It is not yet possible to estimate the proportion of patients with COVID-19 who present distinguishable classical neurological symptoms and syndromes.
The objective of this study is to estimate the incidence of sensory symptoms (hypoaesthesia, paraesthesia, and hyperalgesia) in physicians who have presented the disease at Hospital Universitario Fundación Alcorcón (HUFA) in Madrid; to establish the relationship between sensory symptoms and the presence of other signs of infection; and to study their association with the severity of COVID-19.
MethodsWe conducted a descriptive, cross-sectional, retrospective, observational study. HUFA physicians who presented SARS-CoV-2 infection between 1 March and 25 July 2020 were included in the study. A voluntary, anonymous survey was distributed via corporate email. Sociodemographic and clinical characteristics were collected from professionals with PCR- or serology-confirmed COVID-19.
ResultsThe survey was sent to 801 physicians and we received 89 responses. The mean age of respondents was 38.28 years. A total of 17.98% presented sensory symptoms. A significant relationship was found between the presence of paraesthesia and cough, fever, myalgia, asthaenia, and dyspnoea. A significant relationship was also found between paraesthesia and the need for treatment and admission due to COVID-19. Sensory symptoms were present from the fifth day of illness in 87.4% of cases.
ConclusionsSARS-CoV-2 infection can be associated with sensory symptoms, mostly in severe cases. Sensory symptoms often appear after a time interval, and may be caused by a parainfectious syndrome with an autoimmunity background.
Aun no es posible estimar la proporción de pacientes con COVID-19 que presentan síntomas y síndromes neurológicos clásicos diferenciables.
Nuestro objetivo es estimar la incidencia de síntomas sensitivos (hipoestesia, parestesias e hiperalgesia), en médicos que han sufrido la COVID19 en el Hospital Universitario Fundación Alcorcón de Madrid (HUFA); relacionar la clínica sensitiva con la presencia de otros síntomas o signos de la infección y estudiar la asociación con la gravedad de la COVID 19.
MétodosEstudio descriptivo, transversal, retrospectivo y de carácter observacional. La población del estudio incluyó a médicos del HUFA que sufrieron infección por SARS-CoV2 entre el 1 de marzo y el 25 de Julio del 2020. Se hizo llegar a través del email corporativo una encuesta voluntaria y anónima donde se recogieron características sociodemográficas y clínicas de aquellos trabajadores con confirmación de COVID 19 por PCR o serología.
ResultadosLa encuesta se envió a 801 médicos. Contestaron 89. La edad media fue de 38,28 años. 17,98% presentó clínica sensitiva. Se encontró una relación significativa entre la presencia de parestesias y tos, fiebre, mialgias, astenia y disnea. Se observó una relación significativa entre las parestesias y la necesidad de recibir tratamientos e ingresar por COVID19. El 87,4% presentó la clínica sensitiva a partir del 5º día de enfermedad.
ConclusionesLa infección por SARS-CoV 2 se puede acompañar de clínica sensitiva que refleje cuadros más graves, apareciendo diferida en el tiempo, pudiendo corresponder con un probable origen parainfeccioso en el que puede influir un trasfondo de autoinmunidad.
31 December 2019 marked the beginning of a pandemic, with the outbreak in Wuhan (China) of an infection caused by a new virus, identified on 7 January 2020 as a novel virus of the Coronaviridae family and initially named “novel coronavirus,” or 2019-nCoV. The virus was subsequently denominated SARS-CoV-2, and the associated disease came to be known as COVID-19. SARS-CoV-2 is a β-coronavirus and is the third zoonotic coronavirus to be identified, after SARS and MERS.
We are not yet able to establish the proportion of patients with COVID-19 who present characteristic neurological symptoms and syndromes. Most of the studies published to date are case series in which the assessment of neurological complications is a secondary objective.
Although no cases have been reported of isolated paraesthesia or hyperalgesia associated with mild SARS-CoV-2 infection, some studies do describe a wide range of neurological alterations.
