Microvascular decompression is considered to be the most effective and only etiological surgical treatment for classical trigeminal neuralgia, relieving the neurovascular compression found in up to 95% of cases. This study aims to report the long-term outcomes and to identify prognostic factors in a series of patients with trigeminal neuralgia treated by microvascular decompression.
MethodsA retrospective observational study of 152 consecutive patients operated by microvascular decompression with at least six months of follow-up. The surgical results, including pain relief according to the Barrow Neurological Institute pain scale, complications and the medical treatment during the follow-up period were reviewed. Binary regression analysis was performed to identify factors associated with a good long-term outcome.
ResultsA total of 152 patients with a mean age of 60 years and a mean follow-up of 43 months were included. At the final follow-up visit, 83% of the patients had achieved significant relief of the pain and 63% could reduce the absolute drug doses by 50% or more. The most frequent complications were wound infection (4.5%) and CSF fistula (7%). Being over 70 years of age and having paroxysmal pain were associated with a long-term pain relief.
ConclusionsOur results support the notion that microvascular decompression is an effective and safe therapy in patients with trigeminal neuralgia. A multidisciplinary approach with an early referral to a neurosurgical unit many be beneficial in patients who are refractory to pharmacological treatment.
La descompresión microvascular se considera el tratamiento quirúrgico etiológico más eficaz de la neuralgia clásica del trigémino, dirigido a aliviar la compresión neurovascular identificada hasta en un 95% de casos. Este estudio tiene como objetivo analizar los resultados quirúrgicos y la evolución a largo plazo de una serie de pacientes con neuralgia del trigémino tratados mediante descompresión microvascular, así como identificar factores pronósticos.
MétodosEstudio observacional retrospectivo de 152 pacientes consecutivos sometidos a descompresión microvascular y con un seguimiento posquirúrgico mínimo de seis meses. Analizamos los resultados quirúrgicos, clasificando el grado de dolor según la escala del Instituto Neurológico de Barrow, las complicaciones y el tratamiento médico requerido durante el período de seguimiento. Realizamos un análisis de regresión binaria para identificar factores asociados con un buen resultado a largo plazo.
ResultadosIncluimos 152 pacientes con una edad media de 60 años y un seguimiento medio de 43 meses. En la última visita de seguimiento, el 83% de los pacientes había logrado un alivio significativo del dolor y el 63% pudo reducir la dosis absoluta de fármacos para la neuralgia en un 50% o más. Las complicaciones más frecuentes fueron infección de la herida (4,5%) y fístula de LCR (7%). La edad superior a 70 años y el dolor de predominio paroxístico se asociaron con un mejor pronóstico.
ConclusionesNuestros resultados apoyan que la descompresión microvascular es una terapia efectiva y segura en pacientes con neuralgia del trigémino. La cirugía temprana puede ser beneficiosa en pacientes refractarios al tratamiento farmacológico.
According to the 3rd edition of the International Classification of Headache Disorders, classical trigeminal neuralgia (CTN) is defined as trigeminal neuralgia that develops without an apparent cause other than neurovascular compression (NVC).1–3 The incidence rate of NVC has been reported to be 80–95% in patients suffering from CTN. The diagnosis is based on anamnesis, although magnetic resonance imaging (MRI) is recommended in all cases of CTN to exclude a symptomatic cause for the pain.3,4 Specific high-resolution sequences have been developed to evaluate the presence of NVC and the possible changes in trigeminal morphology, including 3D T2-weighted (DRIVE: driven equilibrium; FIESTA: fast imaging employing steady-state; CISS: constructive interference in steady-state), 3D TOF-MRA, and 3D T1-Gadolinium.5,6
The international guidelines recommend conservative treatment as the initial strategy for CTN. This mainly relies on the administration of antiepileptic drugs (AEDs), with carbamazepine (CBZ) and other sodium channel blockers such as oxcarbazepine (OXC) and eslicarbazepine (ESL) being the preferred choice.4 Other drugs such as gabapentin (GBP), pregabalin (PGB) and baclofen, are also recommended in mono- or polytherapy regimes.4 In the early stages of the disease, approximately 70% of patients will experience pain control with conservative treatment. Unfortunately, the efficacy of medication declines over time and, in some cases, medical therapy has to be discontinued due to adverse effects. Consequently, about 50% of patients with CTN eventually require surgical intervention in order to achieve pain relief.7,8 There is presently not a clear consensus regarding the best time for surgery, and the current guidelines usually recommend considering invasive treatments when the pain is not sufficiently controlled medically or when the patient experiences drug intolerance.3,4,9
The pathophysiology underlying CTN has not yet been fully elucidated. It is, however, widely accepted that vascular compression near the entry of the trigeminal root into the pons (REZ), where Schwann cells are replaced by oligodendroglia, causes focal demyelination of primary afferents. As a result, aberrant, ectopic, neural afferent impulses develop, triggering acute neuralgic attacks.10–12 NVC has been described in numerous people who do not develop TN, thus the current view is to determine the degree of compression in patients with TN and not just any kind of neurovascular contac.13–15 Likewise, genetic factors related with anatomical skull variability, hypertension (HTN), both conditions promoting NVC, and mutations in the genes coding for sodium or calcium channels may contribute to the onset of pain.15,16 A gain-of-function mutation in the voltage gated sodium channel Nav1.6 has been described in a patient suffering from CTN.17 Disregularion of the voltage gated sodium channels has been reported in TN animal models and may be involved in the generation of ectopic activity in trigeminal afferents.15,17
In light of the widely accepted notion that NVC plays a key role, microvascular decompression (MVD) is considered to be the only etiological and non-ablative therapy for CTN. Other surgical procedures commonly used for CTN are stereotactic radiosurgery (SRS) and percutaneous rhizotomy (PR), including balloon compression (BC), glycerol rhizotomy (GR), and radiofrequency thermocoagulation (RF). Compared with these surgical alternatives, MVD offers a higher rate of long-term pain relief, and it is considered to be the surgical technique of choice for CTN.4,18–20 However, as this procedure is associated with a non-negligible rate of complications, including cerebellar hematoma or permanent cranial nerve damage, some physicians are reluctant to use this therapeutic option, especially for older people. Consequently, and despite its widely proven efficacy, MVD is often reserved for just a small percentage of patients suffering from CTN.21
The aim of this study was to report our experience with MVD as a surgical treatment for CTN in a large series, from the perspective of a multidisciplinary approach. We describe the clinical and the radiological characteristics, the disease natural history, and the postoperative long-term outcomes in terms of the efficacy and safety. In addition, factors related to better long-term results were analyzed.
