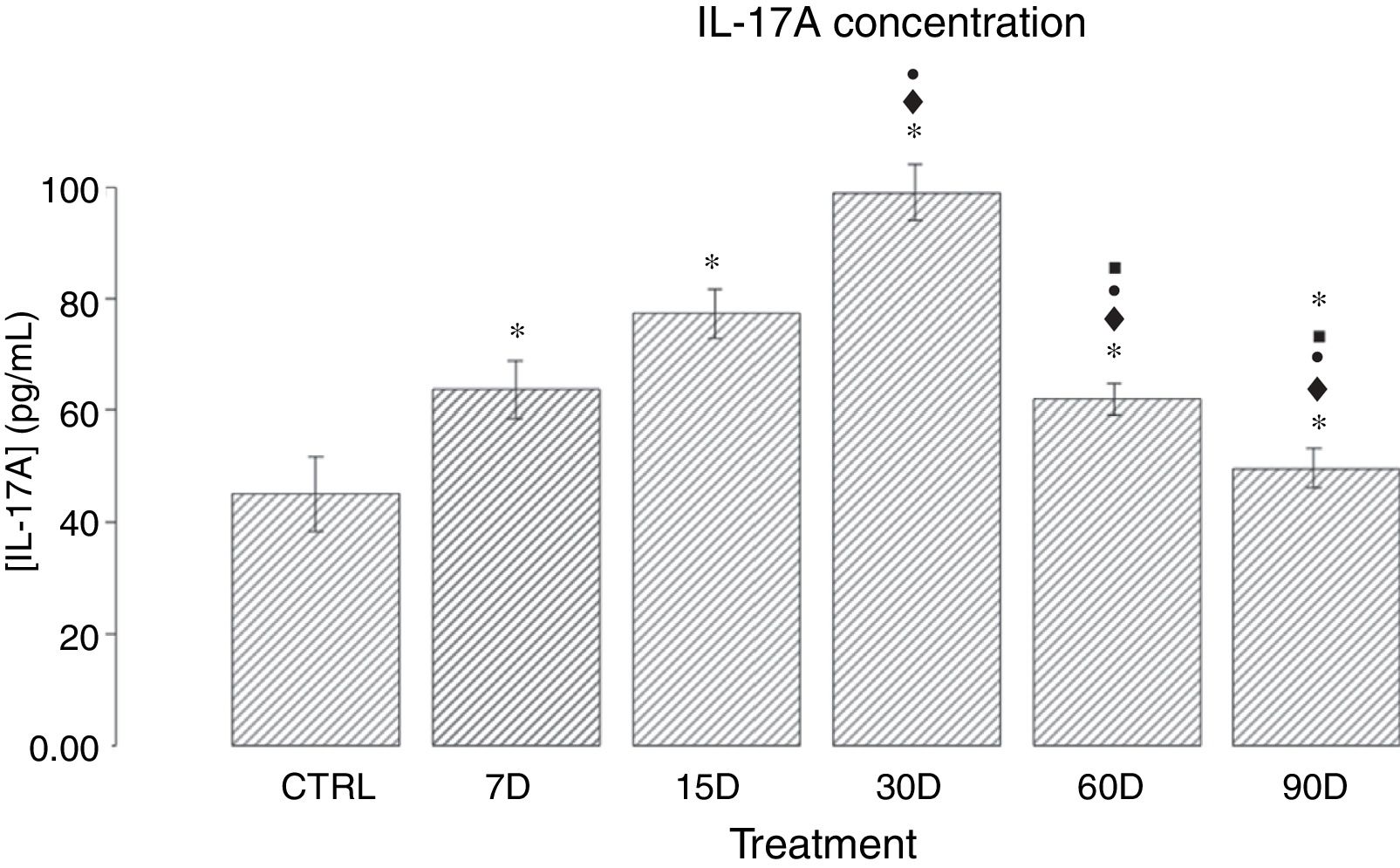
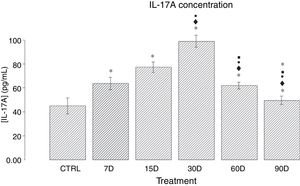
ResultsIL-17A concentration in the hippocampal tissue of rats chronically exposed to low doses of ozoneFig. 1 shows the quantitative analysis of IL-17A concentration in the different study groups. We observed that the groups exposed to ozone for 7, 15, 30, and 60 days presented a significant increase in IL-17A levels when compared to the control group (P<.05). The results also show an increase in IL-17A expression between 7 and 30 days of ozone exposure; the group exposed for 30 days presents the highest expression, and a significant increase with regards to all other groups (P<.05). Furthermore, we observed a gradual decrease in IL-17A concentration in the groups exposed for 60 and 90 days.
