The Barratt Impulsiveness Scale (BIS) is a self-administered instrument designed to assess the personality/behavioural construct of impulsiveness. Impulsiveness has been associated with several psychiatric disorders, including attention-deficit/hyperactivity disorder (ADHD). This study assesses the progression of impulsive behaviour in children with ADHD after an 8-week dietary intervention with the Mediterranean diet and/or omega-3 fatty acid supplementation, by using a version of the 11-item BIS adapted for children (BIS-11c).
MethodsThis cross-sectional study includes 60 children with ADHD from the region of Madrid, Spain. Participants were divided into 4 groups, with one control group and 3 intervention groups (Mediterranean diet; omega-3 supplementation; and Mediterranean diet plus omega-3 supplementation). A personalised Mediterranean diet was designed for members of groups 2 and 4. The BIS-11c was administered to determine the level of impulsiveness, and the KIDMED test was used to assess adherence to the Mediterranean diet.
ResultsThe supplementation group showed a fairly significant decrease in the total BIS-11c (P = .049). Total cognitive score slightly decreased in the diet and supplementation groups. Only the control group showed a considerable decrease in the total motor score. Total nonplanning scores were lower in all groups after the intervention. Baseline and final BIS-11c scores were positively correlated with treatments (r > 0.9).
ConclusionAn intake of 550 mg EPA fatty acid and 225 mg DHA fatty acid per day for 8 weeks is associated with less marked impulsive behaviour in children with ADHD. A Mediterranean diet may improve BIS scores, although our results are not conclusive in this population.
La Escala de Impulsividad de Barratt (BIS) es un instrumento de autoinforme diseñado para evaluar la construcción de personalidad y comportamiento de la impulsividad. La impulsividad se ha asociado con varios trastornos psiquiátricos, como el trastorno por déficit de atención e hiperactividad (TDAH). Este estudio evalúa el progreso de la conducta de impulsividad en niños con TDAH después de una intervención dietética de 8 semanas con dieta mediterránea y/o suplemento de ácidos grasos omega-3, mediante el uso de la BIS-11 adaptada para niños (BIS-11c).
MétodosEste estudio transversal incluyó a 60 niños españoles con TDAH de la provincia de Madrid, España. Los participantes se dividieron en 4 grupos, un grupo de control (G1) y 3 grupos de intervención (dieta mediterránea [G2], suplemento de omega-3 [G3] y dieta mediterránea + suplemento de omega-3 [G4]). Se diseñó una dieta mediterránea personalizada para los grupos 2 y 4. Se administró BIS-11c para determinar los niveles de impulsividad y se usó el KIDMED para evaluar la adherencia a la dieta mediterránea.
ResultadosEl grupo Suplemento mostró una caída bastante significativa (p = 0.049) en la puntuación total de Barratt después del seguimiento. La puntuación cognitiva total disminuyó ligeramente en los grupos de Dieta y Suplemento. Solo el grupo Control tuvo una disminución notable con respecto a la puntuación total de la impulsividad motora. Las puntuaciones totales de ‘falta de planificación’ fueron menores en todos los grupos tras la intervención. Las asociaciones entre las puntuaciones iniciales y finales del BIS-11c y los tratamientos presentaron una correlación positiva (r > 0,9).
ConclusiónUna ingesta de 550 mg de EPA y 225 mg de DHA por día durante 8 semanas se asocia con niveles más bajos de conductas impulsivas en niños con TDAH. Un patrón dietético mediterráneo podría mejorar las puntuaciones de la Escala de Impulsividad de Barratt, pero los resultados de este estudio no son concluyentes en esta población.
Impulsiveness is a complex phenotype that is diagnosed clinically, based on observation of an individual’s behaviour.1 Impulsive behaviour frequently involves quick, unplanned action, and risk-taking.2 From a clinical perspective, impulsiveness is an important diagnostic characteristic of several psychiatric disorders, including attention-deficit/hyperactivity disorder (ADHD).1 Children with ADHD present a variety of deficits in several cognitive functions, particularly executive function (working memory, planning, selective and divided attention, response inhibition, time processing, and set-shifting).3
ADHD is a neurodevelopmental disorder characterised by a persistent pattern of lack of attention and/or hyperactivity and impulsiveness. It is one of the most frequent neurodevelopmental disorders in children,4 persisting until adulthood in over 50% of cases.5 The prevalence of ADHD in children in Spain is estimated at 5%,6 ranging from 4.9% to 8.8% according to different studies.7 Some studies have described an association between unhealthy dietary patterns (eg, diets high in refined sugar and saturated fats and low in fruit and vegetables) and ADHD.8,9 Lower adherence to a Mediterranean diet has also been associated with diagnosis of ADHD. Patients with ADHD more often miss a second serving of vegetables daily, present reduced intake of fish and pulses, and eat pasta or rice nearly every day, as compared to controls.10 Greater frequency of skipping breakfast and eating at fast food restaurants is also associated with diagnosis of ADHD.11 There is growing interest in dietary approaches to the treatment of ADHD, and especially in the use of omega-3 supplements. Omega-3 polyunsaturated fatty acids (n-3 PUFA) include docosahexaenoic acid (DHA or 22:6[n-3]) and eicosapentaenoic acid (EPA or 20:5[n-3]); these essential fatty acids cannot be efficiently synthesised by the human body and must therefore be obtained from food. Dietary approaches to the treatment of ADHD include fatty acid supplementation,12 particularly with n-3 PUFA, since these have recently been shown to be effective.13 Patients who do not respond to conventional treatment or whose parents refuse pharmacological treatment may benefit from omega-3 supplements.
