Susac syndrome (SS) continues to represent a diagnostic challenge since its form of presentation overlaps with those of other conditions. The syndrome is usually misdiagnosed as demyelinating diseases including multiple sclerosis or acute disseminated encephalomyelitis due to the similar clinical presentation and paraclinical findings.1
Occlusion secondary to autoimmune endotheliopathy affects precapillary arterioles of the brain, retina, and inner ear (cochlea and semicircular canals),2 which may cause neurological, auditory, and visual alterations; impaired visual acuity is caused by branch retinal artery occlusion. However, not all patients present this triad of symptoms.
Neurological alterations include impaired level of consciousness and cognitive and behavioural alterations, which may be accompanied by such focal neurological signs as ataxia, paresis, and dysarthria. During this stage of subacute encephalopathy, MRI studies may reveal lesions to the corpus callosum, which help diagnose the condition.
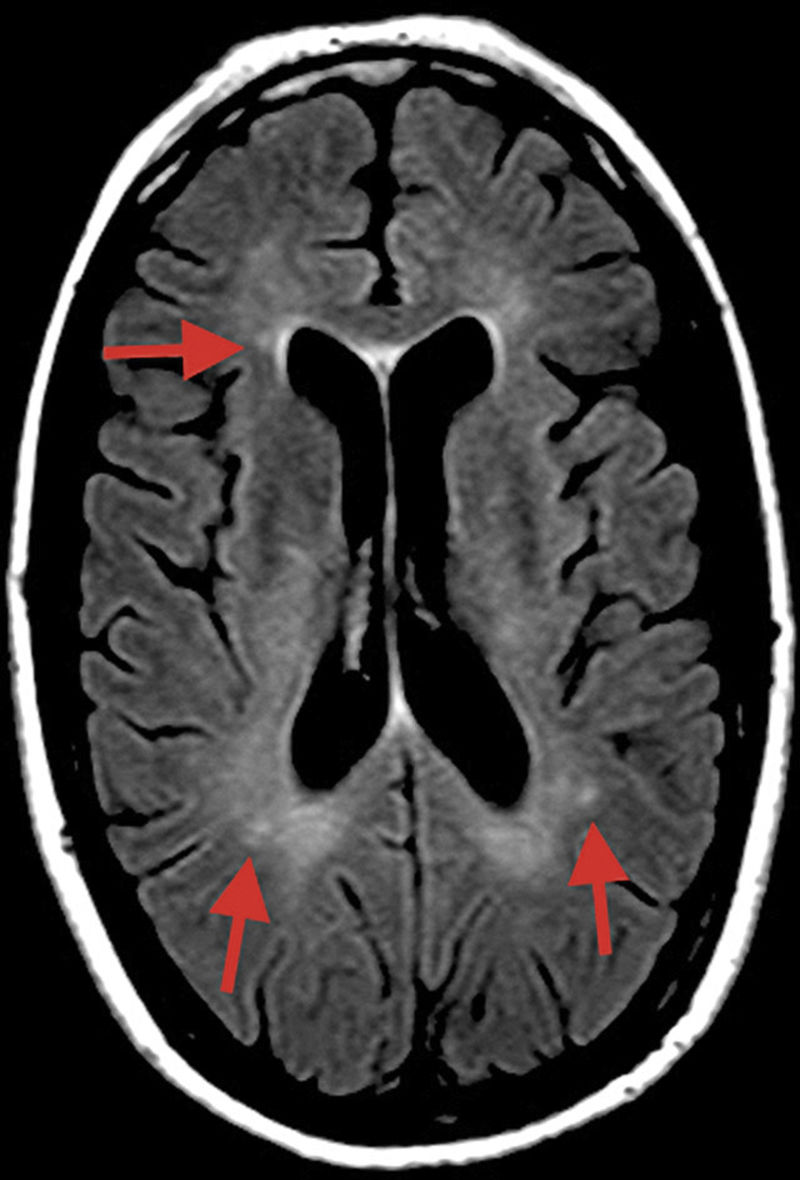
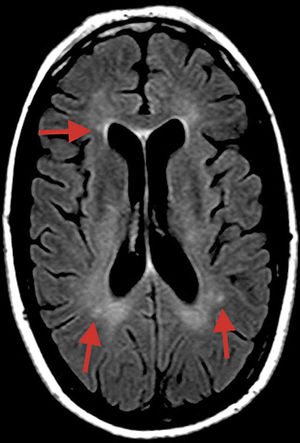
MRI may be helpful for early diagnosis in some cases, revealing microinfarcts in the central region of the corpus callosum in sagittal FLAIR and T2-weighted sequences; lesions typically resemble “snowballs,” and subsequently progress to black holes and atrophy. Lesions measure 3-7mm, suggesting occlusion of precapillaries of less than 0.1mm (100μm) in diameter. Microinfarcts may take the form of linear or “spoke” lesions. Diffusion sequences may also reveal hyperdense lesions to the internal capsule, resembling a “string of pearls.” The presence of these imaging findings in addition to lesions to the central region of the corpus callosum is pathognomonic of SS.2 However, most patients with SS show no MRI alterations.3