Patients and methodsPatient selection and clinical assessmentWe retrospectively studied 152 consecutive patients from a prospectively kept database suffering from refractory CTN who underwent MVD between early 1997 and late 2018 in our center.
MVD can be considered a demanding microsurgical procedure that requires a certain expertise. Since the number of CTN cases referred to surgery is limited, it has been established in our department that only certain neurosurgeons should perform this procedure in order to acquire and maintain surgical skills. The present series reports cases that were all operated by two senior neurosurgeons from 1995 to 2009, while since 2010 the senior author FRJ has operated on 95% of the reported cases.
All of the patients fulfilled the diagnostic criteria for CTN based on the 3rd edition of the International Classification of Headache Disorders criteria,1 and they suffered from severe pain, they were non-responsive to AEDs, or they did exhibit drug intolerance. Charts of patients included prior to this latest version were retrospectively reviewed, mainly to rule out criteria for secondary trigeminal neuralgia. From 2004 onwards, high-resolution 3DT2 DRIVE sequences (Philips Achieva 1.5T) were used to preoperatively evaluate the NVC, defined as the absence of CSF signal between the trigeminal nerve and a vascular structure at any point along the nerve's course in the cerebellopontine cistern,6,13 with a total of 106 patients undergoing this procedure.
The clinical and demographic characteristics included gender, age (< or ≥70 years of age), hypertension; the nature of the neuralgia (paroxysmal or continuous pain), the pain duration (< or ≥4 years), the response to medication, and the medical treatment regimen. Data regarding the NVC such as the severity of the compression, the site, and the responsible vessel were recorded by MRI and during microsurgical inspection of the cerebellopontine angle (CPA). The radiological results from the patients who underwent specific cysternography sequences were compared with the intraoperative findings in order to estimate the sensitivity and the specificity of the high-resolution 3DT2 DRIVE MRI.
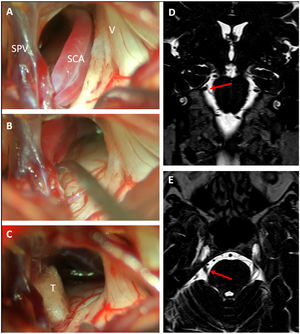
Surgical techniqueThe operations were performed under general anesthesia with the patient in a three-quarter prone position. A standard lateral suboccipital retrosigmoid craniectomy exposing the limits of the transverse and sigmoid sinus was employed to gain access to the CPA. Microsurgical inspection of the CPA with careful dissection of arachnoid attachments allowed for ample exposure of the subarachnoid course of the trigeminal root and its vascular relations. Afferents to the superior petrous vein complex were spared whenever possible, although in cases where exposure of the nerve was severely limited by veins, some afferents were selectively coagulated and sectioned. MCV was performed following the principles described by Jannetta.21,22 In cases of arterial compression, a vessel transposition away from the nerve was attempted and one or more Teflon pledgets were interposed between the artery and the nerve. In cases of venous compression, Teflon decompression, or venous coagulation and sectioning were chosen depending on the caliber of the vein and its disposition. In cases that did not exhibit any vascular compression after thorough exploration, a partial sensory rhizotomy (PSR) was performed (Fig. 1). PSR is an ablative procedure that intentionally damages the nerve providing effective and lasting pain relief in a rate at least comparable to other ablative procedures such as percutaneous trigeminal gangliolysis.23 It may be an acceptable option when no vascular compression can be proven. Since there is no evidence that PSR enhances the results of MVD and because of its ablative nature, it always implies some degree on sensory deficit which in rare cases may cause anesthesia dolorosa,23 we only consider PSR for cases without neurovascular compression or for repeat operations after failed MVD.
