Status epilepticus (SE) is a neurological emergency associated with high morbidity and mortality. One prognostic factor is the type of SE. The purpose of this review is to analyse the most recent recommendations of different scientific societies and expert groups on the treatment of SE, and the latest studies, to assess the literature on the management of focal SE.
MethodsWe searched PubMed for studies published between 1 August 2008 and 1 August 2018 on the pharmacological treatment of focal SE and its different types in adults.
ResultsWe identified 29 publications among reviews, treatment guidelines, meta-analyses, clinical trials, and case series on the treatment of SE. Only 3 of them accounted for whether SE was focal or generalised; 4 focused exclusively on focal SE, and 7 differentiated between convulsive and non-convulsive SE and also record the presence of focal seizures. Treatment recommendations for focal SE do not differ from those of generalised SE in stages I and II: initially intravenous lorazepam or diazepam, if the intravenous route is available, and otherwise intramuscular midazolam, followed by intravenous phenytoin, valproate, levetiracetam, or lacosamide if seizures persist. Use of anaesthetic drugs should be delayed for as long as possible in patients with refractory focal SE.
ConclusionsThe available scientific evidence is insufficient to claim that pharmacological treatment of focal SE should be different from treatment for generalised SE. More studies with a greater number of patients are needed.
El estatus epiléptico (EE) es una emergencia neurológica asociada a una elevada mortalidad y morbilidad. Uno de los factores pronósticos es el tipo de EE. El objetivo de este trabajo es analizar las últimas recomendaciones de las distintas sociedades científicas y grupos de expertos sobre el tratamiento del EE, así como los estudios más recientes, para evaluar las referencias sobre el manejo del EE de tipo focal.
MétodosSe realizó una búsqueda en PubMed entre el 01/08/2008 y el 01/08/2018 sobre el tratamiento farmacológico del EE focal y sus distintos tipos en adultos.
ResultadosSe encontraron 29 publicaciones entre revisiones, guías terapéuticas, metaanálisis, ensayos clínicos y estudios de casos sobre el tratamiento del EE. De éstas, solamente 3 tiene en cuenta si el EE es focal o generalizado, 4 se centran exclusivamente en EE focales y 7 diferencian entre EE convulsivo o no convulsivo especificando si incluyen crisis focales. Las recomendaciones terapéuticas para un EE focal no difieren de las de un EE generalizado en las fases I y II: inicialmente lorazepam o diazepam intravenoso si hay acceso venoso o midazolam intramuscular en caso contrario, seguido de fenitoína, valproato, levetiracetam o lacosamida intravenosos si persisten las crisis. En EE focales refractarios se recomienda retrasar en lo posible el inicio de fármacos anestésicos.
ConclusionesActualmente no disponemos de suficiente evidencia científica para afirmar que el tratamiento farmacológico del EE focal debe ser distinto al del EE generalizado. Son necesarios más registros con un amplio número de pacientes.
Status epilepticus (SE) is a neurological emergency requiring immediate medical attention. According to studies conducted in Europe and the United States, incidence rates range between 6.2 and 41 cases per 100 000 person-years.1–3 Prognosis depends on several factors: type of SE, aetiology, duration, level of consciousness at onset, age, and treatment response. Taking these factors into account, the global mortality rate is estimated at 8% to 65%.4
The most recent semiological classification of SE establishes 2 broad groups according to whether the patient presents motor symptoms; within each group, SE may be of focal or generalised origin (Table 1).5 While the latest classification of seizures replaces the term “partial” with “focal,” and these seizures are classified as “aware” or “impaired awareness” rather than “simple” or “complex,”6 we maintain the terms “partial” and “complex” in this review as the majority of articles reviewed were published before this classification.
