Manual therapy has been shown to reduce self-reported symptoms in patients with chronic tension-type headache (CTTH). However, simultaneous application of suboccipital muscle inhibition and interferential current has not previously been investigated. This study evaluates the effectiveness of combined treatment with suboccipital muscle inhibition and interferential current compared to standard treatment for pain, disability, and headache impact in patients with CTTH.
MethodsPatients were randomly allocated to receive either standard treatment (n = 13) or the experimental treatment (n = 12), consisting of 20 minutes of suboccipital muscle inhibition plus interferential current twice weekly for 4 weeks. The primary outcome was improvement in pain, and secondary outcomes included improvement in headache-related disability and reduction in headache impact, which were assessed at baseline and at 4 weeks by a blinded rater.
ResultsStatistical analysis showed improvements in the experimental treatment group at 4 weeks for headache-related disability (Neck Disability Index: Hedges’ g = 1.01, P = .001; and Headache Disability Inventory: Hedges’ g = 0.48, P = .022) and headache impact (6-item Headache Impact Test: Hedges’ g = 0.15, P = .037) but not for self-reported pain (numerical rating scale: Hedges’ g = 1.13, P = .18).
ConclusionsCombined treatment with suboccipital muscle inhibition and interferential current in patients with CTTH did not significantly improve self-reported pain but did reduce disability and the impact of headache on daily life at 4 weeks. These improvements exceed the minimum clinically important difference, demonstrating the clinical relevance of our findings.
Se ha demostrado que la terapia manual reduce los síntomas autoreportados en pacientes con cefalea tensional crónica (CTC). Sin embargo, la aplicación simultánea de la técnica de inhibición muscular suboccipital y corriente interferencial no se ha investigado previamente. Este estudio evalúa la efectividad de la inhibición muscular suboccipital y la corriente interferencial en comparación con los cuidados habituales sobre el dolor, la discapacidad y el impacto de la cefalea en pacientes con CTC.
MétodosLos pacientes se asignaron al azar al grupo de cuidados habituales (n = 13) o experimental (n = 12) que consistió en 20 minutos de inhibición muscular suboccipital y corriente interferencial dos veces por semana durante cuatro semanas. El resultado primario fue el dolor, y los resultados secundarios incluyeron la discapacidad producida por el dolor de cabeza y el impacto del dolor de cabeza que se valoraron por un evaluador cegado al inicio y después de cuatro semanas.
ResultadosLos análisis mostraron diferencias entre los grupos a favor del grupo experimental a las cuatro semanas para la discapacidad producida por el dolor de cabeza (Neck Disability Index: g-Hedges = 1,01; p = 0,001; Headache Disability Inventory: g-Hedges = 0,48; p = 0,022) e impacto del dolor de cabeza (HIT-6: g-Hedges = 0,15; p = 0,037) pero no para el dolor autoreportado (Numerical Rating Scale: g-Hedges = 1,13; p = 0,18).
ConclusionesLa aplicación simultánea de inhibición muscular suboccipital y corriente interferencial en pacientes con CTC no reduce significativamente el dolor autoreportado a las cuatro semanas. Sin embargo, mejora la discapacidad y el impacto del dolor de cabeza en la vida diaria. Estas mejoras superaron el mínimo cambio clínicamente importante de las mediciones, destacando su relevancia clínica.
Chronic tension-type headache (CTTH) is characterised by recurrent episodes of headache occurring on 15 or more days per month for at least 3 months (≥ 180 days per year). Pain is usually pressing or tightening (non-pulsating), bilaterally located, of mild to moderate intensity, and not aggravated by routine activity.1
The pathogenesis of CTTH remains unclear. Some authors have suggested pathophysiological theories based on central or peripheral sensitisation.2 Impairment of the musculoskeletal function of the head and neck has been observed, as well as forward head posture, impaired neck mobility, or activation of myofascial trigger points in the muscles of the suboccipital region.2–4 Although treatment strategies for CTTH are still non-specific,5 a recently published systematic review of 6 randomised controlled clinical trials has highlighted manual therapy interventions as an effective tool for managing CTTH, which are equally effective as prophylactic medication based on tricyclic antidepressants.6 However, the great variety of treatment options, together with the lack of well-designed studies,6,7 makes it difficult to draw definitive conclusions.