Neurological manifestations of viral infection have also been observed in other coronavirus outbreaks. In the case of SARS, cases have been reported of axonal polyneuropathy at 2–4 weeks after onset of viral symptoms; it has also been suggested that peripheral nervous system alterations may be attributed to post-infectious immune reactions, although direct viral invasion of the nerves and ganglia cannot be ruled out.1 The immune dysfunction observed in patients with SARS may be caused by the production of various proinflammatory mediators that damage not only the lungs but also the nerves.2,3 Furthermore, acute infection has been observed to damage cells by cytolysis, but also by an immunopathological process.1
Loss of the senses of smell and taste has been reported as a mild neurological manifestation of SARS-CoV-2 infection.4 Cases have also been reported of Guillain-Barré syndrome associated with COVID-19,5 suggesting a parainfectious mechanism6; a case of COVID-19–related viral encephalitis has also been reported.7
In a study including 214 patients with confirmed COVID-19 from Wuhan, neurological symptoms were observed in 36.4% of the sample (78 patients), mostly involving the central nervous system.8 Manifestations of peripheral nervous system involvement were reported in 8.9% of patients (n = 19). Twelve of these presented mild COVID-19 symptoms, whereas the remaining 7 met criteria for severe SARS-CoV-2 infection.
In a study conducted at 2 intensive care units in Strasbourg (France), 12% of critically ill patients with COVID-19 presented encephalopathy, agitation, confusion, and signs of corticospinal tract dysfunction.9 The incidence of neurological signs and symptoms is probably underestimated due to patient characteristics (intubation, sedation, neuromuscular blockade). The researchers were unable to determine whether these symptoms were due to the patients’ critical status or the result of the cytokine storm, medication, or direct action of the virus.9
The Spanish Society of Neurology created a registry in which physicians from 12 Spanish autonomous communities reported cases of SARS-CoV-2 infection manifesting with neurological symptoms; a total of 131 cases had been identified as of 12 April 2020. The most frequent symptoms are confusional syndrome and mild-to-moderate encephalopathy (28.3%), stroke (22.8%), and polyradiculoneuropathy (7.6%). Cases have also been reported of peripheral nervous system involvement, in the form of neuropathy, radiculopathy, and plexopathy. Cerebrospinal fluid (CSF) PCR testing for SARS-CoV-2 yielded negative results in all 27 patients undergoing the test.10
The pathogenic mechanisms of COVID-19–related neurological complications are currently unknown. Similarly to other viral infections, COVID-19 may present with direct infection of the nervous system, acute toxic encephalopathy secondary to severe systemic infection, or postinfectious demyelinating lesions. These patients present high D-dimer levels, thrombocytopenia, and pathological signs of microangiopathy, which may cause stroke.11 Furthermore, systemic inflammatory response syndrome may cause immune-mediated neuronal damage,12 and massive release of cytokines may alter vascular permeability and cause coagulopathy and a deregulated inflammatory response.13
The purpose of this study is to estimate the incidence of sensory symptoms (hypoaesthesia, paraesthesia, and hyperalgesia) in healthcare professionals with history of SARS-CoV-2 infection at our centre.
As a secondary objective, we evaluated the association between sensory symptoms and presence of other signs or symptoms of COVID-19, and between these 2 factors and the severity of SARS-CoV-2 infection.
Few cases have been reported of sensory symptoms in patients with COVID-19. Therefore, our study is interesting in that it gathers cases of sensory symptoms with a view to determining their incidence in COVID-19 and studying their association with sociodemographic, clinical, or treatment variables for future studies.
Material and methodsWe conducted a descriptive, cross-sectional, retrospective, observational study into sensory neurological symptoms in patients with SARS-CoV-2 infection.
We included physicians and residents of any specialty working at Hospital Universitario Fundación Alcorcón, in Madrid, who presented SARS-CoV-2 infection between 1 March and 25 July 2020. The study was approved by our centre’s research ethics committee. The authors have no conflicts of interest to declare.