As is the case with the concept of impulsiveness, which has several definitions, different assessment instruments have also been developed to evaluate and measure impulsive behaviour. The Barratt Impulsiveness Scale (BIS-11) is one of the most widely used tools for evaluating impulsiveness.14 It evaluates the main 3 dimensions of impulsive behaviour: cognitive impulsiveness (inability to focus on the task at hand), motor impulsiveness (acting without thinking), and lack of planning (focus on the present rather than the future).15 The BIS-11 has been adapted to different populations16 and translated into at least 11 languages17; all adaptations are reliable (high internal consistency and test-retest reliability) and valid.
Assessment of impulsive behaviour is crucial both in clinical practice and in research in neuroscience and related fields. Dietary approaches to the treatment of ADHD must focus not only on specific nutrients but also on the diet as a whole. In the light of the above, this study aimed to evaluate the progression of impulsive behaviour in children with ADHD after a dietary intervention with the Mediterranean diet and/or omega-3 supplementation, using a version of the BIS-11 adapted to children (BIS-11c). We tested the hypothesis that adherence to the Mediterranean diet reduces ADHD symptoms and impulsive behaviour. Omega-3 fatty acid supplementation with EPA and DHA contributes to the normal function of the heart and helps preserve normal brain function and sight with a daily intake of 250 mg of EPA/DHA.18
ObjectivesThe main purpose of this study was to analyse changes in BIS-11c scores in children with ADHD after an 8-week intervention with the Mediterranean diet, omega-3 fatty acid supplementation, or Mediterranean diet plus omega-3 fatty acid supplementation, as compared to a control group.
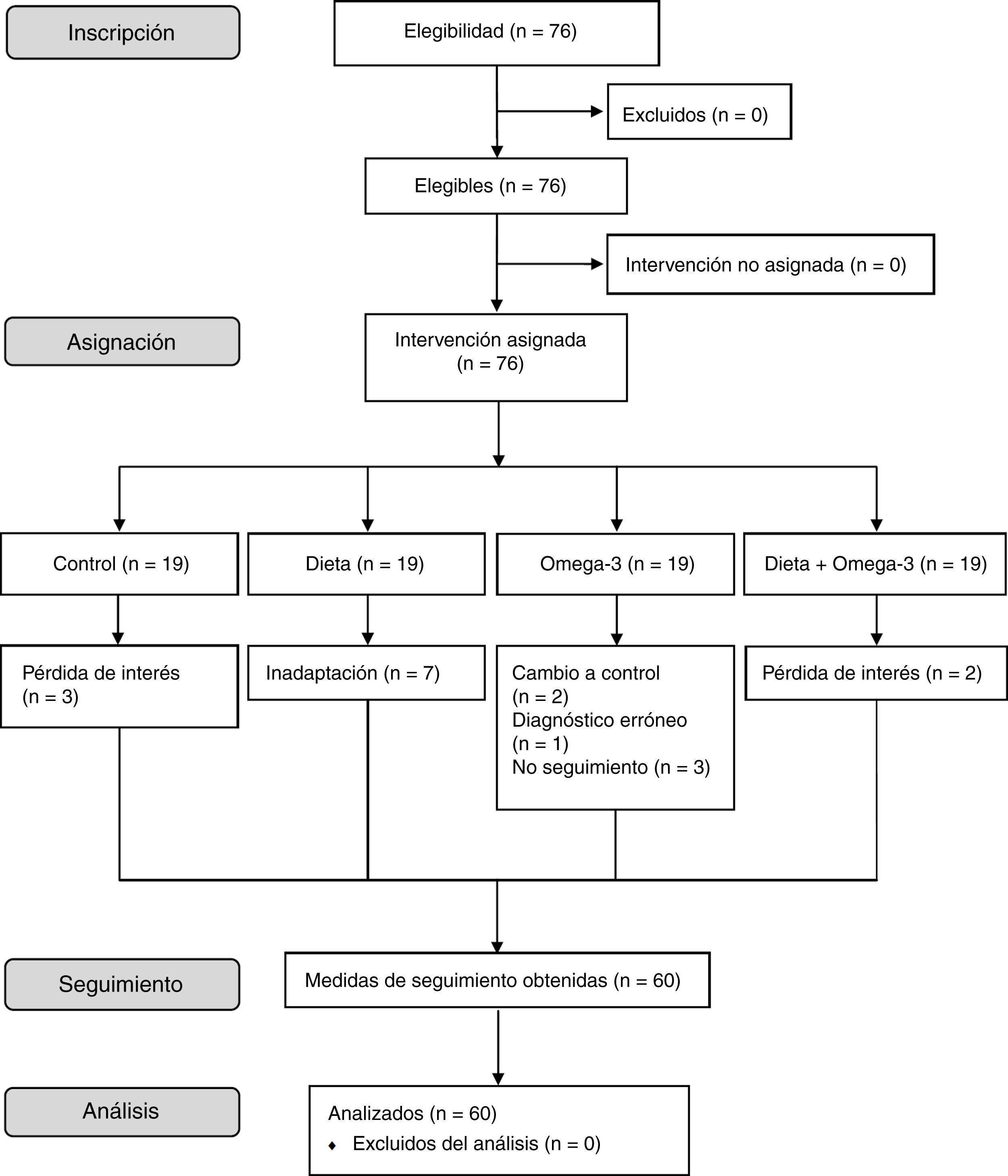
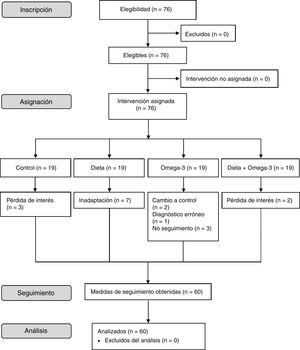
Material and methodsStudy designWe conducted a cross-sectional, observational cohort study of an 8-week dietary intervention in children with ADHD. Eligible patients were divided into 4 groups, with a control group and 3 intervention groups. Group 1 (controls) followed their usual diet. Group 2 (Mediterranean diet) adopted a Mediterranean diet according to a series of recommendations. Group 3 (omega-3) received omega-3 fatty acid supplements. Group 4 (Mediterranean diet + omega-3) adopted the same diet as group 2 and also received omega-3 fatty acid supplements. Fig. 1 summarises the patient selection process.
