Epilepsy is most frequent in children and elderly people. Today’s population is ageing and epilepsy prevalence is increasing. The type of epilepsy and its management change with age.
MethodsWe performed a retrospective, observational study comparing patients aged ≥ 65 years with epilepsy diagnosed before and after the age of 65, and describing epilepsy characteristics and comorbidities in each group.
ResultsThe sample included 123 patients, of whom 61 were diagnosed at < 65 years of age (group A), 62 at ≥ 65 of age (group B). Sex distribution was similar in both groups, with 39 men (62.9%) in group A and 37 (60.7%) in group B. Mean age was 69.97 ± 5.6 years in group A and 77.29 ± 6.73 in group B. The most common aetiology was cryptogenic in group A (44.3%, n = 27) and vascular in group B (74.2%, n = 46). History of stroke was present in 12 patients from group A (19.7%) and 32 (51.6%) in group B. Antiepileptic drugs were prescribed at lower doses in group A. Statistically significant differences were found between groups for history of ischaemic stroke, cognitive impairment, psychiatric disorders, and diabetes mellitus; degree of dependence; and number of antiepileptic drugs.
ConclusionAge of onset ≥ 65 years is closely related to cardiovascular risk factors; these patients require fewer antiepileptic drugs and respond to lower doses. Some cases initially present as status epilepticus.
La epilepsia afecta más frecuentemente a niños y personas ancianas. La edad media de la población está aumentando al igual que la prevalencia de la epilepsia. El tipo de epilepsia y su manejo cambia con la edad.
MétodoPresentamos un estudio observacional retrospectivo en el que comparamos pacientes epilépticos mayores de 65 años, con diagnóstico de epilepsia antes y después de los 65 años. Estudiamos las características de la epilepsia de estos pacientes y las comorbilidades.
ResultadosIncluimos 123 pacientes, 61 fueron diagnosticados de epilepsia antes de los 65 años (grupo A) y 62 después de los 65 (grupo B). La distribución en cuanto al género fue similar en ambos grupos, en el A fueron hombres el 62,9% (N = 39) y 60,7% (N = 37) en el B. La edad media fue 69,97 ± 5,6 años en el A y 77,29 ± 6,73 en el B. La etiología más prevalente fue desconocida en el A (44,3%, N = 27) y estructural en el B (74,2%, N = 46). Se hallaron antecedentes de ictus en el 19,7% (N = 12) en el A y 51,6% (N = 32) en el B. La dosis de fármacos antiepilépticos fue menor en el grupo B. Se encontraron diferencias estadísticamente significativas entre los grupos respecto al antecedente de ictus isquémico, deterioro cognitivo, enfermedades psiquiátricas, diabetes mellitus, grado de dependencia y número de fármacos antiepilépticos.
ConclusiónLa epilepsia que se inicia después de los 65 años tiene una estrecha relación con factores de riesgo cardiovascular, precisa para su control un menor número de fármacos y dosis más bajas, aunque en algunos casos puede iniciarse con estatus epiléptico.
Epilepsy, defined as a predisposition to present epileptic seizures, is a common neurological disorder affecting approximately 1% of the population.1 This tendency to present seizures is observed at all ages, especially in childhood and old age,2,3 although the aetiology of epilepsy in these 2 age groups is different. Alterations in cortical development and genetic causes are more frequent in children.4 At older ages, epilepsy is more frequently associated with degeneration or structural alterations secondary to tumours, stroke, or brain trauma.5 The Royal Spanish Academy defines elderly individuals as “people of old age,”6 and the World Health Organization considers adults aged 60 years and older to be elderly.7 This age may seem young; in fact, due to increased life expectancy, the cut-off age for defining “elderly individuals” has changed, with this population now considered to comprise people aged 65 years or older.
