Headache is one of the most common neurological complaints, and is most frequent during reproductive age. As a result, we are routinely faced with pregnant or breastfeeding women with this symptom in clinical practice. It is important to know which pharmacological choices are the safest, which should not be used, and when we should suspect secondary headache. To this end, the Spanish Society of Neurology’s Headache Study Group has prepared a series of consensus recommendations on the diagnostic and therapeutic algorithms that should be followed during pregnancy and breastfeeding.
DevelopmentThis guide was prepared by a group of young neurologists with special interest and experience in headache, in collaboration with the Group’s Executive Committee. Recommendations focus on which drugs should be used for the most frequent primary headaches, both during the acute phase and for prevention. The second part addresses when secondary headache should be suspected and which diagnostic tests should be performed in the event of possible secondary headache during pregnancy and breastfeeding.
ConclusionsWe hope this guide will be practical and useful in daily clinical practice and that it will help update and improve understanding of headache management during pregnancy and breastfeeding, enabling physicians to more confidently treat these patients.
La cefalea es uno de los motivos de consulta más comunes en neurología, siendo más frecuente durante la edad reproductiva. Por ello, es habitual encontrar en nuestras consultas pacientes embarazadas o en periodo de lactancia con dicha queja. Es importante conocer las opciones farmacológicas más seguras, cuáles no se deben emplear, así como cuándo sospechar cefaleas secundarias. Por este motivo, el Grupo de Estudio de Cefaleas de la Sociedad Española de Neurología (GECSEN) ha elaborado una guía con las recomendaciones consensuadas acerca de los algoritmos diagnósticos y terapéuticos que se deben emplear durante el embarazo y la lactancia.
DesarrolloEsta guía ha sido redactada por un grupo de jóvenes neurólogos con especial interés y experiencia en cefaleas en colaboración con la Junta Directiva del GECSEN. Las recomendaciones se centran en los fármacos aconsejados en las cefaleas primarias más frecuentes, tanto en su fase aguda como preventiva. En una segunda parte se aborda cuándo sospechar y qué pruebas realizar ante una posible cefalea secundaria durante el embarazo y la lactancia.
ConclusionesEsperamos que esta guía resulte de utilidad y permita su aplicación práctica en la consulta diaria. Asimismo, que sirva para actualizar y mejorar el conocimiento del manejo de las cefaleas durante estas etapas, para actuar con mayor confianza ante estas pacientes.
Headache is one of the most frequent reasons for consultation with neurology departments.1 Special attention should be paid to headache during pregnancy, given that the condition is most prevalent in women of child-bearing age. We must be familiar with the behaviour of primary headache during pregnancy and be able to rule out the presence of secondary headache, which is more frequent in this population group.
The Spanish Society of Neurology’s Headache Study Group has prepared a consensus statement aiming to improve the diagnosis and treatment of headache during pregnancy and breastfeeding, given the potential risks to the fetus of pharmacological treatment and the considerable burden of pregnancy and breastfeeding.
The document was drafted by a group of neurologists specialising in headache, and is based on a systematic literature review and on the members’ clinical experience.
Clinical characteristics and management of the main primary headaches during pregnancyMigraineThe natural history of migraine is greatly influenced by hormonal changes. In fact, prepubertal boys and girls show similar prevalence, with prevalence increasing in girls after menarche.2 Pregnancy and breastfeeding are therefore believed to act as modulators, reducing the frequency of migraine or even preventing it. These changes are linked to changes in oestrogen and progesterone levels, which increase during pregnancy but remain stable in comparison to the fluctuations occurring outside of pregnancy. Migraine improves during pregnancy in most women, although this is not always true for chronic forms or migraine with aura.3 Improvements are more marked in the second and third trimesters, with a recurrence rate of up to 90% after delivery.4 Debut of migraine rarely occurs during pregnancy; when it does, it is most frequent during the first trimester.5 Pregnancy is associated with high levels of stress, a classic trigger factor for migraine that should therefore be managed in these patients.
It has also been suggested that breastfeeding plays a protective role as it stabilises the levels of oestrogen, prolactin, and oxytocin, which may have antinociceptive effects.6
For ethical reasons, few data are available on the safety of migraine medications for pregnant women; however, management of these patients is similar to that of other patients with migraine, with some exceptions. We should also reflect on which medications to prescribe to women of childbearing age given the possibility of an unplanned pregnancy. The recommendations included in these guidelines are based on the latest safety studies, expert consensus statements, and registries on the use of migraine medications during pregnancy and breastfeeding.
Symptomatic treatment of migraineSymptomatic treatment is classified according to the severity of migraine attacks (Table 1). Mild-to-moderate episodes will be managed with simple analgesics, preferably paracetamol, as this is the safest molecule for use during pregnancy and breastfeeding.7,8 Non-steroidal anti-inflammatory drugs (NSAID) constitute the second line of treatment; these agents should only be used during the second trimester, as they may have teratogenic effects when administered during the first trimester and increase the risk of premature closure of the ductus arteriosus after week 30. These drugs are safe to use during breastfeeding.7
Recommended drugs for the treatment of migraine during pregnancy.
| 1. Treatment of mild-to-moderate migraine attacks |
| Oral paracetamol/oral NSAIDs in the second trimester (ibuprofen, naproxen, diclofenac) |
| and/or |
| Antiemetics: |
| Metoclopramide |
| Pyridoxine + doxylamine |
| 2. Treatment of moderate-to-severe migraine attacks |
| Oral/IN/SC sumatriptan (other triptans) |
| and/or |
| Antiemetics: |
| Metoclopramide |
| (Ondansetron: 2nd option) |
| 3. Treatment options for status migrainosus |
| a) Anaesthetic nerve block (lidocaine: 1st option) |
| b) IV NSAIDs (diclofenac 75 mg IV) |
| c) Sumatriptan 6 mg SC |
| d) Chlorpromazine 12.5-25 mg IV |
| e) Metoclopramide 10-20 mg IV |
| f) Methylprednisolone 60-120 mg IV/dexamethasone 20-40 mg IV |
| 4. Preventive treatment of first choice |
| a) Non-pharmacological measures (regular schedules, sufficient sleep, avoiding fasting, relaxation, etc) |
| b) Propranolol or metoprolol: 1st choice |
| c) Anaesthetic nerve block: 1st choice |
| d) Lamotrigine: 1st choice for very frequent auras |
| e) Amitriptyline: 2nd choice |
IN: intranasal; IV: intravenous; NSAID: non-steroidal anti-inflammatory drug; SC: subcutaneous.