Semiological classification of status epilepticus.
| A. Prominent motor symptoms |
| A.1. Convulsive SE (CSE; synonym: tonic-clonic SE) |
| A.1.a. Generalised convulsive |
| A.1.b. Focal onset evolving to bilateral convulsive SE |
| A.1.c. Unknown whether focal or generalised |
| A.2. Myoclonic SE (prominent epileptic myoclonic jerks) |
| A.2.a. With coma |
| A.2.b. Without coma |
| A.3. Focal motor |
| A.3.a. Repeated focal motor seizures (Jacksonian) |
| A.3.b. Epilepsia partialis continua (EPC) |
| A.3.c. Adversive status |
| A.3.d. Oculoclonic status |
| A.3.e. Ictal paresis (focal inhibitory SE) |
| A.4. Tonic SE |
| A.5. Hyperkinetic SE |
| B. Without prominent motor symptoms (non-convulsive SE; NCSE) |
| B.1. NCSE with coma (including “subtle” SE) |
| B.2. NCSE without coma |
| B.2.a. Generalised |
| B.2.a.a. Typical absence status |
| B.2.a.b. Atypical absence status |
| B.2.a.c. Myoclonic absence status |
| B.2.b. Focal |
| B.2.b.a. Without impairment of consciousness (continuous aura, without autonomic, sensory, visual, olfactory, gustatory, auditory, or emotional/psychic/experiential symptoms) |
| B.2.b.b. Aphasic status |
| B.2.b.c. With impairment of consciousness |
| B.2.c. Unknown whether focal or generalised |
| B.2.c.a. Autonomic SE |
Source: adapted from Fisher et al.6
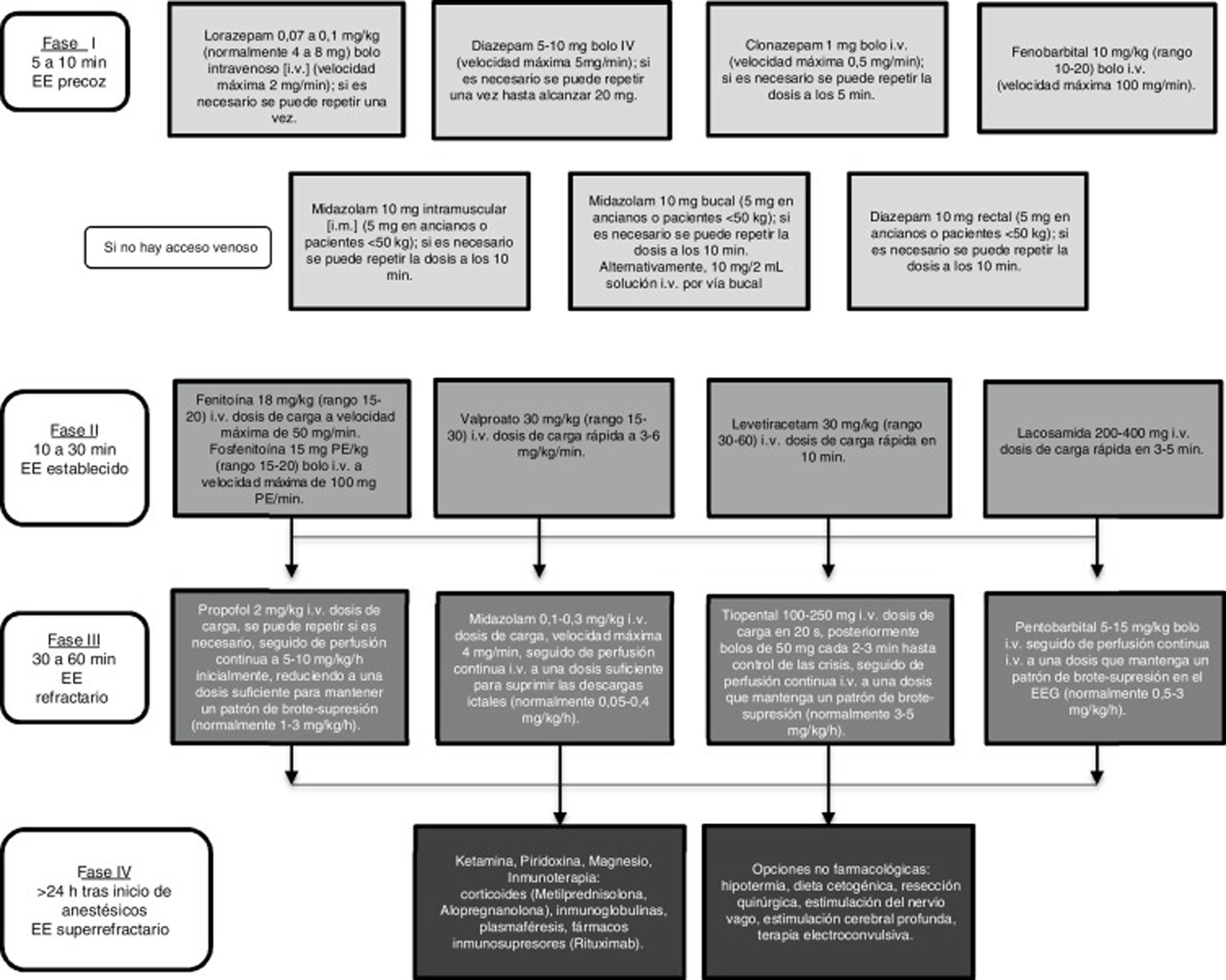
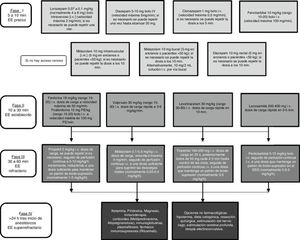
There is broad consensus that in an emergency such as SE, regardless of seizure type, diagnostic and therapeutic measures must be implemented as rapidly as possible. Controlling SE begins with a stabilisation phase, in which general measures are applied, followed by several phases of pharmacological treatment, in line with the patient’s progression (Fig. 1).1,7,8
In recent years, despite progress in the definition and classification of SE, the understanding of its pathophysiology, and in electroencephalography techniques (which are increasingly able to distinguish between SE of focal and generalised origin), few advances have been made in the therapeutic management of the condition. For instance, the majority of publications on the treatment of SE either address convulsive SE (CSE) or do not specify the type. Given that the different available antiepileptic drugs (AED), with their different action mechanisms, often present different levels of efficacy in the treatment of different types of seizure, it seems reasonable to consider that the same may be true in the treatment of different types of SE.
