Myelitis can appear as an initial symptom in the context of demyelinating diseases, systemic inflammatory diseases, and infectious diseases. We aim to analyse the differences between myelitis associated with multiple sclerosis (MS) and myelitis resulting from other aetiologies.
MethodsSingle-centre, retrospective analysis of patients with initial myelitis (2000-2013). Demographic, aetiological, clinical, radiological and prognostic variables were analysed and compared between patients with myelitis from MS and those with myelitis due to other aetiologies.
ResultsWe included 91 patients; mean follow-up was 7 years. Diagnoses were as follows: MS 57 (63%), idiopathic transverse myelitis 22 (24%), associated systemic diseases 6 (7%), and other diagnoses (6%). Myelitis due to MS was associated with younger age of onset (35±11 vs 41±13; P=.02), more pronounced sphincter involvement (40.4% vs 27.3%; P=.05), greater multifocal involvement in spinal MRI (77.2% vs 26.5%; P=.001), shorter lesion extension (2.4 vs. 1.4 vertebral segments; P=.001), cervical location (82.5% vs 64.7%; P=.05) and posterior location (89.5% vs 41.2%; P=.001). Myelitis due to other aetiologies more frequently showed anterior location (47.1% vs 24.6%; P=.02), and central cord involvement (47.1% vs 14.1%; P=.001), with better recovery at one year of follow up (EDSS 2.0 vs 1.5; P=.01). Multivariate analysis showed that multifocal spinal cord involvement (OR 9.38, 95% CI: 2.04-43.1) and posterior cord involvement (OR 2.16, 95% CI: 2.04-2.67) were independently associated with the diagnosis of MS.
ConclusionsA high percentage of patients with an initial myelitis event will be diagnosed with MS. The presence of multifocal and posterior spinal cord lesions was significantly associated with the diagnosis of MS.
Un primer brote de mielitis puede ocurrir en el contexto de enfermedades desmielinizantes, inflamatorias sistémicas o infecciosas. Nuestro objetivo fue analizar las diferencias entre mielitis asociadas a esclerosis múltiple (EM) y mielitis por otras etiologías.
MétodosAnálisis retrospectivo, unicéntrico, de pacientes con primer brote de mielitis (2000-2013). Se analizaron variables demográficas, etiológicas, clínicas, radiológicas y pronósticas, y se compararon entre mielitis por EM y mielitis por otras etiologías.
ResultadosSe incluyó un total de 91 pacientes. Tiempo medio de seguimiento: 7 años. Diagnósticos: EM 57 (63%), mielitis transversa idiopática 22 (24%), asociada a enfermedades sistémicas 6 (7%), otros diagnósticos (6%). Mielitis por EM: menor edad de inicio (35±11 vs .41±13; p=0,02), mayor afectación esfinteriana (40,4 vs 27,3%; p=0,05), mayor afectación multifocal en la RM medular (77,2 vs 26,5%; p=0,001), menor extensión de la lesión (segmentos vertebrales 2,4 vs 1,4; p=0,001), localización cervical (82,5 vs 64,7%; p=0,05) y localización posterior (89,5 vs 41,2%; p=0,001). Mielitis por otras etiologías: mayor localización anterior (47,1 vs 24,6%; p=0,02) y centromedular (47,1 vs 14,1%; p=0,001) y mejor recuperación al año (EDSS 2,0 vs 1,5; p=0,01). Análisis multivariante: la afectación multifocal medular (OR 9,38; IC95%: 2,04-43,1) y del cordón posterior (OR 2,16; IC95%: 2,04-2,67) se asociaron de forma independiente al diagnóstico de EM.
ConclusionesUn alto porcentaje de pacientes con un primer brote de mielitis serán diagnosticados de EM. La presencia de lesiones medulares multifocales y en el cordón posterior se asocian de forma significativa a este diagnóstico.