Moderate-to-severe attacks should be managed with triptans. A meta-analysis of over 4000 women identified no differences in teratogenicity, the rate of miscarriage, and the number of premature births between pregnant women taking and not taking triptans.9 These findings were confirmed in a recent observational study.10 Wherever possible, sumatriptan should be the first choice since it is the triptan with the most published evidence on its use and demonstrating its safety. Sumatriptan is also safe to use during breastfeeding.9,11–13
Metoclopramide is the antiemetic of choice during pregnancy and breastfeeding. Domperidone should be avoided as it increases the risk of prolonged QT interval. Other safe options include chlorpromazine, or combinations of doxylamine and pyridoxine for the treatment of hyperemesis gravidarum.14
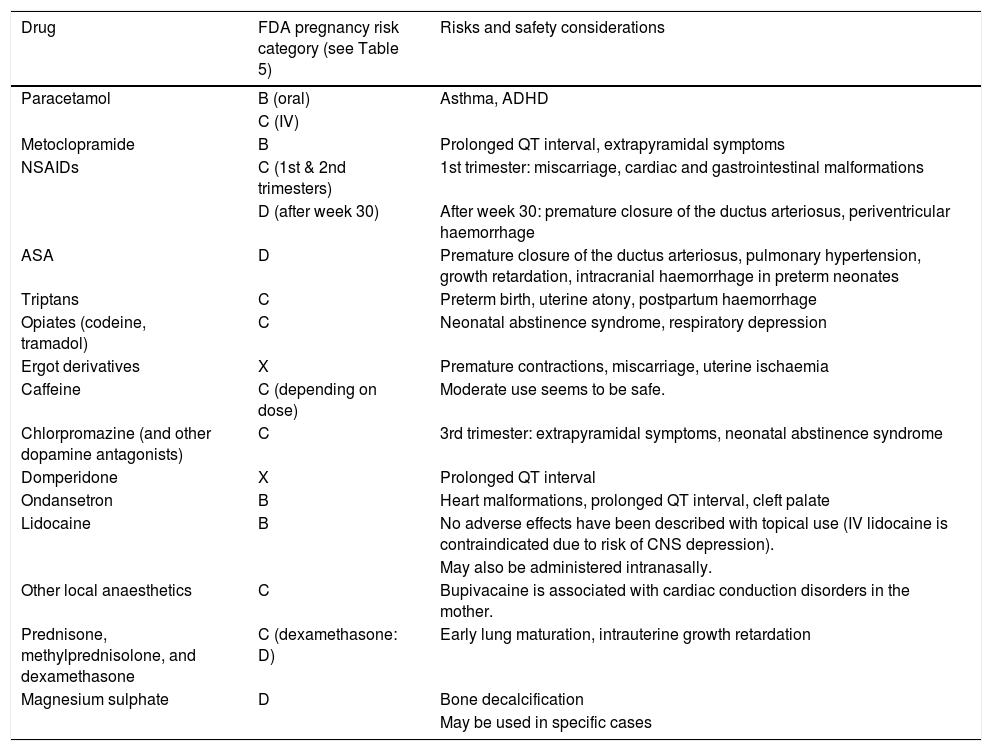
Tables 2 and 3 summarise the indications and potential risks of the drugs used for acute treatment of primary headache during pregnancy and breastfeeding.
Food and Drug Administration pregnancy risk categories and safety considerations for the main drugs used for acute treatment of primary headache during pregnancy.
| Drug | FDA pregnancy risk category (see Table 5) | Risks and safety considerations |
|---|---|---|
| Paracetamol | B (oral) | Asthma, ADHD |
| C (IV) | ||
| Metoclopramide | B | Prolonged QT interval, extrapyramidal symptoms |
| NSAIDs | C (1st & 2nd trimesters) | 1st trimester: miscarriage, cardiac and gastrointestinal malformations |
| D (after week 30) | After week 30: premature closure of the ductus arteriosus, periventricular haemorrhage | |
| ASA | D | Premature closure of the ductus arteriosus, pulmonary hypertension, growth retardation, intracranial haemorrhage in preterm neonates |
| Triptans | C | Preterm birth, uterine atony, postpartum haemorrhage |
| Opiates (codeine, tramadol) | C | Neonatal abstinence syndrome, respiratory depression |
| Ergot derivatives | X | Premature contractions, miscarriage, uterine ischaemia |
| Caffeine | C (depending on dose) | Moderate use seems to be safe. |
| Chlorpromazine (and other dopamine antagonists) | C | 3rd trimester: extrapyramidal symptoms, neonatal abstinence syndrome |
| Domperidone | X | Prolonged QT interval |
| Ondansetron | B | Heart malformations, prolonged QT interval, cleft palate |
| Lidocaine | B | No adverse effects have been described with topical use (IV lidocaine is contraindicated due to risk of CNS depression). |
| May also be administered intranasally. | ||
| Other local anaesthetics | C | Bupivacaine is associated with cardiac conduction disorders in the mother. |
| Prednisone, methylprednisolone, and dexamethasone | C (dexamethasone: D) | Early lung maturation, intrauterine growth retardation |
| Magnesium sulphate | D | Bone decalcification |
| May be used in specific cases |
ADHD: attention-deficit/hyperactivity disorder; CNS: central nervous system; FDA: Food and Drug Administration; IV: intravenous; NSAID: non-steroidal anti-inflammatory drug.