Effect of chronic exposure to low doses of ozone on IL-17A concentration in the rat hippocampus. The x-axis shows the treatments used: control (CTRL: rats exposed to an ozone-free air flow for 30 days) and ozone exposure (7, 15, 30, 60, and 90 days). The y-axis shows IL-17A concentration (pg/mL). Bars represent the mean concentration for each group and the standard error of the mean. Groups exposed to ozone showed significant differences in comparison to the control group (P<.05). Significant differences were observed for the following comparisons: *: study group vs controls; ♦: study group vs group exposed to ozone for 7 days; •: study group vs group exposed to ozone for 15 days; ■: study group vs group exposed to ozone for 30 days; ★: study group vs group exposed to ozone for 60 days. Statistical significance was set at P<.05.
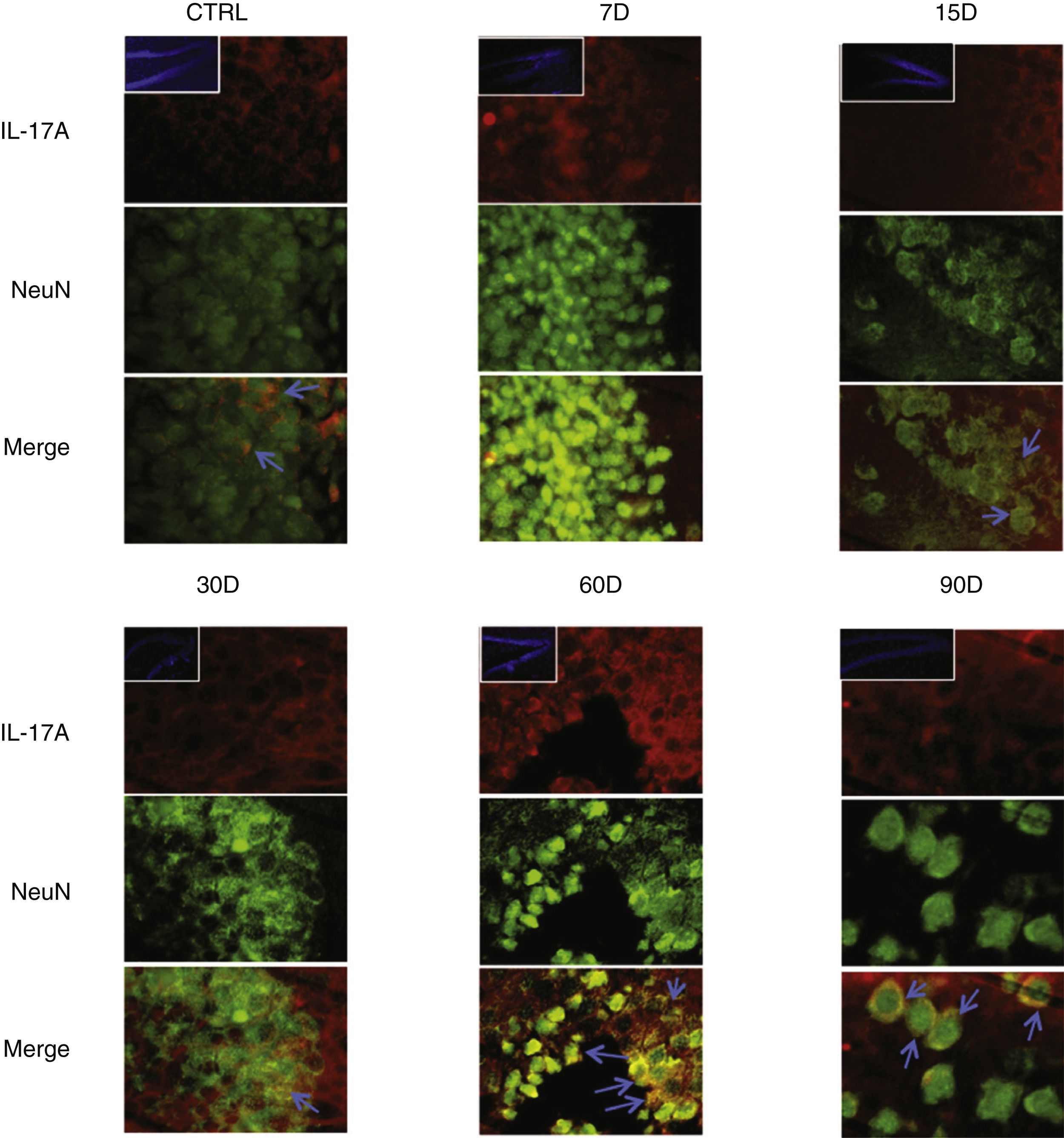
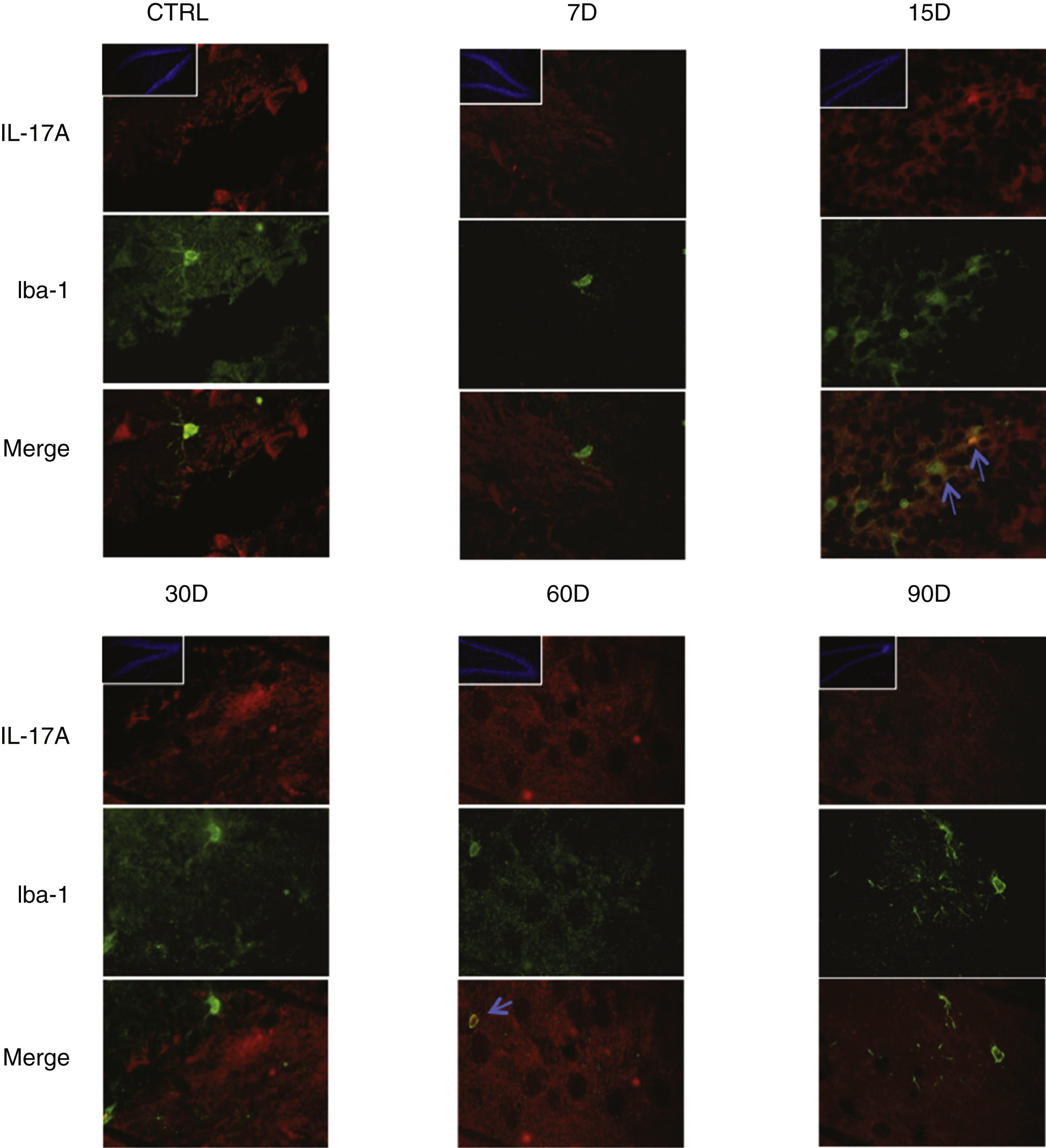
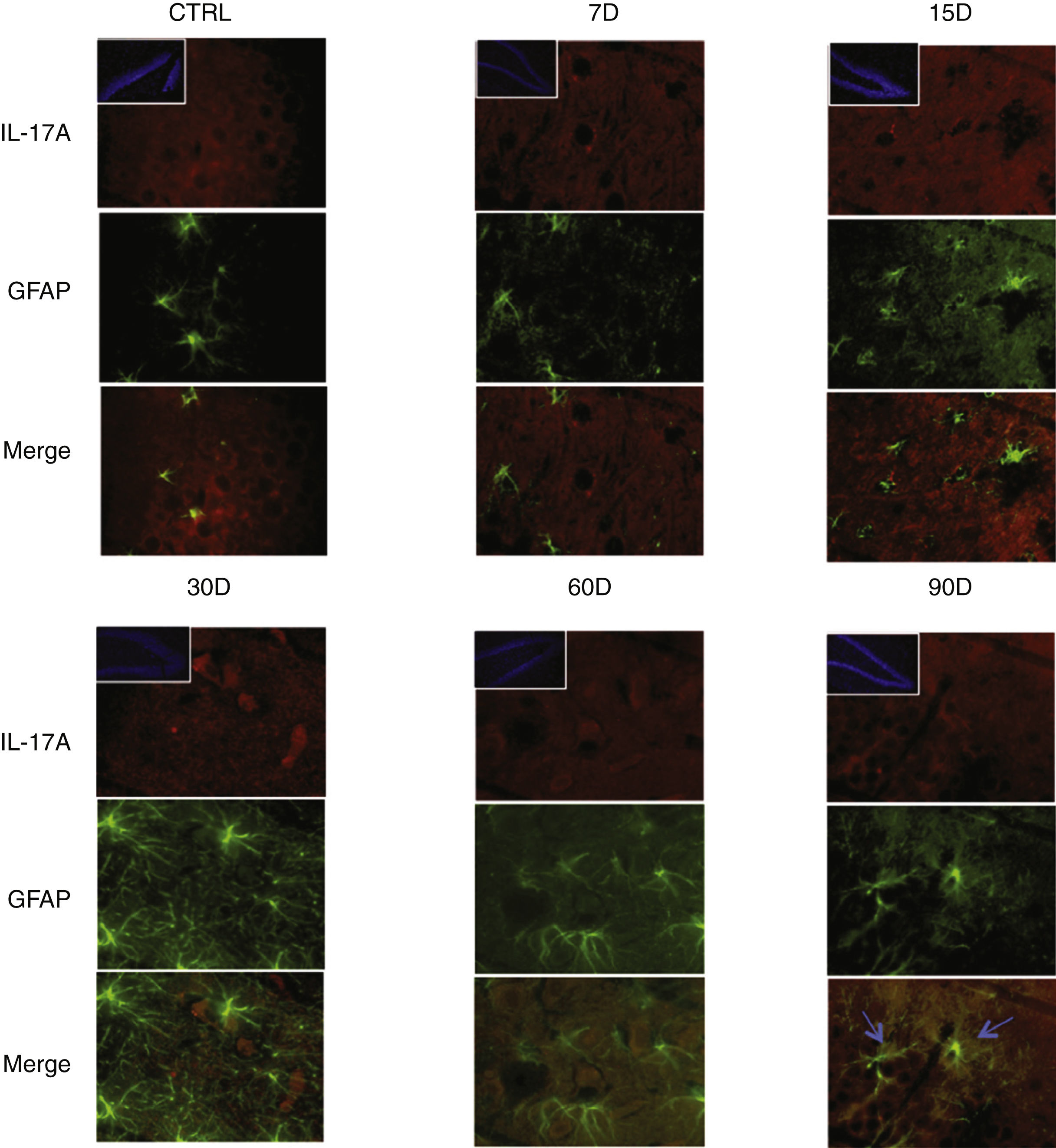
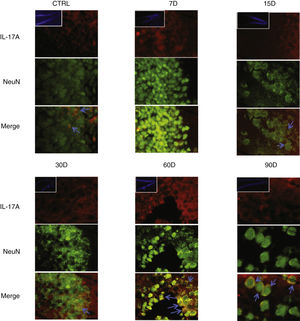
