Fibromyalgia syndrome (FM) is a chronic pathology characterised by widespread pain commonly associated with psychological distress affecting quality of life. In recent years, transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS) have been investigated to treat chronic pain. The aim of the current review is to determine the effects of tDCS and TMS on the main symptoms of patients with FM.
DevelopmentA systematic review based on PRISMA guidelines was carried out. The search strategy was performed in MEDLINE, SCOPUS, PEDro and Cochrane Library. Randomised controlled trials based on the effects of tDCS and TMS on pain, pressure pain threshold (PPT), fatigue, anxiety and depression, catastrophising and quality of life in patients with FM were analysed. Fourteen studies were included.
ConclusionsThe application of tDCS to the motor cortex is the only intervention shown to decrease pain in the short and medium-term in patients with FM. The application of both interventions showed improvements in PPT, catastrophising and quality of life when applied to the motor cortex, and in fatigue when applied to the dorsolateral prefrontal cortex. The effects of these interventions on anxiety and depression are unclear.
La fibromialgia (FM) es una patología crónica caracterizada por la presencia de dolor musculoesquelético generalizado que se asocia a trastornos psicológicos que afectan a la calidad de vida. En los últimos años, la estimulación transcraneal con corriente directa (tDCS) y la estimulación magnética transcraneal (TMS) se han estudiado para el abordaje del dolor crónico. El objetivo de esta revisión es determinar los efectos de la tDCS y la TMS en los síntomas característicos de los pacientes con FM.
DesarrolloSe realizó una revisión sistemática acorde a los criterios PRISMA. Se realizaron búsquedas en las bases de datos MEDLINE, SCOPUS, PEDro y Cochrane Library. Se seleccionaron ensayos clínicos aleatorios que analizaran los efectos de estas intervenciones en el dolor, el umbral de dolor a la presión (UPD), la fatiga, la ansiedad y depresión, el catastrofismo y la calidad de vida en pacientes con FM. Se incluyeron 14 estudios.
ConclusionesLa aplicación de tDCS en el córtex motor es la única intervención que ha mostrado disminuir el dolor a corto y medio plazo en pacientes con FM. La aplicación de ambas intervenciones ha mostrado mejoras en el UDP, el catastrofismo y la calidad de vida cuando se aplica en el córtex motor, y de la fatiga cuando se aplica en la corteza dorsolateral prefrontal. Los efectos de estas intervenciones en la ansiedad y depresión no son concluyentes.
Fibromyalgia (FM) is a chronic rheumatic disease characterised by generalised musculoskeletal pain and reduced pressure pain thresholds (PPT).1–3 The main symptom of FM is pain, which is often accompanied by such other manifestations as fatigue, anxiety, depression, and catastrophising, leading to a reduction in patients’ quality of life.4 Prevalence is estimated at 2.1% globally5 and 2.4% in Spain, with women being affected more frequently than men.6 No clear objective method has been established for the detection and diagnosis of the disease; the criteria currently used are those described by the American College of Rheumatology.7,8
The pathophysiology of FM is not fully understood, although central sensitisation and impaired control of pain pathways are understood to be key mechanisms.9–11 This hypothesis has led to the development of numerous treatment methods.
The most recent clinical guidelines of the European League Against Rheumatism for the management of FM12 recommend the use of conservative, non-pharmacological treatments as the first line of action. These include exercise therapy, education, and cognitive behavioural therapy, among others.13,14 In recent years, advances in neurorehabilitation have led to the development of new approaches, such as transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS).15
Both of these neurophysiological techniques are non-invasive and aim to modulate brain activity and correct alterations.16 In tDCS, the effect is achieved by generating a continuous, low-intensity electric current (0.5−2.0 mA), which is applied through electrodes placed on the scalp over a specific brain area.17 In TMS, a magnetic field passes through the scalp and induces a current in the brain; therefore, the treatment is considered to constitute electric stimulation via electromagnetic induction.18
In recent years, an increasing number of studies have addressed these treatment techniques, observing analgesic effects in chronic diseases.15 However, their effects on patients with FM have not been clearly established.
This article presents a systematic review on the effects of conservative, non-pharmacological treatment of FM with tDCS and TMS, analysing the effects of treatment on pain, PPT, fatigue, anxiety, depression, catastrophising, and quality of life.
Material and methodsSearch strategyWe conducted a systematic literature review following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.19
Literature searches were performed between October 2019 and February 2020 on the MEDLINE (PubMed), Physiotherapy Evidence Database (PEDro), Scopus, and Cochrane Library databases. Searches included the combination of the following MeSH terms: “fibromyalgia,” “transcranial direct current stimulation,” and “transcranial magnetic stimulation.” Terms were combined with the Boolean operators AND and OR. The search was not limited by date of publication. The search strategy is shown in detail in the Appendix.
Selection of articlesThe inclusion criteria applied were based on the PICOS method:
- •
Population: patients diagnosed with FM by a rheumatologist, according to the American College of Rheumatology clinical criteria.
- •
Intervention: treatment with tDCS or TMS.
- •
Comparison: another conservative treatment, sham treatment, or no intervention.