The study by Sillanpää et al.8 on an epilepsy registry conducted in Finland between 1986 and 2002 revealed a decrease in the incidence of new-onset epilepsy in children and a significant increase in the elderly population. Besocke et al.9 observed an age-dependent increase in the incidence of epilepsy, with 40 cases per 100 000 population among individuals aged 40–45 years, 80 cases per 100 000 population in those aged 60–65, and 140 cases per 100 000 population in individuals aged over 80. These findings have considerable implications in view of population ageing, especially in Europe and Spain. According to demographic estimates, 70% of the Spanish population is expected to be older than 64 years in the following 30 years.10
Furthermore, epilepsy management is associated with more limitations in elderly individuals than in younger patients due to the higher incidence of comorbidities and concomitant treatments in the former group. Furthermore, some age-related physiological changes (lower levels of free plasma proteins, decreased kidney or liver function, memory impairment) may have a negative impact on treatment.11
Elderly patients with epilepsy may be classified into those developing the condition during old age and those presenting the condition many years before the 65-year mark. The purpose of this study was to analyse the profile of epileptic patients aged ≥ 65 years and to determine whether it differs from that of elderly patients diagnosed with epilepsy before age 65.
Patients and methodsWe conducted a retrospective observational study of all patients attended at the epilepsy unit of Hospital Clínico Universitario Lozano Blesa between June 2013 and December 2014. We included all patients with an established diagnosis of epilepsy according to the definition of the International League Against Epilepsy12 and aged ≥ 65 years. Patients not meeting the inclusion criteria were excluded. The study complies with the ethical principles of the Declaration of Helsinki; informed consent was not obtained from the included patients given the retrospective observational design of the study.
We gathered demographic data (age, sex), clinical data (age at diagnosis of epilepsy, type of seizure [focal, focal impaired awareness, focal to bilateral tonic-clonic, generalised tonic-clonic], seizure frequency over the past 3 months, epilepsy aetiology), complementary test results (head CT, brain MRI, EEG), and antiepileptic drug (AED) treatment (active ingredient, dose, treatment duration).
Patients were classified into 2 groups: group A, including all patients who presented epilepsy before the age of 65 years, and group B, with all patients who developed epilepsy at age ≥ 65 years.
