Multiple sclerosis (MS) is a demyelinating autoimmune disease of the central nervous system (CNS), in which astrocytes play an important role as CNS immune cells. However, the activity of astrocytes as antigen-presenting cells (APC) continues to be subject to debate.
DevelopmentThis review analyses the existing evidence on the participation of astrocytes in CNS inflammation in MS and on several mechanisms that modify astrocyte activity in the disease.
ConclusionAstrocytes play a crucial role in the pathogenesis of MS because they express toll-like receptors (TLR) and major histocompatibility complex (MHC) class I and II. In addition, astrocytes participate in regulating the blood-brain barrier (BBB) and in modulating T cell activity through the production of cytokines. Future studies should focus on the role of astrocytes in order to find new therapeutic targets for the treatment of MS.
La esclerosis múltiple (EM) es una enfermedad autoinmune desmielinizante del sistema nervioso central (SNC) en la que los astrocitos tienen una participación importante como células inmunes del SNC, aunque su actividad como células presentadoras de antígeno (APC) aún es discutida.
DesarrolloEn la presente revisión se analiza la evidencia existente sobre la participación de los astrocitos en la inflamación del SNC en la EM, así como diversos mecanismos que modifican su actividad en la enfermedad.
ConclusionesLos astrocitos desempeñan un papel trascendental en la patogénesis de la EM, debido a que expresan receptores TLR, así como proteínas del complejo principal de histocompatibilidad (MHC) clase I y II. Además, participan en la regulación de la barrera hemato-encefálica (BHE) y la modulación de la actividad de los linfocitos T mediante la producción de citocinas. Futuros estudios deberán enfocarse en el papel de los astrocitos con el objetivo de encontrar nuevos blancos terapéuticos para el tratamiento de la EM.
Multiple sclerosis (MS) is a demyelinating autoimmune disease of the central nervous system (CNS)1–3 presenting great clinical heterogeneity.4 It has been associated with numerous genetic and environmental risk factors.5 The disease is characterised by the appearance of lesions or plaques6 formed by CNS cells and immune system cells, which migrate across the blood-brain barrier (BBB) and induce CNS inflammation.7 T cells and macrophages are the main mediators of CNS inflammation; these cells invade the CNS and modulate the activity of glial cells and neurons through different cell mechanisms, leading to demyelination and cell death in patients with MS.8
Astrocytes are the most abundant type of glial cells in the CNS9 and play a dual role in MS pathogenesis10: firstly, they contribute to disease progression, acting as CNS immune cells and secreting chemokines that promote immune cell migration from the peripheral blood to the CNS10; and secondly, they promote the migration, proliferation, and differentiation of oligodendrocyte precursor cells,11 favouring remyelination. The available evidence suggests that the role of astrocytes should be reevaluated, as these cells not only provide support and contribute to maintaining homeostasis, but also play a major role in the pathogenesis of CNS autoimmune diseases, particularly MS.
In the light of these considerations, the purpose of this study is to review, summarise, and discuss the available evidence on the role of astrocytes in MS pathogenesis. This review addresses the following points: 1) the role of astrocytes as CNS immune cells in the MS inflammatory process; 2) the role of astrocytes as antigen-presenting cells (APC); 3) the role of astrocytes in BBB integrity and some mechanisms altering BBB permeability, which enables immune cell migration into the CNS; and 4) the role of astrocytes in T cell regulation.
Astrocytes as immune cells in multiple sclerosisAs is the case with microglia, astrocytes express pattern recognition receptors (PRR), which bind to pathogen-associated molecular patterns (PAMP).12 PRRs recognise PAMPs derived from different pathogenic microorganisms, such as bacteria13,14 and viruses,15,16 as well as damage-associated molecular patterns (DAMP), which are considered to be host-derived and are released after cell damage or death.17 Astrocytes express toll-like receptors TLR3 and TLR4.12 TLR3 recognises double-stranded RNA released by damaged or virus-infected cells, inducing a proinflammatory response in the immune system18 through TRIF-, NF-κB-, RIP1-, and TRAF6-dependent signalling.19 However, Bsibsi et al.20 showed that TLR3-mediated astrocyte activation in the CNS induces overproduction of anti-inflammatory cytokines interleukin (IL)-9, IL-10, and IL-11, and decreases the expression of the p40 subunit of IL-12 and IL-13. Furthermore, TLR3 expression by astrocytes may be enhanced by interferon (IFN)-γ, IFN-β, or IL-1β.21 This suggests that TLR3 expression in patients with MS modulates inflammation through the expression of anti-inflammatory cytokines, which limit the activity of immune cells.