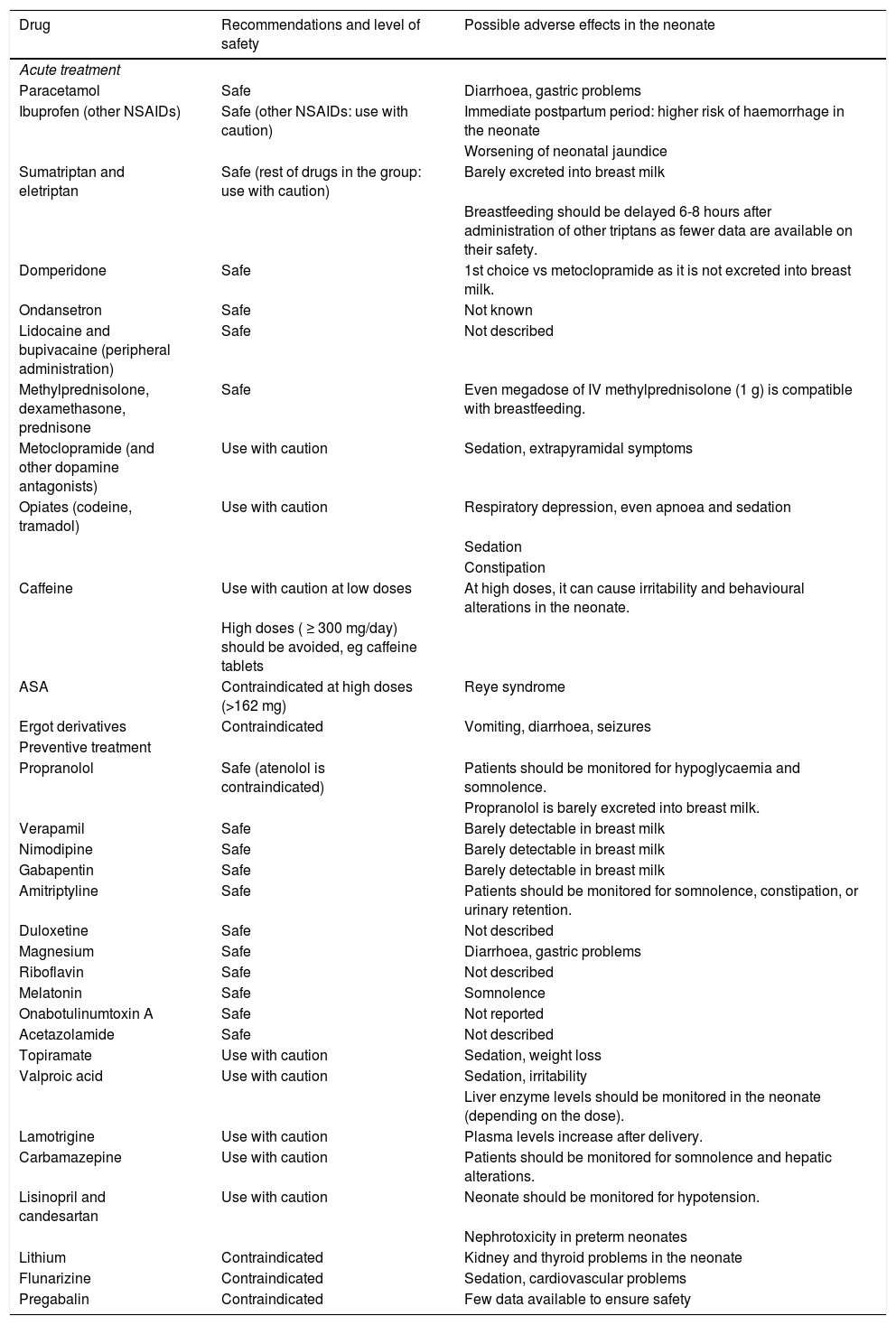
Level of safety and recommendations for the main drugs used for primary headache during breastfeeding.