ParticipantsParticipants were recruited from the paediatrics department at Hospital El Escorial, in Madrid (Spain). We selected children aged 6 to 16 years, of either sex, with a diagnosis of ADHD. We excluded patients with medical conditions that may interfere with food intake (eg, coeliac disease) or with chronic diseases (chronic kidney disease; chronic liver disease; chronic neurological, rheumatic, or autoimmune diseases), and those taking nutritional supplements (vitamins or minerals). The study included 76 patients with ADHD aged 6 to 16 years; 60 of these completed the intervention. Two patients were diagnosed with predominantly hyperactive-impulsive ADHD, 30 with predominantly inattentive ADHD, and 28 with combined-type ADHD. Sixteen patients also presented such comorbidities as dyslexia (n = 9), obesity (n = 3), developmental delay (n = 2), and oppositional defiant disorder (n = 2). Three controls dropped out due to loss of interest. Seven patients in group 2 could not adapt to the diet. Two patients in group 3 were finally included in the control group due to intolerance to the supplement, one patient from that same group was excluded from the study due to misdiagnosis (dyslexia), and 3 additional patients were lost to follow-up. Lastly, 2 patients from group 4 dropped out due to loss of interest (Fig. 1).
ProcedureAll participants were informed about the study verbally and in writing. The patients’ parents signed informed consent forms, and participants gave oral consent to participate in the study. The informed consent forms provided detailed information on the purpose of the study, procedures, study duration, lack of certainty on the safety and efficacy of treatment, and details of the lead researchers. We gathered demographic, anthropometric, and clinical data from all participants and their parents. The KIDMED questionnaire19 was administered to evaluate adherence to the Mediterranean diet. The BIS-11c was administered to every child individually. The application of all assessment tools was supervised by healthcare professionals and qualified researchers. The intervention, from the first to the last visit, lasted a maximum of 8 weeks.
Anthropometric dataAnthropometric data (height and weight) were gathered by the same researcher both at baseline and during follow-up (Table 1). Height and weight were measured with the participants only wearing light clothes (to a precision of 0.1 cm and 0.1 kg, respectively) using a SECA 216 measuring station (Hamburg, Germany), with a range of 3.5-230 cm, and a Tanita BP-601 bioelectric impedance body composition monitor (Amsterdam, The Netherlands), with a range of 0.1-150 kg.
Baseline characteristics of our participants, by intervention group.
| Control | Diet | Supplementation | Diet + supplementation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | P | |
| Age (years) | 10.72 | 2.606 | 11.50 | 2.168 | 11.27 | 2.533 | 10.07 | 2.738 | .577 |
| Height (m) | 1.43 | 0.164 | 1.44 | 0.134 | 1.47 | 0.179 | 1.49 | 0.126 | .763 |
| Weight (kg) | 37.55 | 12.817 | 37.01 | 11.067 | 40.35 | 14.218 | 37.19 | 14.439 | .934 |
| KIDMED score (baseline/8-week) | 6.96 | 2.525 | 6 | 1.604 | 5.45 | 1.635 | 4.80 | 2.274 | .028 |
| 7.08 | 2.159 | 8.57 | 2.440 | 6.91 | 1.814 | 8.20 | 2.077 | – | |
SD: standard deviation.
The BIS-11c is a scale designed to evaluate impulsiveness (Table 2), and was adapted from the Spanish-language version created by Cosi et al.20 It comprises 26 items, distributed in 3 subscales: motor impulsiveness (13 items: 2, 5, 8, 13, 15-18, 20, 21, 23-25), cognitive impulsiveness (5 items: 3, 4, 6, 9, 14), and lack of planning (8 items: 1, 7, 10-12, 19, 22, 26). The BIS-11c has the same response format as the BIS-11, with each item providing 4 response options (0: rarely or never; 1: occasionally; 2: often; 3: always or nearly always).21 The scale may be self-administered or administered by a healthcare professional. From a clinical viewpoint, the quantitative value of the total score is most relevant. Items 1, 7, 9-12, 19, 22, and 26 are reverse-scored. The score of each subscale is obtained by adding the scores of all items included in that subscale. The total score is the combination of the scores of each subscale. No cut-off point has been established for the scale.