Presence of SARS-CoV-2 infection was established by either laboratory (PCR testing of nasopharyngeal exudate) or serological results for viral infection (ELISA test) in a seroprevalence study including all healthcare professionals working at our hospital. The only exception was a respondent who did not undergo PCR testing and presented negative serology test results, but whose symptoms were highly suggestive of SARS-CoV-2 infection (respiratory and gastrointestinal symptoms), and members of whose household presented positive PCR test results. In this participant, negative IgG results were interpreted as negativisation (in some cases, serological studies were not performed until several months after symptom resolution) and negative PCR test results as a false negative result.
We gathered data on clinical symptoms compatible with COVID-19 in the context of the pandemic: fever associated with respiratory symptoms (coughing, nasal congestion, sneezing, dyspnoea, bronchospasm) or other symptoms that may also be associated with SARS-CoV-2 infection (asthenia, myalgia, lower back pain, nausea or vomiting, diarrhoea, anosmia, ageusia, or headache). We also gathered sociodemographic variables, diagnostic data (PCR and serology test results), treatment variables (treatment protocol applicable at the time in our hospital), and progression variables (duration of COVID-19 symptoms, duration of sensory symptoms, time from onset of COVID-19 symptoms to onset of sensory symptoms).
Data were gathered through a Google Docs survey that was sent to the hospital e-mail address of all physicians and residents of any specialty working at Hospital Universitario Fundación Alcorcón; participation was voluntary and anonymous. The survey can be consulted in Appendix 1. The survey was designed in accordance with the Checklist for Reporting Results of Internet E-Surveys. It was sent to 801 healthcare professionals, and completed by 89.
The survey gathered the clinical and sociodemographic characteristics of healthcare professionals who presented COVID-19 symptoms and had PCR or serology confirmation of the disease. Among physicians at our centre, the prevalence of COVID-19 was 39.6%.14
Qualitative data are expressed as absolute and relative frequencies, and quantitative data as means (standard deviation) or median (interquartile range). The main outcome measure was frequency of sensory symptoms; 95% confidence intervals were calculated with the Wilson method. We analysed differences between participants with and without symptoms with a univariate analysis: qualitative variables were compared with the chi-square test or the Fisher exact test, whereas quantitative variables were compared with the t test for independent samples or the non-parametric Mann-Whitney U test, depending on data distribution. We also studied the association between presence of symptoms and disease severity. As a measure of association, we calculated odds ratios (OR) with unadjusted data and with data adjusted for age, sex, and comorbidities, using logistic regression models. All tests were 2-tailed; the threshold for statistical significance was set at P < .05.
ResultsOur study included 89 healthcare professionals from our centre with PCR- or serology-confirmed SARS-CoV-2 infection. Mean age (SD) was 38.28 (11.16) years. The sample included 54 women (60.7%) and 35 men (39.3%).