- •
Outcome: pain intensity, PPT, fatigue, anxiety, depression, catastrophising, and quality of life.
- •
Study: randomised clinical trials.
- •
Language of publication: English or Spanish.
Studies were excluded if: patients presented such concomitant diseases as rheumatoid arthritis, autoimmune diseases, neurological or oncological diseases, or other systemic diseases; pharmacological treatment control was not explained, if present; patients received multimodal treatment; the intervention was compared against pharmacological or surgical treatment; a randomised clinical trial study design was not followed; or methodological quality scored lower than 5 on the PEDro scale.
Two authors independently reviewed the titles and abstracts of the articles retrieved from databases, and applied the selection criteria to identify potentially relevant studies. The same reviewers independently extracted data from each study. Disagreements were resolved with recourse to a third reviewer. To avoid overlooking potentially eligible studies, we also manually reviewed the references cited in each of the included studies.
Data analysis and synthesisThe PRISMA checklist was used to gather data, including information on study design, sample size, patient characteristics, treatment protocol, dependent variables, measurement tools, and results.
Methodological quality was assessed with the PEDro scale, based on the Delphi list developed by Verhagen et al.,20 of Maastricht University’s Department of Epidemiology. This Delphi list includes criteria for characterising the quality of clinical trials for the development of systematic reviews according to the Delphi consensus. The PEDro scale is an 11-item scale, scored 0-10 according to the number of criteria that are met. Lower scores indicate poorer methodological quality. Scores of 7 or higher denote high quality, whereas scores of 5-6 indicate acceptable quality and scores of 4 or less reflect poor quality. The PEDro scale is a valid measure of the methodological quality of clinical trials, with high internal consistency (0.53), inter-rater reliability (0.4−0.75), and test-retest reliability (r = 0.99).21
We also applied the Oxford Centre of Evidence-Based Medicine scale to determine the level of evidence of each study reviewed. This scale assesses the evidence presented according to the thematic area and type of study. It presents the advantage that evidence can be graded according to the best design for each clinical scenario.22 The extraction of data and assessment of study quality were conducted independently by the same 2 authors.
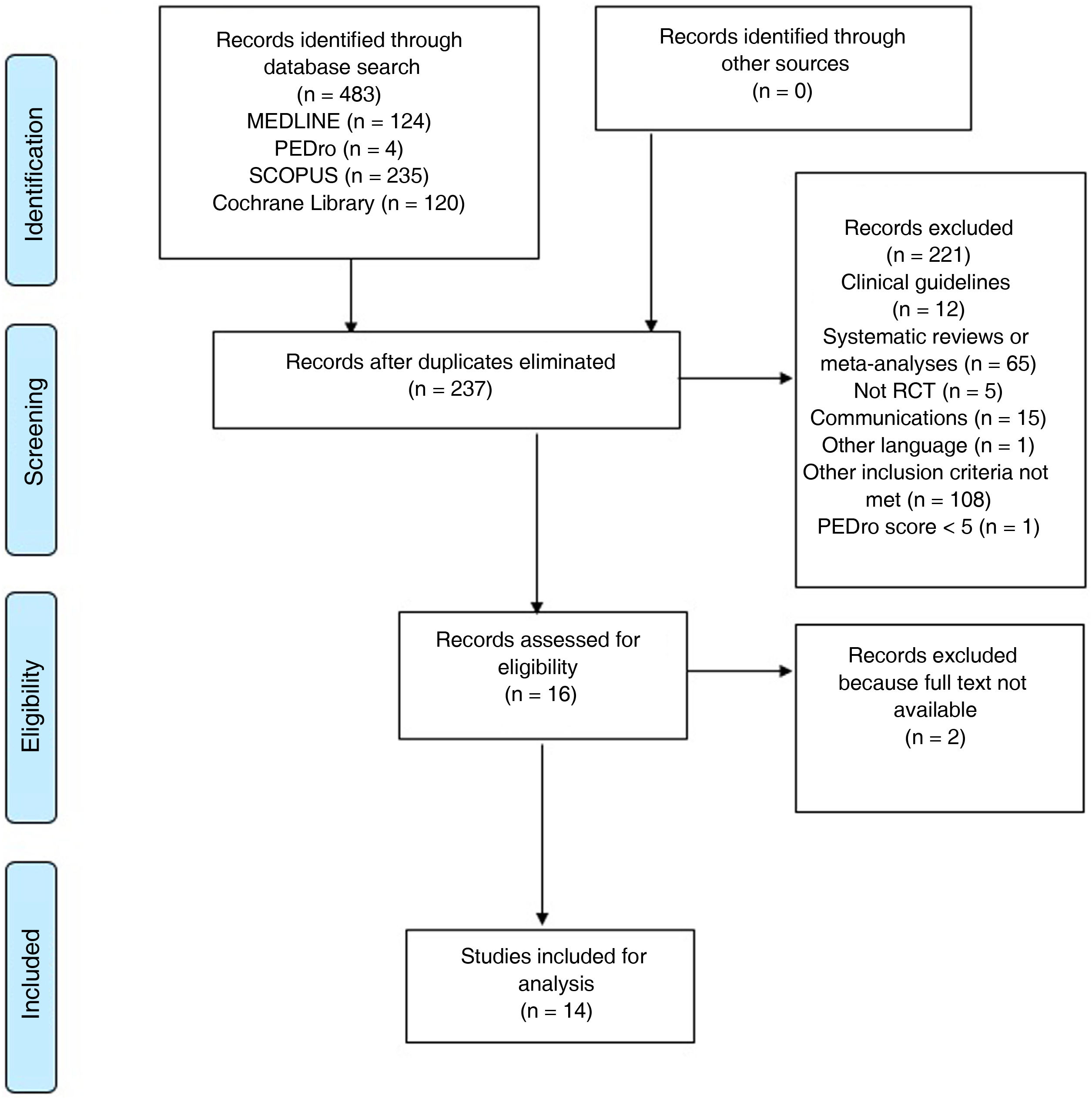
ResultsA total of 483 studies were identified in the different databases searched (124 in MEDLINE, 4 in PEDro, 235 in Scopus, and 120 in Cochrane Library). After eliminating duplicate studies, the title and abstract of each study was reviewed, and a total of 16 relevant studies were reviewed in full text. Finally, 14 studies meeting the selection criteria were included for review. The article selection process is shown in Fig. 1.
