End-of-life care frequently involves the treatment of difficult-to-manage symptoms. Patients with central nervous system involvement (tumour, stroke) may present difficult-to-control epileptic seizures, which have significant physical and psychological consequences and a negative impact on the patient’s and his/her family’s quality of life. In palliative care, oral administration of drugs to treat seizures is usually preferable in the interest of patient well-being, but some circumstances may prevent the use of these drugs, making it necessary to use alternative routes, such as subcutaneous administration.
We present our experience in the use of subcutaneous levetiracetam (LEV) in the palliative care of patients with epileptic seizures attended at a chronic care and long-stay hospital.
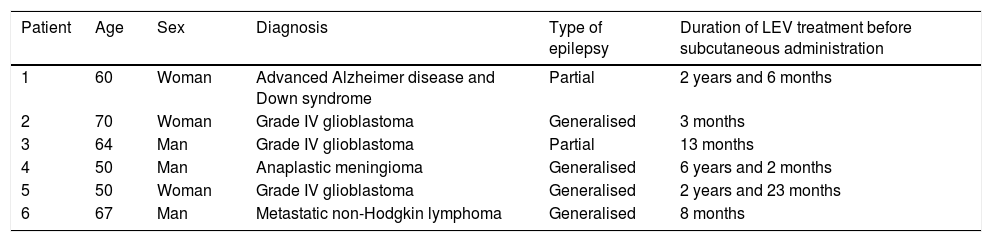
Six patients were treated between July 2016 and December 2019. Table 1 describes the patients’ characteristics. Subcutaneous treatment was started because oral administration was impossible and due to difficulties placing a venous catheter.
Clinical characteristics of our patient sample.
| Patient | Age | Sex | Diagnosis | Type of epilepsy | Duration of LEV treatment before subcutaneous administration |
|---|---|---|---|---|---|
| 1 | 60 | Woman | Advanced Alzheimer disease and Down syndrome | Partial | 2 years and 6 months |
| 2 | 70 | Woman | Grade IV glioblastoma | Generalised | 3 months |
| 3 | 64 | Man | Grade IV glioblastoma | Partial | 13 months |
| 4 | 50 | Man | Anaplastic meningioma | Generalised | 6 years and 2 months |
| 5 | 50 | Woman | Grade IV glioblastoma | Generalised | 2 years and 23 months |
| 6 | 67 | Man | Metastatic non-Hodgkin lymphoma | Generalised | 8 months |
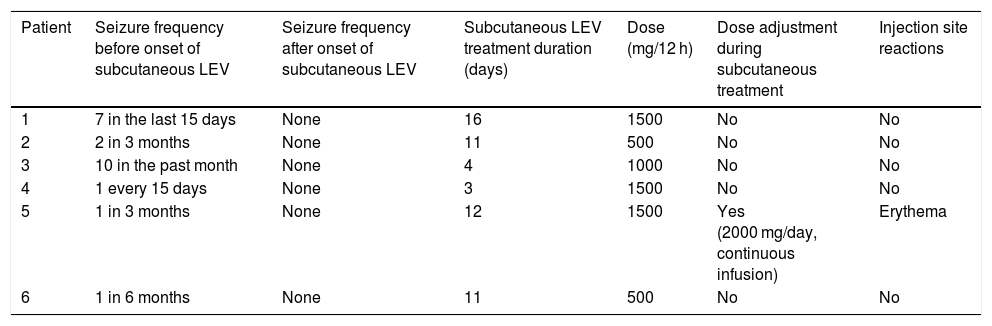
Administration was intermittent, every 12 hours, with the drug diluted in 100 cc of saline solution, using a system with a 22 G butterfly needle. The system was placed in the lower hemiabdomen, with the injection site being rotated. The dose administered was the same as that previously taken by the patient, between 500 and 1500 mg every 12 hours. One patient presented cutaneous irritation at the administration site with doses of 1500 mg every 12 hours, so we reduced the dose to 2000 mg a day, with continuous subcutaneous administration; no further events were reported. Mean duration of subcutaneous LEV administration was 9.5 days (range: 3-16), and the total number of doses administered amounted to 103. No patient presented seizures after onset of subcutaneous treatment. Table 2 provides detailed information regarding treatment response.
Clinical response after subcutaneous levetiracetam administration.
| Patient | Seizure frequency before onset of subcutaneous LEV | Seizure frequency after onset of subcutaneous LEV | Subcutaneous LEV treatment duration (days) | Dose (mg/12 h) | Dose adjustment during subcutaneous treatment | Injection site reactions |
|---|---|---|---|---|---|---|
| 1 | 7 in the last 15 days | None | 16 | 1500 | No | No |
| 2 | 2 in 3 months | None | 11 | 500 | No | No |
| 3 | 10 in the past month | None | 4 | 1000 | No | No |
| 4 | 1 every 15 days | None | 3 | 1500 | No | No |
| 5 | 1 in 3 months | None | 12 | 1500 | Yes (2000 mg/day, continuous infusion) | Erythema |
| 6 | 1 in 6 months | None | 11 | 500 | No | No |
LEV: levetiracetam.
Epileptic seizures may occur at some point in disease progression in up to 70% of patients with brain tumours, as well as in patients with some advanced neurodegenerative diseases.1,2 In recent years, LEV has been used more frequently than such other antiepileptic drugs as phenytoin and valproate, due to its favourable safety and tolerability profile. LEV causes less sedation than other drugs used in the treatment of seizures, such as benzodiazepines or phenobarbital; this is important in palliative care patients who do not want to limit their cognition or ability to interact with their surroundings during the final stages of their disease. Several authors have published their experience in the use of subcutaneous LEV (continuous infusion or every 12 hours) as an off-label indication for compassionate use.3–7 Therefore, our patients and their families were duly informed and consented to the treatment. Regarding tolerability and clinical response, published case series4,6 report good tolerability, safety, and effectiveness. In our series, after onset of subcutaneous LEV, all patients were seizure-free and we observed only one case of erythema at the infusion site, which immediately resolved after reducing the dose and switching to continuous administration. Publications determining the levels of LEV during the treatment period have shown that they always remain within therapeutic ranges.8,9 This technique is not available at our centre; however, as no seizures were observed in our patients, we believe that the doses administered were adequate.
Subcutaneous treatment lasted several days (between 3 and 16 days), until the death of the patient. We did not find treatment periods longer than one month in the literature3–7; therefore, it is unclear whether effectiveness or safety would be maintained for more prolonged periods.
To conclude, we consider that subcutaneous LEV is a safe and effective alternative in palliative care patients with epileptic seizures.
Please cite this article as: Más-Sesé G, Martín-Bautista D, Navarro-Catalá A. Experiencia de uso de levetiracetam subcutáneo en pacientes paliativos. Neurología. 2021;36:474–475.