Data were analysed using SPSS statistical software, version 22.0. In the descriptive study, quantitative variables are expressed as means and standard deviations (SD) and qualitative variables as frequencies and percentages.
Quantitative variables were analysed with the t test or with the Mann-Whitney U test, in the case of variables not following a normal distribution. Qualitative variables were analysed with the chi-square test; when the number of cases was less than 5, we used the Fisher exact test. The Bonferroni correction was applied to minimise the risk of type 1 errors. Finally, a multivariate logistic regression analysis was performed. Values of P < .05 were considered to indicate statistically significant differences.
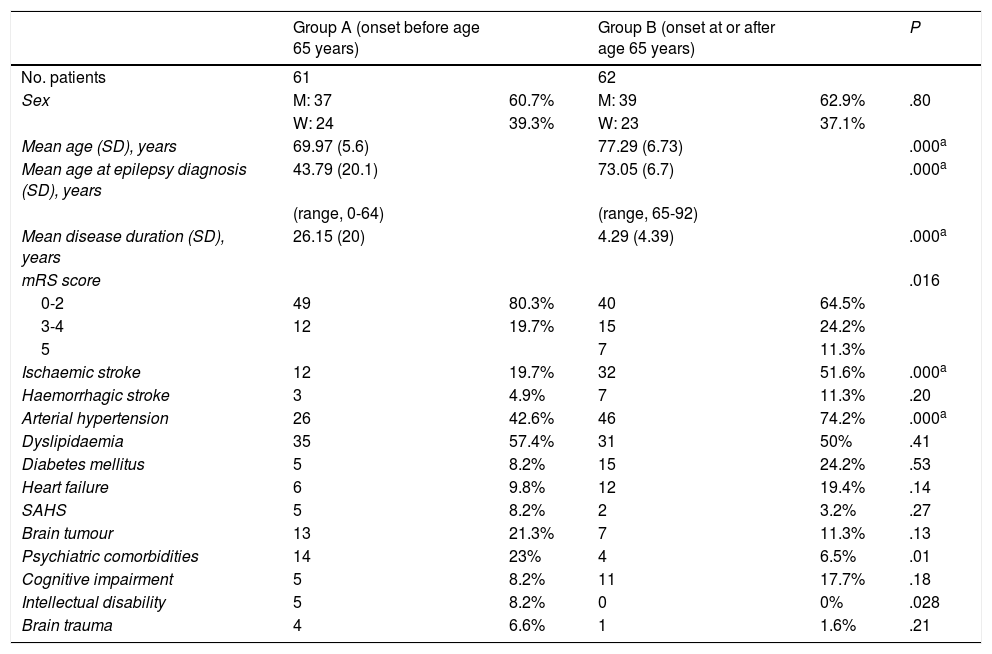
ResultsA total of 123 patients were included in our study: 61 in group A and 62 in group B. Both groups included a similar number of patients and presented similar age and sex distributions, with men accounting for 60.7% (n = 37) of the sample in group A and 62.9% (n = 39) in group B. As expected, mean age and age at diagnosis were lower in group A than in group B (Table 1).
Demographic data, modified Rankin Scale scores, and risk factors in our sample (N = 123), by group.
| Group A (onset before age 65 years) | Group B (onset at or after age 65 years) | P | |||
|---|---|---|---|---|---|
| No. patients | 61 | 62 | |||
| Sex | M: 37 | 60.7% | M: 39 | 62.9% | .80 |
| W: 24 | 39.3% | W: 23 | 37.1% | ||
| Mean age (SD), years | 69.97 (5.6) | 77.29 (6.73) | .000a | ||
| Mean age at epilepsy diagnosis (SD), years | 43.79 (20.1) | 73.05 (6.7) | .000a | ||
| (range, 0-64) | (range, 65-92) | ||||
| Mean disease duration (SD), years | 26.15 (20) | 4.29 (4.39) | .000a | ||
| mRS score | .016 | ||||
| 0-2 | 49 | 80.3% | 40 | 64.5% | |
| 3-4 | 12 | 19.7% | 15 | 24.2% | |
| 5 | 7 | 11.3% | |||
| Ischaemic stroke | 12 | 19.7% | 32 | 51.6% | .000a |
| Haemorrhagic stroke | 3 | 4.9% | 7 | 11.3% | .20 |
| Arterial hypertension | 26 | 42.6% | 46 | 74.2% | .000a |
| Dyslipidaemia | 35 | 57.4% | 31 | 50% | .41 |
| Diabetes mellitus | 5 | 8.2% | 15 | 24.2% | .53 |
| Heart failure | 6 | 9.8% | 12 | 19.4% | .14 |
| SAHS | 5 | 8.2% | 2 | 3.2% | .27 |
| Brain tumour | 13 | 21.3% | 7 | 11.3% | .13 |
| Psychiatric comorbidities | 14 | 23% | 4 | 6.5% | .01 |
| Cognitive impairment | 5 | 8.2% | 11 | 17.7% | .18 |
| Intellectual disability | 5 | 8.2% | 0 | 0% | .028 |
| Brain trauma | 4 | 6.6% | 1 | 1.6% | .21 |
M: men; mRS: modified Rankin Scale; SAHS: sleep apnoea-hypopnoea syndrome; SD: standard deviation; W: women.
Regarding clinical data, focal impaired awareness seizures were the most frequent type in both groups (41% in group A and 53% in group B), with no statistically significant differences between groups.