Study characteristicsThe articles reviewed reported a total of 565 patients. Sample sizes varied greatly, with the majority including 30-50 patients16,23–30; only 3 studies included more than 50 patients,31–33 with 2 including fewer than 30.34,35 Half of the studies established the sample size required according to a calculation of the minimum clinically important difference for at least one of the primary outcome variables.16,23–26,31,35 Studies were conducted in Europe,16,23–26,31,32 the Americas,29,30 Asia,27,33,35 Africa,28 and Australia.34 Participants were mainly recruited through hospital databases16,23,28–31 from physical medicine and rehabilitation departments,24,27,33 pain units and nuclear medicine departments,25 research centres,34 and patients’ associations,23,32 and by publishing advertisements in the local press.23
The intervention analysed was tDCS in 6 studies and TMS in 8.
The frequency and number of sessions varied between studies. Most treatment protocols included 10-15 sessions over a period of 2-3 weeks.16,25–29,33,35 In 3 studies, stimulation was applied in 8 sessions over 4 weeks24,31 or 8 weeks32; 2 scheduled 5 sessions over 5 consecutive days23,30; and in one study patients underwent 20 sessions over 4 weeks.34 In trials of tDCS, treatment sessions were 20 minutes long in all cases,23,24,28–31 whereas session duration ranged from 8.5 minutes33 to 30 minutes27,32,34 in trials of TMS. Twelve studies characterised the target region with the International 10-20 EEG system.16,23,24,26–34
With respect to outcome variables and measurement instruments, 13 studies evaluated pain intensity using the numeric pain rating scale (NPRS),16,23–26,31,34 the visual analogue scale (VAS),27,33,35 the short-form McGill Pain Questionnaire (SF-MPQ),16,26,34 the Widespread Pain Index (WPI) and Symptom Severity (SS) scale,28 or the Brief Pain Inventory (BPI).16,26,34 Four studies assessed PPT using algometry16,25,32 or Von Frey monofilaments.28 Three studies evaluated fatigue, using the Modified Fatigue Impact Scale (MFIS),24 Multidimensional Fatigue Inventory (MFI-20),34 or Fatigue Severity Scale (FSS).27 Twelve studies assessed anxiety and/or depression, using the Hospital Anxiety and Depression Scale (HADS),16,23,25,26 Beck Depression Inventory (BDI),16,25–27,29–31,34,35 Beck Anxiety Inventory (BAI),34 Hamilton Depression Rating Scale (HAM-D),28 Hamilton Anxiety Rating Scale (HAM-A),28 Geriatric Depression Scale (GDS),29 Montgomery-Åsberg Depression Rating Scale (MADRS),33 and State-Trait Anxiety Scale (STAI).29 Three studies used the Pain Catastrophizing Scale to evaluate catastrophising.24,26,34 Twelve studies evaluated quality of life, using the Fibromyalgia Impact Questionnaire (FIQ),16,23,25–27,29–32,34,35 36-item Short Form Health Survey (SF-36),23,25,27,30,34 and the abbreviated World Health Organization Quality of Life scale (WHOQOL-BREF).33
Treatment effectsPainSix studies found that the application of tDCS over the motor cortex (M1 region) improved pain intensity in the short23,24,28–31 and medium term,28–30 compared to sham treatment. The application of tDCS over the dorsolateral prefrontal cortex (DLPFC) was controversial, with 2 studies observing a short-term improvement in pain intensity, compared to sham treatment,24,29 and 2 reporting no such effect.30,31
Conflicting results are reported for TMS applied over the M1 region. Five studies found that this treatment improved pain intensity in the short16,26,27,32,33 and medium term,16,26 compared to sham treatment, with 2 observing no improvement.25,35 TMS applied over the DLPFC did not improve pain intensity.27,34
Pressure pain thresholdOne study found that tDCS applied over the M1 region improved PPT, compared to sham treatment.28