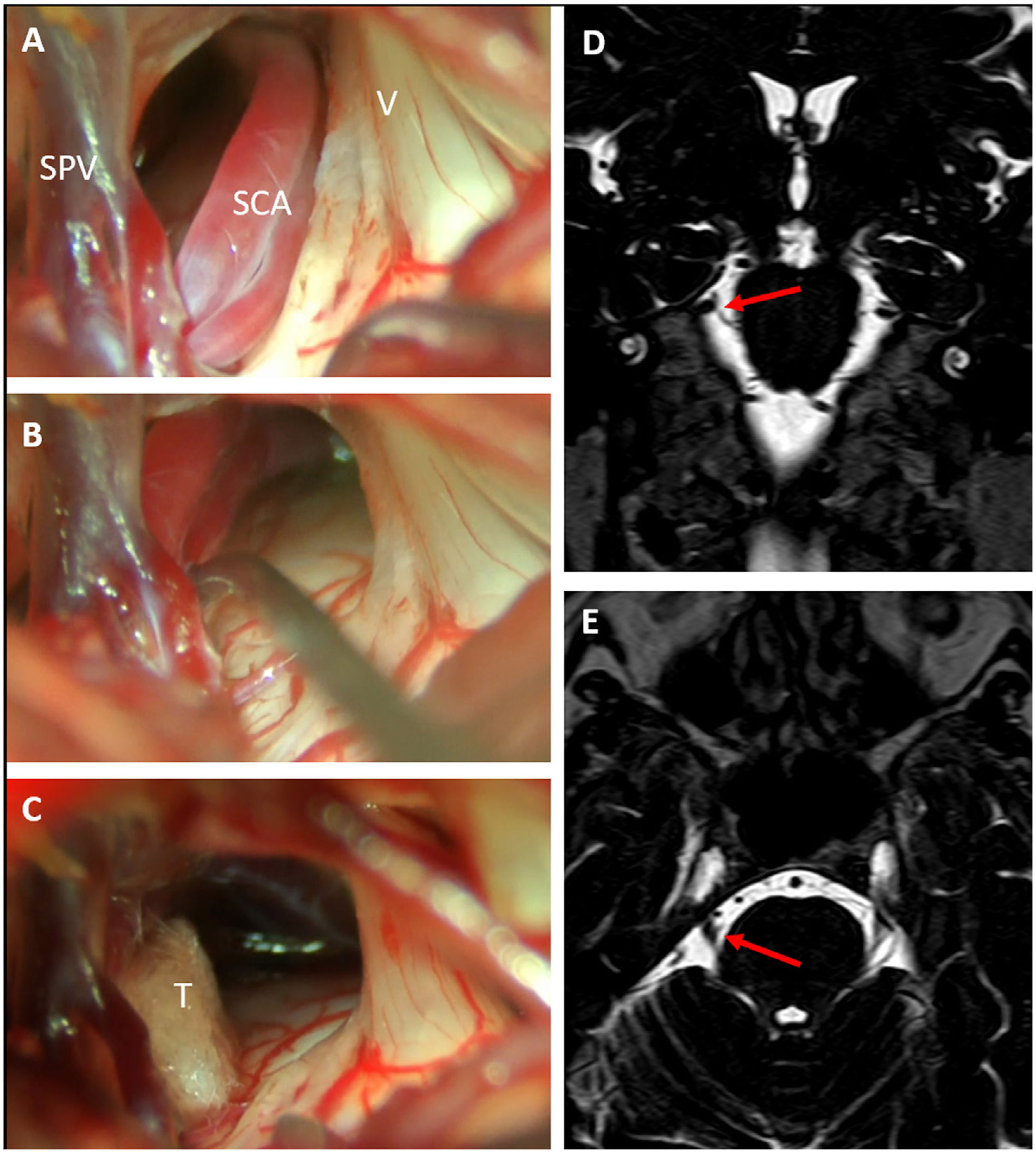
Right trigeminal neuralgia caused by a descending loop of the superior cerebellar artery (SCA) as seen on preoperative coronal (D) and axial (E) DRIVE-MRI sequences. Microsurgical inspection revealed a double arterial loop corresponding to the superior and inferior branches of the SCA compressing the superomedial portion of the trigeminal nerve (A). After dissection of the arachnoid adhesions, the arteries could be transposed cranially (B) and held in place with a Teflon pledget (C). SPV: Superior petrosal vein; SCA: Superior cerebellar artery; V: Fifth cranial nerve; T: Teflon pledget.
Only patients with CTN who underwent MVD and a minimum period of 6 months of follow-up were included. Most of the patients were checked at one month, 3 months, 6 months, and yearly after the surgery.
The pain outcome was assessed with the Barrow Neurological Institute pain scale (BNI score), classified as either pain-free and no medication (BNI I-II), pain-free with medication (BNI III), or not pain-free (BNI score IV-V). BNI scores of I to III were considered to represent therapeutic success.24 Recurrence was defined as the presence of facial pain at BNI grade IV or V after the initial pain relief.
The main efficacy outcome measures were early (first month after discharge) and long-term (last follow-up visit) postoperative significant pain relief (BNI score of I to III). Taking advantage of the multidisciplinary management between neurology and neurosurgery provided at our institution, medication dose reduction and the percentage of patients on monotherapy after surgery were recorded as secondary efficacy outcomes.
Statistical analysisThe statistical analysis was performed using IBM SPSS Statistics for Windows, Version 22.0, software (IBM Corp. Armonk, NY, USA). Descriptive analysis of the clinical and the paraclinical data was performed. Paired nominal data were compared using McNemar's test. The probability of being pain-free without medication (BNI I) and the rates of severe pain relapse (BNI IV-V) over time were assessed with Kaplan-Meier analyses. Correlations between the clinical characteristics and the outcomes were evaluated by Kaplan–Meier curves and uni- and multivariate binary logistic regressions. Odds ratios (OR) and 95% confidence intervals (CI) were used to evaluate the association strength. A p-value of <0.05 was considered statistically significant.
Ethical considerationsThis study was performed following the Helsinki Declaration principles, approved by the Ethics Committee of our center and the Spanish Agency of Medicines and Medical Devices (AEMPS).
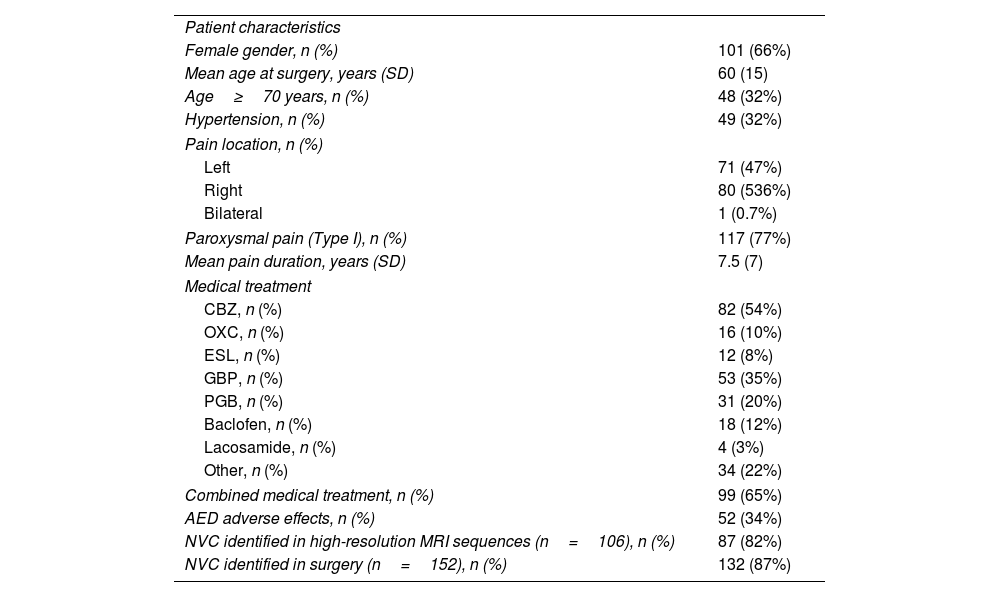
ResultsDemographic and clinical characteristicsThe clinical characteristics of the patients are presented in Table 1. Of the 152 cases, 101 (66%) were women, with a mean age of 60 years at the time of the surgery (24–85). Forty-eight (32%) were 70 years of age or older, and 32% had previously been diagnosed with arterial hypertension (HTN). In terms of the pain characteristics, 117 (77%) patients had typical paroxysmal pain (type I), while 35 (23%) suffered concomitant persistent facial pain (type II). The right side of the face was affected in 80 cases (53%) and the left side in 71 (47%). The mean duration of the symptoms until the surgery was 7.5 years (range 2 months to 30 years).