| Drug | Recommendations and level of safety | Possible adverse effects in the neonate |
|---|---|---|
| Acute treatment | ||
| Paracetamol | Safe | Diarrhoea, gastric problems |
| Ibuprofen (other NSAIDs) | Safe (other NSAIDs: use with caution) | Immediate postpartum period: higher risk of haemorrhage in the neonate |
| Worsening of neonatal jaundice | ||
| Sumatriptan and eletriptan | Safe (rest of drugs in the group: use with caution) | Barely excreted into breast milk |
| Breastfeeding should be delayed 6-8 hours after administration of other triptans as fewer data are available on their safety. | ||
| Domperidone | Safe | 1st choice vs metoclopramide as it is not excreted into breast milk. |
| Ondansetron | Safe | Not known |
| Lidocaine and bupivacaine (peripheral administration) | Safe | Not described |
| Methylprednisolone, dexamethasone, prednisone | Safe | Even megadose of IV methylprednisolone (1 g) is compatible with breastfeeding. |
| Metoclopramide (and other dopamine antagonists) | Use with caution | Sedation, extrapyramidal symptoms |
| Opiates (codeine, tramadol) | Use with caution | Respiratory depression, even apnoea and sedation |
| Sedation | ||
| Constipation | ||
| Caffeine | Use with caution at low doses | At high doses, it can cause irritability and behavioural alterations in the neonate. |
| High doses ( ≥ 300 mg/day) should be avoided, eg caffeine tablets | ||
| ASA | Contraindicated at high doses (>162 mg) | Reye syndrome |
| Ergot derivatives | Contraindicated | Vomiting, diarrhoea, seizures |
| Preventive treatment | ||
| Propranolol | Safe (atenolol is contraindicated) | Patients should be monitored for hypoglycaemia and somnolence. |
| Propranolol is barely excreted into breast milk. | ||
| Verapamil | Safe | Barely detectable in breast milk |
| Nimodipine | Safe | Barely detectable in breast milk |
| Gabapentin | Safe | Barely detectable in breast milk |
| Amitriptyline | Safe | Patients should be monitored for somnolence, constipation, or urinary retention. |
| Duloxetine | Safe | Not described |
| Magnesium | Safe | Diarrhoea, gastric problems |
| Riboflavin | Safe | Not described |
| Melatonin | Safe | Somnolence |
| Onabotulinumtoxin A | Safe | Not reported |
| Acetazolamide | Safe | Not described |
| Topiramate | Use with caution | Sedation, weight loss |
| Valproic acid | Use with caution | Sedation, irritability |
| Liver enzyme levels should be monitored in the neonate (depending on the dose). | ||
| Lamotrigine | Use with caution | Plasma levels increase after delivery. |
| Carbamazepine | Use with caution | Patients should be monitored for somnolence and hepatic alterations. |
| Lisinopril and candesartan | Use with caution | Neonate should be monitored for hypotension. |
| Nephrotoxicity in preterm neonates | ||
| Lithium | Contraindicated | Kidney and thyroid problems in the neonate |
| Flunarizine | Contraindicated | Sedation, cardiovascular problems |
| Pregabalin | Contraindicated | Few data available to ensure safety |
ASA: acetylsalicylic acid; IV: intravenous; NSAID: non-steroidal anti-inflammatory drug.
Nerve blocks constitute a safe and effective alternative to oral and parenteral medications for the treatment of status migrainosus during pregnancy and breastfeeding (Table 1). Lidocaine is the local anaesthetic of choice (Food and Drug Administration [FDA] pregnancy risk category B), although low-dose bupivacaine and mepivacaine may also be used for peripheral nerve blocks.15,16
Parenteral diclofenac and subcutaneous sumatriptan may also be administered in specific cases. Intravenous metoclopramide and chlorpromazine are safe options during pregnancy and breastfeeding.
Parenteral corticosteroids (preferably methylprednisolone) may be used during pregnancy in specific cases, though never during the third trimester. Corticosteroids can also be administered during breastfeeding, although breastfeeding should be delayed for 2-8 hours, or milk should be extracted before the treatment is administered.17
Preventive treatmentDue to the low prevalence of migraine during the second and third trimesters, use of preventive drugs is uncommon; only patients with frequent attacks will require preventive treatment. In these patients, special emphasis should be placed on non-pharmacological treatment, focusing on avoiding any potential migraine triggers.
Beta blockers, and particularly propranolol and metoprolol, are the drugs of first choice in pregnant and breastfeeding women (Table 1). Patients receiving these drugs should undergo ultrasound studies every 2 weeks and, wherever possible, discontinue treatment several days or weeks before the estimated due date, due to the risk of neonatal bradycardia, hypoglycaemia, and respiratory depression.18 Amitriptyline is the second option; administration during the first trimester should be avoided and the lowest possible dose should be used. Treatment with amitriptyline also requires more frequent ultrasound monitoring. The drug is safe to use during breastfeeding, although it may cause somnolence or have anticholinergic effects on the neonate.19 Lamotrigine can be used during pregnancy and breastfeeding, and is therefore a valid option for preventive treatment of migraine with aura.
Nerve blocks constitute a good treatment option and are increasingly used in pregnant women. As mentioned previously, lidocaine is preferred over other anaesthetic agents due to its good safety profile.20
Botulinum toxin type A was classified as a category C drug according to the old FDA pregnancy risk categories. It is not recommended for use during pregnancy, although no study has demonstrated its teratogenicity and it is unclear whether it may have deleterious effects on the fetus in pregnant women with systemic botulism. Data from over 300 women exposed to botulinum toxin type A during at least the first trimester (the drug was only administered for migraine prevention in 22 cases) are included in the registries of several pharmaceutical companies; the rates of miscarriage and teratogenicity in this sample were similar to those recorded in the general population.4,21 Therefore, patients with refractory migraine who showed good response to botulinum toxin type A before pregnancy may continue with this treatment.22,23
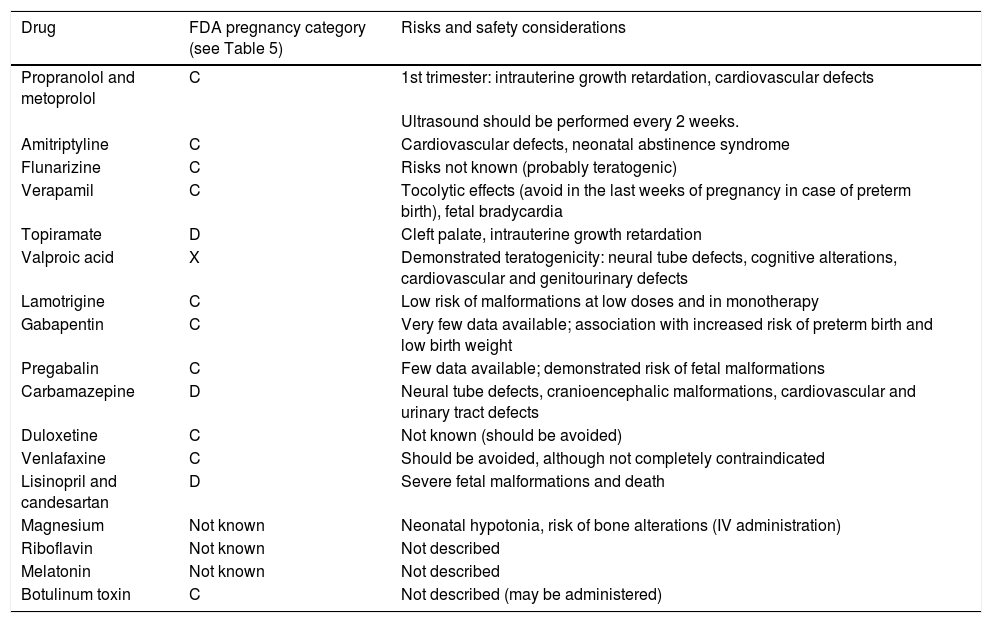
Tables 3 and 4 summarise the indications and potential risks of the main drugs used for preventive treatment of primary headache during pregnancy and breastfeeding; Table 5 summarises the FDA pregnancy risk categories.