Three studies evaluated the effects of TMS on PPT. Two found that TMS applied over the M1 region increased PPT in the short but not in the medium term, compared to sham treatment.16,32 One study found no improvement in PPT.25
FatigueOne study found that tDCS over the DLPFC improved fatigue in the short term, compared with occipital stimulation and with sham treatment.24
Another study reported that TMS applied over the DLPFC improved fatigue in the short and in the medium term, compared to sham treatment.34
Anxiety and depressionThe reported effects of tDCS on anxiety and depression in FM are contradictory. Two studies found that tDCS applied over the M1 and DLPFC regions improved anxiety and/or depression, compared to sham treatment.28,31 On the other hand, 3 studies observed no improvement associated with this treatment.23,29,30
Five studies found no short- or medium-term improvement in anxiety or depression after application of TMS over the M1 or DLPFC regions, compared to sham treatment.16,25,26,33,34 In 2 studies, TMS improved depression in the short but not in the medium term.27,35
CatastrophisingOne study found that tDCS applied over the M1 or DLPFC regions reduced catastrophising in the short term, compared to sham treatment.24
According to one study, application of TMS over the M1 region improves catastrophising in the short and medium term, compared to sham treatment26; another found that TMS applied over the DLPFC did not improve catastrophising.34
Quality of lifeFour studies observed that tDCS applied over the M1 or DLPFC regions improved quality of life in the short term,23,29–31 with stimulation of the M1 region also associated with a medium-term improvement.29,30
Seven studies reported that TMS over the M1 region improved some subdomains of quality of life scales in the short term, compared to sham treatment.16,25–27,32,33,35 One study found that TMS over the DLPFC improved some subdomains of quality of life scales in the short term, compared to sham treatment27; another study observed no such effect.34Table 1 summarises the results of the studies reviewed.
Results of the articles included in the review.
| Author | Sample (N) | Intervention | Intensity | Number/frequency/duration of sessions | Study variables | Results | Follow-up |
|---|---|---|---|---|---|---|---|
| tDCS | |||||||
| Fagerlund et al.23 | G1: 49.04 ± 8.63 years(n = 24)G2:48.17 ± 10.56 years(n = 24) | G1: tDCS over M1G2: sham tDCS | G1: intensity of 2 mAG2: intensity of 2 mA | G1: 5 20-min sessions on 5 consecutive daysG2: same procedure, but only 30 s’ active stimulation | NPRSFIQHADSSF-36 | NPRS and FIQ improved in G1, compared to G2 (P < .05). No statistically significant differences were observed in the remaining variables (P > .05). | Not reported |
| Fregni et al.30 | G1: 54.2 ± 7.4 years(n = 11)G2: 54.8 ± 9.3 years (n = 11)G3: 50.8 ± 10.2 years(n = 10) | G1: tDCS over DLPFCG2: tDCS over M1G3: sham tDCS | G1: intensity of 2 mAG2: intensity of 2 mAG3: intensity of 2 mA | G1 and G2: 5 20-min sessions on consecutive daysG3: same procedure, but only 30 s’ active stimulation | VASFIQSF-36BDIVAS-anxiety | G2 improved more than G1 and G3 in FIQ and the physical functioning and bodily pain subdomains of the SF-36 (P < .05). G2 improved more in the VAS than G3 (P < .05). No differences were observed between G1 and G3 (P > .05). No differences between the 3 groups were observed in the remaining variables (P > .05). | Improvements persisted at 3 weeks of follow-up. |
| Yoo et al.31 | G1: 47.81 ± 8.23 years (n = 21)G2: 45.76 ± 10.80 years (n = 21)G3: 47.19 ± 8.14 years (n = 16) | G1: tDCS over occipital nerveG2: tDCS over DLPFC before occipital stimulationG3: sham tDCS | G1: intensity of 1.5 mAG2: intensity of 2 mAG3: intensity of 1.5 mA | G1 and G2: 2 sessions per week, 3 days apart, for 4 weeks. Total of 8 sessionsDuration: 20 min for G1 and 40 min for G2G3: same procedure, but only 30 s’ active stimulation | FIQBDINPRS | NPRS and FIQ improved in G1 compared to G3 (P < .05). No differences between G2 and G3BDI improved in G1 and G2 compared to G3 (P < .05). | Not reported |