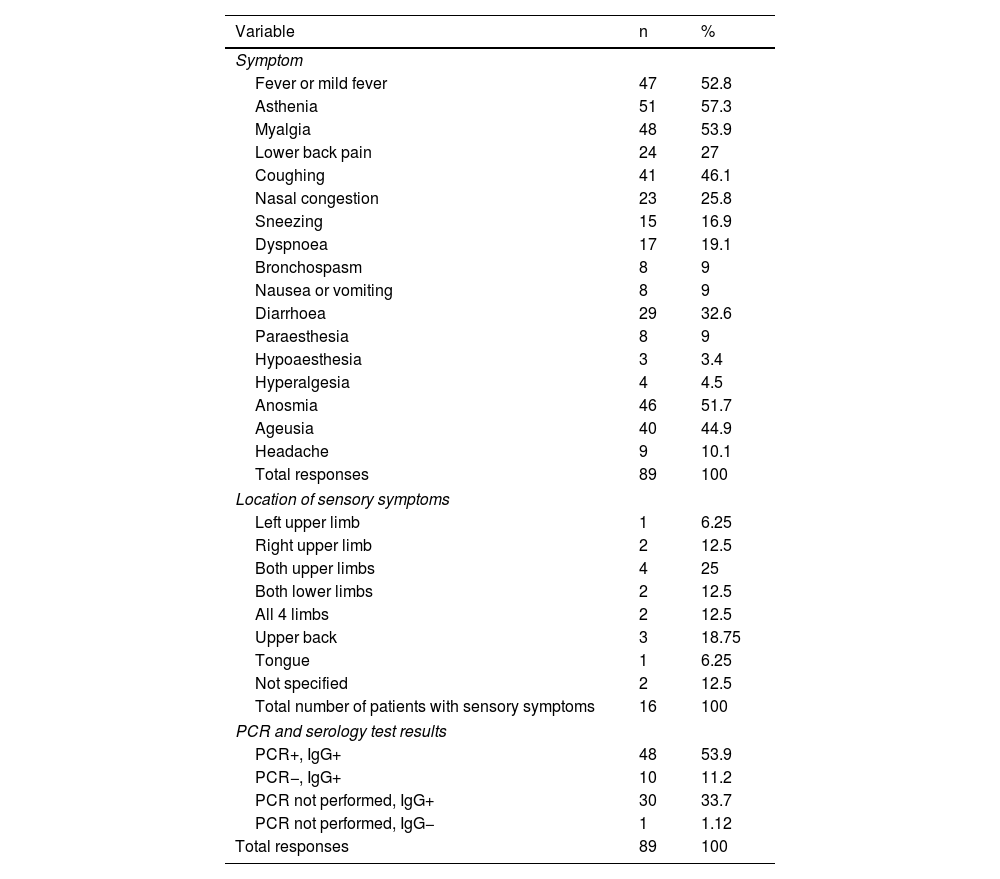
A total of 86 respondents (96.6%) reported symptoms associated with SARS-CoV-2 infection. Table 1 presents the main symptoms reported, and their frequencies.
Clinical characteristics of the survey respondents who presented symptoms compatible with SARS-CoV-2 infection.
| Variable | n | % |
|---|---|---|
| Symptom | ||
| Fever or mild fever | 47 | 52.8 |
| Asthenia | 51 | 57.3 |
| Myalgia | 48 | 53.9 |
| Lower back pain | 24 | 27 |
| Coughing | 41 | 46.1 |
| Nasal congestion | 23 | 25.8 |
| Sneezing | 15 | 16.9 |
| Dyspnoea | 17 | 19.1 |
| Bronchospasm | 8 | 9 |
| Nausea or vomiting | 8 | 9 |
| Diarrhoea | 29 | 32.6 |
| Paraesthesia | 8 | 9 |
| Hypoaesthesia | 3 | 3.4 |
| Hyperalgesia | 4 | 4.5 |
| Anosmia | 46 | 51.7 |
| Ageusia | 40 | 44.9 |
| Headache | 9 | 10.1 |
| Total responses | 89 | 100 |
| Location of sensory symptoms | ||
| Left upper limb | 1 | 6.25 |
| Right upper limb | 2 | 12.5 |
| Both upper limbs | 4 | 25 |
| Both lower limbs | 2 | 12.5 |
| All 4 limbs | 2 | 12.5 |
| Upper back | 3 | 18.75 |
| Tongue | 1 | 6.25 |
| Not specified | 2 | 12.5 |
| Total number of patients with sensory symptoms | 16 | 100 |
| PCR and serology test results | ||
| PCR+, IgG+ | 48 | 53.9 |
| PCR−, IgG+ | 10 | 11.2 |
| PCR not performed, IgG+ | 30 | 33.7 |
| PCR not performed, IgG− | 1 | 1.12 |
| Total responses | 89 | 100 |
IgG: immunoglobulin G; PCR: polymerase chain reaction.