Risk and safety considerations for the main preventive drugs for primary headache during pregnancy.
| Drug | FDA pregnancy category (see Table 5) | Risks and safety considerations |
|---|---|---|
| Propranolol and metoprolol | C | 1st trimester: intrauterine growth retardation, cardiovascular defects |
| Ultrasound should be performed every 2 weeks. | ||
| Amitriptyline | C | Cardiovascular defects, neonatal abstinence syndrome |
| Flunarizine | C | Risks not known (probably teratogenic) |
| Verapamil | C | Tocolytic effects (avoid in the last weeks of pregnancy in case of preterm birth), fetal bradycardia |
| Topiramate | D | Cleft palate, intrauterine growth retardation |
| Valproic acid | X | Demonstrated teratogenicity: neural tube defects, cognitive alterations, cardiovascular and genitourinary defects |
| Lamotrigine | C | Low risk of malformations at low doses and in monotherapy |
| Gabapentin | C | Very few data available; association with increased risk of preterm birth and low birth weight |
| Pregabalin | C | Few data available; demonstrated risk of fetal malformations |
| Carbamazepine | D | Neural tube defects, cranioencephalic malformations, cardiovascular and urinary tract defects |
| Duloxetine | C | Not known (should be avoided) |
| Venlafaxine | C | Should be avoided, although not completely contraindicated |
| Lisinopril and candesartan | D | Severe fetal malformations and death |
| Magnesium | Not known | Neonatal hypotonia, risk of bone alterations (IV administration) |
| Riboflavin | Not known | Not described |
| Melatonin | Not known | Not described |
| Botulinum toxin | C | Not described (may be administered) |
IV: intravenous.
Food and Drug Administration pregnancy risk categories. This classification has now been replaced by a more descriptive version, but is still in use.
| Category | |
|---|---|
| A | Safe. Adequate and well-controlled studies have failed to demonstrate a risk to the fetus in the first trimester of pregnancy (and there is no evidence of risk in later trimesters). |
| B | Animal reproduction studies have failed to demonstrate a risk to the fetus and there are no adequate and well-controlled studies in pregnant women. |
| C | Animal reproduction studies have demonstrated a risk to the fetus, but there are no adequate and well-controlled studies in pregnant women. |
| D | There is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks. |
| X | Contraindicated. Studies in animals or humans have demonstrated fetal abnormalities and/or there is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience, and the risks involved in use of the drug in pregnant women clearly outweigh potential benefits. |
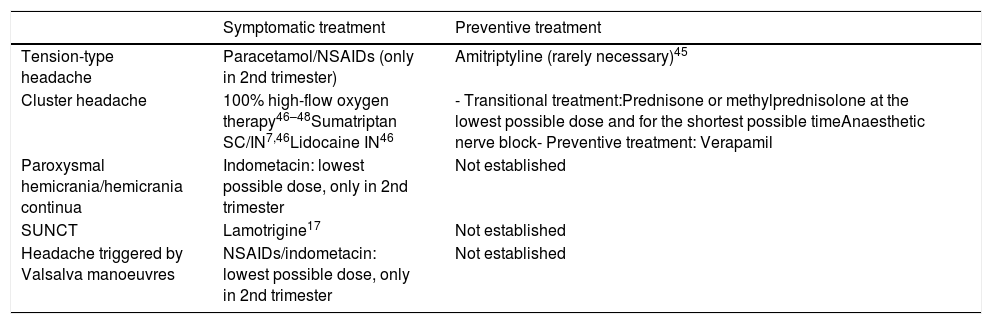
Table 6 lists the main drugs recommended for the treatment of other primary headaches during pregnancy and breastfeeding.
Drugs recommended for the treatment of other primary headaches during pregnancy and breastfeeding.
| Symptomatic treatment | Preventive treatment | |
|---|---|---|
| Tension-type headache | Paracetamol/NSAIDs (only in 2nd trimester) | Amitriptyline (rarely necessary)45 |
| Cluster headache | 100% high-flow oxygen therapy46–48Sumatriptan SC/IN7,46Lidocaine IN46 | - Transitional treatment:Prednisone or methylprednisolone at the lowest possible dose and for the shortest possible timeAnaesthetic nerve block- Preventive treatment: Verapamil |
| Paroxysmal hemicrania/hemicrania continua | Indometacin: lowest possible dose, only in 2nd trimester | Not established |
| SUNCT | Lamotrigine17 | Not established |
| Headache triggered by Valsalva manoeuvres | NSAIDs/indometacin: lowest possible dose, only in 2nd trimester | Not established |
IN: intranasal; NSAID: non-steroidal anti-inflammatory drug; SC: subcutaneous; SUNCT: short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing.