In the light of these facts, the purpose of this study is to review and analyse the latest recommendations of different scientific societies, expert panels, and publications on the treatment of SE in order to evaluate the references on the treatment of focal SE and to assess the possibility of establishing a series of treatment recommendations for use in clinical practice.
Material and methodsWe conducted a systematic review of documents, publications, and studies by different scientific bodies that address the treatment of focal SE. References were gathered from PubMed, using the following search terms: “partial/focal status epilepticus,” “drug therapy,” “repeated focal motor seizures,” “epilepsia partialis continua,” “aphasic status epilepticus,” “versive status epilepticus,” “oculoclonic status epilepticus,” “inhibitory status epilepticus,” and “secondarily generalized status epilepticus.” The following filters were applied:
- •
Time of publication: 01/08/2008 to 01/08/2018
- •
Types of study: reviews, treatment guidelines, meta-analyses, clinical trials, and case reports
- •
Languages: English, French, German, Portuguese, and Spanish
- •
Age range: older than 13 years
- •
Human studies only.
We also analysed the reference sections of the articles selected in order to identify additional studies potentially eligible for analysis.
The main inclusion criterion applied to articles identified in the search was that they focused on the pharmacological treatment of SE.
The exclusion criteria were as follows:
- -
Exclusively addressing non-therapeutic aspects of SE (eg, definitions, classification, diagnosis)
- -
Addressing a specific type of epilepsy (eg, epilepsy secondary to Rasmussen encephalitis)
- -
Addressing pharmacological treatments that target seizures of a specific aetiology or are used in very advanced stages of SE, such as immunotherapy, pyroxidine, magnesium, etc
- -
Addressing non-pharmacological treatments, such as neurosurgery or ketogenic diet.
The initial search identified 789 studies, which were subsequently reviewed for inclusion in the study.