Of the total sample of 89 respondents, 16 (17.98%) presented sensory symptoms, either in isolation or combined with other symptoms. Of the 16 participants reporting sensory symptoms, 8 presented paraesthesia (50%), 3 presented hypoaesthesia (18.75%), and 4 reported hyperalgesia (25%). The locations of sensory symptoms are reported in Table 1.
We studied the association between sensory symptoms and other COVID-19 symptoms, observing statistically significant associations between paraesthesia and coughing (P = .014), dyspnoea and paraesthesia (P = .001), dyspnoea and sensory symptoms including paraesthesia, hypoaesthesia, and hyperalgesia (P = .014), asthenia and sensory symptoms (P = .001), fever and paraesthesia (P = .042), fever and sensory symptoms (P = .033), and myalgia and sensory symptoms (P = .044).
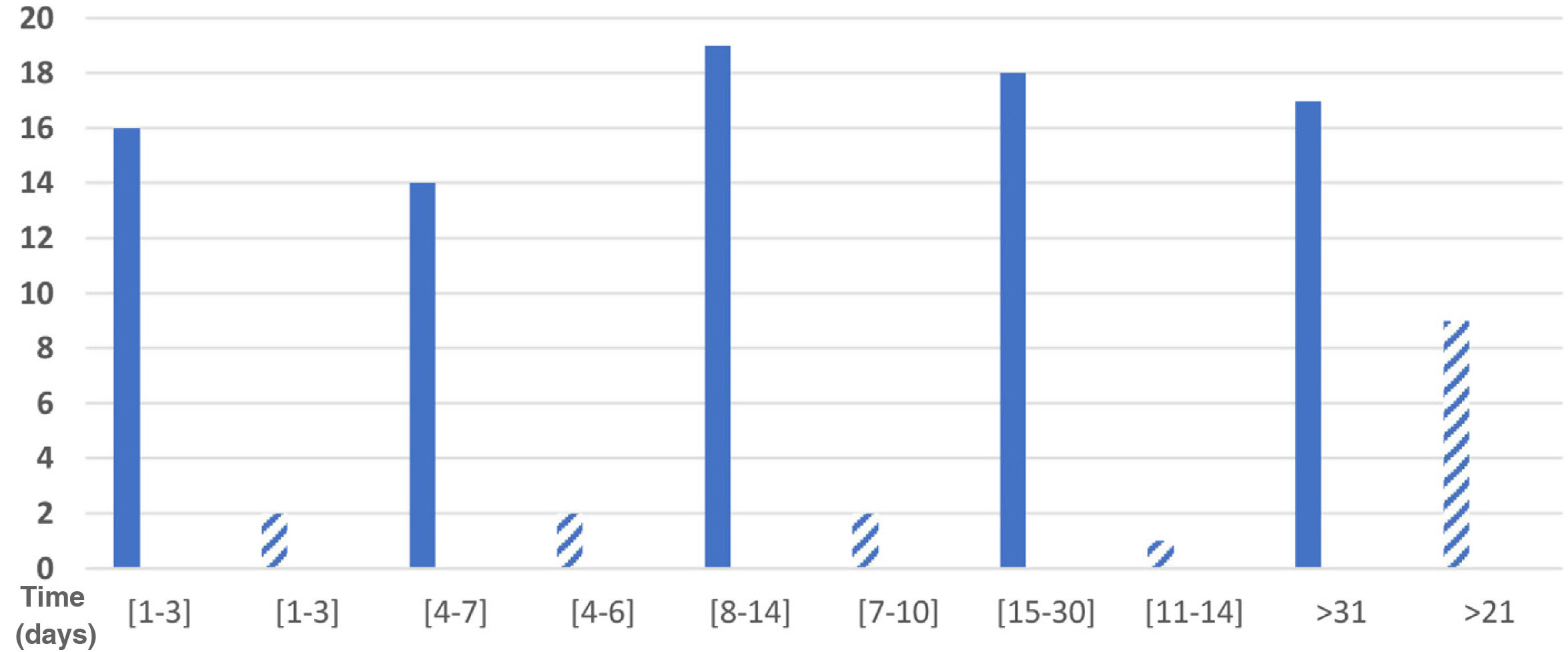
Eighty-four respondents reported the duration of their symptoms. Total symptom duration was 1–3 days in 19.0% (16 respondents), 4–7 days in 16.7% (14), 8–14 days in 22.6% (19), 15–30 days in 21.4% (18), and over a month in 20.2% (17); 5 respondents did not provide this information. Fig. 1 shows the distribution of participants by duration of sensory symptoms.