| Khedr et al.28 | G1: 31.3 ± 10.9 years(n = 18)G2: 33.9 ± 11.2 years(n = 18) | G1: tDCS over M1G2: sham tDCS | G1: intensity of 2 mAG2: intensity of 2 mA | G1: 5 consecutive sessions per week for 2 weeks. Total of 10 20-min sessionsG2: same procedure, but only 30 s’ active stimulation | WPISSVASPPTHAM-DHAM-A | At the end of treatment, G1 showed improvements in WPI, VAS, PPT, HAM-D, and HAM-A, compared to G2 (P < .05). | All differences observed between G1 and G2 persisted at 2 and 4 weeks of follow-up (P < .05). |
| Valle et al.29 | G1: 54.8 ± 9.6 years(n = 14)G2: 54.8 ± 9.6 years(n = 13)G3: 54.8 ± 9.6 years(n = 14) | G1: tDCS over M1G2: tDCS over DLPFCG3: sham tDCS | G1: intensity of 2 mAG2: intensity of 2 mAG3: intensity of 2 mA | G1 and G2: 5 consecutive sessions per week for 2 weeks. Total of 10 20-min sessionsG3: same procedure, but only 30 s’ active stimulation | VASFIQBDIGDSSTAI | VAS and FIQ showed differences between the 3 groups (P < .05). G1 and G2 showed statistically significant improvements (P < .05). No differences between the 3 groups were observed in the remaining variables (P > .05). | The improvement in VAS only persisted at 1 and 2 months of follow-up in G1 (P < .05). |
| To et al.24 | G1: 47.13 ± 10.01 years (n = 15)G2: 47.81 ± 10.17 years (n = 11)G3: 46.19 ± 10.49 years(n = 16) | G1: tDCS over occipital nerveG2: tDCS over DLPFCG3: sham tDCS | G1: intensity of 1.5 mAG2: intensity of 1.5 mAG3: intensity of 1.5 mA | G1 and G2: 2 sessions per week for 4 weeks. Total of 8 20-min sessionsG3: same procedure, but only 10 s’ active stimulation | NPRSPCSMFIS | Statistically significant differences were observed between the 3 groups. G1 and G2 showed improvements in NPRS and PCS, compared to G3 (P < .05), with no differences between both groups (P > .05). G2 improved more in the MFIS than G1 or G3 (P < .05). | Not reported |
| TMS | |||||||
| Passard et al.16 | G1: 52.6 ± 7.9 years(n = 15)G2: 55.3 ± 8.9 years(n = 15) | G1: TMS over M1G2: sham TMS (coil producing a similar sound to the active coil) | G1: 25 series of 10 Hz; 2000 pulses | 5 consecutive sessions per week for 2 weeks. Total of 10 sessions. Duration of sessions not specified | NPRSPPTSF-MPQFIQBPIHADSBDIHAM-D | G1 improved in NPRS, PPT (epicondyle and trochanter), SF-MPQ, FIQ, and BPI (walking, sleep, and general activity), compared to G2 (P < .05). No differences were observed in the remaining variables (P > .05). | Improvements persisted in NPRS and BPI at 2 weeks of follow-up (P < .05) and in NPRS at 5 weeks (P < .05). |
| Boyer et al.25 | G1: 49.1 ± 10.6 years(n = 19)G2: 47.7 ± 10.4 years(n = 19) | G1: TMS over M1G2: sham TMS (coil producing a similar sound to the active coil) | G1: 20 series of 10 Hz; 2000 pulses | 5 consecutive sessions per week for 2 weeks, then 1 session every 2 weeks. Total of 14 sessions. Duration of sessions not specified | FIQSF-36NPRSPPTHADSBDI | No differences between groups were observed after treatment (P > .05). | At 11 weeks of follow-up, G1 scored better than G2 for FIQ and SF-36 (mental health) (P < .05). |
| Tekin et al.33 | G1: 42.4 ± 7.63 years (n = 27)G2: 46.5 ± 8.36 years(n = 25) | G1: TMS over M1G2: sham TMS (coil producing a similar sound to the active coil) | G1: 30 series of 10 Hz; 1500 pulses | 10 8.5-min sessions on consecutive days | VASMADRSWHOQOL-BREF | Compared to G2, G1 showed improvements in VAS and in the physical health domain of the WHOQOL-BREF (P < .05). No differences were observed in the remaining variables (P > .05). | Not reported |