Suboccipital muscle inhibition belongs to the group of manual therapy techniques aimed at myofascial areas compromised by restriction.8 The application of this technique as an isolated therapy has been found to improve pain in patients with CTTH.9,10 However, to our knowledge, the simultaneous application of suboccipital muscle inhibition and interferential current in patients with CTTH has not previously been analysed. As interferential currents have been shown to induce analgesia by reducing muscle electrical activity and muscular tension,11 the simultaneous application of both techniques may lead to an improvement in these patients’ self-reported symptoms.
The aim of this study is to analyse the effectiveness of simultaneous application of suboccipital muscle inhibition and interferential current in reducing pain, disability, and the impact of headache on the daily life of patients with CTTH. We proposed the hypothesis that simultaneous application of manual therapy and interferential current may be more effective than standard treatments in patients with CTTH.
Material and methodsParticipantsWe conducted a single-blind randomised controlled clinical trial (prospectively registered on 21 July 2014 at www.clinicaltrials.gov, NCT02195648). All procedures complied with the Declaration of Helsinki and the study was approved by the ethics committee of Universidad Católica de Murcia (Spain). Secondary psychosocial endpoints (anxiety, depression, and healthcare-related quality of life) will be reported separately.
We selected patients by non-probability and convenience sampling. We recruited patients attended at a regional hospital (Hospital General Universitario Morales Meseguer, Murcia, Spain) and diagnosed with CTTH by a neurologist according to the International Headache Society (ICHD-third edition, beta version) criteria.12Table 1 shows the inclusion and exclusion criteria used.
Inclusion and exclusion criteria.
| Inclusion criteria |
| Recurrent episodes of headache on more than 15 days per month for at least 3 months (≥ 180 days per year) |
| Pain episodes lasting 30 minutes to 7 days |
| 2 or more of the following characteristics: |
| Bilateral location of pain |
| Non-pulsating pain upon pressure |
| Mild to moderate pain |
| Headache not exacerbated by routine activity |
| Headache may be associated with pericranial pain sensitivity |
| Pharmacologically controlled |
| Exclusion criteria |
| Patients with other types of primary or secondary headache |
| Pain aggravated by head movement |
| Metabolic and musculoskeletal problems with symptoms similar to headache |
| History of trauma to the cervical spine |
| Vertigo, dizziness, uncompensated neck tension |
| Cardiac devices |
| Photophobia, phonophobia, nausea, or vomiting |
| Pregnancy |
Patients could withdraw from the study at any time, and could withdraw their consent without repercussion. The patients leaving the study did not undergo any additional follow-up and were not replaced; however, we used an intention-to-treat analysis. To determine the necessary sample size using ANOVA, we performed calculations based on previous studies9,10 in order to detect differences in treatment with an effect size of f = 0.5, an alpha level of 0.05, and a desired power (beta) of 80%, for a two-tailed test. The estimated sample size was 13 patients per group. However, we anticipated a drop-out rate of 10%, and therefore considered a total sample size of 29 subjects to be sufficient.