Head CT was performed in 96.7% of patients (59 from group A and 60 from group B) and brain MRI was performed in 80.3% (n = 49) of patients from group A and 61.3% (n = 38) of patients from group B. Brain MRI findings were normal in 32.8% (n = 16) of patients from group A and 11.3% (n = 4) of patients from group B. MRI revealed encephalomalacia secondary to stroke or other lesions (eg, brain trauma) in 24.6% (n = 12) of patients from group A and 64.5% (n = 25) of patients from group B. Mesial temporal sclerosis was found in one patient from group A (1.6%) and in none of the patients in group B. Statistically significant differences (P < .001) were observed between groups in neuroimaging results. Temporal lobe involvement was observed in 25.6% (n = 13) of patients in group A and 18% (n = 17) of patients in group B. Only 10.3% (n = 5) of patients in group A presented parietal lobe involvement, as compared to 24% (n = 9) of patients in group B. Basal ganglia involvement was observed in 28.2% of group A patients and 38% of group B patients. Again, the differences between the 2 groups were statistically significant (P = .003).
Awake EEG revealed no alterations in 27.9% (n = 17) of group A patients and 40.3% (n = 25) of group B patients. Abnormalities were generalised in 8.2% (n = 5) of group A patients and 6.5% (n = 4) of group B patients, and focal in 26.2% (n = 16) of group A patients and 14.5% (n = 9) of group B patients, although these differences were not statistically significant.
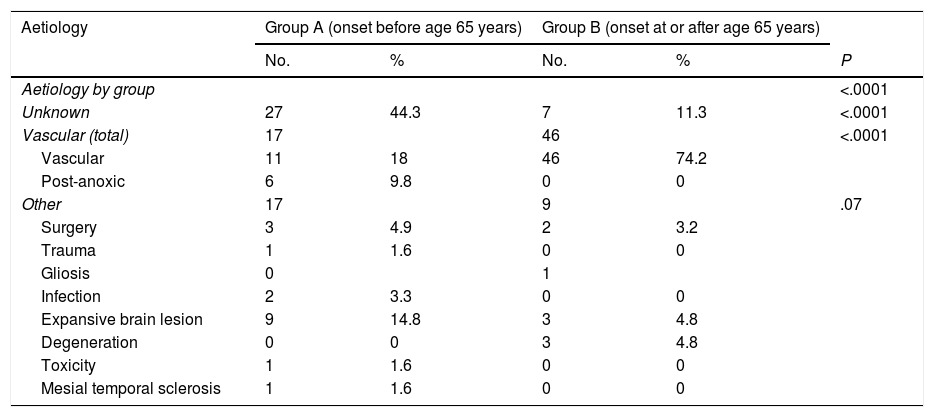
The aetiology of epilepsy was unknown in 44.3% (n = 27) of group A patients and 11.3% (n = 7) of group B patients. The most frequent aetiology in group B was vascular, accounting for 74.2% (n = 46) of patients, vs 18% (n = 11) of patients in group A. Interestingly, post-anoxic epilepsy and epilepsy secondary to lesions compatible with intraparenchymal tumour were observed in 9.8% and 14.8% of group A patients, respectively, but were rarely observed in group B patients (0% and 4.8%, respectively; P < .001). Tables 2 and 3 present detailed information on epilepsy aetiology in our sample.
Aetiology and subtype of epilepsy in our sample, by group.