TLR4 is upregulated in brain lesions of animals with experimental autoimmune encephalomyelitis (EAE), an experimental model of MS, as well as in blood cells from patients with relapsing-remitting MS (RRMS) and secondary progressive MS (SPMS).22 Crowley et al.23 report that TLR4 and TLR3 activation with an inflammatory stimulus in patients with MS enhances NF-κB expression, increasing TNF-α levels. This finding supports the hypothesis that astrocytes play a dual role in MS: on the one hand, TLR3 activation in astrocytes releases anti-inflammatory cytokines that limit neuroinflammation, and on the other hand, TLR3 and TLR4 stimulation may promote a proinflammatory process secondary to increased TNF-α production.
Astrocytes as antigen-presenting cells in multiple sclerosisAstrocytes are non-traditional APCs,24 since they only serve an immune function if CNS damage is present.25 Astrocytes constitutively express major histocompatibility complex (MHC) class I molecules.26 However, MHC class II expression in astrocytes is controversial: while some studies suggest that MHC class II molecules are constitutively expressed in astrocytes,27 others report minimal expression28 or suggest that expression is cytokine-dependent.29 There is also controversy around the expression of co-stimulatory molecules: some studies suggest that astrocytes can express co-stimulatory molecules of the B7 family,30,31 whereas others have found no evidence of B7 molecule expression.32,33 In any case, it seems clear that such infectious processes as viral infections induce MHC class I and II expression in astrocytes,34,35 and that expression of both classes of MHC molecules changes according to the predominant cytokine profile in the CNS.27,36–38
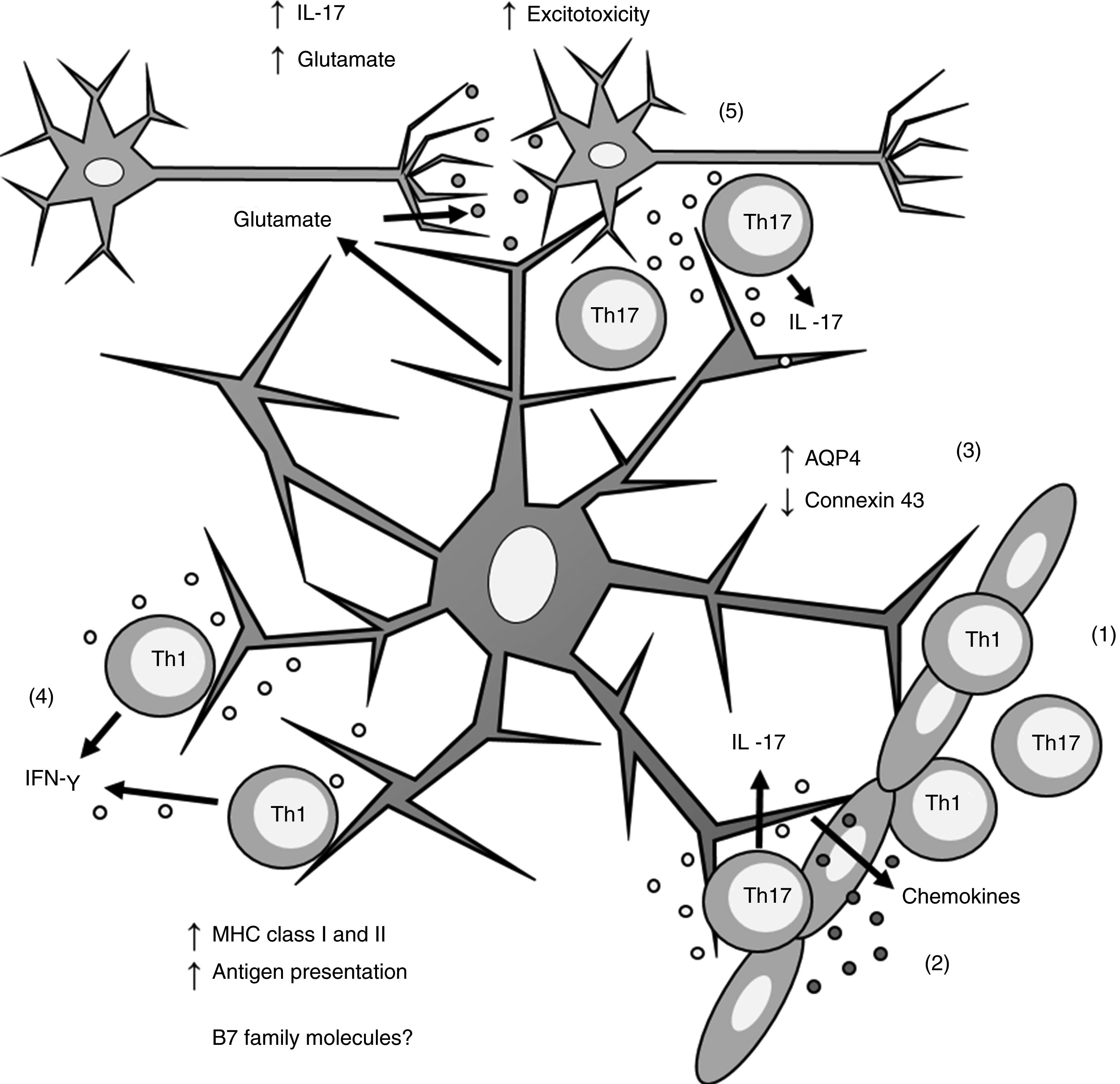
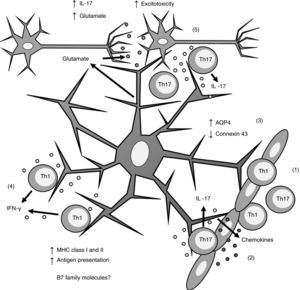
Biopsy studies of post mortem brain tissue from patients with MS have detected MHC class I39 and II40,41 molecules in astrocytes (Fig. 1). T cells present in MS lesions induce MHC class I overexpression through IFN-γ production,42 which is regulated by NF-κB activity.26 IFN-γ production also increases MHC class II expression27,36,43 through increased activity of protein kinase C and IFN-gamma enhanced factor X; the latter interacts with the X box of the DRA promoter.44 Furthermore, IFN-γ increases the mobility of MHC class II compartments, which is intermediate filament- dependent, allowing faster delivery of MHC molecules to the surface of astrocytes.36 This suggests that astrocytes may constitutively express MHC class II molecules, which remain in intracellular compartments until astrocytes are stimulated by IFN-γ.