Myelitis is an inflammation of the spinal cord characterised by the acute or subacute onset of sensory, motor, and autonomic symptoms (sphincter and sexual dysfunction) combined to varying extents.1,2
Aetiologies are very diverse, but mainly appear in the context of demyelinating diseases of the central nervous system, systemic inflammatory diseases, and infectious diseases.1,2 In the case of the first scenario, myelitis can be the initial manifestation of multiple sclerosis (MS), disorders in the spectrum of neuromyelitis optica (NMO), or of other immune-mediated demyelinating diseases. These are different diseases that require distinct diagnostic procedures and specific treatments in order to prevent new episodes.
In 2002, the Transverse Myelitis Consortium Working Group proposed international criteria to define idiopathic acute transverse myelitis with the aim of improving differential diagnosis and standardising terminology for future studies.3 In accordance with these criteria, a significant percentage of cases of myelitis appearing as an isolated neurological syndrome are classified as idiopathic in normal clinical practice. However, subsequent progression of myelitis may result in a change in diagnosis.4,5
The aim of our study was to analyse the demographic, aetiological, clinical, radiological, and prognostic characteristics of patients who presented an initial myelitis episode and compare characteristics of MS-related myelitis to those of myelitis of other aetiologies.
Material and methodsWe performed a descriptive and retrospective analysis of data gathered prospectively from the iMed database. We included patients with at least one year of clinical follow-up at the demyelinating diseases unit due to an initial myelitis episode (January 2000-December 2013).
Study variables were as follows: (1) demographic: sex, age at onset; (2) clinical: type of involvement (sensory, motor, or sphincter), disability during the episode measured by the Expanded Disability Status Scale (EDSS); (3) laboratory: IgM oligoclonal bands (IgM OCBs), anti-aquaporin 4 antibodies (AQP-4), other autoantibodies (ANA, intrinsic factor, parietal cell antibody, anti-TPO, ECA, anti-smooth muscle); (4) radiological: spinal magnetic resonance imaging (MRI) (location, size, longitudinally extensive transverse myelitis [LETM] affecting≥3 vertebral segments, unifocal or multifocal lesion), brain MRI (normal, unspecific, Barkhof or Swanton criteria); (5) prognostic: EDSS at one year, time to the second episode; and (6) aetiological (diagnostic).
IgM OCBs were measured using isoelectric focusing. MRI scans were performed using a 1.5 Tesla scanner (Philips Intera). We analysed T1- and T2-weighted sequences, FLAIR sequences, and postcontrast T1-weighted sequences. Spinal MRI in sagittal planes with T1-weighted spin echo, postcontrast T1-weighted, dual TSE PD/T2-weighted sequences, and particularly STIR sequences with 3mm slices were also analysed, as well as images in the axial plane of the lesion.
The study included a descriptive analysis, a chi-square univariate analysis, t-test, Mann-Whitney U test, and a multivariate logistic regression analysis to study the factors associated with myelitis and related to a higher probability of developing MS. We used SPSS version 21 for statistical analysis.
ResultsWe identified 286 patients whose first neurological sign appeared during the study period. From this pool, we analysed the 91 patients who had initial myelitis. Mean follow-up time (SD) was 7 years (± 5).