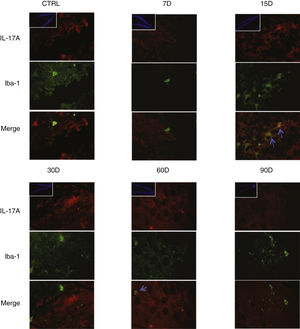
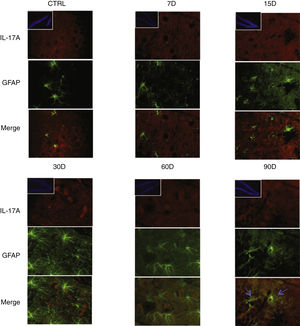
Immunoreactivity results, shown as double-labelled fluorescence for IL-17A in neurons, microglia, astrocytes, and T cells in the rats’ hippocampi, suggest that immunoreactivity to IL-17A predominates in different cell populations depending on the time of exposure: neurons (Fig. 2) at 60 and 90 days, astrocytes (Fig. 3) at 90 days, and microglia (Fig. 4) at 15 days. Immunofluorescence images of T cells (CD3+) are not reproduced here, as we found very few or no T cells in the dentate gyrus, regardless of the duration of exposure to ozone; neither did we identify an association between these cells and IL-17A expression in this hippocampal structure. Furthermore, the immunofluorescence images are consistent with the quantitative analysis of IL-17A levels, as they show increased expression of IL-17A in the groups exposed to ozone for 15, 30, and 60 days.