We gathered data on the time between onset of COVID-19 symptoms and onset of sensory symptoms: 1–2 days in one respondent, 3–4 days in one respondent, 5–7 days in 4, 8–10 days in 4, 11–14 days in 4, 15–20 days in 2, 21–30 days in one, and more than 30 days in 3.
Sensory symptoms lasted 1–3 days in 2 of the 16 respondents with these symptoms (12.5%), 4–6 days in 2, 7–10 days in 2, 11–14 days in one (6.25%), and 21 days or more in 9 respondents (56.25%); therefore, we were unable to calculate the mean duration of sensory symptoms. These data are presented in Fig. 1.
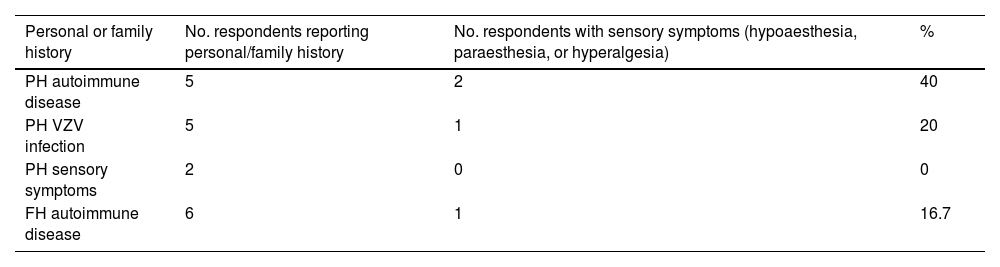
Table 2 summarises the medical history of survey respondents. Regarding history of autoimmune disease, 3 participants had psoriatic arthritis, one had Hashimoto thyroiditis, and another had Graves disease.
Personal and family history of survey respondents.
| Personal or family history | No. respondents reporting personal/family history | No. respondents with sensory symptoms (hypoaesthesia, paraesthesia, or hyperalgesia) | % |
|---|---|---|---|
| PH autoimmune disease | 5 | 2 | 40 |
| PH VZV infection | 5 | 1 | 20 |
| PH sensory symptoms | 2 | 0 | 0 |
| FH autoimmune disease | 6 | 1 | 16.7 |
FH: family history; PH: personal history; VZV: varicella zoster virus.
Sensory symptoms were only reported by one respondent with history of psoriatic arthritis (paraesthesia and hypoaesthesia) and by the patient with Graves disease (hypoaesthesia). Therefore, 2 of the 16 respondents with sensory symptoms (12.5%) had history of autoimmune disease.
No statistically significant association was observed between sensory symptoms and history of autoimmune disease (P > .05), although history of autoimmune disease did show a significant association with hypoaesthesia during SARS-CoV-2 infection (2 of 5 patients [40%]; P = .007). None of them had previously presented sensory symptoms.
Furthermore, one participant with family history of autoimmune disease (hypothyroidism) presented sensory symptoms (paraesthesia), 5 had history of herpes zoster infection, and 2 respondents with no history of autoimmune disease had experienced sensory symptoms in the past but not during SARS-CoV-2 infection. These data are summarised in Table 2.