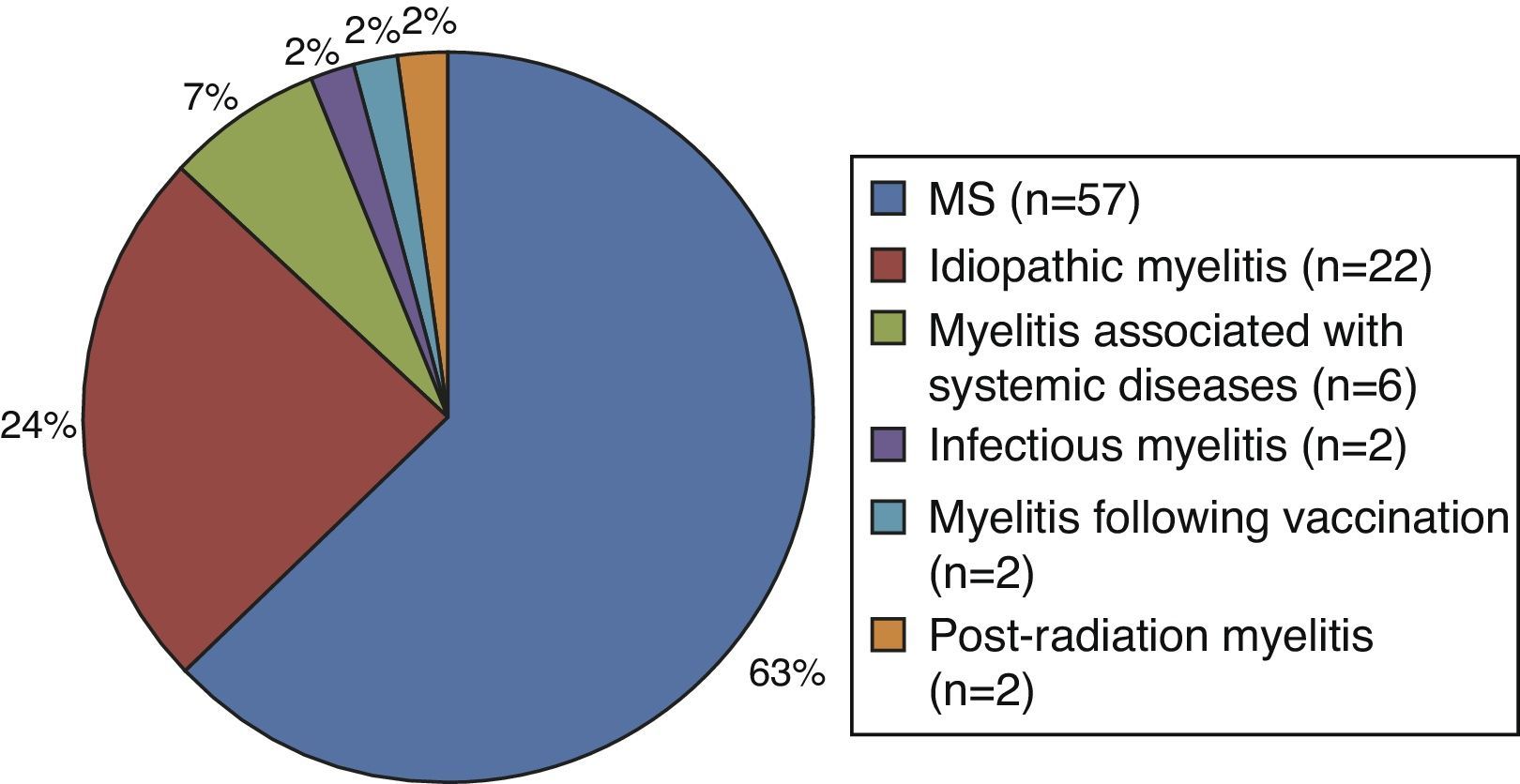
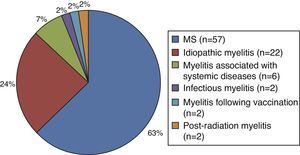
Of the patients with myelitis, 63% (n=57) were ultimately diagnosed with MS after a mean time of 12 months (range, 4-48). The remaining patients (n=34) were diagnosed with other diseases: 24% with idiopathic myelitis, 7% with myelitis associated with other systemic diseases (vasculitis, vitamin B12 deficiency anaemia, scleroderma, and systemic lupus erythematosus), 2% with parainfectious myelitis (CMV IgM antibody in CSF), 2% with myelitis following vaccination, and 2% with post-radiation myelitis (Fig. 1). We used AQP-4 antibody assays in 43% of the patients, but all cases were negative.