Patient characteristics (n=152).
| Patient characteristics | |
| Female gender, n (%) | 101 (66%) |
| Mean age at surgery, years (SD) | 60 (15) |
| Age≥70 years, n (%) | 48 (32%) |
| Hypertension, n (%) | 49 (32%) |
| Pain location, n (%) | |
| Left | 71 (47%) |
| Right | 80 (536%) |
| Bilateral | 1 (0.7%) |
| Paroxysmal pain (Type I), n (%) | 117 (77%) |
| Mean pain duration, years (SD) | 7.5 (7) |
| Medical treatment | |
| CBZ, n (%) | 82 (54%) |
| OXC, n (%) | 16 (10%) |
| ESL, n (%) | 12 (8%) |
| GBP, n (%) | 53 (35%) |
| PGB, n (%) | 31 (20%) |
| Baclofen, n (%) | 18 (12%) |
| Lacosamide, n (%) | 4 (3%) |
| Other, n (%) | 34 (22%) |
| Combined medical treatment, n (%) | 99 (65%) |
| AED adverse effects, n (%) | 52 (34%) |
| NVC identified in high-resolution MRI sequences (n=106), n (%) | 87 (82%) |
| NVC identified in surgery (n=152), n (%) | 132 (87%) |
SD: Standard deviation, CBZ: Carbamazepine, OXC: Oxcarbazepine, ESL: Eslicarbazepine, GBP: Gabapentin, PGB: Pregabalin.
All of the patients had received medical therapy to which they had become refractory or intolerant. CBZ was the main AED prescribed (54%), followed by GBP (35%), PGB (20%), and baclofen (12%). At the time of the surgery, 99 (65%) of our patients were under a polytherapy regime and the rate of drug intolerance was 34%. Severe adverse effects, such as hepatitis, cutaneous reactions, or symptomatic hyponatremia, occurred in 9% of cases.
In terms of the neuroimaging findings, high-resolution 3DT2 DRIVE MRI sequences were performed in 106 individuals. These revealed a vascular structure in contact with the trigeminal root in 87 cases (82%). Comparing these radiological results with the surgical findings in this subgroup of patients (NVC found intraoperatively in 97 cases; 92%), the specific cisternography 3DT2 DRIVE MRI had a sensitivity of 90% and a specificity of 100%. Considering the 46 patients that were not preoperatively studied with 3DT2 MRI sequences, 28% did not show neurovascular compression intraoperatively. The overall rate of NVC found during the surgery was 87% (Fig. 1).
Postoperative outcomesThe mean follow-up duration was 43 months, ranging from 6 months to 15 years. At the time of the study, 38 patients had been evaluated for more than 5 years, 11 of whom had completed a 10-year follow-up period. At the first follow-up visit, which was in the first month±2 weeks after discharge, significant pain relief (BNI I-III) was noted in 97% of cases. There were four patients who did not improve initially and were offered a second MVD as well as the percutaneous treatment options short after surgery. Three patients underwent a second MVD that included PSR within the first month after the first MVD, while the fourth patient preferred a RF. Two of the three patients who underwent MVD combined with PSR showed a significant improvement of pain.