Effect of chronic exposure to low doses of ozone on IL-17A expression in neurons. Double-labelled fluorescence analysis of the hippocampus from rats exposed to low doses of ozone for 7, 15, 30, 60, and 90 days. Neurons are labelled in green and IL-17A in red. The main images show the dentate gyrus at 100× magnification; boxes show the same structure at 10× magnification. Blue arrows indicate marker colocalisation. Note that neurons in the dentate gyrus present higher immunoreactivity to IL-17A at 60 and 90 days of exposure. Scale bar=20microns.
Effect of chronic exposure to low doses of ozone on IL-17A expression in astrocytes. Double-labelled fluorescence analysis of the hippocampus from rats exposed to low doses of ozone for 7, 15, 30, 30, 60, and 90 days. Astrocytes are labelled in green and IL-17A in red. The main images show the dentate gyrus at 100× magnification; boxes show the same structure at 10× magnification. Blue arrows indicate marker colocalisation. Note that astrocytes in the dentate gyrus present higher immunoreactivity to IL-17A at 90 days of exposure. Note also the increase in the number of astrocytes and the length of their projections from 15 to 90 days of exposure. Scale bar=20microns.
Effect of chronic exposure to low doses of ozone on IL-17A expression in microglia. Double-labelled fluorescence analysis of the hippocampus from rats exposed to low doses of ozone for 7, 15, 30, 60, and 90 days. Microglial cells are labelled in green and IL-17A in red. The main images show the dentate gyrus at 100× magnification; boxes show the same structure at 10× magnification. Blue arrows indicate marker colocalisation. Note that microglia in the dentate gyrus present higher immunoreactivity to IL-17A at 15 days of exposure. Scale bar=20microns.
Fig. 3 also shows a greater number of cells expressing Iba1 (microglia) after 15 days of exposure to ozone. We also observed an increase in the number of astrocytes (Fig. 4) in the groups exposed to ozone for 15, 30, and 60 days. Furthermore, we also observed more extensive astrocyte filaments in the above mentioned groups.