| Maestú et al.32 | G1: 40.7 ± 6.7 years (n = 34)G2: 40.7 (6.7)(n = 33) | G1: low-intensity TMS across entire cortexG2: sham TMS (coil disconnected) | G1: number of series and pulses not specified. Frequency: 8 Hz | One session per week for 8 consecutive weeks. Total of 8 20-min sessions | PPTFIQ (adapted VAS) | G1 showed improvements in VAS for daily activities, sleep quality, and perceived pain, compared to G2 (P < .05). No differences were observed in the remaining domains (P > .05). | Not reported |
| Yağci et al.35 | G1: 45.25 ± 9.33 years (n = 12)G2: 43 ± 7.63 years (n = 13) | G1: TMS over M1G2: sham TMS (coil at 90° angle to M1) | G1: number of series not specified. Frequency: 10 Hz; 1200 pulses | 5 sessions per week for 2 weeks. Total of 10 sessions. Duration of sessions not specified | VASFIQBDI | FIQ and BDI improved in G1, compared to G2 (P < .05). No differences were observed in the remaining variables (P > .05). | At 4 and 12 weeks of follow-up, no differences were observed between groups (P> .05). |
| Mhalla et al.26 | G1: 51.8 ± 11.6 years(n = 20)G2: 49.6 ± 10.0 years(n = 20) | G1: TMS over M1G2: sham TMS (coil producing a similar sound to the active coil) | G1: 15 series of 10 Hz; 1500 pulses | 5 sessions on consecutive days, followed by 1 session per week for 3 weeks, then 1 session every 2 weeks for 6 weeks, then 1 session per month for 3 months. Total of 14 sessions. Duration of sessions not specified | NPRSSF-MPQBPIFIQHADSBDIPCS | G1 showed improvements in NPRS, SF-MPQ (affective subscale), BPI (walking, relations, enjoyment of life, sleep, and general activity), FIQ (fatigue, stiffness, and tiredness), and PCS, compared to G2 (P < .05). No differences were observed in the remaining subscales or in HADS or BDI (P > .05) | All differences observed between G1 and G2 persisted at 4 weeks of follow-up (P < .05). |
| Fitzgibbon et al.34 | G1: 45.07 ± 11.02 years(n = 14)G2: 46.25 ± 15.04 years(n = 12) | G1: TMS over DLPFCG2: sham TMS (coil producing a similar sound to the active coil) | G1: 75 series of 10 Hz; 3000 pulses | G1: 5 30-min sessions were performed per week for 4 weeks. Total of 20 sessions | SF-MPQBPINPRSFIQSF-36MFI-20PCSBDIBAI | G1 improved in the MFI-20, compared to G2 (P < .05). No differences between groups were observed in the remaining variables (P > .05). | The difference observed between G1 and G2 persisted at 4 weeks of follow-up (P < .05). |
| Altas et al.27 | G1: 46.3 ± 9.01 years (n = 10)G2: 47.9 ± 7.89 years (n = 10)G3: 48.2 ± 9.38 years (n = 10) | G1: TMS over M1G2: TMS over DLPFCG3: sham TMS (coil placed in reverse) | G1: 60 series of 10 Hz; 1200 pulses.G2: 60 series of 10 Hz; 1200 pulses.G3: 60 series of 10 Hz; 1200 pulses. | 30-min sessions on 5 consecutive days per week, for 3 weeks. Total of 15 sessions | VASFIQFSSSF-36BDI | Differences between groups were observed for the VAS, SF-36, and BDI. G1 improved in the VAS, compared to G3, and in the emotional role functioning domain of the SF-36, compared to G2 and G3 (P < .05). G1 and G2 improved in the BDI and in the physical role functioning and general health perceptions domains of the SF-36. No differences between groups were observed in the FIQ or FSS (P > .05). | Not reported |
BAI: Beck Anxiety Inventory; BDI: Beck Depression Inventory; BPI: Brief Pain Inventory; DLPFC: dorsolateral prefrontal cortex; FIQ: Fibromyalgia Impact Questionnaire; FSS: Fatigue Severity Scale; G: group; GDS: Geriatric Depression Scale; HADS: Hospital Anxiety and Depression Scale; HAM-A: Hamilton Anxiety Rating Scale; HAM-D: Hamilton Depression Rating Scale; M1: primary motor cortex; MADRS: Montgomery–Åsberg Depression Rating Scale; MFI-20: Multidimensional Fatigue Inventory; MFIS: Modified Fatigue Impact Scale; NPRS: numeric pain rating scale; PCS: Pain Catastrophizing Scale; PPT: pressure pain threshold; SF-36: 36-item Short Form Health Survey; SF-MPQ: short-form McGill Pain Questionnaire; SS: Fibromyalgia Symptom Severity Scale; STAI: State-Trait Anxiety Inventory; tDCS: transcranial direct current stimulation; TMS: transcranial magnetic stimulation; VAS: visual analogue scale; WHOQOL-BREF: abbreviated World Health Organization Quality of Life scale; WPI: Widespread Pain Index.