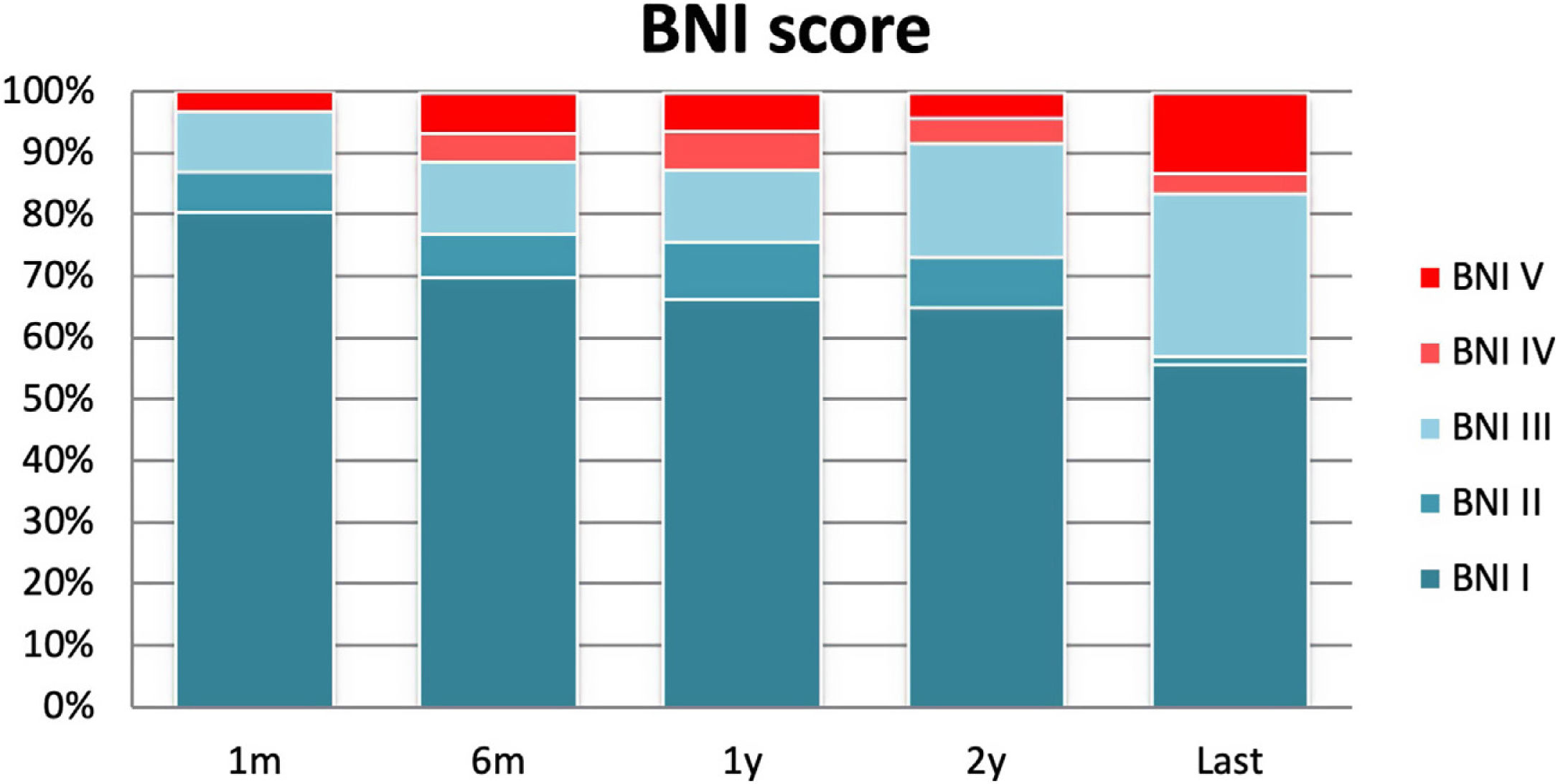
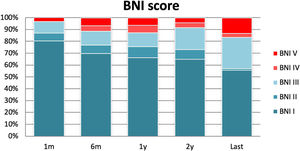
At the final evaluation, 83.4% of the patients achieved long-term pain relief (BNI I–III) after MVD; with excellent pain control (BNI I) in 84 patients (56%) and satisfactory pain control (BNI II–III) in 42 (28%). Pain control was absent (BNI IV and V) in 25 patients (16%) (Fig. 2). During the follow-up period, 13 patients (9%) with previously controlled pain developed recurrent pain that was refractory to medication (BNI IV–V), with a mean time of 13.5 months until the recurrence (Table 2). Among these, a second surgical procedure aimed at relieving the pain was performed in ten cases: 7 patients had MVD, and 3 underwent RF.
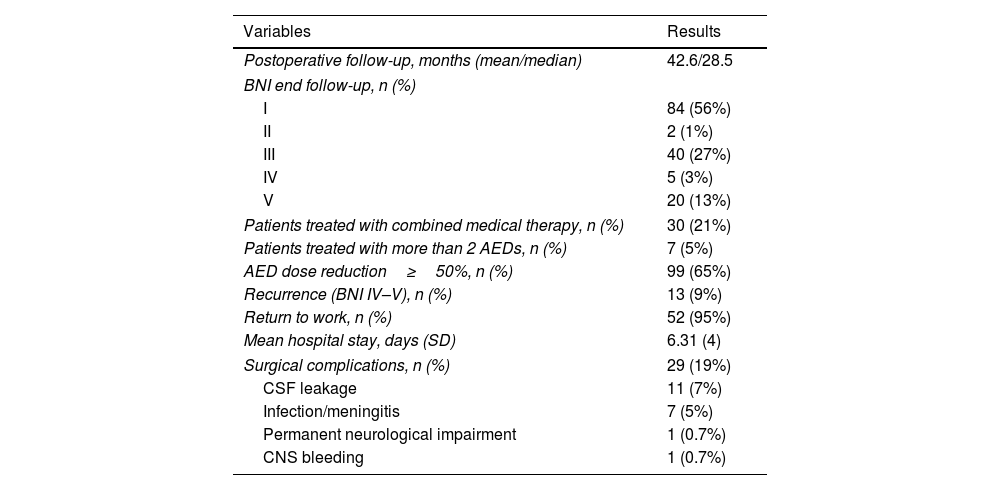
Postoperative outcomes at the final follow-up visit (mean 43 months).
| Variables | Results |
|---|---|
| Postoperative follow-up, months (mean/median) | 42.6/28.5 |
| BNI end follow-up, n (%) | |
| I | 84 (56%) |
| II | 2 (1%) |
| III | 40 (27%) |
| IV | 5 (3%) |
| V | 20 (13%) |
| Patients treated with combined medical therapy, n (%) | 30 (21%) |
| Patients treated with more than 2 AEDs, n (%) | 7 (5%) |
| AED dose reduction≥50%, n (%) | 99 (65%) |
| Recurrence (BNI IV–V), n (%) | 13 (9%) |
| Return to work, n (%) | 52 (95%) |
| Mean hospital stay, days (SD) | 6.31 (4) |
| Surgical complications, n (%) | 29 (19%) |
| CSF leakage | 11 (7%) |
| Infection/meningitis | 7 (5%) |
| Permanent neurological impairment | 1 (0.7%) |
| CNS bleeding | 1 (0.7%) |
AEDs: Antiepileptic drugs, CSF: Cerebrospinal fluid, CNS: Central nervous system.
Regarding the secondary outcomes of medication dose reduction and the percentage of patients on monotherapy at the last follow-up visit, 99 patients (65%) were able to reduce the AEDs absolute dosage by at least 50%, including people in monotherapy regimes, and 84 patients (56% of the cohort) were without medication. The pain could be controlled in 33 patients with monotherapy after surgery, and only 30 patients (21%) needed a combination of medications to control the pain, compared with 99 (65%) who were on polytherapy prior to the surgery (p<0.001). Of these patients, only 5% needed three or more AEDs to control the pain at the end of the follow-up.