Structure and scoring system of the Barratt Impulsiveness Scale for children.
| Type of impulsiveness | No. | Item | Control | Diet | Supplementation | Diet + supplementation | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 8 weeks | Baseline | 8 weeks | Baseline | 8 weeks | Baseline | 8 weeks | |||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Total | 43.39 | 14.323 | 44.35 | 13.374 | 50.33 | 4.719 | 51.67 | 10.172 | 49.00 | 12.095 | 45.10 | 10.867 | 42.38 | 9.473 | 43.50 | 8.361 | ||
| Cognitive | 3 | Decido rápidamente. | 1.36 | 1.221 | 1.35 | 1.265 | 1.62 | 1.302 | 1.14 | 1.464 | 1.82 | 1.168 | 1.45 | 1.036 | 1.93 | 0.884 | 1.93 | 1.033 |
| 4 | Cuando mis amigos me preguntan algo, puedo responder rápidamente. | 2.04 | 0.889 | 1.96 | 0.935 | 2.00 | 0.756 | 1.71 | 1.254 | 1.82 | 0.982 | 1.45 | 0.934 | 1.87 | 0.915 | 2.00 | 0.926 | |
| 6 | Pienso con rapidez. | 1.52 | 0.963 | 1.48 | 0.963 | 1.50 | 0.926 | 1.14 | 1.345 | 1.00 | 1.000 | 1.36 | 1.206 | 1.64 | 1.008 | 2.00 | 1.000 | |
| 9 | Me puedo concentrar rápidamente.a | 0.56 | 0.768 | 0.60 | 0.816 | 0.38 | 0.744 | 0.29 | 0.488 | 0.64 | 1.206 | 0.55 | 0.934 | 0.73 | 0.594 | 1.27 | 0.961 | |
| 14 | En el colegio, soy de los primeros en levantar la mano cuando el profesor hace una pregunta. | 0.88 | 1.013 | 0.92 | 0.997 | 0.75 | 0.707 | 1.29 | 1.380 | 0.91 | 0.944 | 0.73 | 0.786 | 1.27 | 0.961 | 1.27 | 1.100 | |
| Cognitive total | 8.24 | 2.758 | 8.26 | 2.816 | 8.50 | 2.726 | 8.00 | 4.690 | 7.91 | 3.961 | 7.45 | 2.876 | 8.79 | 2.359 | 8.93 | 2.631 | ||
| Motor | 2 | Hago las cosas sin pensarlas. | 1.72 | 0.980 | 1.32 | 0.988 | 2.13 | 0.991 | 2.14 | 0.690 | 2.18 | 0.982 | 2.27 | 0.647 | 1.93 | 1.033 | 2.00 | 0.756 |
| 5 | Me cuesta trabajo estar atento. | 2.24 | 0.779 | 2.32 | 0.690 | 2.25 | 0.886 | 2.57 | 0.535 | 2.55 | 0.688 | 2.27 | 1.009 | 2.13 | 0.640 | 2.27 | 0.458 | |
| 8 | Me desespero con facilidad. | 2.00 | 1.000 | 1.96 | 0.935 | 2.13 | 1.356 | 2.57 | 0.787 | 1.36 | 1.206 | 2.09 | 1.136 | 1.80 | 0.941 | 1.93 | 0.961 | |
| 13 | Digo cosas sin pensar. | 1.68 | 1.069 | 1.68 | 1.069 | 2.13 | 0.835 | 2.57 | 0.535 | 2.00 | 1.155 | 2.09 | 0.944 | 1.73 | 0.961 | 1.79 | 1.122 | |
| 15 | Cambio con facilidad mi manera de pensar. | 1.00 | 1.000 | 1.00 | 1.080 | 1.88 | 1.126 | 1.43 | 1.397 | 1.36 | 1.120 | 1.18 | 1.168 | 1.40 | 1.121 | 1.07 | 0.961 | |
| 16 | Actúo sin pensar. | 1.76 | 1.012 | 1.60 | 1.041 | 2.00 | 1.069 | 2.57 | 0.787 | 2.09 | 0.831 | 2.18 | 0.603 | 1.47 | 0.915 | 2.14 | 0.864 | |
| 17 | Cuando estoy haciendo algo que requiere concentración, me distraigo con facilidad. | 2.20 | 0.816 | 2.16 | 0.987 | 2.50 | 0.756 | 2.86 | 0.378 | 2.45 | 0.688 | 2.55 | 0.688 | 2.40 | 0.737 | 2.20 | 0.862 | |
| 18 | Me dejo llevar por mis impulsos. | 2.20 | 1.041 | 2.00 | 1.041 | 2.13 | 0.991 | 2.86 | 0.378 | 2.09 | 0.831 | 1.64 | 0.674 | 1.87 | 0.990 | 2.07 | 0.961 | |
| 20 | Cambio con frecuencia de amigos. | 0.32 | 0.852 | 0.24 | 0.723 | 0.50 | 0.756 | 0.57 | 1.134 | 0.50 | 0.707 | 0.27 | 0.467 | 0.40 | 0.737 | 0.40 | 0.737 | |
| 21 | Digo cosas sin pensar. | 1.00 | 1.216 | 0.80 | 1.041 | 1.14 | 1.345 | 1.57 | 1.272 | 0.70 | 1.059 | 1.00 | 1.247 | 0.43 | 0.756 | 0.92 | 1.256 | |
| 23 | Gasto más de lo que tengo. | 0.83 | 1.129 | 0.92 | 1.187 | 0.29 | 0.756 | 1.29 | 1.604 | 0.70 | 1.059 | 0.60 | 1.075 | 0.43 | 0.938 | 0.58 | 0.996 | |
| 24 | Cuando estoy pensando en algo me distraigo con facilidad. | 1.92 | 1.152 | 2.12 | 1.013 | 2.25 | 0.707 | 2.71 | 0.756 | 2.36 | 0.505 | 2.27 | 1.009 | 2.21 | 0.802 | 2.13 | 0.834 | |
| 25 | Me cuesta trabajo quedarme quieto en clase. | 1.56 | 1.121 | 1.44 | 1.294 | 1.57 | 1.272 | 1.71 | 1.113 | 2.00 | 1.095 | 1.82 | 1.079 | 1.40 | 1.121 | 1.67 | 1.047 | |
| Motor total | 20.25 | 8.664 | 19.56 | 8.307 | 22.29 | 6.900 | 27.43 | 4.962 | 22.25 | 7.906 | 22.20 | 7.772 | 19.71 | 7.740 | 20.83 | 7.321 | ||
| Lack of planning | 1 | Planeo las cosas que hago.a | 1.12 | 1.054 | 0.96 | 0.978 | 0.75 | 0.886 | 0.57 | 0.787 | 0.73 | 1.009 | 1.27 | 0.905 | 1.20 | 0.862 | 1.33 | 0.900 |