| Aetiology | Group A (onset before age 65 years) | Group B (onset at or after age 65 years) | |||
|---|---|---|---|---|---|
| No. | % | No. | % | P | |
| Aetiology by group | <.0001 | ||||
| Unknown | 27 | 44.3 | 7 | 11.3 | <.0001 |
| Vascular (total) | 17 | 46 | <.0001 | ||
| Vascular | 11 | 18 | 46 | 74.2 | |
| Post-anoxic | 6 | 9.8 | 0 | 0 | |
| Other | 17 | 9 | .07 | ||
| Surgery | 3 | 4.9 | 2 | 3.2 | |
| Trauma | 1 | 1.6 | 0 | 0 | |
| Gliosis | 0 | 1 | |||
| Infection | 2 | 3.3 | 0 | 0 | |
| Expansive brain lesion | 9 | 14.8 | 3 | 4.8 | |
| Degeneration | 0 | 0 | 3 | 4.8 | |
| Toxicity | 1 | 1.6 | 0 | 0 | |
| Mesial temporal sclerosis | 1 | 1.6 | 0 | 0 | |
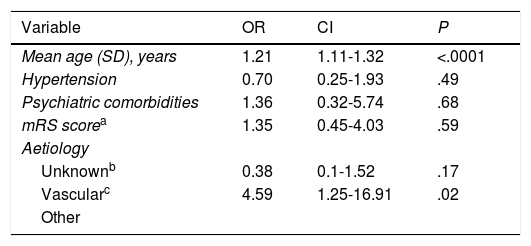
Multivariate analysis of the risk factors selected.
| Variable | OR | CI | P |
|---|---|---|---|
| Mean age (SD), years | 1.21 | 1.11-1.32 | <.0001 |
| Hypertension | 0.70 | 0.25-1.93 | .49 |
| Psychiatric comorbidities | 1.36 | 0.32-5.74 | .68 |
| mRS scorea | 1.35 | 0.45-4.03 | .59 |
| Aetiology | |||
| Unknownb | 0.38 | 0.1-1.52 | .17 |
| Vascularc | 4.59 | 1.25-16.91 | .02 |
| Other |
CI: confidence interval; mRS: modified Rankin Scale; OR: odds ratio; SD: standard deviation.
The main comorbidities observed in both groups were arterial hypertension (42.6% in group A vs 74.2% in group B), history of ischaemic stroke (19.7% vs 51.6%), history of haemorrhagic stroke (4.9% vs 11.3%), dyslipidaemia (57.4% vs 50%), diabetes mellitus (8.2% vs 24.2%), cognitive impairment (8.2% vs 17.7%), and psychiatric disorders (23% vs 6.5%). Further information on risk factors is provided in Tables 1 and 3. We observed statistically significant differences between groups in the prevalence of ischaemic stroke (P = .003), cognitive impairment (P = .045), psychiatric disorders (P = .027), and diabetes mellitus (P = .016). However, after application of the Bonferroni correction, we only found significant inter-group differences in hypertension, ischaemic stroke, and age (Table 3).
Patients from both groups were classified according to the degree of dependence, as measured with the modified Rankin Scale (mRS): mRS 0−2 (no or minimal disability, able to perform daily living activities), mRS 3−4 (moderate or moderate-to-severe disability), and mRS 5 (severe disability); statistically significant differences were observed between groups (P = .016), but the post hoc test did not detect significant differences. Our results are summarised in Tables 1 and 3.
Regarding seizure frequency, 78.3% (n = 47) of group A patients and 74.2% (n = 46) of group B patients had presented no seizures during the 3 months prior to study inclusion. More group B patients than group A patients presented 2 or fewer seizures during that period (93.5% [n = 58] vs 90% [n = 54]), although this difference was not significant. History of status epilepticus was more frequent in group A (16.1% [n = 10]) than in group B (1.6% [n = 1]).