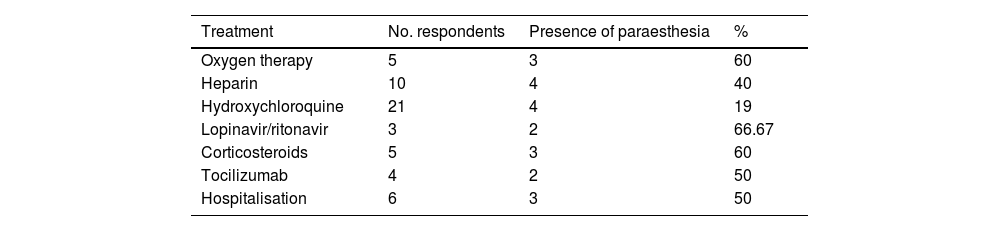
The treatments used and need for hospitalisation are shown in Table 3. A significant association was observed between need for treatment and hospitalisation. A subgroup analysis by type of sensory symptom (paraesthesia, hypoaesthesia, or hyperalgesia) revealed a significant association between paraesthesia and treatment with heparin (P = .005), oxygen therapy (P = .005), lopinavir/ritonavir (P = .02), corticotherapy (P = .005), and tocilizumab (P = .039). No significant association was observed between any treatment and presence of hyperalgesia or hypoaesthesia.
The results of PCR and serology (IgG) testing for SARS-CoV-2 are presented in Table 1. One participant presented negative IgG results and had not undergone PCR testing; however, her symptoms were compatible with SARS-CoV-2 infection and members of her household had presented positive PCR results for SARS-CoV-2.
Of all participants reporting sensory symptoms, 5 (31.3%) had not undergone PCR testing, 7 (43.8%) presented positive PCR results, and 4 (25%) presented negative PCR results.
DiscussionWe e-mailed the survey to 801 physicians and residents of any specialty working at our hospital; the estimated incidence of COVID-19 in our sample was 39.6%, according to the seroprevalence study (312 individuals).14 The survey was completed by 89 individuals, 16 (17.98%) of whom reported sensory symptoms. To date, no study has analysed the incidence of sensory symptoms; therefore, we cannot compare our results to any other data. The Spanish Society of Neurology’s “COVID-19 manual for the general neurologist” provides data on the incidence of peripheral nerve disease (e.g., Guillain-Barré syndrome) in these patients. However, it does not estimate the incidence of such symptoms as paraesthesia, hypoaesthesia, or hyperalgesia.10
Our study found a significant association between presence of mild sensory symptoms (paraesthesia) at different locations and such other symptoms of COVID-19 as coughing, fever, myalgia, asthenia, and dyspnoea, suggesting an association between presence of sensory symptoms and highly symptomatic COVID-19, manifesting with such severe symptoms as dyspnoea.
We should underscore that only 5 of the 17 patients reporting dyspnoea received oxygen therapy. Dyspnoea is a subjective symptom and may not result in hypoxaemia; this may may result in bias when comparing clinical severity of COVID-19 and sensory symptoms. However, of these 17 patients with dyspnoea, 5 needed oxygen therapy, 4 presented bronchospasm, and 6 were hospitalised; we may conclude that, in this case, the subjective feeling of dyspnoea is indicative of clinical severity. Furthermore, we found a significant association between presence of paraesthesia and need for treatment for COVID-19 or hospitalisation; this suggests that the presence of paraesthesia is significantly associated with more severe COVID-19. Therefore, severe COVID-19 according to clinical criteria (dyspnoea) and objective data (need for such specific treatments as oxygen therapy, corticotherapy, lopinavir/ritonavir, tocilizumab) was significantly associated with presence of sensory symptoms during SARS-CoV-2 infection. These results are consistent with the available evidence, according to which neurological symptoms are more common in more severe cases and in critically ill patients; however, little evidence is available on mild sensory symptoms, such as paraesthesia, hypoaesthesia, or hyperalgesia.15
Sensory symptoms appeared 5–10 days after COVID-19 symptom onset in 50% of participants, with latencies of 1–4 days in 12.6% and over 11 days in 37.4%. Other authors have also noted a delay in the onset of sensory symptoms after onset of COVID-19 symptoms, reporting mean latencies of 5–10 days.15
We gathered data on the time between onset of COVID-19 symptoms and onset of sensory symptoms, observing times of 1–2 days in one respondent, 3–4 days in one, 5−4 days in 4, 8−7 days in 4, 11–14 days in 4, 15–20 days in 2, 21–30 days in one, and more than 30 days in 3 respondents. Sensory symptoms appeared 5–10 days after COVID-19 symptom onset in 50% of participants, while only 12.6% presented a latency of 1–4 days and 37.4% showed a latency of over 11 days.