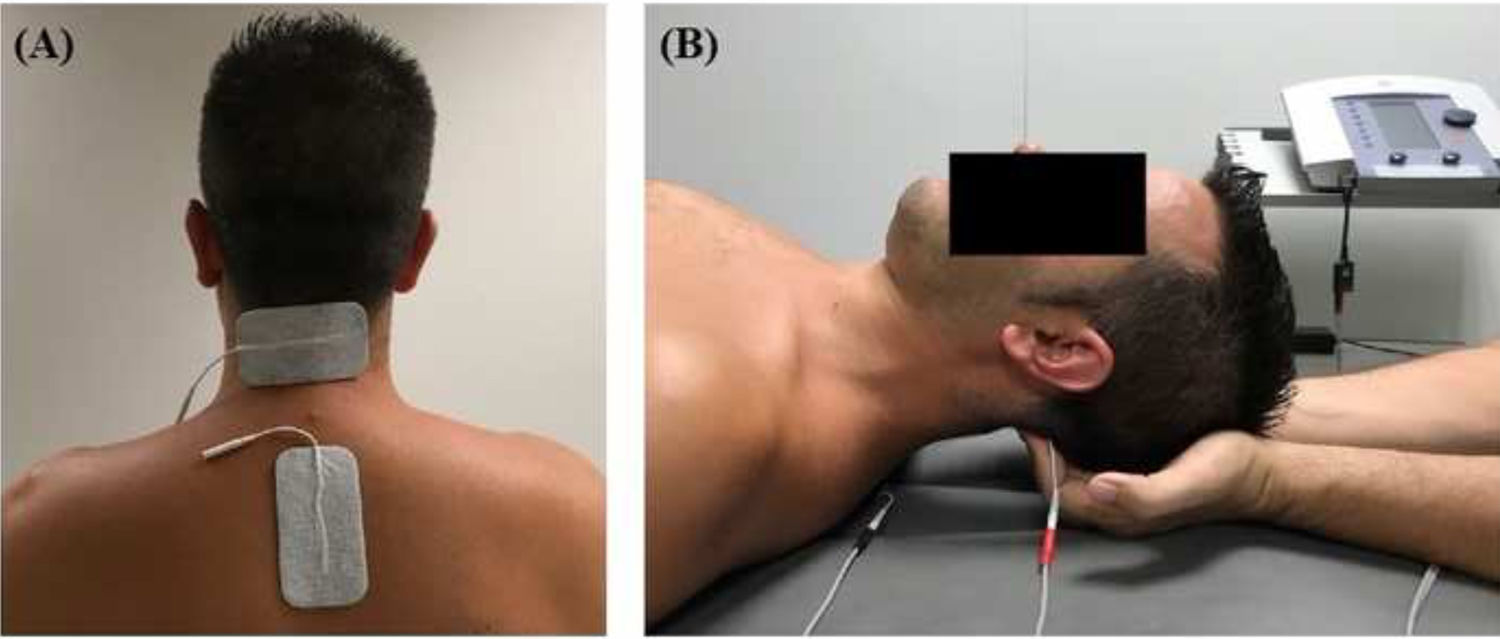
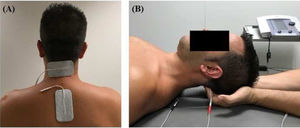
InterventionsTreatment was applied by a physiotherapist with more than 10 years’ clinical experience. Patients were randomly assigned to the intervention or the control group (standard treatment) by a blinded researcher using a software tool. Both groups received pharmacological treatment prescribed by a neurologist. The intervention group attended a 20-minute session (5 minutes to receive the patient, 10 minutes of treatment, and 5 minutes for rest and haemodynamic stabilisation) twice a week for 4 weeks; during sessions, patients underwent muscle inhibition and interferential currents applied to the suboccipital muscles. Suboccipital muscle inhibition was applied with the patient in the supine position with the eyes closed and the physiotherapist’s hands placed under the patient’s head, in contact with the suboccipital muscles. The physiotherapist progressively increased the pressure exerted during the 10 minutes of treatment.9,10 Simultaneously, an interferential current was applied; the current was generated with a Sonopuls 492® device (Sonopuls 492 TM Enraf-Nonius, Rotterdam, the Netherlands) through one channel, with 2 adhesive electrodes applied over the suboccipital muscles (Fig. 1). The parameters selected were carrier frequency of 4000 Hz and amplitude modulated frequency of 100 Hz for a duration of 10 minutes. Current intensity was gradually increased during the first minute, until the physiotherapist noticed tension in the patient’s muscles.
ResultsWe assessed the primary and secondary endpoints at baseline and at 4 weeks (end of intervention) using a paper form administered by a researcher blinded to treatment allocation.
The primary endpoint studied was the change in self-reported pain measured with a numerical pain rating scale (NPRS) scored from 0 to 10 points. Secondary endpoints were disability caused by headache and the impact of headache on daily life. The disability caused by headache was assessed with the Spanish-language version of the Headache Disability Inventory (HDI) and the Neck Disability Index (NDI), which have shown a high degree of consistency and reliability,13–15 as well as the Headache Impact Test–6 (HIT-6). The HDI includes 25 items that assess the emotional (13 items) and functional domains (12 items), with 4 possible answers on each item. The score ranges from 0 to 52 points for the emotional domain and from 0 to 48 points for the functional domain, with higher scores indicating greater disability. The NDI includes 10 questions that measure functional disability, with 6 possible answers for each item. The total NDI score ranges from 0 to 50, with scores from 0 to 4 indicating no disability, 5 to 14 mild disability, 15 to 25 moderate disability, 25 to 34 severe disability, and 35 to 50 complete disability. We used the HIT-6 to measure the impact of headache on daily life (Cronbach’s alpha of 0.89; test-rest reliability of 0.78-0.90).16 The total HIT-6 score ranges from 36 to 78 points, with higher scores indicating greater impact.