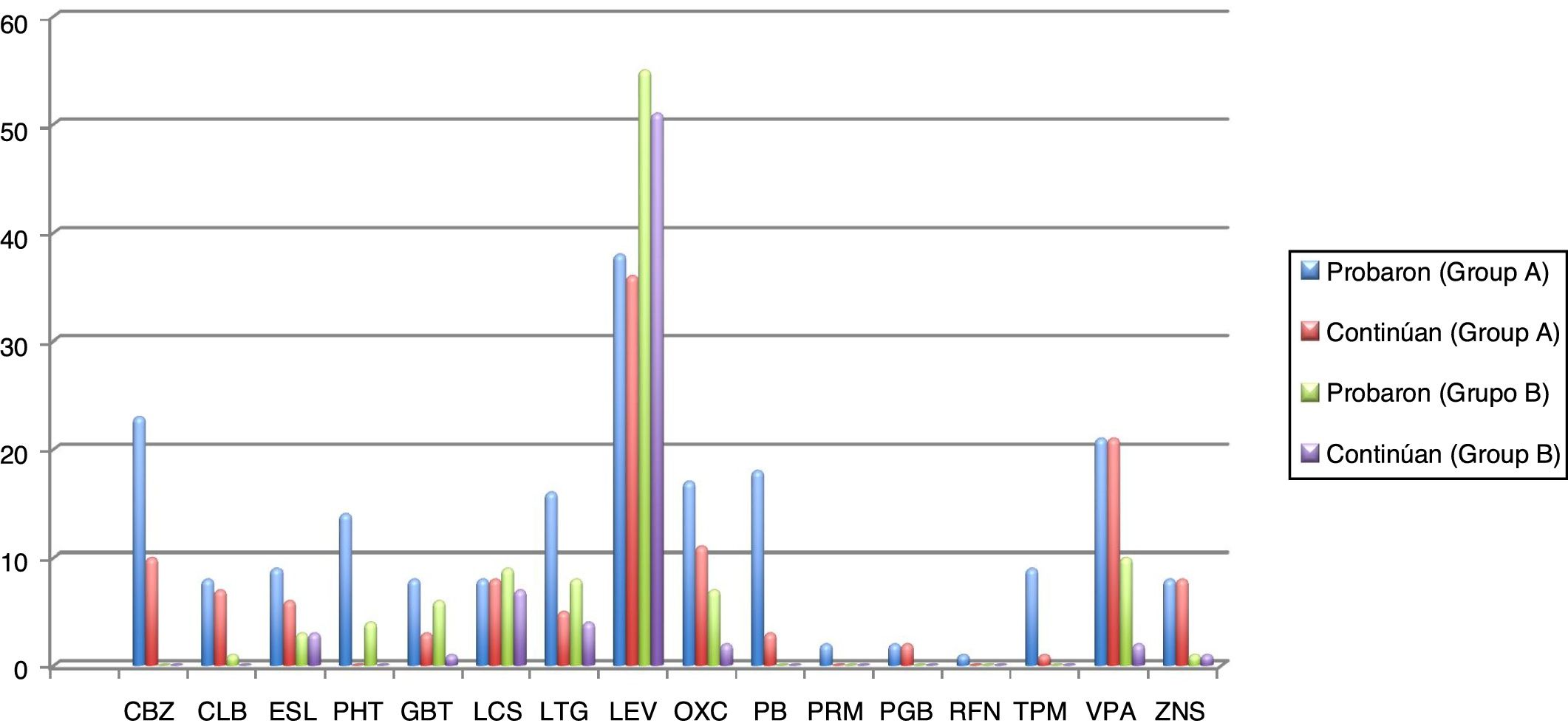
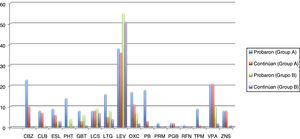
Seizures were more easily managed with a single AED in group B (59.7% [n = 37]) than in group A (16.4% [n = 10]). Similarly, the proportion of patients requiring 3 AEDs was significantly higher in group A (18% [n = 11]) than in group B (16.1% [n = 10]) (P < .001). The AEDs most frequently used in group A were levetiracetam, oxcarbazepine, carbamazepine, and valproic acid, and the best tolerated were lacosamide, levetiracetam, zonisamide, and clobazam. In group B, in turn, the most frequently used AEDs were levetiracetam, valproic acid, lacosamide, and lamotrigine, and the best tolerated were eslicarbazepine, zonisamide, levetiracetam, and lacosamide. Fig. 1 and Table 4 show data on AED use in each group. Group B patients used lower doses than group A patients, although differences are not significant.