The most common postoperative complications were cerebrospinal fluid (CSF) leakage (11 cases; 7%) and wound infection (7 cases; 5%). A single patient developed permanent neurological impairment (hypoacusia), and only one patient experienced a postoperative cerebellar hematoma that required urgent evacuation surgery who then recovered completely. There were no deaths related to the surgical procedure. Comparison of the incidence of neurological complications in patients older than 70 years versus younger patients did not reveal a statistically significant difference (9% vs. 11%, respectively; p=0.36). The incidence of medical postoperative complications (e.g., urinary tract infection, pneumonia, heart failure, deep vein thrombosis, or pulmonary embolism) was also found to be similar in both age groups, i.e., 7% in the ≥70 years of age group and 11% in younger patients (p=0.6).
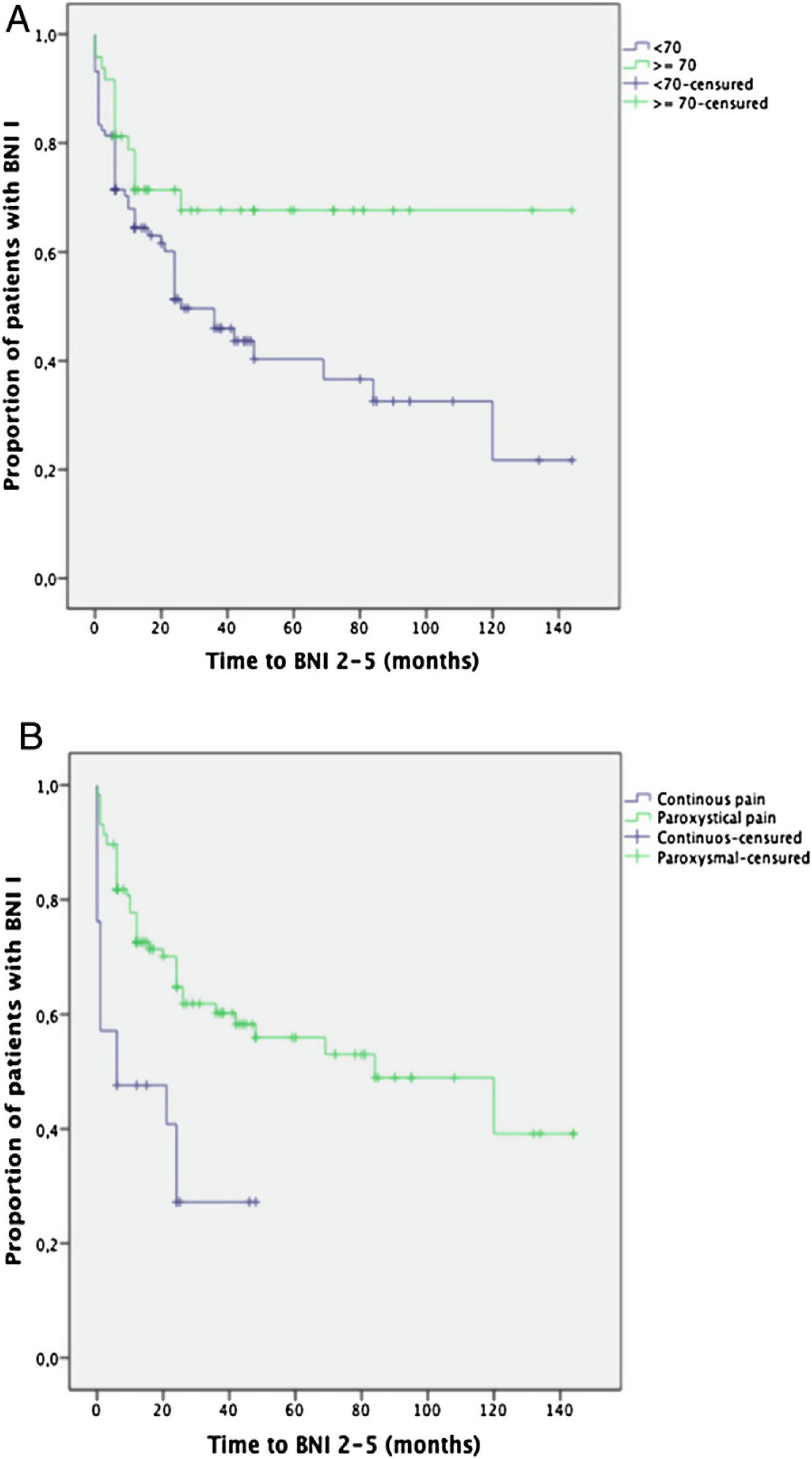
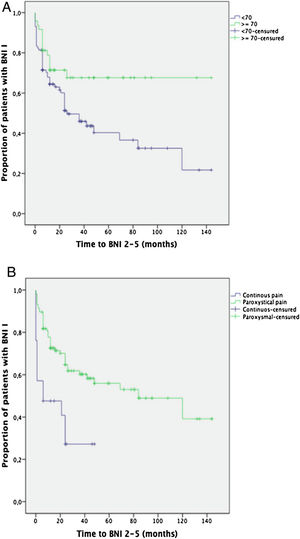
Prognostic factorsKaplan-Meier survival curves comparing the age groups (< or ≥70 years of age) and the pain characteristics (paroxysmal or continuous pain) showed a significantly better long-term outcome for those over 70 years of age (p=0.02) and for the patients suffering from paroxysmal pain (p<0.001) (Fig. 3).
In order to identify factors associated with long-term pain relief (BNI I-III) and an excellent outcome (BNI I) at the final follow-up visit, binary logistic regression was performed, analyzing gender, age, disease duration, presence of HTN, pain characteristics, initial pain response to medical treatment, and the presence of NVC in MRI sequences.