Statistical analysisStatistical analysis was performed with the IBM SPSS Statistics software, version 19.0 (IBM Corporation; Armonk, NY, USA). Data were tested for normal distribution using the Shapiro-Wilks test and for homoscedasticity with the Levene test.
We used a one-way analysis of variance to compare clinical and sociodemographic variables. We calculated the effect size with the Hedges’ g, which corrects bias of the Cohen’s d for small samples,17 and also calculated the common language (CL) effect size statistic, which is interpreted as the probability that a value sampled at random from the first population will be greater than a value sampled at random from the second. Therefore, values close to 50% indicate similarity between groups and values close to 100% suggest significant differences between groups.18 Categorical variables were analysed with the chi-square test.
We used the repeated measures ANOVA to compare the effect of time (pre- and post-treatment) and the intergroup factor (control and intervention group) on scores in the NPRS, NDI, HDI, and HIT-6.
We performed univariate post hoc t tests for each dependent measurement with regard to baseline (intragroup comparisons) and to controls (intergroup comparisons). Significance was established at P < .05 (two-tailed tests) and corrected with the Duncan–Bonferroni method for multiple comparisons. The intention-to-treat analysis was performed using the last-observation-carried-forward method.
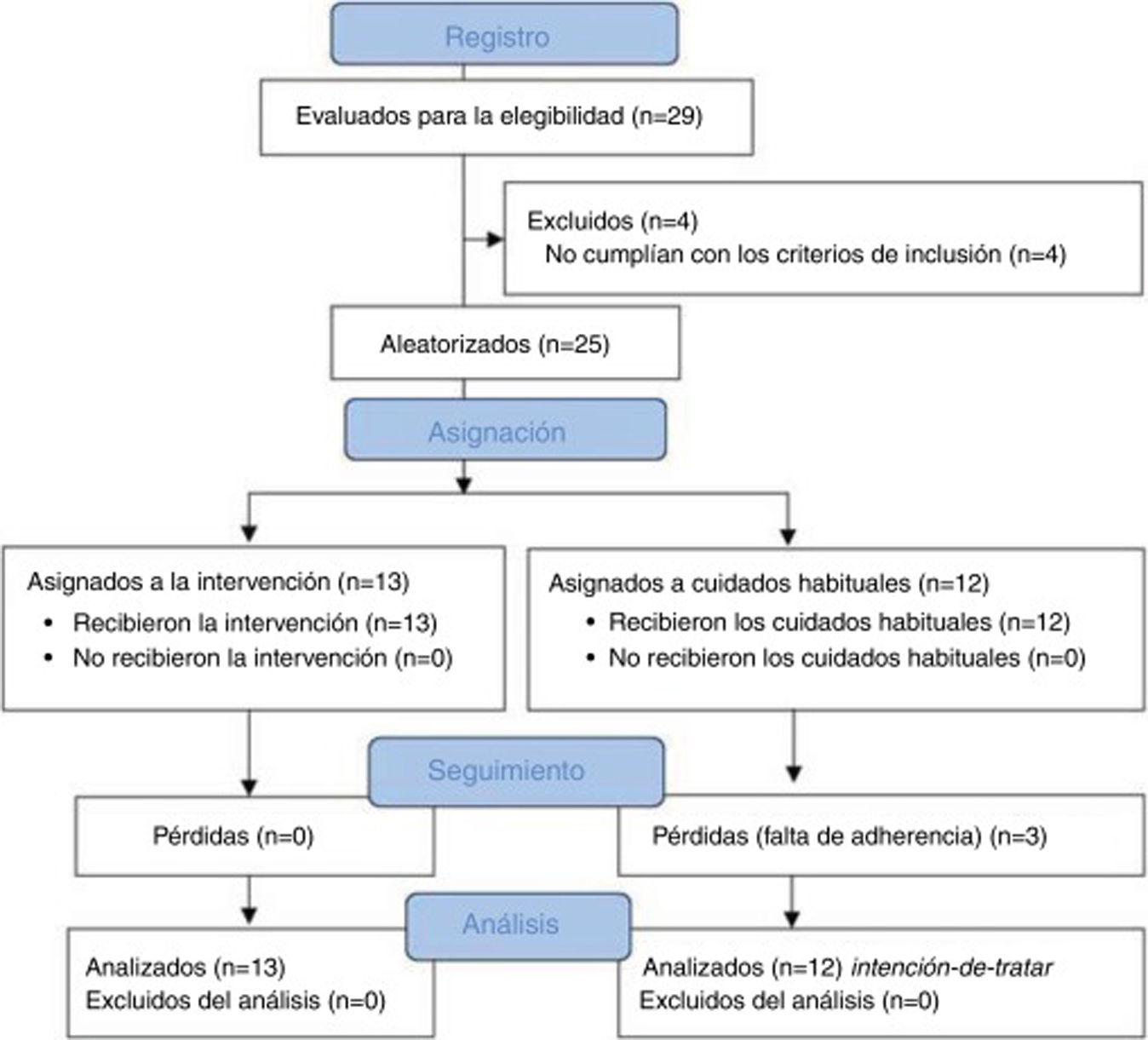
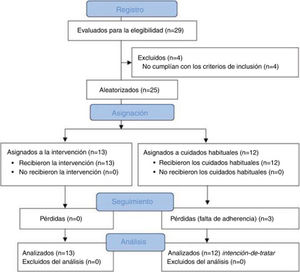
ResultsSampleA total of 29 patients with CTTH were assessed to participate in the study; 25 met all eligibility criteria and were randomly assigned to the intervention (n = 13) or control group (n = 12). After the randomisation process, no intergroup differences were observed in sociodemographic (Table 2) or clinical