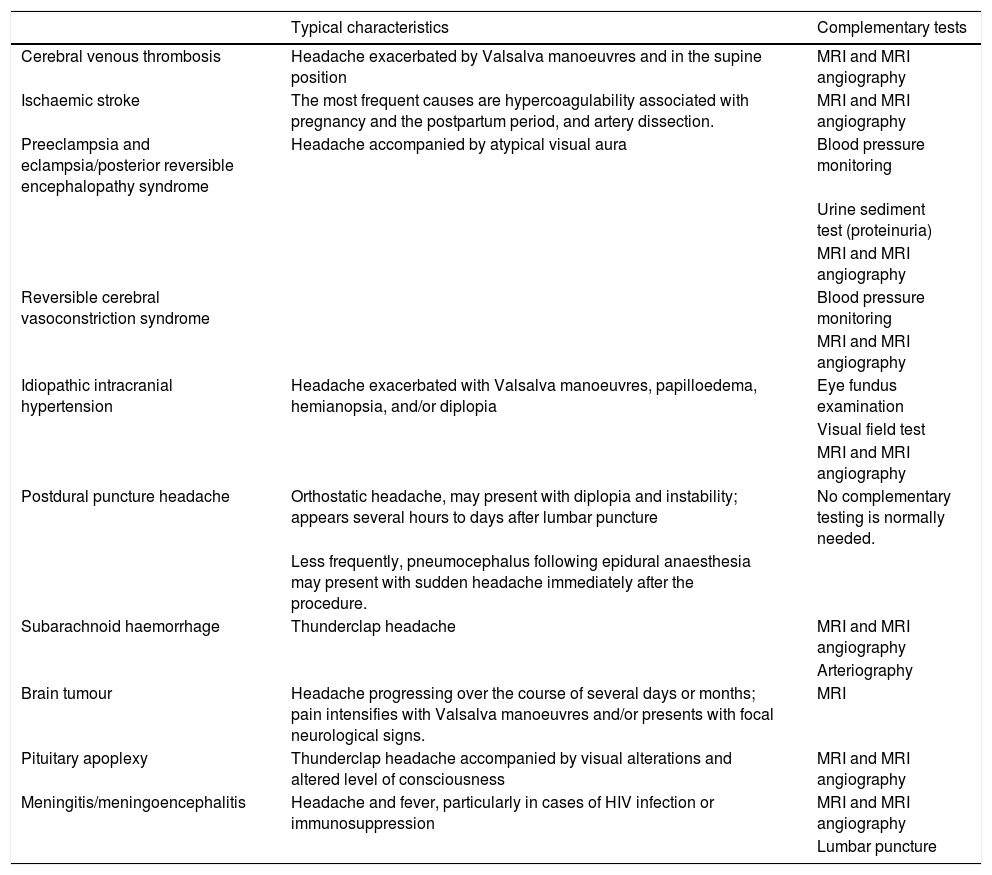
It is essential to understand the main secondary headaches in pregnant and breastfeeding women, since their frequency increases in this population group, accounting for up to one-third of headaches in some series.24 The most relevant findings are occurrence of a first episode of headache during pregnancy or breastfeeding, particularly in patients with high blood pressure. Table 7 lists the most common secondary headaches and their main clinical characteristics.
Main causes of secondary headache during pregnancy and breastfeeding.
| Typical characteristics | Complementary tests | |
|---|---|---|
| Cerebral venous thrombosis | Headache exacerbated by Valsalva manoeuvres and in the supine position | MRI and MRI angiography |
| Ischaemic stroke | The most frequent causes are hypercoagulability associated with pregnancy and the postpartum period, and artery dissection. | MRI and MRI angiography |
| Preeclampsia and eclampsia/posterior reversible encephalopathy syndrome | Headache accompanied by atypical visual aura | Blood pressure monitoring |
| Urine sediment test (proteinuria) | ||
| MRI and MRI angiography | ||
| Reversible cerebral vasoconstriction syndrome | Blood pressure monitoring | |
| MRI and MRI angiography | ||
| Idiopathic intracranial hypertension | Headache exacerbated with Valsalva manoeuvres, papilloedema, hemianopsia, and/or diplopia | Eye fundus examination |
| Visual field test | ||
| MRI and MRI angiography | ||
| Postdural puncture headache | Orthostatic headache, may present with diplopia and instability; appears several hours to days after lumbar puncture | No complementary testing is normally needed. |
| Less frequently, pneumocephalus following epidural anaesthesia may present with sudden headache immediately after the procedure. | ||
| Subarachnoid haemorrhage | Thunderclap headache | MRI and MRI angiography |
| Arteriography | ||
| Brain tumour | Headache progressing over the course of several days or months; pain intensifies with Valsalva manoeuvres and/or presents with focal neurological signs. | MRI |
| Pituitary apoplexy | Thunderclap headache accompanied by visual alterations and altered level of consciousness | MRI and MRI angiography |
| Meningitis/meningoencephalitis | Headache and fever, particularly in cases of HIV infection or immunosuppression | MRI and MRI angiography |
| Lumbar puncture |
HIV: human immunodeficiency virus; MRI: magnetic resonance imaging.
Classic trigeminal neuralgia rarely affects young individuals. No data are available on the progression or presentation of these disorders during pregnancy. Management of trigeminal neuralgia is more complex in these patients due to the therapeutic limitations associated with pregnancy.25 Neuromodulators should be avoided, or administered at the lowest possible dose, and patients should be informed of the associated risks. A consensus should be reached between doctor and patient. Lamotrigine is the neuromodulator presenting the best safety profile; we should therefore avoid the use of other neuromodulators with less favourable safety profiles, even it they show greater effectiveness. Conventional analgesics usually have little effect on this type of pain, although pain intensity decreases in some patients. These patients may be treated with very low doses of opiates in specific cases. Most drugs are safer to use while breastfeeding than during pregnancy.16,26
Secondary painful trigeminal neuropathy is also rare during pregnancy; suspected cases should be evaluated with brain MRI studies. In patients with trigeminal post-herpetic neuralgia, immunosuppression should be ruled out. Acute herpesvirus infection is treated with aciclovir; this treatment is safe for use during pregnancy.27 The risk-benefit balance of neuromodulatory treatment should be assessed in patients developing secondary neuralgia.