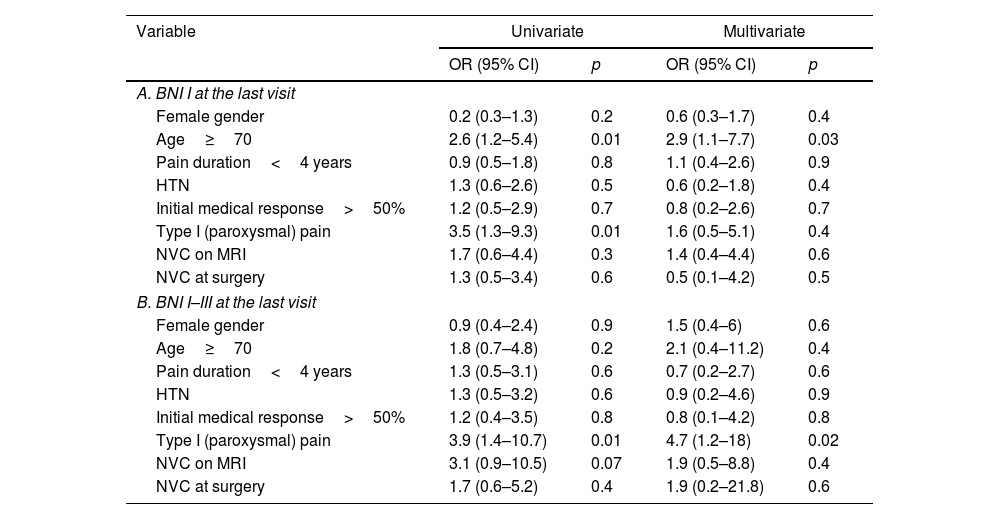
Being over 70 years of age was found to be the only independent factor significantly associated with an excellent long-term outcome (BNI I) at the end of the follow-up period (OR=2.9, 95% CI: 1.1–7.7; p=0.03).
Paroxysmal pain (OR: 4.7, 95% CI 1.2–18; p=0.02) was found to be the only independent factor significantly associated with postoperative pain relief (BNI I–III) at the last visit (Table 3).
Binary logistic regression uni- and multivariate analysis to identify factors associated with an excellent (BNI I) result at the last follow-up visit (A) and with a good result at 12 months of follow-up (B). The multivariate analysis was adjusted for age and type of pain.
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| A. BNI I at the last visit | ||||
| Female gender | 0.2 (0.3–1.3) | 0.2 | 0.6 (0.3–1.7) | 0.4 |
| Age≥70 | 2.6 (1.2–5.4) | 0.01 | 2.9 (1.1–7.7) | 0.03 |
| Pain duration<4 years | 0.9 (0.5–1.8) | 0.8 | 1.1 (0.4–2.6) | 0.9 |
| HTN | 1.3 (0.6–2.6) | 0.5 | 0.6 (0.2–1.8) | 0.4 |
| Initial medical response>50% | 1.2 (0.5–2.9) | 0.7 | 0.8 (0.2–2.6) | 0.7 |
| Type I (paroxysmal) pain | 3.5 (1.3–9.3) | 0.01 | 1.6 (0.5–5.1) | 0.4 |
| NVC on MRI | 1.7 (0.6–4.4) | 0.3 | 1.4 (0.4–4.4) | 0.6 |
| NVC at surgery | 1.3 (0.5–3.4) | 0.6 | 0.5 (0.1–4.2) | 0.5 |
| B. BNI I–III at the last visit | ||||
| Female gender | 0.9 (0.4–2.4) | 0.9 | 1.5 (0.4–6) | 0.6 |
| Age≥70 | 1.8 (0.7–4.8) | 0.2 | 2.1 (0.4–11.2) | 0.4 |
| Pain duration<4 years | 1.3 (0.5–3.1) | 0.6 | 0.7 (0.2–2.7) | 0.6 |
| HTN | 1.3 (0.5–3.2) | 0.6 | 0.9 (0.2–4.6) | 0.9 |
| Initial medical response>50% | 1.2 (0.4–3.5) | 0.8 | 0.8 (0.1–4.2) | 0.8 |
| Type I (paroxysmal) pain | 3.9 (1.4–10.7) | 0.01 | 4.7 (1.2–18) | 0.02 |
| NVC on MRI | 3.1 (0.9–10.5) | 0.07 | 1.9 (0.5–8.8) | 0.4 |
| NVC at surgery | 1.7 (0.6–5.2) | 0.4 | 1.9 (0.2–21.8) | 0.6 |
Gender, disease duration, the presence of HTN, the number of drugs prior to the surgery, and the presence of NVC in MRI (either conventional or high-resolution sequences) were not found to be predictive factors.
DiscussionOur results for early and long-term postoperative pain relief after MVD in patients with CTN are in line with those published previously. The rate of initial pain control for our patients was 97%, which is consistent with other series that have reported early success rates of 80–96%.25–29
Regarding the long-term outcomes, the follow-up period of our patients was heterogeneous, ranging from 6 months to 15 years. However, 25% of our cohort had a 5-year or longer follow-up period at the time of the study. At the last follow-up visit, 83% of our patients had experienced significant pain relief (BNI I–III) and 56% of them were completely pain-free (BNI I). These results are comparable with previous series, which had BNI I rates of 54–84% at the final visit and a mean follow up of 2–8 years.25–29
Initial pain relief after MVD is as high as 95–100% in most series, especially in patients with clear cut arterial compression, while venous compression or absence of compression show poorer results.25–29 Recurrence occurs in 3–30% with a yearly rate of about 4% that drops to 1–2% yearly after the first six years.30 In our study, 9% of the patients with initial pain relief experienced uncontrolled pain (BNI IV–V) over time after surgery. Seven of them underwent a second MVD and three benefited from RF. There is no established management of pain recurrence after successful MVD. Most series dealing with recurrent CTN have found satisfactory results with reoperations including re-do MVD and PSR, but also with RF.30–32 In accordance to other authors, we have found that microsurgical reexploration and renewed MVD if compression is found or PSR, in case NVC is not identified, is a safe option that contributes to pain relief in a substantial number of patients.30–32 Therefore, at our institution, reoperation is proposed in most cases of surgical failure as first option, though percutaneous Gasserian ganglion ablation is offered alternatively in all cases. Likewise, patients failing to improve after a second procedure may be considered for neuromodulation with motor cortical stimulation after a successful trial with transcranial magnetic stimulation.33
The goal of surgical treatment for CTN is not only to completely relieve the pain but also to reduce medical treatment when the neuralgia is not fully controlled. As a secondary efficacy outcome, we evaluated the need for medical treatment after surgery. The proportion of people requiring polytherapy regimes decreased significantly (from 65% to 21%, p<0.001), and 65% of the patients could reduce the absolute drug doses by 50% or more. To our knowledge, this is the only study to date to evaluate the effect of MVD in medical treatment as an efficacy endpoint. Patients with incomplete pain relief after surgery need lower doses of AEDs to control the neuralgia, with a lower rate of adverse effects and better tolerance.