The usefulness of MRI is therefore limited in small-vessel diseases such as SS (vessels with a diameter below 0.1mm); alterations in small-calibre vessels of the white matter are frequently difficult to detect, even with conventional arteriography.4 MRI is useful for detecting occlusion, stenosis, or dilation of medium- or large-calibre vessels (>0.5mm).3
We present the case of a 34-year-old woman who was referred to our centre due to motor instability, tinnitus, hearing impairment, and impaired visual acuity in the right eye.
A T2-weighted FLAIR sequence obtained at symptom onset revealed small, nonspecific hyperintensities in the subcortical white matter, corpus callosum, and cerebellar peduncles (Fig. 1).
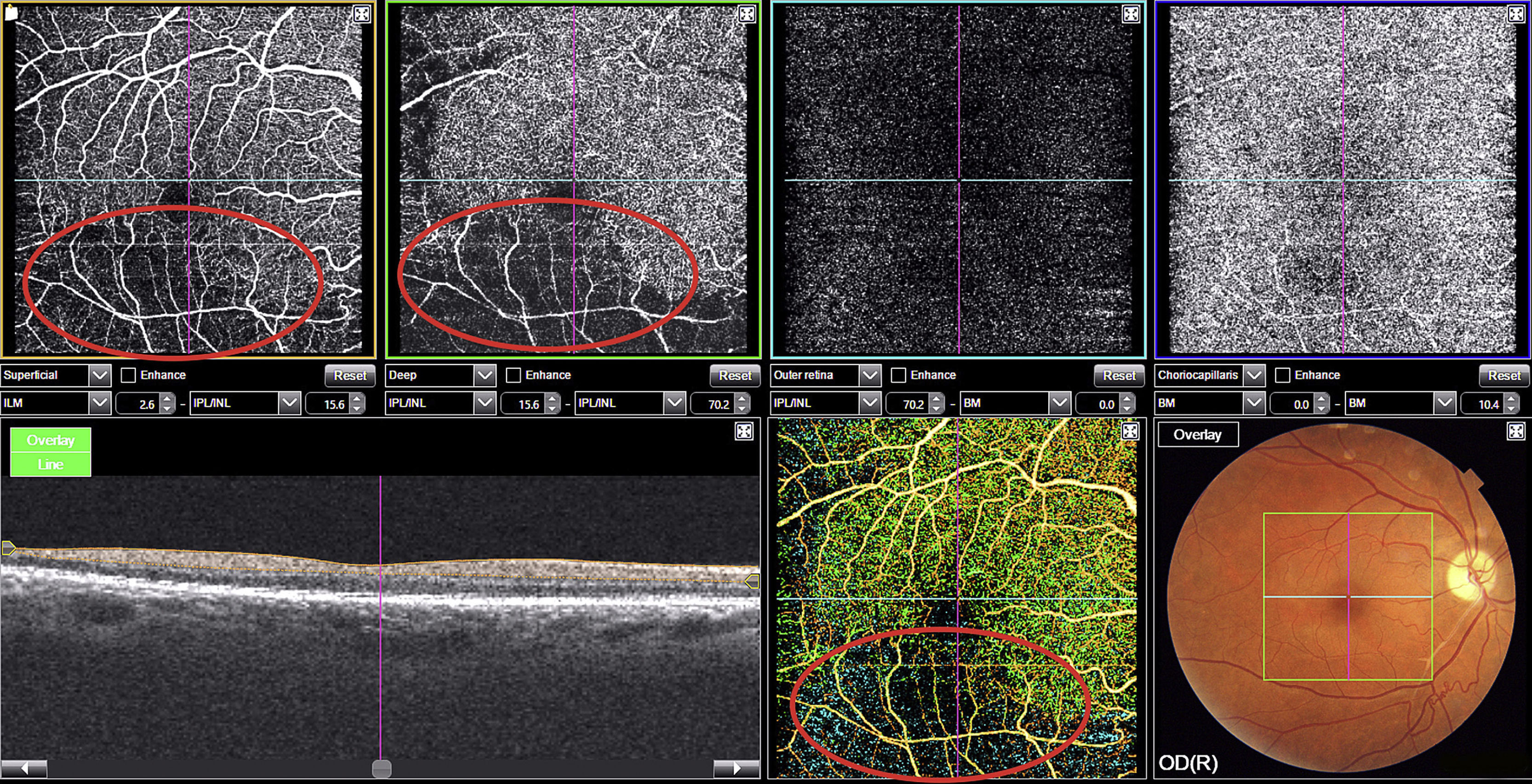
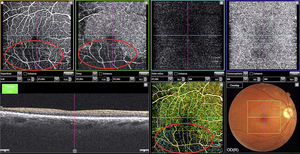
The right eye fundus examination revealed Gass plaques and opacification, thickening, and partial occlusion of an artery in the inferior temporal region. Optical coherence tomography angiography (OCT-A) of the posterior pole revealed loss of the superficial and deep retinal capillary plexuses of the right eye, with a decrease in the number of arterial branches and areas of peripheral ischaemia in the inferior temporal region (Fig. 2).
OCT-A is a new imaging technique that employs motion contrast to differentiate retinal blood vessel cells from the surrounding tissue, which is static. Measuring the signal amplitude of moving structures is a safe, non-invasive method of generating eye fundus angiograms