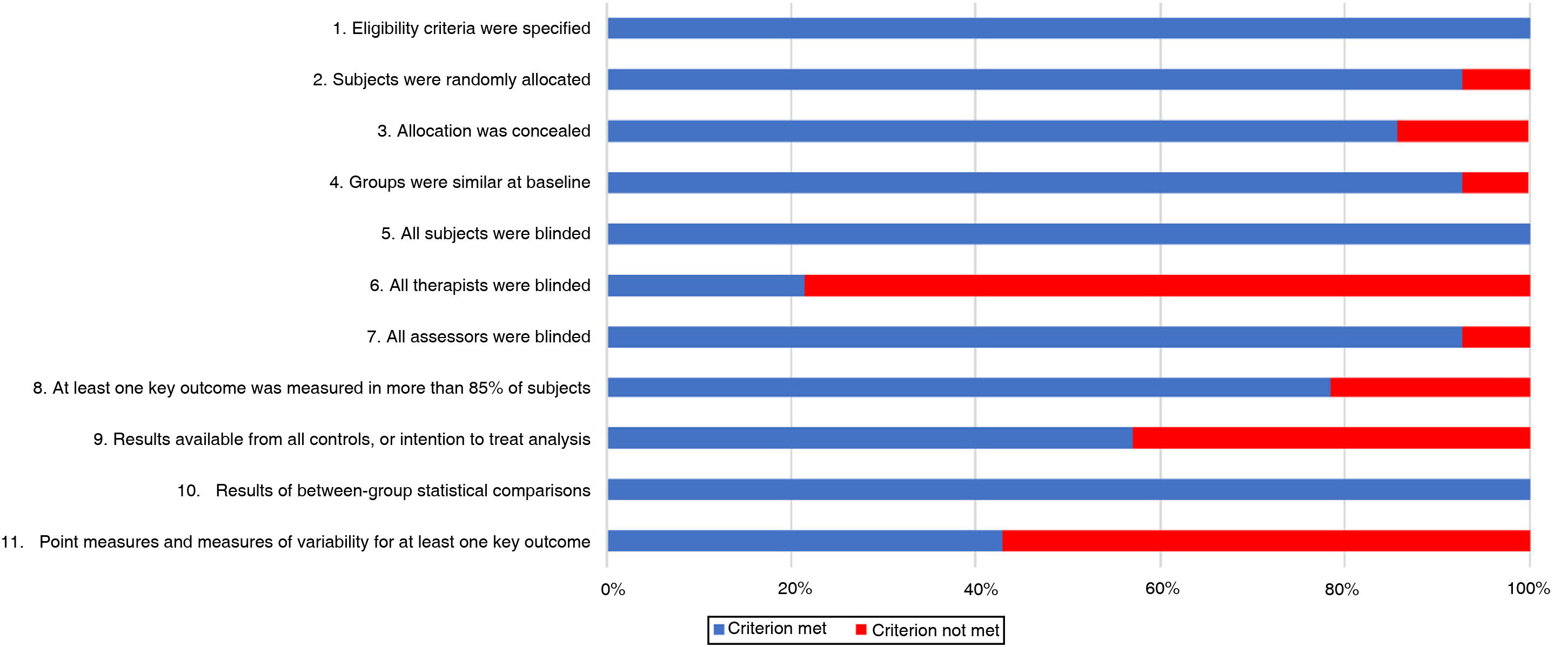
On the PEDro scale (Table 2), 5 trials of tDCS scored 7 or more,23,28–31 with the other scoring 6.24 All trials of TMS were of high methodological quality, scoring 7 or higher.16,25–27,32–35 Subject blinding was appropriate in all studies, as were between-group statistical comparisons for at least one key outcome variable. However, therapists were not blinded in the majority of studies. The risk of bias is shown in Fig. 2.
PEDro scale scores of the studies reviewed.
| Study | Items | Total | Study quality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |||
| Khedr et al.28 | Y | Y | Y | Y | Y | N | Y | Y | N | Y | N | 7/10 | High |
| To et al.24 | Y | Y | Y | Y | Y | N | N | N | N | Y | Y | 6/10 | Acceptable |
| Fagerlund et al.23 | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | 9/10 | High |
| Boyer et al.25 | Y | Y | Y | Y | Y | N | Y | N | Y | Y | N | 7/10 | High |
| Mhalla et al.26 | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | 9/10 | High |
| Passard et al.16 | Y | Y | N | Y | Y | N | Y | Y | Y | Y | N | 7/10 | High |
| Fregni et al.30 | Y | Y | N | Y | Y | N | Y | Y | Y | Y | N | 7/10 | High |
| Maestú et al.32 | Y | Y | Y | Y | Y | Y | Y | N | N | Y | N | 7/10 | High |
| Altas et al.27 | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | N | 8/10 | High |
| Tekin et al.33 | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | 8/10 | High |
| Valle et al.29 | Y | N | Y | Y | Y | N | Y | Y | Y | Y | N | 7/10 | High |
| Yoo et al.31 | Y | Y | Y | N | Y | N | Y | Y | Y | Y | Y | 8/10 | High |
| Yağci et al.35 | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | 8/10 | High |
| Fitzgibbon et al.34 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | 9/10 | High |
1. Eligibility criteria were specified.
Not counted in the final score.
Out of 10: N: criterion is not met; Y: criterion is met.
2. Subjects were randomly allocated to groups.
3. Allocation was concealed.
4. The groups were similar at baseline regarding the most important prognostic indicators.
5. There was blinding of all subjects.
6. There was blinding of all therapists who administered the therapy.
7. There was blinding of all assessors who measured at least one key outcome.
8. Measures of at least one key outcome were obtained from more than 85% of the subjects initially allocated to groups.
9. All subjects for whom outcome measures were available received the treatment or control condition as allocated or, where this was not the case, data for at least one key outcome was analysed by “intention to treat.”
10. The results of between-group statistical comparisons are reported for at least one key outcome.
11. The study provides both point measures and measures of variability for at least one key outcome.
Furthermore, all the studies reviewed presented a level of evidence of 1b, corresponding to a consideration of “recommendable” with a moderate level of evidence. This denotes that the measure is effective and that its benefits outweigh the costs.22
DiscussionThis systematic review evaluates the effects of tDCS and TMS on pain, PPT, fatigue, anxiety, depression, catastrophising, and quality of life in patients diagnosed with FM.
Our results seem to show that the application of tDCS over the M1 region causes short-23,24,28–31 and medium-term improvements in pain intensity28–30 and short-term improvements in PPT.28 Evidence of the analgesic effects of tDCS and TMS over the DLPFC is inconsistent.
