Moebius syndrome (OMIM 157900) is characterised by facial paralysis with impairment of ocular abduction. The facial nerve (cranial nerve VII) and the abducens nerve (cranial nerve VI) are the ones most commonly involved, but other cranial nerves can also be affected. The phenotype is variable and may include congenital, orofacial and limb defects.1
The Poland syndrome (OMIM 173800) is characterised by the presence of unilateral brachysyndactyly and ipsilateral aplasia of the sternal portion of the pectoralis major muscle. It is sometimes called Poland sequence, as it was initially described in Poland.2
The combination of Moebius and Poland syndrome is rarely seen. It has been estimated to occur in 1 in 500,000 people; in the literature we reviewed, we did not find this association with cases of prenatal exposition to misoprostol.3
We present a report on Moebius and Poland syndrome in a newborn prenatally exposed to misoprostol.
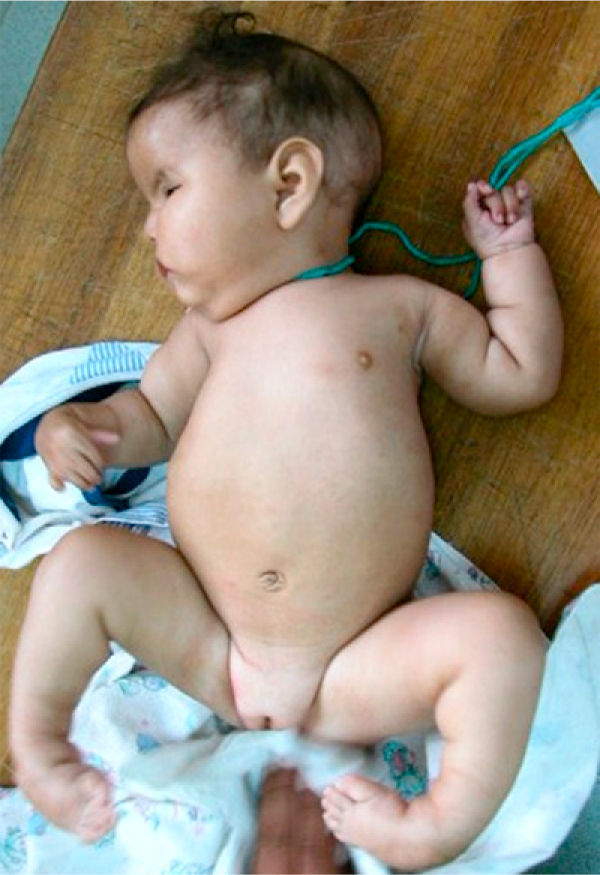
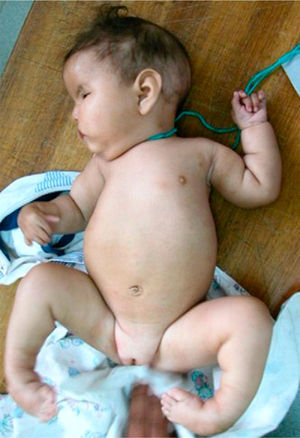
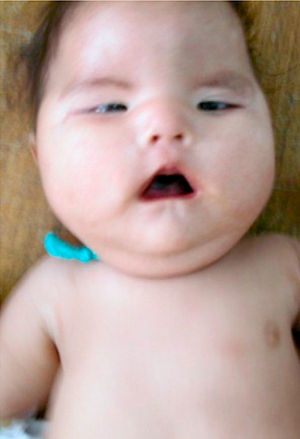
The patient, who was the son of an 18-year-old mother, was brought to consult for facial dysmorphism. However, on examination he was found to have bilateral facial paralysis, a rounded face, narrow palpebral fissures, cupid's bow lips, arched palate, micrognathia and hypoplasia with absence of pectoralis major nipple (Atelier), as well as proximal syndactyly of second and third fingers and bilateral clubfoot (Figs. 1 and 2). As an important background fact, we found that his mother had used 400g of misoprostol orally and vaginally at 5 weeks of gestation to bring about an abortion, but had had only slight bleeding.
Misoprostol is a synthetic analogue of prostaglandin E1 approved by drug regulatory agencies in many countries for the prevention and treatment of gastric ulcers associated to the use of non-steroidal anti-inflammatories, due to its gastric acid antisecretory effect. Prenatal exposure to misoprostol has been associated to vascular disruption defects, mainly Moebius sequence and terminal transverse type limb defects.4,5
The abnormalities in the vascular structure could be secondary to teratogenic effects. Teratogens can act directly by reducing blood flow or blood vessel development, changing anatomy and/or structure.6,7 The vascular abnormalities of the right subclavian artery observed in Poland syndrome could be related to a vascular disruption caused by misoprostol during a critical period.5,8
The presentation of a case of Moebius and Poland syndromes associated to prenatal exposure to misoprostol has not been reported. However, this combination of two congenital defects has been reported as being associated to another vascular disruptor (such as cocaine).3
The presentation of these two defects in a same patient provides evidence that both pathologies present similar mechanisms, which are probably those of a vascular disruption.
Defects due to a vascular disruption are structural disturbances in development produced by vascular problems, such as severe intermittent vasoconstriction, abnormal vessel regression during the remodelling of the vascular system, arterial thrombosis or any other phenomenon that produces a lack of O2.9
The aetiology of Moebius and Poland syndromes is unknown; a possible vascular origin has been suggested for the two pathologies. Hypotheses suggest a transient ischemia, particularly in the vertebral arteries.10,11 Premature obstruction or regression of the terminal arteries of the trigeminal (V) nerve and/or delayed formation of the vertebral basilar system could lead to anomalies in cranial nerve development.12 In children with Moebius syndrome, cerebral necrosis has been reported together with capillary telangiectasia in the midbrain and bridge.13 Disruption of the subclavian artery occurs around week 6 of gestation and is related to Moebius–Poland syndrome; this same phenomenon is related to transversal limb defects and arthrogryposis.14 The disruption phenomenon can be secondary to a blood flow interruption secondary to an arterial spasm during the sensitive embryonic phase.15,16
Please cite this article as: Pachajoa H, Isaza C. Primer caso de síndrome de Moebius-Poland en niño expuesto prenatalmente a misoprostol. Neurología. 2011;26:502-3.