with no need for contrast agents (Fig. 2).5 With lateral resolution of 20μm and depth resolution of 2.6μm,6 OCT-A enables visualisation of macular capillaries, enabling early, non-invasive detection of retinal capillary loss. In our patient, the technique was extremely helpful in diagnosing SS before any clear ischaemic lesions to the brain parenchyma were visible on MR images.
No treatment for SS has been evaluated in controlled clinical trials due to the small number of cases of the condition.7 Therefore, patients are usually treated with different immunomodulatory agents, with no standardised approach.
High-dose corticosteroid therapy continues to be a pillar of treatment, although patients frequently require complementary treatment with intravenous immunoglobulins, mycophenolate mofetil, or cyclophosphamide.8 Anticoagulant, antiplatelet, and anti-vasospastic agents should also be considered.9
Early diagnosis of SS is needed to provide intensive, long-term treatment, which may improve prognosis.2 The course of the disease is unpredictable10 and no reliable biomarkers have been identified.7 OCT-A generates images with a mean resolution of 8-10μm, showing ischaemic processes11 in small-calibre vessels (<100μm) of the retinal parenchyma and their progression, and complements such other imaging techniques as MRI, in which some planes may not display endothelial lesions during the early stages of the disease.
Please cite this article as: García-Serrano JL, Muñoz de Escalona-Rojas JE, Callejas-Rubio JL, Barrero-Hernández FJ. Angiografía por tomografía de coherencia óptica en el diagnóstico precoz del síndrome de Susac. Neurología. 2020;35:62–63.