variables (Table 3) at baseline. No differences were observed between groups in prophylactic and symptomatic medication. Six patients in each group were taking antidepressants, whereas 2 patients in the control group were receiving antidepressants and anxiolytics vs 5 patients in the intervention group. Furthermore, 4 patients in the control group reported no pharmacological prescription, vs 2 in the intervention group. Regarding analgesic drugs, all patients were receiving paracetamol and non-steroidal anti-inflammatory drugs (NSAID).
Baseline sociodemographic variables.
| Variables | Control group (n = 12) | Intervention group (n = 13) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Minimum | Q1 | Median (IQR) | Q3 | Maximum | Mean (SD) | Minimum | Q1 | Median (IQR) | Q3 | Maximum | Hedges’ g (CL%) | P | |
| Age (years) | 46.2 (16.37) | 28.7 | 34.3 | 41.4 (24.43) | 58.8 | 82.4 | 43.3 (18.07) | 19.2 | 27.0 | 43 (30.45) | 57.4 | 73.8 | 0.16 (55%) | .679 |
| Weight (kg) | 68.9 (11.07) | 50.0 | 62.8 | 67.5 (14.75) | 77.5 | 89.0 | 67.5 (13.12) | 48.0 | 58.0 | 64 (20.5) | 78.5 | 90.0 | 0.11 (53%) | .777 |
| Height (m) | 1.63 (0.109) | 1.45 | 1.54 | 1.63 (0.2) | 1.74 | 1.80 | 1.65 (0.1) | 1.52 | 1.55 | 1.68 (0.18) | 1.73 | 1.81 | 0.19 (55%) | .54 |
| BMI (kg/m2) | 26.3 (5.46) | 19.1 | 22.7 | 25.9 (4.19) | 26.9 | 40.5 | 24.7 (4.64) | 18.4 | 21.1 | 24.8 (4.48) | 25.6 | 36.3 | 0.31 (59%) | .44 |
| Sex (women, %) | 11 (92%) | – | – | – | – | – | 10 (77%) | – | – | – | – | – | – | .65 |
BMI: body mass index; CL%: interpreted as the probability that a value sampled at random from the first population will be greater than a value sampled at random from the second; IQR: interquartile range; Q1: first quartile; Q3: third quartile; SD: standard deviation.
Hedges’ g with grouped standard deviation. P-value for one-way ANOVA and chi-square for the variable “sex.”