Nerve blocks are recommended for treatment of other pericranial neuralgias; the use of oral drugs should be avoided wherever possible.
Characteristics and management of the main secondary headaches during pregnancy and breastfeedingIdiopathic intracranial hypertensionIdiopathic intracranial hypertension may appear in pregnant patients, typically during the first half of pregnancy, whereas recurrences normally occur around week 20, coinciding with the period of maximum weight gain.28 It usually presents with daily episodes of progressive, holocranial headache, which is exacerbated by Valsalva manoeuvres and associated with papilloedema, visual alterations, tinnitus, or sixth nerve palsy.17 A non-contrast brain MRI angiography study should be conducted to rule out cerebral venous thrombosis (CVT); other tests include lumbar puncture and visual field test. The latter is a useful tool for patient follow-up.29 Weight gain should be monitored, and some patients may need to undergo lumbar cerebrospinal fluid drainage. Acetazolamide and other diuretic agents should be avoided in these patients.30
Eclampsia and preeclampsiaEeclampsia and preeclampsia usually occur after week 20 and may also occur in the postpartum period. These conditions present with progressive, bilateral, pulsating headache that is exacerbated by physical activity and does not respond to symptomatic treatment; they are also frequently associated with visual alterations (aura), seizures (70%), and confusion. Patients with suspected eclampsia/preeclampsia should undergo an MRI scan and their blood pressure and urine protein levels should be tested. Management includes administration of beta blockers (labetalol is the safest option) and such calcium channel blockers as nicardipine,28 as well as pregnancy termination.31
Cerebral venous thrombosisCerebral venous thrombosis is more frequent during pregnancy due to a prothrombotic state appearing during pregnancy (mainly the third trimester) and the postpartum period.32 In 90% of cases, CVT presents with progressive holocranial headache, although 10% of patients present thunderclap headache.28 In the postpartum period, presence of thunderclap headache that worsens in the supine position, in the absence of preeclampsia, is suggestive of CVT.28
Patients should undergo brain MRI angiography and a coagulation study, to be repeated 6 weeks after delivery, due to the effects of pregnancy on protein S, and one month after discontinuation of anticoagulant treatment; patients should also be monitored for vasculitis.33
Treatment is based on low molecular weight heparin; after delivery, patients may either continue with this drug or switch to vitamin K antagonists. No evidence is available on the use of factor Xa inhibitors. In subsequent pregnancies, patients should receive preventive treatment with low molecular weight heparin.33 The long-term prognosis of CVT is more favourable in pregnant women than in other patients.
Subarachnoid haemorrhageThe risk of subarachnoid haemorrhage is greater in the peripartum (2 days before and the day after delivery) and postpartum periods.34 The condition presents with thunderclap headache induced by exertion, in addition to the typical symptoms. The risks of head CT should be weighed against its benefits; in the event of normal CT findings, a lumbar puncture and brain MRI angiography should be performed. Management of subarachnoid haemorrage in pregnant women is similar to that in other patients.
Ischaemic strokeIschaemic stroke most frequently occurs in the postpartum period, particularly within 8 days of delivery.35 The aetiology of postpartum stroke varies greatly: coagulation disorders, emboligenic heart disease, artery dissection, pregnancy-related hypercoagulability, etc. Some of the most relevant cardioembolic causes are peripartum cardiomyopathy and patent foramen ovale.36 In addition to the standard aetiological study and coagulation study, a brain MRI angiography should also be performed.
Thrombolysis may be considered during pregnancy and the postpartum period if the benefits of the procedure outweigh the potential risk of intrauterine haematoma.37 The decision to perform thrombectomy should be made on a case-by-case basis, although some authors support this approach.38
Reversible cerebral vasoconstriction syndromePregnancy is a predisposing factor for reversible cerebral vasoconstriction syndrome, although most cases occur during the postpartum period (postpartum angiopathy).39 The condition typically appears during the first week postpartum, presenting in the form of recurrent thunderclap headache that is exacerbated by physical activity, Valsalva manoeuvres, or emotional stimuli, with angiographic evidence of vasoconstriction.17,26 It usually resolves within 3 months. Differential diagnosis includes central nervous system vasculitis.17 Treatment focuses on managing pain; the use of nimodipine may be considered if necessary.40