Number of elderly patients in groups A (disease onset before 65 years of age) and B (onset at or after 65 years) in whom different antiepileptic drugs were tried and who continued treatment with these drugs (results are expressed as absolute numbers). Mean doses (mg/24 h) with standard deviations and ranges (mg/24 h) are also indicated.
CBZ: carbamazepine; CLB: clobazam; ESL: eslicarbazepine; GBT: gabapentin; LCS: lacosamide; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PB: phenobarbital; PGB: pregabalin; PHT: phenytoin; PRM: primidone; RFN: rufinamide; TPM: topiramate; VPA: valproic acid; ZNS: zonisamide.
Antiepileptic drugs and mean daily dose (mg/24 h) in our sample, by group.
| AED | Group A (onset before age 65 years) | Group B (onset at or after age 65 years) | P | ||
|---|---|---|---|---|---|
| mg/24 h (SD) | mg/24 h (SD) | Doseb | Tried | Continuedc | |
| CBZ | 773 (344.06) | ||||
| CLB | 13.75 (5.18) | 10 | .33 | .017 | .027 |
| ESL | 800 (346.41) | 800 | 1 | .75 | .32 |
| GBT | 1500 (555.49) | 1216.67 (856.54) | .5 | .55 | .37 |
| LCS | 212.5 (99.1) | 222.22 (66.67) | .81 | .82 | .76 |
| LEV | 1789.47 (731.82) | 1545 (680.57) | .1 | .001a | .005 |
| LTG | 329.69 (229.35) | 150 (106.9) | .05 | .06 | .74 |
| OXC | 788.32 (284.79) | 842.86 (335.94) | .69 | .02 | .009 |
| PHT | 271.43 (46.88) | 300 | .25 | .01 | |
| TPM | 277.78 (183.9) | 134.85 | .000a | .12 | |
| VPA | 1342.85 (459.97) | 1210 (404) | .44 | .24 | |
| ZNS | 300 (92.58) | 300 | .87 | .24 | .24 |
AED: antiepileptic drug; CBZ: carbamazepine; CLB: clobazam; ESL: eslicarbazepine; GBT: gabapentin; LCS: lacosamide; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHT: phenytoin; TPM: topiramate; VPA: valproic acid; ZNS: zonisamide.
Epilepsy is frequent in adult patients; its incidence increases with age and is linked to increased incidence of other diseases that can cause epilepsy, particularly tumours, ischaemic or haemorrhagic stroke, and dementia.13 Our results show that age in itself is a risk factor for epilepsy in old age. In patients developing epilepsy at younger ages (< 65 years), the condition may be associated with idiopathic generalised epilepsy, malformations of cortical development, post-anoxic episodes during prenatal development or delivery, and mesial temporal sclerosis, among other aetiologies.
Our study compared 2 groups of elderly patients with epilepsy: those presenting epilepsy before the age of 65 years, and those developing the condition at 65 years of age or older. Hesdorffer et al.14 estimate the risk of status epilepticus in elderly patients at 0.4% at age 75 years; subsequent studies report an incidence rate of 86 cases per 100 000 population among elderly people, with an upward trend, reaching 100 cases per 100 000 population among individuals aged 70–79 years.15 In our study, only one patient from group B presented history of status epilepticus, whereas group A included several cases.
The study by Stefan et al.13 established 3 groups according to age of epilepsy onset: onset at ages 50–65, onset after age 65 years, and onset at ages 18–50 years. The first 2 groups showed a higher incidence of focal seizures (26.9% and 21.5%, respectively) than groups A and B from our study (10% and 14%, respectively). The prevalence of focal impaired awareness seizures was 64.2% and 48.1% in the first 2 groups from the study by Stefan et al.,13 compared to 41% and 53% in groups A and B from our study. However, the percentage of patients with generalised tonic-clonic seizures was lower in our group B than in the study by Stefan et al.13 (15% vs 46.8%), in line with the data reported by Hughes and Zialcita16 in 1999. We recommend classifying patients into 2 single age groups to better assess disease progression and the associated comorbidities.