Baseline clinical variables.
| Variable | Control group | Intervention group | Total | P | |
|---|---|---|---|---|---|
| Sex | Women | 11 (91.7%) | 10 (76.9%) | 21 (95.5%) | .65 |
| Men | 1 (8.3%) | 3 (23.1%) | 4 (18.2%) | ||
| Pericranial pain sensitivity | No | 5 (41.7%) | 9 (69.2%) | 14 (63.6%) | .33 |
| Yes | 7 (58.3%) | 4 (30.8%) | 11 (50%) | ||
| Pain with activity | No | 4 (33.3%) | 6 (46.2%) | 10 (45.5%) | .81 |
| Yes | 8 (66.7%) | 7 (53.8%) | 15 (68.2%) | ||
| Pain with neck movement | No | 6 (50%) | 9 (69.2%) | 15 (68.2%) | .57 |
| Yes | 6 (50%) | 4 (30.8%) | 10 (45.5%) | ||
| Pain upon pressure | No | 5 (41.7%) | 7 (53.8%) | 12 (54.5%) | .84 |
| Yes | 7 (58.3%) | 6 (46.2%) | 13 (59.1%) | ||
| Restricted neck mobility | No | 4 (33.3%) | 7 (53.8%) | 11 (50%) | .53 |
| Yes | 8 (66.7%) | 6 (46.2%) | 14 (63.6%) | ||
| Mean (SD) | Mean (SD) | Hedges’ g (CL%) | P | ||
| Pain in the last month (0-10) | 6.9 (1.33) | 6.5 (1.33) | 0.29 (58%) | .460 |
Data are reported in absolute values and relative frequencies; P-values are for the chi-square and for one-way ANOVA for the variable “pain in the last month.”
BMI: body mass index; CL%: interpreted as the probability that a value sampled at random from the first population will be greater than a value sampled at random from the second; Hedges’ g: effect size for the grouped standard deviation.
During follow-up, 3 patients from the control group dropped out of the study due to lack of adherence to the study protocol. We observed no adverse events during the study. Fig. 2 shows a flow diagram of the recruitment and follow-up processes.
Primary endpointsSelf-reported pain decreased by 2.6 points (P = .011) in the intervention group, whereas the control group showed no changes at the end of the 4 weeks of intervention. However, in the intergroup comparison, we observed no significant differences at the end of the study but a large effect size (Hedges’ g = 1.13) and a CL effect size of 79% (Table 4), which may suggest a significant real effect not detected due to the small size of the groups.
Intragroup and intergroup effects for pain, disability, and headache impact.
| Variables | Intervention group (n = 13) | Intragroup | Control (n = 12) | Intragroup | Intergroup | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | 95% CI | Min | Max | Hedges’ g (CL%) | P | Mean (SD) | 95% CI | Min | Max | Hedges’ g (CL%) | P | Hedges’ g (CL%) | P | |
| Pre-treat. pain (0-10) | 4.6 (2.87) | 6.4-2.9 | 0 | 9 | 4.7 (2.99) | 6.3-2.6 | 0 | 8 | 0.03 (51%) | .96 | ||||
| Post-treat. pain (0-10) | 2 (0.29) | 3.7-2.8 | 0 | 8 | 1.23 (82%) | .011 | 3.5 (1.83) | 5.3-2.7 | 0 | 10 | 0.47 (63%) | .27 | 1.13 (79%) | .18 |
| Pre-treat. NDI (0-100) | 26.9 (15.74) | 38.1-18.5 | 6 | 68 | 38.8 (28.86) | 48.8-15.7 | 22 | 78 | 0.50 (64%) | .097 | ||||
| Post-treat. NDI (0-100) | 17 (6.99) | 26.9-16.5 | 0 | 40 | 0.78 (72%) | .003 | 41.2 (32.63) | 49.7-13.4 | 24 | 66 | 0.08 | .46 | 1.01 (77%) | .001 |
| Pre-treat. HDI emotional (0-52) | 24.5 (17.39) | 31.5-11.7 | 4 | 44 | 28.3 (19.58) | 37.1-13.8 | 6 | 50 | 0.20 (56%) | .46 | ||||
| Post-treat. HDI emotional (0-52) | 19 (13.4) | 24.7-9.3 | 6 | 38 | 0.34 (60%) | .019 | 28.3 (20.91) | 35.8-11.7 | 6 | 50 | 0.00 (50%) | .99 | 0.52 (65%) | .037 |
| Pre-treat. HDI functional (0-48) | 28.5 (21.95) | 35.0-10.8 | 6 | 44 | 34.7 (28.29) | 41.0-10.0 | 18 | 48 | 0.24 (57%) | .15 | ||||
| Post-treat. HDI functional (0-48) | 22.6 (16.26) | 28.9-10.4 | 8 | 36 | 0.29 (59%) | .005 | 33.3 (26.52) | 40.2-10.7 | 14 | 48 | 0.05 (51%) | .51 | 0.48 (63%) | .018 |
| Pre-treat. total HDI (0-100) | 53.4 (39.31) | 67.5-23.3 | 10 | 92 | 62.5 (48.95) | 76.0-21.3 | 26 | 98 | 0.20 (56%) | .32 | ||||
| Post-treat. total HDI (0-100) | 41.7 (29.83) | 53.6-19.6 | 18 | 74 | 0.32 (59%) | .006 | 61.5 (48.34) | 74.7-20.8 | 26 | 98 | 0.02 (51%) | .81 | 0.48 (64%) | .022 |