The authors of these studies propose that the central sensitisation that characterises chronic pain may involve a dysfunction of sensory processing, with maladaptive neuroplastic changes of cortical activity.36 These physiological techniques based on the application of current to the cerebral cortex appear to increase cortical excitability, promoting changes in areas associated with pain modulation.30
It has been suggested that the analgesic effects may be induced by activation of pain modulatory systems.18,37 Specifically, recent studies have demonstrated that these techniques result in activation of the endogenous opioid system and the release of β-endorphins.38 Furthermore, stimulation of the M1 region may directly inhibit the thalamus via thalamocortical fibres, potentially modulating thalamic nuclei.39,40 In addition, the potential analgesic effects attributed to stimulation of the DLPFC (which, as we have seen, are controversial), may be explained by modulation of the limbic system and anterior cingulate cortex26,28,31; these structures are related to emotions, affective states,30 and emotional aspects of pain.41,42
As reflected in the results of the studies reviewed, both tDCS and TMS appear to reduce fatigue when applied over the DLPFC, with TMS showing a more prolonged effect.24,34
The definition and assessment of fatigue is complex, as the condition involves multidimensional components associated with functional and structural changes in the prefrontal cortex.43 It should be stressed that these treatments have only been shown to reduce fatigue when applied over the DLPFC. These results may be due to the fact that stimulation of this region can cause modulation of the limbic system and other networks related to sensory, affective, and cognitive processing, which may affect the perception of fatigue.43
The results of the studies reviewed demonstrate the considerable controversy around the potential psychosocial effects of these techniques on anxiety and depression. Four studies report favourable results,27,28,31,35 whereas another 8 observed no changes after application of tDCS or TMS.16,23,25,26,29,30,33,34 Regarding catastrophising, tDCS was associated with short-term improvements, and TMS applied over the DLPFC was associated with short- and medium-term changes.24,26
The improvements achieved may be due to activation of the endogenous opioid system and the release of β-endorphins, which have demonstrated analgesic effects.38 Similarly, close links have been demonstrated between pain intensity and symptoms of anxiety and depression in patients with chronic musculoskeletal pain.44 As catastrophising refers to patients’ thoughts and sensations related to pain,26 a reduction in pain intensity may explain the improvements observed in these variables. The studies that did not find improvements in depression/anxiety with these interventions attribute this to the characteristics of the patients included. Both techniques, and particularly TMS, have been used to treat psychiatric disorders and severe depression.45 Therefore, the authors suggest that the lack of improvement in these variables may be because the patients included only presented mild or moderate depressive symptoms.30
The studies reviewed report that both applications are associated with short- and medium-term benefits related to quality of life, including physical function, sleep quality, and activities of daily living.16,24–27,29–34 Although such symptoms as pain, fatigue, and catastrophising have been linked to poorer quality of life in these patients, the improvements observed in these typical symptoms of FM may result in an improvement in quality of life.26
Regarding tDCS protocols, the authors seem to agree that anodal stimulation should be applied in sessions of 20 minutes’ duration, at an intensity of 1.5-2 mA,23,24,28–31 with a minimum of 5 sessions23,30; with higher numbers of sessions, effects seem to persist in the medium term.28,29 Stimulation with the anodal electrode results in increased excitability.46 The TMS protocols used show greater variability. Many studies used treatment sessions of 8.5-30 minutes’ duration, with stimulation applied at a frequency of 10 Hz, typically with 5 sessions per week on consecutive days, for 2,16,25,33,35 3,27 or 4 weeks.34 High-frequency stimulation protocols increase cortical excitability.18 Both treatment protocols are similar to those described by other authors in the management of other chronic conditions.15
Our review presents some limitations. Firstly, the heterogeneity of the assessment instruments used to measure different variables hinders the comparison of results between studies. Secondly, we only included studies published in English or Spanish, and may have overlooked potentially relevant studies in other languages. Finally, we only included studies of patients with FM, which prevents extrapolation of our findings to other diseases characterised by chronic pain.
Future studies should seek to establish the long-term effects of these interventions, the most beneficial intervention time, and the optimal session duration, and explore the effects of combining this stimulation with other treatments.
ConclusionThe results of this review show that TMS applied to the M1 region reduces pain in the short and medium term in patients with FM. The application of tDCS or TMS improves PPT, catastrophising, and quality of life when stimulation is applied over the M1 region, and fatigue when the DLPFC is targeted. However, the effects of these interventions on anxiety and depression are inconclusive.
FundingThis study has received no specific funding from any public, commercial, or non-profit organisation.
Conflicts of interestThe authors have no conflicts of interest to declare.
MEDLINE (PubMed): (“fibromyalgia”[MeSH Terms] OR “fibromyalgia”[All Fields]) AND ((“transcranial direct current stimulation”[MeSH Terms] OR (“transcranial”[All Fields] AND “direct”[All Fields] AND “current”[All Fields] AND “stimulation”[All Fields]) OR “transcranial direct current stimulation”[All Fields]) OR (“transcranial magnetic stimulation”[MeSH Terms] OR (“transcranial”[All Fields] AND “magnetic”[All Fields] AND “stimulation”[All Fields]) OR “transcranial magnetic stimulation”[All Fields])).
PEDro: fibromyalgia AND transcranial direct current stimulation; fibromyalgia AND transcranial magnetic stimulation.
Scopus: fibromyalgia AND (transcranial AND direct AND current AND stimulation OR transcranial AND magnetic AND stimulation).
Cochrane Library: fibromyalgia AND (transcranial direct current stimulation OR transcranial magnetic stimulation).