This suggests that delayed onset of sensory symptoms is more common (87% of respondents presented sensory symptoms at least 5 days after onset of COVID-19 symptoms, and nearly 40% at least 11 days later); this may be linked to a host inflammatory response to infection rather than the direct action of the virus.16
Our study found a significant association between history of autoimmune disease and presence of hypoaesthesia during COVID-19 (P = .007), which suggests that history of autoimmune disease predisposes to the development of sensory symptoms. However, we are unable to draw robust conclusions due to the small number of patients with autoimmune diseases in our sample (n = 5).
According to the parainfectious theory, we would expect most patients to display negative PCR results at the time of sensory symptom onset, as this stage would be associated with a low viral load or resolved infection. However, only 25% of our participants (n = 4) reported negative PCR results, while 43.8% (n = 7) reported positive results; in any case, we were unable to gather data on the time elapsed from onset of COVID-19 symptoms and onset of sensory symptoms (longer latencies would suggest a parainfectious origin).
An alternative hypothesis suggests that positive PCR results indicate viral infection of the central nervous system, which would be the cause of sensory symptoms (direct viral invasion), rather than a parainfectious explanation. However, SARS-CoV-2 is rarely detected in the CSF. Researchers from the virology laboratory of the University Hospital of Lyon analysed the CSF of 555 patients, with all but 2 samples yielding negative results; in these 2 patients, post mortem examination found no evidence of the virus in brain tissue, suggesting contamination of the CSF with blood. The authors attribute sensory symptoms to a host immune response rather than to direct action of the virus. This is consistent with our theory of a parainfectious mechanism, whether due to autoimmunity or associated with a host inflammatory response.17
None of the healthcare professionals who completed the survey proposed an alternative explanation for their sensory symptoms; neither did we find a significant association between history of varicella zoster virus infection and presence of sensory symptoms during COVID-19. Therefore, history of infection was regarded as the most probable cause of the sensory symptoms.
The fact that our study population was made up of healthcare professionals makes the self-reported data (identification of sensory symptoms and alternative causes) more reliable.
Our study is not without limitations. It may be affected by a response bias, as data gathering relied on the voluntary participation of the study population, and individuals with sensory symptoms may have been more motivated to complete the survey than those without these symptoms. Furthermore, as participants were healthcare professionals, our results may not be applicable to other populations (external validity).
In conclusion, our sample is not sufficiently large to draw definitive conclusions, which underscores the need for further studies with greater statistical power. However, our data suggest that SARS-CoV-2 infection may be accompanied by sensory symptoms, which may in turn reflect more severe COVID-19. Onset of sensory symptoms is delayed. The pathogenic mechanisms are unknown, although our results suggest a parainfectious origin rather than direct viral invasion, with autoimmunity potentially playing a role.18
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Gayoso Cantero D, Cantador Pavón E, Pérez Fernández E, Novillo López ME. Estudio de clínica sensitiva leve durante la infección por SARS-CoV-2 en población sanitaria. Neurología 2022. https://doi.org/10.1016/j.nrl.2021.06.008