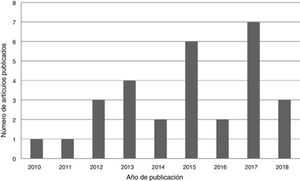
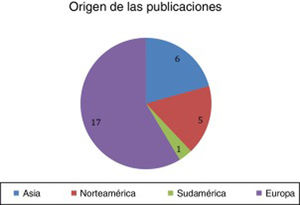
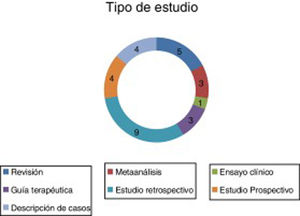
ResultsA total of 29 articles met the selection criteria (Figs. 2–4).
These articles included various types of studies on the pharmacological treatment of different stages of SE (Table 2).
Overview of the characteristics of the reviewed studies.
| Stage of SE | Type of study | Year | Author | SE episodes (n) | Type of SE | Conclusion |
|---|---|---|---|---|---|---|
| All | Treatment guidelines | 2010 | Meierkord et al.9 | – | CSE, NCSE, partial SE | IV LZP or DZP+PHT (level A rating) |
| Recommend delaying sedation in patients with complex partial seizures | ||||||
| All | Treatment guidelines | 2012 | Brophy et al.10 | – | CSE | IV LZP or IM MDZ (class category I, level of evidence A) + VPA (level of evidence A) |
| MDZ as first choice for therapeutic coma (class category IIa, level of evidence B) | ||||||
| All | Treatment guidelines | 2016 | Mercadé Cerdá et al.11 | – | CSE, NCSE, partial SE | IV LZP or IV DZP+PHT or PB for CSE (level of evidence IV) |
| Recommend delaying sedation in NCSE | ||||||
| Stage 1 | Double-blind randomised clinical trial | 2012 | Silbergleit et al.12 | 893 | CSE | IM MDZ controls more seizures than IV LZP. |
| Stage 1 | Meta-analysis | 2016 | Brigo et al.13 | 656 | CSE | IV LZP and IV DZP present similar efficacy as first-line treatments. |
| Stage 2 | Retrospective study | 2011 | Alvarez et al.14 | 187 | Not specified | LEV presents poorer efficacy than PTH and VPA. |
| Stage 2 | Prospective randomised controlled study | 2015 | Mundlamuri et al.15 | 150 | CSE | PHT, VPA, and LEV present similar efficacy as second-line treatments after LZP. |
| Stage 2 | Prospective randomised study | 2015 | Chakravarthi et al.16 | 44 | Not specified | LEV and PHT present similar efficacy for SE refractory to LZP. |
| Stage 2 | Meta-analysis | 2014 | Yasiry and Shorvon17 | 798 | CSE | PHT presents poorer efficacy than VPA, PB, and LEV. |
| Stage 2 | Prospective study | 2015 | Atmaca et al.18 | 30 | CSE, NCSE, and EPC | LEV is well tolerated and effective for both focal and generalised SE. |
| Stages I and II | Retrospective study | 2013 | Rantsch et al.19 | 167 | CSE, NCSE, and EPC | CLP is superior to DZP, MDZ, LEV, and VPA in generalised CSE; no differences were observed for NCSE or EPC. |
| Stages I and II | Meta-analysis | 2014 | Prasad et al.20 | 2755 | Not specified | IV LZP is better than IV DZP or PHT in controlling SE. |
| In pre-hospital management, IM MDZ seems more effective than IV LZP in controlling seizures. | ||||||
| Stage 3 | Retrospective study | 2013 | Höfler and Trinka21 | 136 | CSE, NCSE, partial SE | LCM may be an alternative to classic AEDs in second-line treatment of established SE. |
| Stage 3 | Case study | 2013 | Hawkes et al.22 | 2 | Partial SE | Oral LCM may be a treatment option for refractory simple partial SE. |
| Stage 3 | Case study | 2013 | Spalletti et al.23 | 1 | EPC | IV LCM improved refractory partial motor SE. |
| Stage 3 | Retrospective study | 2017 | Newey et al.24 | 84 | CSE, NCSE | IV LCM seems promising for SE management. |
| Stage 3 | Retrospective study | 2018 | Santamarina et al.25 | 165 | CSE, NCSE | Response to LCM is better in SE refractory to benzodiazepines and with a loading dose>5.3mg/kg. |
| Stage 3 | Retrospective study | 2015 | Redecker et al.26 | 10 | CSE, NCSE, partial SE | PER dosed at 6mg in 10 cases of refractory SE favoured resolution of seizures in 2-6 episodes (depending on efficacy criteria). |
| Stage 3 | Prospective randomised study | 2018 | Masapu et al.27 | 23 | Not specified | Propofol is similar to MDZ in terms of seizure control, complications, hospital stays, and mortality. |
| Stage 3 | Retrospective study | 2015 | Marchi et al.28 | 467 | CSE, NCSE, partial SE | Therapeutic coma is associated with poor prognosis, especially in SE with complex partial seizures. |