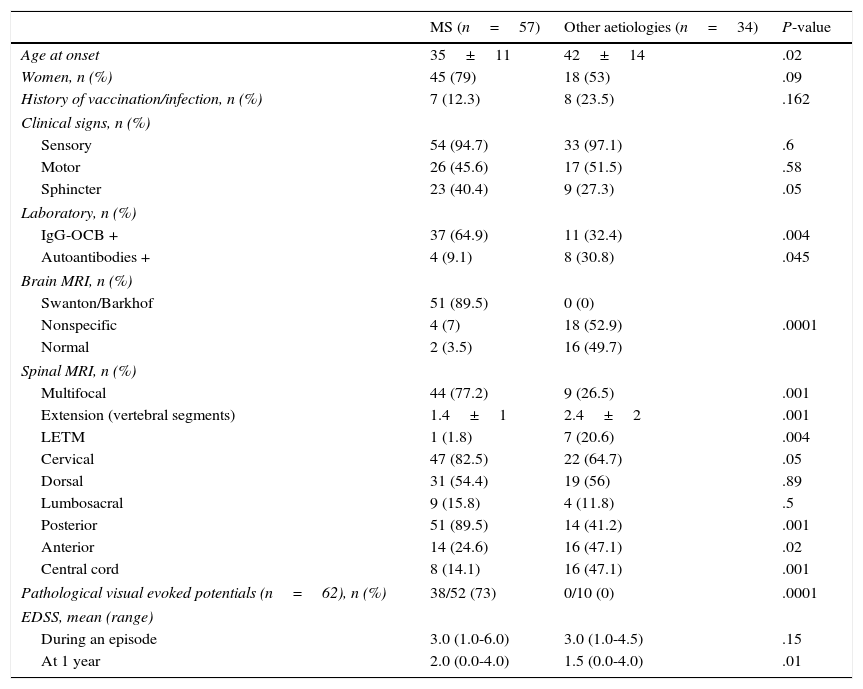
Table 1 shows the differences between MS-related myelitis and myelitis due to other aetiologies. Patients with MS-related myelitis had an earlier age of onset (35±11 vs 42±14; P=.02). Sensory involvement was the most prevalent in both groups (94.7% vs 97.1%). Patients with MS-related myelitis showed a higher rate of sphincter dysfunction (40.4% vs 27.3%; P=.05).
Differences between MS-related myelitis and myelitis due to other aetiologies.
| MS (n=57) | Other aetiologies (n=34) | P-value | |
|---|---|---|---|
| Age at onset | 35±11 | 42±14 | .02 |
| Women, n (%) | 45 (79) | 18 (53) | .09 |
| History of vaccination/infection, n (%) | 7 (12.3) | 8 (23.5) | .162 |
| Clinical signs, n (%) | |||
| Sensory | 54 (94.7) | 33 (97.1) | .6 |
| Motor | 26 (45.6) | 17 (51.5) | .58 |
| Sphincter | 23 (40.4) | 9 (27.3) | .05 |
| Laboratory, n (%) | |||
| IgG-OCB + | 37 (64.9) | 11 (32.4) | .004 |
| Autoantibodies + | 4 (9.1) | 8 (30.8) | .045 |
| Brain MRI, n (%) | |||
| Swanton/Barkhof | 51 (89.5) | 0 (0) | |
| Nonspecific | 4 (7) | 18 (52.9) | .0001 |
| Normal | 2 (3.5) | 16 (49.7) | |
| Spinal MRI, n (%) | |||
| Multifocal | 44 (77.2) | 9 (26.5) | .001 |
| Extension (vertebral segments) | 1.4±1 | 2.4±2 | .001 |
| LETM | 1 (1.8) | 7 (20.6) | .004 |
| Cervical | 47 (82.5) | 22 (64.7) | .05 |
| Dorsal | 31 (54.4) | 19 (56) | .89 |
| Lumbosacral | 9 (15.8) | 4 (11.8) | .5 |
| Posterior | 51 (89.5) | 14 (41.2) | .001 |
| Anterior | 14 (24.6) | 16 (47.1) | .02 |
| Central cord | 8 (14.1) | 16 (47.1) | .001 |
| Pathological visual evoked potentials (n=62), n (%) | 38/52 (73) | 0/10 (0) | .0001 |
| EDSS, mean (range) | |||
| During an episode | 3.0 (1.0-6.0) | 3.0 (1.0-4.5) | .15 |
| At 1 year | 2.0 (0.0-4.0) | 1.5 (0.0-4.0) | .01 |
Presence of IgM OCBs was more frequent in the MS group (64.9% vs 32.4%; P=.004) and was significantly related to a shorter time before recurrence (P=.04). The CSF biochemical profile prepared for 27 patients did not show any intergroup differences. Detection of other ANA was more frequent in patients with myelitis not due to MS (9.1% vs 30.8%, P=.045). Visual evoked potential tests performed in 62 patients yielded pathological results in 73% of the patients with MS-related myelitis. No pathological results were found in the group with other aetiologies (P<.0001).