| 7 | Organizo mi tiempo libre.a | 1.04 | 1.197 | 1.00 | 1.155 | 0.75 | 0.886 | 1.50 | 1.378 | 0.36 | 0.674 | 0.55 | 0.820 | 1.07 | 1.033 | 1.07 | 0.730 | |
| 10 | Ahorro lo que más puedo.a | 1.08 | 1.222 | 1.44 | 1.227 | 1.38 | 1.302 | 0.57 | 0.787 | 1.18 | 1.250 | 1.45 | 1.368 | 1.93 | 1.207 | 1.75 | 1.215 | |
| 11 | Me gusta pensar bien las cosas.a | 1.20 | 1.080 | 1.28 | 0.980 | 0.88 | 0.641 | 1.43 | 1.134 | 0.55 | 1.036 | 0.73 | 0.786 | 1.13 | 0.990 | 1.47 | 0.834 | |
| 12 | Hago planes para el futuro.a | 1.12 | 0.971 | 1.08 | 0.954 | 0.86 | 0.690 | 1.57 | 0.787 | 0.64 | 1.027 | 1.09 | 0.944 | 1.13 | 0.743 | 0.93 | 0.884 | |
| 19 | Me gusta pensar las cosas.a | 1.16 | 1.143 | 1.32 | 1.108 | 0.88 | 0.835 | 0.86 | 1.069 | 0.55 | 0.820 | 0.82 | 0.982 | 1.00 | 0.655 | 1.00 | 0.655 | |
| 22 | Soluciono los problemas uno por uno.a | 0.80 | 0.957 | 0.92 | 1.115 | 0.63 | 1.188 | 0.29 | 0.756 | 0.45 | 0.820 | 0.90 | 1.197 | 1.00 | 0.877 | 1.14 | 1.027 | |
| 26 | Soluciono los problemas uno por uno.a | 0.96 | 1.172 | 0.96 | 1.060 | 0.57 | 1.134 | 0.57 | 0.787 | 0.64 | 1.027 | 1.09 | 1.044 | 0.57 | 0.756 | 1.00 | 0.926 | |
| Lack of planning total | 15.83 | 6.005 | 15.04 | 5.763 | 18.67 | 2.804 | 16.33 | 5.391 | 18.909 | 4.392 | 15.60 | 6.186 | 14.77 | 3.539 | 14.00 | 4.199 | ||
SD: standard deviation.
The Mediterranean diet is characterised by a high ratio of monounsaturated to saturated fats; high consumption of pulses, fruit, vegetables, whole grains and minimally processed whole grains, fish, and nuts; low consumption of red meat; and moderate consumption of dairy products and alcohol.22 Adherence to the Mediterranean diet was evaluated with the KIDMED questionnaire,19 a tool designed for the PREDIMED trial.23 Scores range from –4 to 12 points, with higher scores indicating better adherence to the Mediterranean diet (Table 1).
Mediterranean dietA group of dietitians designed a normocaloric Mediterranean diet and tailored it to each participant’s energy requirements. To promote adherence, we considered personal preferences and the place where participants usually ate their meals (home, school, or restaurants). Likewise, all patients and their families were provided with a book of recipes and cooking techniques, and were educated on healthy eating.
Dietitians provided participants with the following general recommendations on the Mediterranean diet24: 1) use of olive oil for cooking and dressings; 2) at least 2 daily servings of vegetables (at least one should be eaten raw, for example in salads), not including garnishes; 3) at least 2-3 daily servings of fruit (including natural fruit juice); 4) at least 3 weekly servings of pulses; 5) at least 3 weekly servings of fish or seafood (at least one should be oily fish); 6) at least 1 weekly serving of nuts or seeds; 7) prioritising white meat (skinless poultry, rabbit) over red or processed meat (hamburgers, sausages); and 8) regular use (at least twice weekly) of tomatoes, garlic, and onion, and dressing vegetables, pasta, rice, and other dishes with a slow-cooked sauce with chopped tomatoes, garlic, onion, and olive oil. Patients and their parents were also advised to avoid or limit consumption of cream, butter, margarine, luncheon meats, pâté, duck meat, carbonated or sugary drinks, industrial baked goods (cakes, doughnuts, biscuits), desserts, pastries and puddings, and potato chips.
Omega-3 fatty acid supplementation“Children’s Omega-3” (New Roots Herbal Inc.; Quebec, Canada) is a sugar-free nutritional supplement containing omega-3 fatty acids, which provides 137.5 g EPA and 56.25 mg DHA per softgel, at the ideal 2:1 ratio. Softgels are available in 3 different flavours: lemon, orange, and blackcurrant. Fish oil is sourced from deep-sea sardines and anchovies by molecular distillation, a purification method that guarantees that the oil is free from environmental pollutants. The recommended dose was 2 softgels half an hour after breakfast, plus 2 softgels half an hour after dinner, that is, 550 mg EPA and 225 mg DHA per day.