As expected, a high percentage of patients from group A showed no alterations on neuroimaging studies, as compared to group B. This finding was associated with a higher frequency of idiopathic epilepsy in group A. Likewise, group B patients more frequently displayed focal lesions (especially due to stroke or trauma), which may have a direct causal role in the aetiology of epilepsy.
Our results on the frequency of comorbidities in both groups are noteworthy. This information is crucial for better understanding of this type of population with a view to optimising treatment. These patients present a high incidence of cardiovascular risk factors, mainly arterial hypertension, diabetes mellitus, and dyslipidaemia; the latter is the most frequent risk factor among patients with epilepsy onset before the age of 65 years. This may be explained by the AEDs used for epilepsy management in this population, which may increase cardiovascular risk and, as a result, the incidence of brain lesions causing epilepsy. In fact, history of ischaemic stroke was found to be much more prevalent in both groups than history of haemorrhagic stroke, and it was twice as frequent in group B as in group A, representing the most frequent aetiology of epilepsy in the elderly population. We should also mention the large number of patients with cognitive impairment in group B. In contrast, psychiatric comorbidities were more frequent among patients with epilepsy onset before 65 years of age. A small subgroup of patients (more numerous in group A than in group B) were diagnosed with sleep apnoea-hypopnoea syndrome.
These patients require comprehensive management, considering not only epilepsy and the associated comorbidities but also cognitive function and independence. Previous studies have not analysed patients’ degree of dependence. In our study, group B patients exhibited a higher degree of dependence. The literature includes extensive data on dependence in this population group. Optimising independence and preventing new seizures may help prevent falls, bone fractures, hospitalisation, and early admission to nursing homes.17
Regarding epilepsy treatment, marked differences were found between groups in the number of AEDs used: 16.4% (n = 10) of patients in group A received a single AED, compared to 59.7% (n = 37) of patients in group B. Furthermore, doses were lower in group B. However, in a study published by Tanaka et al.18 in 2013, the doses administered to elderly patients with epilepsy were considerably lower than in our study. Unlike in the study by Phabphal et al.,19 and in line with the evidence reported by Stefan et al.,13 we found a clear preference for new-generation AEDs to minimise the risk of adverse reactions. We gathered data not only on the AEDs used but also on drug and treatment discontinuation. Although insufficient evidence is available, our data suggest that new-generation AEDs are better tolerated, even at relatively high doses, as they present fewer drug interactions and a better biopharmacokinetic profile.
In the light of the above, presence of comorbidities associated with epilepsy should be assessed before prescribing an AED. A sizeable percentage of patients with epilepsy present cardiovascular risk factors; certain AEDs may directly or indirectly increase the risk of cardiovascular events.20 Wherever possible, we should avoid prescribing AEDs that may cause such psychiatric symptoms as depression or psychosis to patients with mental disorders, in order to avoid exacerbating the underlying psychological conditions and to ensure proper adherence to antiepileptic treatment.
Our study has several limitations. The patients included were attended at our centre’s epilepsy unit, which may have resulted in selection bias, as these patients may be particularly refractory to treatment. Furthermore, data were obtained retrospectively by reviewing medical records. Further studies with larger samples should be conducted to gather more information about epilepsy in this population group, as the incidence and the burden of the condition on the healthcare system is increasing.
FundingThis study has received no specific funding from any public, private, or non-profit organisation.
Conflicts of interestThe author has no conflicts of interest to declare.
Please cite this article as: Suller Marti A, Bellosta Diago E, Vinueza Buitron P, Velázquez Benito A, Santos Lasaosa S, Mauri Llerda JÁ. Epilepsia en el anciano: ¿la edad de inicio marca la diferencia? Neurología 2022;37:171–177.