Of the patients with MS-related myelitis, 77.2% presented multifocal spinal lesions vs 26.5% in the group with other aetiologies (P=.001). We observed more impairment of both the anterior region (24.6% vs 47.1%; P=.02) and the central cord region (14.1% vs 47.1%; P=.0001) in the group with myelitis not due to MS. Among patients with myelitis due to other aetiologies, 20% (n=7) presented LETM compared to only one patient from the MS-related myelitis group (P=.0004).
Brain MRIs from almost 90% of the patients with MS-related myelitis fulfilled Barkhof or Swanton criteria; none of the patients with myelitis due to other aetiologies met those criteria. MRI scans showed nonspecific demyelinating lesions in 7% of the patients with MS and in 53% of the patients with myelitis due to other aetiologies. Findings were normal in 5.3% and 50% of each group, respectively. These differences were statistically significant (P<.001).
Results from the initial brain MRI were considered normal (n=2) or nonspecific (n=4) in 10% of the patients diagnosed with MS (n=6). Their condition progressed to MS after a mean of 18 months (4-48). Spinal MRI scans showed that 100% of the patients had impairment in the posterior column: 50% in the cervical area, and 50% in the dorsal area. Four patients presented a lesion that extended over one vertebral segment; 2 showed multifocal impairment.
Baseline EDSS was similar in both groups (3.0 [1.0-6.0] vs 3.0 [1.0-4.5]). EDSS scores at one year were lower for the group with myelitis due to other aetiologies than for the group with MS-related myelitis (1.5 [0-4] vs 2.0 [0-4]; P=.01). Mean time until the second episode in patients with MS was 18 months (range, 1-120), vs 48 months (range, 6-180) for patients with idiopathic recurrent myelitis (n=3).
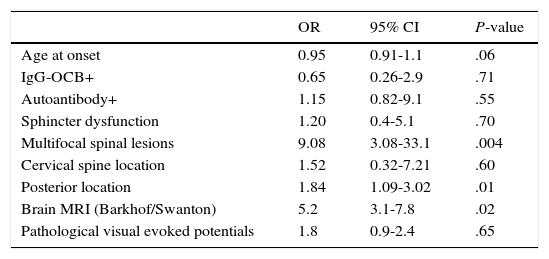
Multivariate analysis showed that multifocal spinal cord impairment (OR 9.38; 95% CI: 2.04-43.1) and posterior column impairment (OR 2.16; 95% CI: 2.04-2.67) were independently associated with MS diagnosis and brain MRI scans showing lesions at typical localisations for MS lesions (Table 2).