| All | Retrospective study | 2017 | De la Morena Vicente et al.29 | 84 | Generalised SE/partial SE | There were no significant differences in mortality or sequelae between types of SE. |
| Stage 3 | Questionnaire sent to randomly selected hospitals | 2015 | Patel et al.30 | – | CSE | Only 21 of 75 British hospitals evaluated had protocols for the treatment of refractory CSE in the ICU. |
| Stages III and IV | Review | 2018 | Holtkamp31 | – | Generalised CSE, complex focal SE | Patients with refractory complex focal SE should receive second-line AEDs not previously administered: LEV, PHT, VPA, LCM. |
| Stages III and IV | Review | 2018 | Rai and Drislane32 | – | Generalised CSE, typical absence SE, complex partial SE, EPC, myoclonic SE | Except in the case of generalised CSE, non-sedative drugs are recommended: IV PHT, fosphenytoin, PB, VPA, LCM, LEV; enteral TPM or CBZ. |
| All | Review | 2017 | Trinka and Kälviäinen7 | – | Not specified | 40% of patients with SE did not respond to early administration of benzodiazepines. It is important to determine the cause of SE. |
| All | Review | 2017 | Bauerschmidt et al.1 | – | Not specified | The authors support early, intensive treatment with benzodiazepines in stage 1 and highlight the outcomes of IV LCM in stage 2 and ketamine and clobazam in stage 3. |
| All | Review | 2017 | Zaccara et al.8 | – | CSE | The authors highlight the importance of early treatment onset, the disadvantages of PHT in stage 2, and the advantages of MDZ in stage 3. |
| All | Case study | 2017 | Qiu et al.36 | 1 | Aphasic SE | The patient improved with OXC. Aphasic SE is often treated with DZP and PHT due to their broad spectrum. |
| All | Review | 2016 | Mameniskiené and Wolf37 | – | EPC | Treatment of EPC depends on aetiology. It is usually treatment-resistant; the best outcomes are associated with TPM and LEV. |
AED: antiepileptic drug; CBZ: carbamazepine; CLP: clonazepam; CSE: convulsive status epilepticus; DZP: diazepam; EPC: epilepsia partialis continua; ICU: intensive care unit; IM: intramuscular administration; IV: intravenous administration; LCM: lacosamide; LEV: levetiracetam; LZP: lorazepam; MDZ: midazolam; NCSE: non-convulsive status epilepticus; OXC: oxcarbazepine; PB: phenobarbital; PER: perampanel; PHT: phenytoin; SE: status epilepticus; TPM: topiramate; VPA: valproic acid.
The oldest document in this group is the European guidelines, published in 2010.9 For patients with CSE, these guidelines recommend administering intravenous (IV) lorazepam (LZP) (level A rating) or, if this drug is not available, IV diazepam (DZP) followed by phenytoin (PHT) (level A rating). The authors found insufficient evidence to recommend valproic acid (VPA) for the first-line treatment of this type of SE. In non-convulsive SE (NCSE) (complex partial or subtle), the initial recommendations are the same as those for CSE. If CSE persists, they recommend immediate infusion of anaesthetics with management in an intensive care unit (ICU). In the event of persistence of SE with complex partial seizures, they recommend postponing sedation as far as possible.
The latest guidelines of the American Epilepsy Society were published in 2012.10 All the recommendations address CSE and do not differentiate between generalised and focal SE. Regarding first-line treatments, the guidelines recommend LZP for IV treatment (class category I, level of evidence A), with midazolam (MDZ) being most suitable for intramuscular (IM), buccal, or intranasal administration (class category I, level of evidence A). In stage 2 of SE, they recommend VPA (class category IIa, level of evidence A), PHT (class category IIa, level of evidence B), or levetiracetam (LEV) (class category IIb, level of evidence C). In stage 3, the most recommended drugs are MDZ (class category IIa, level of evidence B), propofol, or pentobarbital (class category IIb, level of evidence B).