Statistical analysisStatistical analysis was performed using the SPSS statistics software, version 24.0 (IBM; Armonk, NY, USA). We conducted a descriptive analysis of sociodemographic variables and lifestyle habits. Continuous variables are expressed as means and standard deviation (SD). Means were compared with ANOVA and the t test for paired samples. All tests were applied to the total population, and to sex, age, and treatment subgroups. Statistical significance was set at P < .05.
ResultsWe finally included 60 patients aged 6-16 years (mean [SD], 10.74 [2.57] years), who were recruited between 2017 and 2018. Of these, 41 (68.4%) were boys and 18 (30%) were girls; sex information was lost for one patient. Baseline demographic and anthropometric data and KIDMED scores are shown in Table 1.
The t test did not reveal statistically significant differences in baseline characteristics between sexes. However, we did find significant differences in baseline KIDMED scores between treatment groups (P = .028) (Table 1). Controls scored a mean of 6.96 (2.53) on the KIDMED scale; this reflects higher adherence to the Mediterranean diet; however, all treatment groups improved adherence by the end of the intervention, particularly the diet + supplementation group.
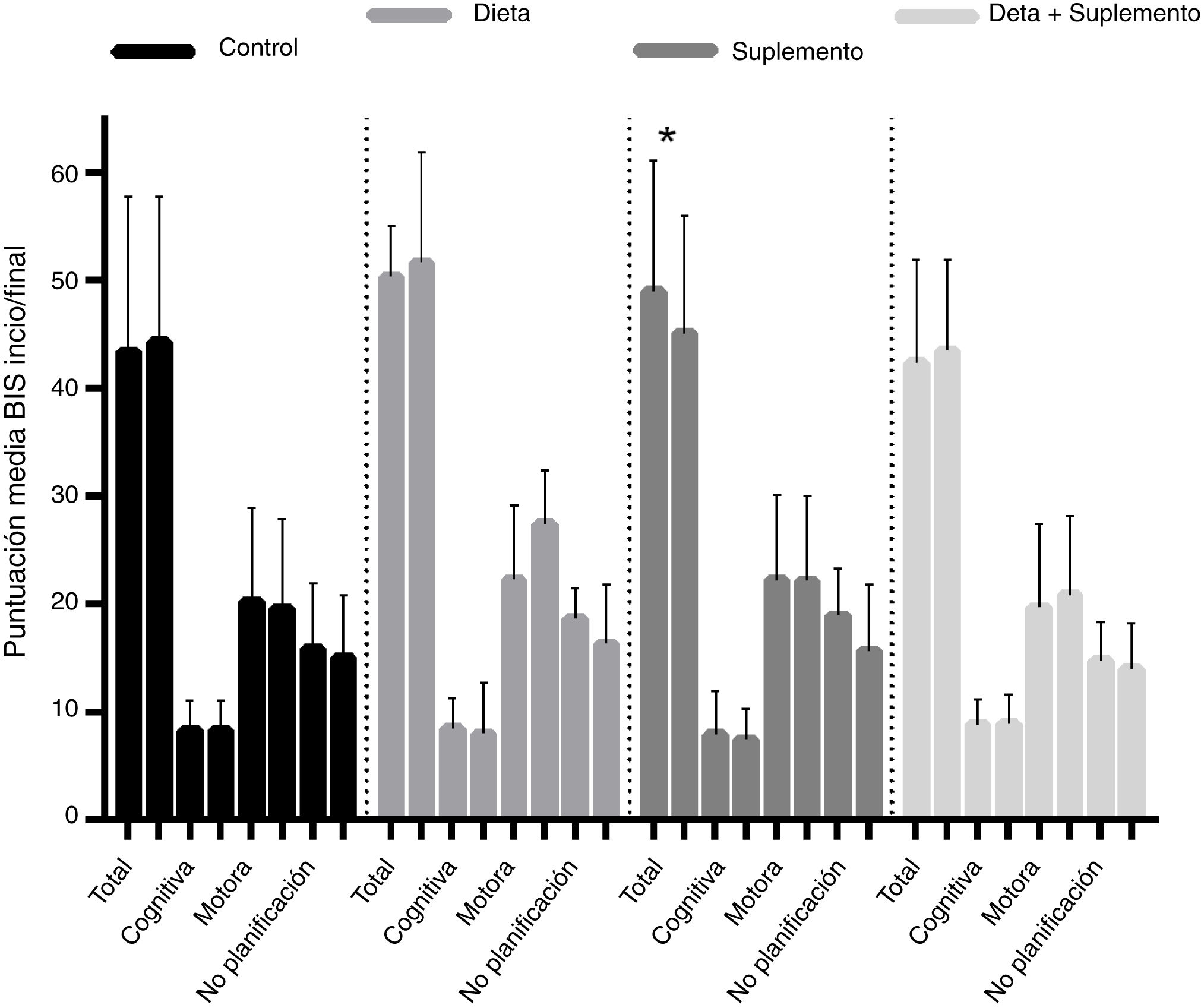
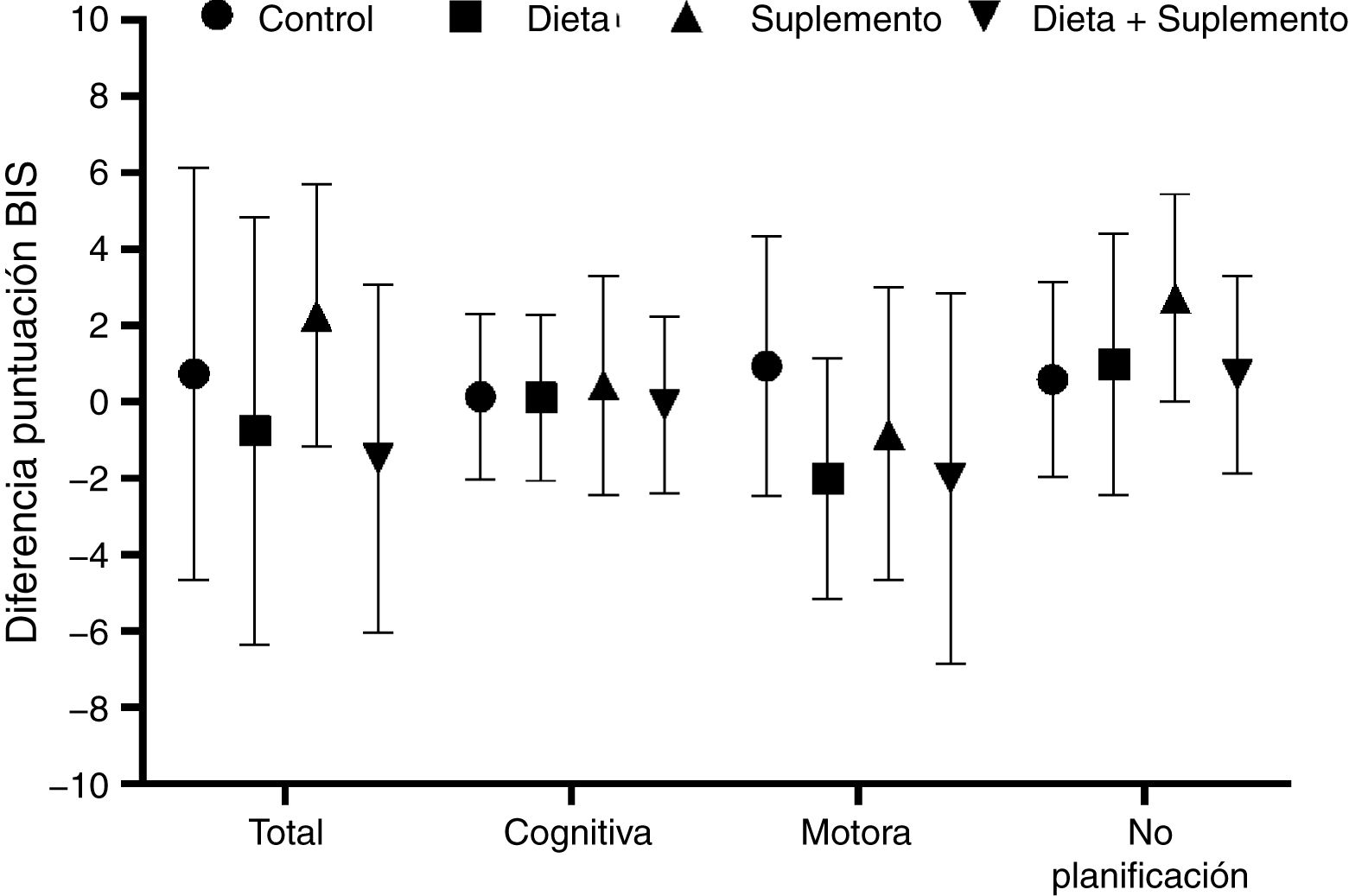
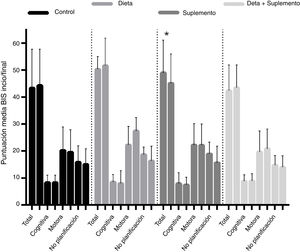
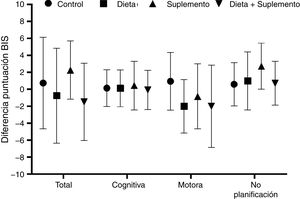
BIS-11c scores for each treatment group are shown in Table 2. Children in the diet and supplementation groups scored higher at baseline than controls and children in the diet plus supplementation group. However, only those children in the supplementation group scored lower on the BIS-11c scale after the intervention. Regarding total cognitive scores, no changes were observed in the control group, whereas children in the diet and supplementation groups presented slight decreases, and