| Pre-treat. HIT-6 (36-78) | 62.5 (58.47) | 66.5-6.6 | 43 | 67 | 64.3 (61.02) | 67.5-5.1 | 54 | 70 | 0.029 (51%) | .46 | ||||
| Post-treat. HIT-6 (36-78) | 53.4 (46.25) | 60.5-11.8 | 36 | 78 | 0.167 (55%) | .001 | 61.7 (58.17%) | 65.1-5.5 | 47 | 68 | 0.04 (51%) | .31 | 0.15 (54%) | .037 |
95% CI: 95% confidence interval; BMI: body mass index; CL%: interpreted as the probability that a value sampled at random from the first population will be greater than a value sampled at random from the second; HDI: Headache Disability Index; Hedges’ g: effect size with grouped standard deviation; HIT-6: Headache Impact Test–6; NDI: Neck Disability Index; SD: standard deviation.
P-value with Duncan–Bonferroni correction.
The intervention group showed greater improvements than the control group in the disability caused by headache at the end of the 4 weeks of intervention. The results showed intergroup differences in mean scores of 24.2 points, 9.3 points, 10.7 points, and 19.8 points in NDI, HDI emotional domain, HDI functional domain, and total HDI scores, respectively (P < .05).
Furthermore, we observed improvements (differences between mean scores of 8.3 points) in the intervention group on the impact of headache on daily life as measured with the HIT-6 questionnaire at the end of the 4 weeks of intervention (P = .037) (Table 4).
DiscussionOur single-blind randomised controlled clinical trial assessed the effectiveness of suboccipital muscle inhibition and interferential current in improving pain and disability in patients with CTTH. Our results indicate improvements in self-reported pain, headache-related disability, and headache impact in the intervention group, whereas no improvements were observed in the control group. These findings support our hypothesis as they suggest that the simultaneous application of suboccipital inhibition and interferential current may be more effective than standard treatment in improving pain and disability in patients with CTTH in the short term.
Similarly to our findings, Espí-López et al.10 found improvements in self-reported pain, headache impact, and disability after 4 weeks of treatment with suboccipital muscle inhibition combined with an osteopathic cervical manipulation technique. Furthermore, the group that received both treatments showed greater improvement than those receiving suboccipital muscle inhibition, cervical manipulation, or sham treatment only, suggesting that a combination of treatments is sometimes more effective than an isolated therapy; this approach bears more similarity to everyday physiotherapy practice. However, the results obtained in that study after 4 weeks of suboccipital muscle inhibition combined with osteopathic manipulation did not exceed the minimum clinically important change (the change in the score on an instrument that reflects modification in a person’s health status over time) or the minimum detectable change (the minimum amount of change that is probably not explained by random variation in the measurement) in self-reported pain,19 headache impact as measured with the HIT-6,20 or disability caused by headache as measured with the HDI questionnaire21 in patients with CTTH, raising questions about the effects of the treatment and the clinical relevance of these findings.
In our study, while we observed no intergroup differences in self-reported pain, the results showed improvements exceeding the minimum clinically important change for disability measured with the NDI (mean change > 3.5 points),22 headache impact measured with the HIT-6 (mean change > 8 points),20 and disability caused by headache measured with the HDI (mean change > 16 points).21 These findings are very promising for the rehabilitation of these patients in daily practice. However, our study does not address long-term effects, so these findings must be interpreted with caution.