The Spanish Society of Neurology’s Epilepsy Study Group (GE-SEN) published its last official guidelines for the diagnosis and treatment of epilepsy in 2012.11 For CSE, the guidelines recommend starting IV LZP or DZP, followed by IV PHT or phenobarbital (PB) if SE is not controlled by the first-line drugs (level of evidence IV). VPA, LEV, or lacosamide (LCM) may be used if PHT is contraindicated, as an alternative to PB, or in the event of refractory SE (level of evidence III). For refractory SE, GE-SEN recommends inducing coma, according to the experience or protocols of the hospital’s ICU. For NCSE, second-line AEDs should be selected according to whether the patient presents absence SE or complex partial seizures. Second-line treatments include PHT, PB, VPA, LEV, LCM, lamotrigine (LTG), and topiramate (TPM); if SE is treatment-resistant, intensive treatment is not recommended without inducing profound coma due to good prognosis.
First-line treatmentsIn a clinical trial comparing IM MDZ against IV LZP in patients with CSE, seizures were controlled in 73.4% of patients receiving MDZ and 63.4% of those receiving LZP, although symptoms resolved quicker with LZP (1.6minutes, vs 3.3minutes for MDZ).12
A meta-analysis compared IV LZP and IV DZP in patients with CSE (both generalised and focal), identifying no significant differences in symptom control or administration of other drugs due to persistence of SE.13
Second-line treatmentsA retrospective study compared PHT, VPA, and LEV in SE (the authors only specify that patients with postanoxic SE were excluded), observing that LEV is significantly less effective than VPA and PHT.14 However, a subsequent prospective study comparing the same drugs in patients with CSE found no significant differences (success rate of 68%, 68%, and 78%, respectively).15 Another prospective study compared PHT and LEV in treating SE (type not specified), finding no significant differences in seizure control, recurrence, adverse reactions, or functional prognosis.16
A meta-analysis comparing the effectiveness of PHT, VPA, PB, LEV, and LCM for treating CSE following treatment failure with benzodiazepines reported that seizures were controlled in 75.7% of cases for VPA, 73.6% for PB, and 68.5% for LEV; PHT presented the lowest rate of seizure control, at 50.2%.17 Insufficient data were available to assess the efficacy of LCM.
The first prospective study evaluating treatment of SE with IV LEV included patients with CSE, NCSE, and epilepsia partialis continua (EPC), and reported that seizures resolved in 76.6% of patients.18
First- and second-line treatmentsA retrospective study comparing clonazepam (CLP), DZP, MDZ, LEV, and VPA reported that CLP presented greater efficacy than the other drugs in controlling generalised CSE but found no significant differences for NCSE or EPC.19
A meta-analysis evaluating the safety and effectiveness of various first- and second-line drugs, comparing the drugs against each other and against placebo, found that IV LZP is superior to IV DZP in terms of seizure control and in reducing the risk of prolonged SE; in pre-hospital management, IM MDZ was at least as effective as IV LZP (and probably more effective) in controlling seizures and reducing the rate of hospitalisation and ICU admissions; IV LZP was superior to IV PHT in reducing the risk of prolonged SE.20
Third- and fourth-line treatmentsSeveral articles have assessed the effectiveness of LCM in SE: a review of studies including patients with CSE (19%), NCSE (50%), and partial SE (31%), reporting a success rate of 56%21; a report of 2 cases of refractory simple partial SE treated satisfactorily with oral LCM (300mg)22; a report of a case of refractory focal motor SE that improved with IV LCM (400mg)23; a retrospective review (59.5% of patients with NCSE) reporting seizure control in 84.4% of cases24; and another recent retrospective review (52.7% of patients with NCSE) reporting greater response to LCM in patients who had received benzodiazepines and with a loading dose greater than 5.3mg/kg.25
Another study found that perampanel may be effective for treating NCSE, EPC, and complex partial SE after treatment failure with a mean of 5 AEDs.26
A prospective study evaluated the efficacy of propolol and MDZ in treating refractory and super-refractory SE (type not specified), finding no difference between the 2 drugs.27
In a retrospective review studying the progression of patients with refractory generalised or partial SE, 51.1% of patients returned to their baseline status, 34.6% presented another episode or disability, 14.3% died, and 10.7% were treated with therapeutic coma.28 The authors conclude that therapeutic coma is associated with poorer outcomes and a considerable increase in the rate of infections and hospitalisation time, with this effect being more pronounced in patients with partial complex seizures than in those with generalised CSE or NCSE.