children in the diet plus supplementation group scored higher after the intervention. Only controls presented a significant decrease in total motor scores. Total lack of planning impulsiveness scores were lower in all groups after the intervention (Fig. 2). Lower scores indicate less marked impulsiveness. Improvements are presented in a separate figure (Fig. 3) to facilitate comprehension.
Scores on the Barratt Impulsiveness Scale at baseline and at 8 weeks of follow-up, by dimension of impulsive behaviour and intervention group. *P < .05. Mean improvements are represented in Fig. 3.
A high positive correlation (r > 0.9) was found between baseline and final Barratt scores in the control, diet, and supplementation groups. This correlation was statistically significant in all 4 groups, although the t test for paired samples showed that the only truly significant change from baseline to end of treatment was observed in the supplementation group, with a significant drop in Barratt scores (from 49 to 45.10 points; P = .049) (Fig. 2).
DiscussionThis is the first study into the effects of the Mediterranean diet and/or n-3 PUFA supplementation as interventions to improve impulsive behaviour in children and adolescents with ADHD; the BIS-11c was used to assess behavioural changes.
The BIS is a well-designed instrument with adequate reliability and validity.17 Previous validations of the BIS-11 in Spanish-speaking adolescents14 seem not to be adapted to the context of this age group. Items 1, 14, 20, 25, and 27 were not rephrased to evaluate the behaviour of children/adolescents. In our study, we used the rephrased items proposed by Chahin et al.,25 according to the recommendations of the authors of the BIS. For example, instead of asking a child about changes in residence, we asked about changes in friendship, which is more representative of their impulsiveness and more appropriate for this population.