Multivariate analysis. Variables associated with MS diagnosis.
| OR | 95% CI | P-value | |
|---|---|---|---|
| Age at onset | 0.95 | 0.91-1.1 | .06 |
| IgG-OCB+ | 0.65 | 0.26-2.9 | .71 |
| Autoantibody+ | 1.15 | 0.82-9.1 | .55 |
| Sphincter dysfunction | 1.20 | 0.4-5.1 | .70 |
| Multifocal spinal lesions | 9.08 | 3.08-33.1 | .004 |
| Cervical spine location | 1.52 | 0.32-7.21 | .60 |
| Posterior location | 1.84 | 1.09-3.02 | .01 |
| Brain MRI (Barkhof/Swanton) | 5.2 | 3.1-7.8 | .02 |
| Pathological visual evoked potentials | 1.8 | 0.9-2.4 | .65 |
In our series, 63% of the patients with an initial myelitis episode were ultimately diagnosed with MS. Patients with MS-related myelitis were younger than the rest, as has also been shown by other studies.4,5 Sensory symptoms were the most prevalent, as is usually the case with acute myelitis regardless of its aetiology.1,2 We observed a higher rate of sphincter dysfunction among patients with MS, a finding which has not been reported by other studies.4 This could be related to the higher frequency of multifocal lesions in these patients. Most patients with MS (64.9%) presented IgG-OCBs in CSF, and we observed a significant correlation between this finding and a shorter time to recurrence (P=.04), as has been reported by other authors.4,6 Unlike in other studies, we did not observe significant differences with regard to sex.4,5
MS-related myelitis had a significantly higher prevalence of smaller lesions (<2 spinal cord segments) and multifocal spinal cord lesions located in the cervical spine and posterior column. These findings resemble those described in the literature.7 A spinal cord lesion of this type, even given a normal baseline brain MRI, indicates a higher risk of MS conversion during follow-up.8 A long-term study showed a 22% risk of progression to MS even in cases with a normal baseline brain MRI, and as much as 88% in cases of nonspecific demyelinating brain lesions.9 In our series, 6 patients who were diagnosed with MS during the follow-up period displayed normal or nonspecific characteristics on the baseline brain MRI.
Several studies describe prognostic factors for MS conversion after myelitis as isolated neurological syndrome,4,5,9,10 but we have not found any assessing the recovery potential of patients according to the aetiology of the myelitis. We have assessed recovery at one year of follow-up and observed that myelitis due to other aetiologies presented a significantly lower degree of disability than MS-related myelitis (EDSS 1.5 [0-4] vs 2 [0-4]; P=.01). This finding would mean better recovery.
Inflammatory LETM is mostly idiopathic and its prognosis tends to be good.11 We observed the same findings in our cohort. Among the 8 patients with LETM, 5 were seronegative for anti-AQP4 and subsequently diagnosed with idiopathic myelitis. Antibodies were not determined in the remaining 3 patients since they had other alternative diagnoses (MS, CNS vasculitis, post-radiation vasculitis). According to current diagnostic criteria,12–14 none of our patients with myelitis developed NMO or an NMO spectrum disorder during the follow-up period.
This retrospective study contains a limited and heterogeneous sample of patients. We have not considered therapeutic responses or their impact on outcomes. Although it has recently become common practice, IgM OCBs were not evaluated, and only a third of the patients were able to undergo a biochemical analysis of CSF. Despite these limitations, we believe that our study achieved its aim since it presents differences between types of myelitis that are helpful in assigning an initial aetiological diagnosis.
According to our series, the most frequent cause of myelitis is MS. Presence of multifocal spinal cord lesions or those located in the posterior column has a significant association with diagnosis of MS. Other factors such as young age, sphincter dysfunction, location in the cervical spine, small lesion size, presence of IgG-OCBs, and pathological results on a visual evoked potential test can also help point to a diagnosis of MS in its initial stages.
Conflicts of interestThis study received no public or private funding. The authors of this study have no conflicts of interest to declare.
Please cite this article as: Presas-Rodríguez S, Grau-López L, Hervás-García JV, Massuet-Vilamajó A, Ramo-Tello C. Mielitis. Diferencias entre esclerosis múltiple y otras etiologías. Neurología. 2016;31:71–75.
This study was presented at the 66th SEN Annual Meeting (2014) and accepted for presentation in poster format at the 67th Annual Meeting of the American Academy of Neurology (2015).