A subsequent retrospective study, also evaluating the progression of these patients, quantifies the different types of SE: 47.6% tonic-clonic, 21.4% complex partial, 17.9% partial motor, 6% simple partial, 3.6% myoclonic, and 3.6% subtle.29 SE resolved in the early phase in 13.1% of cases, in the established phase in 20.2%, in the refractory phase in 41.7%, and in the super-refractory phase in 13.1%; 11.9% of patients died before SE was controlled. ICU admission and systemic complications were most frequent in patients with tonic-clonic SE, but no significant differences were observed in the rate of sequelae or mortality between different types of SE.
Another study showed that of 75 randomly selected hospitals in the United Kingdom, only 21 had protocols in place for the treatment of CSE in the ICU.30 Furthermore, there were considerable differences in the contents of the protocols, and they lacked information on the most suitable drugs and doses, when drugs should be administered, and the most appropriate time interval before starting sedation. This results in delayed seizure control and a potentially preventable risk to patients.30
A recent review of the pharmacological management of refractory and super-refractory SE established specific treatment schedules for refractory complex focal SE and generalised CSE.31 For the former, anaesthetics are not recommended as they may involve more complications than those associated with this type of SE.31 The same recommendation is made in a similar review, which addresses different types of SE: on the one hand, generalised CSE (and the subsequent “subtle” or non-convulsive form), and on the other hand typical absence SE, complex partial seizures, EPC, and myoclonic SE, for which a less aggressive approach is advised.32
Reviews of the treatment of all stages of status epilepticusThree reviews published in 2017 address the management of all stages of SE:
The first does not specify the type of SE, although the majority of recommendations refer to CSE (Fig. 1).7 In the initial phase, early administration of IV benzodiazepines (LZP, DZP, CLP) or IM MDZ are recommended due to their efficacy. However, 40% of patients with generalised CSE do not respond to these first-line drugs. Therefore, IV PHT, PB, VPA, LEV, or LCM are recommended in stage 2. Level I evidence is not available to recommend any of these drugs over the others.7,12,33,34 The most frequently used anaesthetic in patients with refractory SE is MDZ (59%), followed by propofol (32%) and barbiturates (8%). Several ongoing studies have demonstrated the efficacy of anaesthetics such as ketamine or neurosteroids such as allopregnanolone for treating super-refractory SE.
The second review found no significant differences in efficacy between the different drugs used in each stage of SE, but also did not take into account the type of SE.1 In stage 1, the authors recommend early, intensive administration of benzodiazepines: IV LZP if intravenous access has been established and IM MDZ if not. Regarding stage 2, they highlight the recent support for IV LCM as an urgent control therapy for partial SE. Clobazam, ketamine, and perampanel are recommended in stage 3.
The last of this group of reviews focuses on CSE.8 According to this study, selection of a specific drug is less important than ensuring early treatment during the initial stage. However, the data reviewed support the effectiveness of MDZ over IV LZP in adult patients with SE without established intravenous access.35 This study also questions the use of PHT, citing the most recent clinical practice guidelines and data from meta-analyses suggesting that it has poorer efficacy than other second-line AEDs, in addition to its poor tolerability and associated adverse reactions.17 Regarding drugs used in stage 3, MDZ seems to be the best tolerated and the most frequently used, followed by propofol and sodium thiopental (pentobarbital in the United States).