The average age of the patients in our series was 60 years, with 48 patients older than 70 years of age, and there was a majority of women (66%). This is similar to what has been reported before, with a female to male ratio from 2:1 to 4:3.3
The disease duration until the surgery has been widely discussed in the literature, with most patients undergoing MVD after at least four years from the onset of symptoms.25–29 In our study, the mean duration of pain shows a surprisingly wide range from 2 months to 30 years with a median of 7.5 years and a slight tendency of an earlier surgical approach in the more recently operated patients. Being aware that many of the patients have been suffering disabling pain in spite of medical therapy for years and that there is a growing evidence that a shorter pain duration may increase the chances of successful pain relief after MVD,34,35 we have tried in recent years to support a multidisciplinary approach of CTN patients at our institution that ensures an early referral to neurosurgery of patients with medically refractory pain. Still, we tend to receive patients from other institutions with unacceptably long histories of uncontrolled pain. Therefore, a wider diffusion among neurologists about the surgical treatment options for CTN and their results is warranted.
Regarding the medical treatment before the surgery, as noted previously,25–29 most of the patients were receiving CBZ at a medium dose, and in many cases higher doses were not well tolerated. New drugs such as ESL and OXC were also frequently prescribed for some of the patients included in the last 5–10 years, and these presented a more favorable adverse effects profile.
Preoperative high-resolution 3DT2 MRI sequences are considered an essential tool for accurate presurgical prediction of NVC and evaluation of the trigeminal nerve morphology.36–38 In this study, NVC was identified in 82% of patients who benefited from these sequences. All of them showed NVC in surgery. High-resolution 3DT2 MRI sequences were found to have a sensitivity and a specificity of 90% and 100%, respectively, to predict the presence of NVC, which is in keeping with the previously reported data.37,39,40
MVD is considered to be a safe procedure, however, complications such as permanent cranial nerve injuries (1–8%), CSF fistula (1.5–5%), central nervous system infarction or hematoma (0.1–0.4%), and death (0.15–2%) have been reported.25–29 In our study, we found similar complication rates: 7% for CSF fistula and 5% for wound infection, and all of these cases fully recovered within the first month after surgery.
The large experience with MVD and the reported efficacy and safety data support this technique as the first surgical choice for CTN.34,41 Despite the lack of randomized controlled trials comparing different surgical options for CTN, evidence derived from multiple observational studies upholds the superiority of MVD in terms of initial and long-term pain relief, as well as preservation of facial sensation.9,42
In terms of complications, higher incidences of minor adverse effects such as temporary facial sensory deficits, have been reported with PR and SRS. However, there have been no reports of mortality with these techniques.18,43 As MVD is more invasive than other surgical procedures for CTN, its safety and efficacy in older patients have been a matter of debate.44–46 As reported before by other authors, the current study also found that old age is not a contraindication for surgery. People over 70 years of age had a greater probability of achieving an excellent outcome (Fig. 3, Table 3) and they had a similar rate of complications. Previous series of cases have been consistent in terms of good pain control and lower recurrence rates in elderly patients, which is probably a result of a greater degree of NVC with age. Furthermore, surgical exposure of the CPA in this group is usually described to be easier due to atrophy.44–46 Aside from age, other potential prognostic factors have been investigated. Male gender, paroxysmal pain, the presence of trigger points, a greater degree of neurovascular compression, and immediate postoperative relief have all been associated with better outcomes in some studies.28,46–48 In the present study, paroxysmal pain correlated with a better long-term outcome as an independent factor in the multivariate analysis. Unlike other series showing a positive prognostic value of NVC identified on preoperative MRI,49,50 we have not found the presence of NVC in MRI (either conventional or high-resolution sequences) to be correlated with response to surgical procedure. The disease duration has also been identified by many authors as an important prognostic factor, as patients with shorter disease durations appear to have better pain control.51,52
These findings may help neurologists and surgeons with choosing more suitable candidates for MVD at an earlier stage of the disease. There is no consensus regarding the best time for surgery, which patients could benefit the most from it, and how many different medications should be tried before classifying a CTN as refractory.9 Based on our clinical experience and previous evidence, we consider that a multidisciplinary approach with an early referral to a neurosurgical unit can benefit both patients and physicians by laying out surgical options that may be addressed whenever the medical therapy should fail or its secondary effects should turn unbearable.
Overall, CTN should not only be viewed as a neurological condition, but also as an emotional, psychological, and social disorder that requires collaboration between specialties as well as a personalized strategy. Detailed knowledge of the surgical options by neurologists, as well as a multidisciplinary approach, are essential in order to achieve a satisfactory outcome for CTN patients.
The present series is limited by its observational nature and the long period of patient recruitment. As in many studies assessing pain, there is a potential bias by the non-blinded interviewer recording the clinical data and a potential response bias as outcomes in pain are always self-rereported.
ConclusionOur study supports the notion that MVD is a safe and effective treatment for patients suffering from refractory CTN. It can provide long-term pain relief as well as a pronounced reduction of AEDs and a low rate of complications. Paroxysmal pain and older age (>70 years) were identified as factors associated with a good prognosis, thus helping physicians to identify the best candidates for MVD.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestsThe authors declare no potential conflicts of interest regarding the research, authorship, and/or publication of this article.