Posterior reversible encephalopathy syndromePosterior reversible encephalopathy syndrome (PRES) is frequently associated with high blood pressure; therefore, PRES occurring during pregnancy is usually secondary to eclampsia or preeclampsia.39,41 Headache is the most frequent symptom of PRES, with pain predominantly affecting the occipital region. The condition may also present with visual symptoms, seizures, and alterations in the level of consciousness. Occasionally, PRES may present in association with reversible cerebral vasoconstriction syndrome.39,42 These patients must be evaluated with head CT or brain MRI.17,42
Central nervous system tumoursThe incidence of intracranial tumours does not increase during pregnancy, but their growth pattern or clinical expression may change.43,44 Meningiomas may increase in size due to hypervolaemia and greater stimulation of hormonal receptors in the tumour.44 In women with pituitary macroadenoma, hypercoagulability and physiological pituitary hyperplasia may lead to pituitary apoplexy,28 which typically presents with thunderclap headache, visual alterations, involvement of the cranial nerves of the cavernous sinus, and impaired consciousness. Therefore, patients with history of pituitary adenoma should be closely monitored, undergoing frequent ophthalmological assessments. Some tumours appear exclusively during pregnancy; one example is choriocarcinoma, which frequently causes haemorragic central nervous system metastasis.17,44
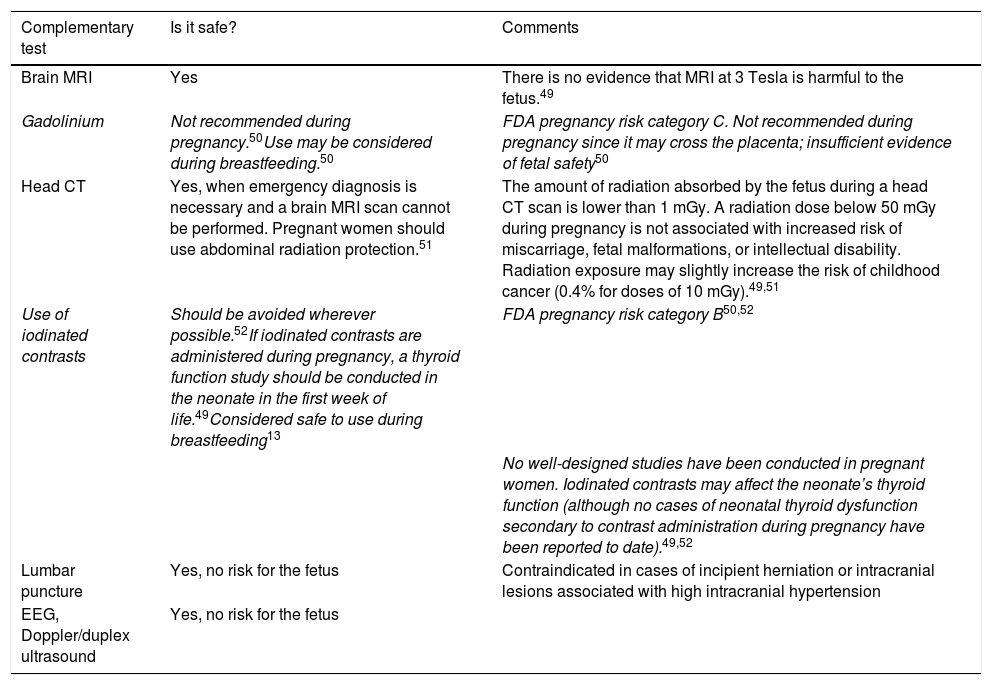
Diagnostic tests for headache during pregnancy and breastfeedingPregnant women experiencing alarm signs of headache should undergo the necessary complementary tests to rule out secondary causes. Brain MRI is preferred over head CT as it does not use ionising radiation, although the fetus is only exposed to very low amounts of radiation during a CT scan.28Table 8 includes several recommendations for the use of diagnostic tests for headache during pregnancy and breastfeeding.
Recommendations for complementary testing in pregnant or breastfeeding women with headache.
| Complementary test | Is it safe? | Comments |
|---|---|---|
| Brain MRI | Yes | There is no evidence that MRI at 3 Tesla is harmful to the fetus.49 |
| Gadolinium | Not recommended during pregnancy.50Use may be considered during breastfeeding.50 | FDA pregnancy risk category C. Not recommended during pregnancy since it may cross the placenta; insufficient evidence of fetal safety50 |
| Head CT | Yes, when emergency diagnosis is necessary and a brain MRI scan cannot be performed. Pregnant women should use abdominal radiation protection.51 | The amount of radiation absorbed by the fetus during a head CT scan is lower than 1 mGy. A radiation dose below 50 mGy during pregnancy is not associated with increased risk of miscarriage, fetal malformations, or intellectual disability. Radiation exposure may slightly increase the risk of childhood cancer (0.4% for doses of 10 mGy).49,51 |
| Use of iodinated contrasts | Should be avoided wherever possible.52If iodinated contrasts are administered during pregnancy, a thyroid function study should be conducted in the neonate in the first week of life.49Considered safe to use during breastfeeding13 | FDA pregnancy risk category B50,52 |
| No well-designed studies have been conducted in pregnant women. Iodinated contrasts may affect the neonate’s thyroid function (although no cases of neonatal thyroid dysfunction secondary to contrast administration during pregnancy have been reported to date).49,52 | ||
| Lumbar puncture | Yes, no risk for the fetus | Contraindicated in cases of incipient herniation or intracranial lesions associated with high intracranial hypertension |
| EEG, Doppler/duplex ultrasound | Yes, no risk for the fetus |
Primary headache, and particularly migraine, is the most frequent and prevalent type of headache during pregnancy. Migraine generally improves in this period due to hormonal factors, although the relief is not as marked in patients with migraine with aura. Little evidence is available on the characteristics or progression of other types of primary headache in pregnant women due to their low prevalence.
It is essential to rule out secondary headache in these patients, as this type of headache may be associated with such conditions as preeclampsia or venous sinus thrombosis secondary to hypercoagulability. Complementary tests can currently be performed with few restrictions, although MRI is preferred over techniques that use ionising radiation.
As a general rule, pharmacological treatment should be administered at the lowest possible dose and for the shortest possible time, and the patient should be informed of the associated risks and benefits. Breastfeeding offers greater flexibility for pharmacological treatment, although medications should be administered several hours before breastfeeding.
We hope these guidelines will help physicians to more safely manage pregnant patients with headache, informing them of the safest treatment options and key data for the differential diagnosis of secondary headache.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: González-García N, Díaz de Terán J, López-Veloso AC, Mas-Sala N, Mínguez-Olaondo A, Ruiz-Piñero M, et al. Cefalea: embarazo y lactancia. Recomendaciones del Grupo de Estudio de Cefaleas de la Sociedad Española de Neurología (GECSEN). Neurología. 2022;37:1–12.