DiscussionIn this study, we quantified IL-17A concentrations in the hippocampi of rats chronically exposed to low doses of ozone. Results show a gradual increase in IL-17A until 30 days of exposure, with this group displaying the highest IL-17A concentration; in line with previous reports from our working group,5 the neurodegenerative process becomes irreversible after this point. The fact that the neurodegenerative process develops in parallel with the increase in hippocampal IL-17A concentration suggests that this cytokine is a factor present during this process. In this line, Sun et al.12 reported that after a challenge with lipopolysaccharide, levels of IL-17A, microglial activation, and cognitive damage increased in the hippocampi of aged rats. However, it is unclear whether IL-17A is a contributing factor or a consequence of the neurodegenerative process.
The findings of our analysis with double-labelled fluorescence corroborate the hypothesis that T cells are not the main cell type secreting IL-17A in the hippocampi of rats exposed to ozone, and that there is not significant flow of these cells to the hippocampus. A study by our working group found a weak correlation between systemic IL-17A secretion and Th17 cells6; therefore, we may hypothesise that cells other than Th17 may be involved in secretion of this cytokine. For this reason, this study aimed to identify which type of cells constitute the main source of IL-17A in the dentate gyrus of rats chronically exposed to low doses of ozone. In this regard, our data suggest that IL-17A is expressed in neurons, astrocytes, and microglia in the dentate gyrus of rats chronically exposed to low doses of ozone; however, this expression is dependent on exposure time and the degree of neurodegeneration. One of the most important findings of our study is that the data suggest a coexpression of NeuN and IL-17A, especially in the groups exposed to ozone for 60 and 90 days. In a previous study, our working group reported that plasma IL-17A increased in the initial stages of exposure to low doses of ozone; however, in the advanced stages and after progressive neurodegeneration becomes irreversible, IL-17A concentration significantly decreases in the plasma but increases in the hippocampus.6 This shows that during the irreversible progressive degeneration, where the inflammatory response is organ-specific, neurons are the main source of the IL-17A present in the hippocampus. Despite the fact that neurons have not classically been characterised as cytokine-secreting cells, some authors do not rule out this possibility.13,14 A study by Schubert et al.,14 which characterised the cytokine-secreting profile of neurons and neuronal precursor cells, found these cells to secrete a greater number and variety of proteins than glial cells. These authors also reported that several of the molecules secreted by neurons belonged to the family of multifunctional stress proteins associated with protein folding, signalling, and cytoprotection inside cells. Although this study evaluated expression of a wide variety of molecules, no cytokine was included; however, it shows that the secretion profile of neurons goes beyond what has been classically described. Therefore, we should not rule out the possibility that in the context of chronic oxidative stress, neurons are capable of paracrine secretion of such cytokines as IL-17A, and therefore provide inflammatory feedback to the CNS.
Finally, we observed that the gradual increase in IL-17A concentration coincides with a higher number of astrocytes, as well as an increase in the size of their intermediate filaments. This is consistent with the results obtained by You et al.,15 who showed that IL-17A can induce astrocyte reactivity, which is characterised by hypertrophy of these cells and high GFAP expression.16
ConclusionsOur results show that exposure to low doses of ozone gradually increases IL-17A expression in the rat hippocampus, coinciding with the onset of an irreversible process of progressive neurodegeneration. The tissue changes induced by IL-17A in the dentate gyrus occur as part of the loss of regulation of the inflammatory response that accompanies the progressive neurodegenerative process.
Furthermore, the main source of IL-17A changes with exposure time; however, our most relevant finding is that neurons represent the main source of IL-17A when the neurodegenerative process has become irreversible. The data also suggest that IL-17A may be associated with an increase in astrocyte reactivity, which favours the development of the neurodegenerative process.
FundingThis research project was funded by the General Directorate of Academic Personnel Affairs (grant no. IN221417, awarded to SRA).
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank Gabino Borgonio-Pérez for his assistance with the handling of the laboratory animals and with sample collection.
Please cite this article as: Solleiro-Villavicencio H, Hernández-Orozco E, Rivas-Arancibia S. Efecto de la exposición a bajas dosis de ozono en la expresión de la interleucina 17A durante el proceso de neurodegeneración progresiva en el hipocampo de ratas. Neurología. 2021;36:673–680.