To our knowledge, few studies have analysed interventions with manual therapy and analgesic electrotherapy to treat CTTH symptoms.6,7 Jay et al.23 reported improvements in headache frequency and intensity after the application of transcutaneous electrical nerve stimulation (TENS) in isolation or combined with manual therapy. Furthermore, although these improvements persisted in both groups after 6 months of follow-up, the group that received both treatments showed a faster response. These findings underscore the relevance of manual therapies combined with analgesic electrotherapy in the short- and medium-term symptomatic management of patients with CTTH. That study addressed the hypothesis that such improvements were the result of decreased muscle spasm and increased blood serotonin, beta-endorphin, ACTH, and GABA levels.23
Suboccipital muscle inhibition and interferential current may induce analgesia and decrease tension in suboccipital muscles by reducing muscle activity,11 thereby decreasing peripheral and central sensitisation. However, our study was not specifically designed to identify the underlying mechanism explaining these improvements. Future studies are needed to better understand the main mechanisms responsible for the decrease in self-reported symptoms in patients with CTTH.
Several studies have been designed to compare different individual treatment approaches, for example, head-neck massage vs ultrasound therapy,24 or myofascial trigger point management vs standard treatment.25 However, in daily practice, most therapists apply more than one treatment during the rehabilitation of patients, and the effect of each individual treatment is therefore not easy to determine. Our study may shed light on the impact of combining the treatment approaches used in physiotherapy sessions on rehabilitation outcomes.
The methodological quality of the studies assessing manual therapy interventions for CTTH is frequently criticised.6,7 It is well known that blinding in physiotherapy studies presents particular difficulties compared to other fields of medicine, as the nature of physiotherapy interventions (such as manual therapy) makes blinding of therapists and patients difficult or even impossible26; however, blinding raters is crucial in randomised clinical trials, especially when such subjective instruments as self-reports are used.27 If this blinding is not applied, results on pain and disability may be overestimated by up to 25%.27
Study limitationsOur study is a randomised controlled clinical trial with blinding of the rater and assessor to minimise the risk of bias. However, despite our strict approach towards study design, data collection, and synthesis, our study still presents some limitations. Firstly, although calculations to determine the sample size were based on data from previous studies,9,10 the sample size in both groups was small, which limited the statistical power of our analysis; furthermore, we observed no statically significant differences in the intergroup analysis of pain, despite the large effect size (Hedges’ g = 1.13). Secondly, as previously mentioned, we only analysed the short-term effects of the intervention, and the medium- and long-term effects remain unknown. Furthermore, headache frequency, one of the primary endpoints proposed by the International Headache Society, was not recorded in our study. However, this variable is particularly useful for assessing the results of pharmacological treatments in large-scale trials and in the long term.28 Finally, the introduction of a sham treatment group may have revealed some mechanisms underlying the improvements reported in the intervention group, considering that manual therapies may involve a significant placebo component; therefore, the improvements observed in this study may not be fully explained by the intervention applied.29
ConclusionThe simultaneous application of suboccipital muscle inhibition and interferential current in patients with CTTH does not significantly reduce self-reported pain at 4 weeks. However, it reduces disability and the impact of headache on the daily lives of these patients. These improvements exceeded the minimum clinically important change for the scales applied, highlighting the clinical relevance of the study. Suboccipital muscle inhibition combined with interferential current may be more effective than standard treatment in improving symptoms in patients with CTTH. However, there is a need for future studies with larger sample sizes and sham treatment groups to determine the effects of this therapy.
FundingThis study received no funding of any kind.
Protocol approvalThe study was approved by the ethics committee at Universidad Católica de Murcia (Spain). This study was designed according to the CONSORT regulations and prospectively recorded in the ClinicalTrial.gov database (NCT02195648).
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors wish to thank all the individuals who participated in the study.
Please cite this article as: Pérez-Llanes R, Ruiz-Cárdenas JD, Meroño-Gallut AJ, Fernández-Calero MI, Ríos-Díaz J. Efectividad de la inhibición suboccipital combinada con corriente interferencial en pacientes con cefalea tensional crónica: un ensayo clínico controlado aleatorizado. Neurología. 2022;37:717–725.