N-3 PUFA deficiency may play a pathogenic role in ADHD.26,27 As approximately 20%-40% of patients with ADHD do not respond to pharmacological treatment,28 developing new treatments and strategies is essential. Interestingly, while some clinical trials of n-3 PUFA supplementation in ADHD have reported improvements in symptoms29,30 and cognitive function,31 others have failed to find beneficial effects.32 This motivated our decision to include omega-3 supplementation in our analysis. The dose administered to patients with ADHD range from 2.7 mg to 640 mg of DHA and from 80 mg to 650 mg EPA, according to a recent meta-analysis.28 All the clinical trials included in the meta-analysis found improvements in inattention and in total scores for ADHD symptoms, regardless of EPA dosage.
However, only studies administering EPA at doses ≥ 500 mg reported improvements in hyperactivity. In our study, patients with ADHD taking n-3 PUFA supplements (550 mg EPA and 225 mg DHA daily) showed significantly lower levels of impulsiveness than those adopting a Mediterranean diet and controls. These patients also scored lower on all subscales of the BIS (cognitive, motor, and lack of planning). However, no differences were found in impulsive behaviour between patients taking omega-3 supplements and those taking supplements and adhering to the Mediterranean diet, which suggests that the combination of both strategies does not provide additional benefits.
Dietary patterns have influenced both physical and mental health.33 Few studies have examined the association between dietary patterns and multifaceted mental states, such as impulsive behaviour.8,34 Toyomaki et al.33 found that low intake of whole grains is associated with more impulsive behaviour, as demonstrated by BIS-11 deliberation and sum scores. Okubo et al.35 suggest that a diet rich in vegetables, soya products, fruit, and fish may have beneficial effects on cognitive function. Some trials of dietary interventions have reported behavioural improvements in children with such other neurodevelopmental disorders as autistic spectrum disorder36 and developmental coordination disorder37; these are not analysed in detail in our study as they did not include children with ADHD.
To our knowledge, this is the first study into the relationship between the Mediterranean diet, or any dietary intervention based on this dietary pattern, and impulsive behaviour in children with ADHD. In our study, no association was found between the Mediterranean diet and lower levels of impulsiveness in children with ADHD. One previous study analysed the association between dietary patterns in school-age Korean children and ADHD, manifesting as impulsive behaviour and inattention.8 The researchers found that the traditional healthy Korean diet was associated with a lower likelihood of ADHD, and therefore with a lower likelihood of presenting impulsive or inattentive behaviour. Likewise, another study found poorer adherence to a Mediterranean diet among children with ADHD. However, this study did not evaluate the changes these children may have presented with proper adherence to the Mediterranean diet.10
Our study is not without limitations. The sample was small for a cross-sectional study, which may have contributed to the negative results; subtle differences in impulsiveness may also have gone undetected. Furthermore, our sample included patients with combined-type ADHD; these patients may be less responsive to any type of intervention. Paediatric patients frequently present dietary problems and/or unhealthy eating habits and lifestyles. Achieving appropriate adherence to a Mediterranean diet was not easy, as in some cases it led to stress, irritability, arguments, and aggressive behaviour.
ConclusionOur results show no statistically significant differences between groups, except for the group of children receiving omega-3 supplementation. Patients with ADHD receiving 550 mg EPA and 225 mg DHA daily present less impulsive behaviour than controls with ADHD and patients who adopted a Mediterranean diet. EPA/DHA supplements may therefore be considered for paediatric patients with ADHD, particularly those with the predominantly hyperactive-impulsive subtype. The impact of a Mediterranean diet combined with omega-3 supplementation on impulsive behaviour in patients with ADHD should be further studied in both clinical and research settings. Studies with larger samples are therefore needed to determine the relationship between BIS scores and treatments; this will deepen our understanding of this topic.
Ethical standardsAll procedures involving humans complied with the ethical standards of the research committee at Hospital Universitario Puerta del Hierro in Majadahonda (Madrid, Spain) (clinicaltrials.gov identifier: NCT02999503), as well as with the principles of the 1964 Declaration of Helsinki and its subsequent amendments, and with comparable ethical standards.
All participants gave informed consent to be included in the study.
FundingThis study was supported by New Roots Herbal Inc. (Vaudreuil-Dorion, Quebec, Canada), which provided us with the “Children’s Omega-3” supplements. New Roots Herbal Inc. did not participate in data collection or study design, and did not analyse or interpret our results, nor has it reported our findings.
Author contributionsISMM and JABO participated in study conception and design; LGC, RCC, and SSR participated in data collection; SSR participated in data analysis and interpretation; and EGV drafted the manuscript. All authors made a critical review of the manuscript and approved the final version for publication.
Conflicts of interestThe authors have no conflicts of interest to declare.
We wish to thank Hospital El Escorial (Madrid, Spain), the APDE Sierra Association (Madrid, Spain), and New Roots Herbal Inc. (Vaudreuil-Dorion, Quebec, Canada) for their expertise and assistance throughout the study.
Please cite this article as: San Mauro Martin I, Sanz Rojo S, González Cosano L, Cotny de la Campa R, Garicano Vilar E, Blumenfeld Olivares JA. Impulsividad en el trastorno por déficit de atención e hiperactividad en niños después de una intervención de 8 semanas con dieta mediterránea y/o ácidos grasos omega-3: ensayo clínico aleatorizado. Neurología. 2022;37:513–523.