Publications addressing status epilepticus of known focal originOnly 2 articles discuss the treatment of some type of SE of focal origin: one describes a case of aphasic SE that improved with oxcarbazepine (OXC)36 and the other is a review addressing the treatment of EPC, and reports that the best outcomes were associated with TPM and LEV.37
DiscussionWhen the literature search was limited to studies addressing pharmacological treatment of focal SE from the last 10 years, which was the objective of our study, we obtained relatively few results (n=29). Of these, only 3 studies took into account whether SE was focal or generalised, and 4 focused exclusively on patients with focal SE. Seven publications differentiated between CSE and NCSE, and also specified whether patients presented episodes of focal seizures. Two studies differentiated between convulsive and non-convulsive, and 7 only included CSE. Six studies did not specify the type of SE. Of the studies including different types of SE, only 6 analysed whether type of SE had an effect on prognosis.
While the majority of articles were published in the last 5 years, the current treatment guidelines have not been updated since 2010 and 2012. The recommendations of these guidelines generally differentiate between CSE and NCSE, although the Spanish11 and European9 guidelines refer to seizures of focal origin: the former document recommends accounting for the type of seizures in selecting a second-line drug to treat NCSE, and the latter recommends exercising caution when sedating patients with complex partial seizures.
We observed a general consensus that benzodiazepines are the most appropriate drugs in the first stage of treatment, although none of the studies addressing this issue account for whether SE is focal or generalised. We did find one study comparing first- and second-line drugs in the treatment of focal and generalised SE, reporting no significant differences.19
Regarding treatment in stage 2, one study demonstrated the efficacy of LEV for focal SE18; several articles support the efficacy of LCM in patients with refractory SE of focal origin,21–23 and the drug’s use as a second-line treatment is increasingly recommended.1,21 Another new drug reported to be successful for treating refractory SE, including focal SE, is perampanel.26
In refractory cases, in which both first- and second-line drugs fail to control SE, even less emphasis is placed on the type of SE when administering sedation. As a result, there currently seems to be no clear answer as to which type of SE benefits most from therapeutic coma, or how and when sedation should be administered in patients with refractory SE; however, the latest studies argue that coma should not be induced in patients with non-convulsive or focal SE, which present more favourable prognosis a priori.31,32
Therefore, most studies on the efficacy of first-, second-, or third-line drugs do not take into account the type of SE; those that do consider type of SE mainly differentiate between convulsive and non-convulsive. However, both CSE and NCSE can be of focal or generalised origin. This may constitute an independent prognostic factor and lead to bias in evaluating the efficacy of AEDs, potentially explaining the great disparities between the conclusions presented in the different studies.
Furthermore, the majority of patient studies, clinical trials, standardised guidelines, and hospital protocols reviewed suggest that early treatment is more important than the type of drug administered.
We also observed that daily clinical practice differs greatly between hospitals, mainly due to differences in the drugs available and staff experience at each centre. Based on these parameters and the available evidence, it is advisable to develop specific hospital protocols for each team to facilitate the management of this great emergency and to reduce delays in administering each drug, ensuring proper dosage.
Our review found that many observational studies and clinical trials comparing different AEDs in the management of SE include few patients, resulting in a lack of strong evidence on which to base therapeutic recommendations on effectiveness, dosage, tolerability, or toxicity.
In the light of all of the above, insufficient scientific evidence is currently available to assert that focal and generalised SE should be treated differently. The recommendations that we may make for stages 1 and 2 of the treatment of SE of confirmed focal origin do not differ from the recommendations for generalised SE: initial administration of IV LZP or DZP if intravenous access is already established or IM MDZ if not, followed by PHT, VPA, LEV, or LCM if seizures are not controlled. The only potential change in the therapeutic approach is delaying as far as possible the onset of intensive treatment with anaesthetics in patients with refractory focal SE.
There is a need for further prospective randomised controlled trials with large patient samples aiming to identify the best drugs for each type of SE and to establish a unified treatment protocol for the emergency management of SE. Until such studies are conducted, observational and retrospective data may be valuable.
FundingThis study has received no funding of any kind.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Huertas González N, Barros González A, Hernando Requejo V, Díaz Díaz J, Estatus epiléptico focal: revisión del tratamiento farmacológico. Neurología. 2022;37:757–766.