Role of astrocytes on the pathogenesis of multiple sclerosis. 1) Th1 and Th17 cells cross the blood-brain barrier through diapedesis; 2) Th17 cells produce IL-17, which induces chemokine production by astrocytes, increasing immune cell recruitment; 3) astrocytes overexpress AQP4 and decrease the expression of connexin 43; 4) Th1 cells produce IFN-γ, which induces MHC class I and II overexpression in astrocytes; this in turn increases antigen presentation; 5) Th17 cells produce IL-17, which induces an increase in glutamate levels in the synaptic region, leading to excitotoxicity and neuronal death.
AQP4: aquaporin-4; IFN-γ: interferon γ; IL-17: interleukin 17; MHC: major histocompatibility complex; Th1: T helper 1 cell; Th17: T helper 17 cell.
However, MHC expression in astrocytes may be inhibited by different molecules. One of the first molecules to be analysed was IFN-β, which blocks IFN-γ- induced MHC class II expression45,46 without modifying MHC class I expression.46 This immunomodulatory effect is used as an action mechanism in IFN-β treatment for MS,47–49 which was approved by the United States Food and Drug Administration in 1993.50 It delays relapses, decreases neurological deterioration, and slows disease progression.50
Other cytokines associated with modulation of MHC class II expression in astrocytes are IL-4,38 IL-1,27 and TGF-β.51 TGF-β, the most extensively studied cytokine, is known to decrease MHC class II expression in astrocytes51,52 and other types of cells.53,54 Dong et al.55 showed that TGF-β inhibits the expression of the MHC class II transactivator (CIITA), which is needed for the induction of EAE,56 and the activity of the CIITA type IV promoter through the Smad3 transcription factor.55
Some studies suggest that MHC expression is regulated by molecules directly involved in nerve transmission. Similarly, it is reported that glutamate,57 norepinephrine,57,58 and gangliosides59,60 may inhibit MHC expression in astrocytes. Furthermore, serotonin may modify the response of immune system cells.61,62 Serotonin receptor agonists are known to inhibit the expression of IFN-γ- induced MHC class II molecules and B7 co-stimulatory molecules in astrocytes.63 Seddighzadeh et al.64 found an interaction between a protective haplotype in serotonin receptor HTR2A alleles and HLA-DRB1 alleles constitutively expressed in synovial fibroblasts from patients with rheumatoid arthritis, a widely studied autoimmune disease. Since astrocytes regulate synapse formation,65 changes in neurotransmitter release may alter MHC expression in astrocytes and their involvement in T cell antigen presentation.
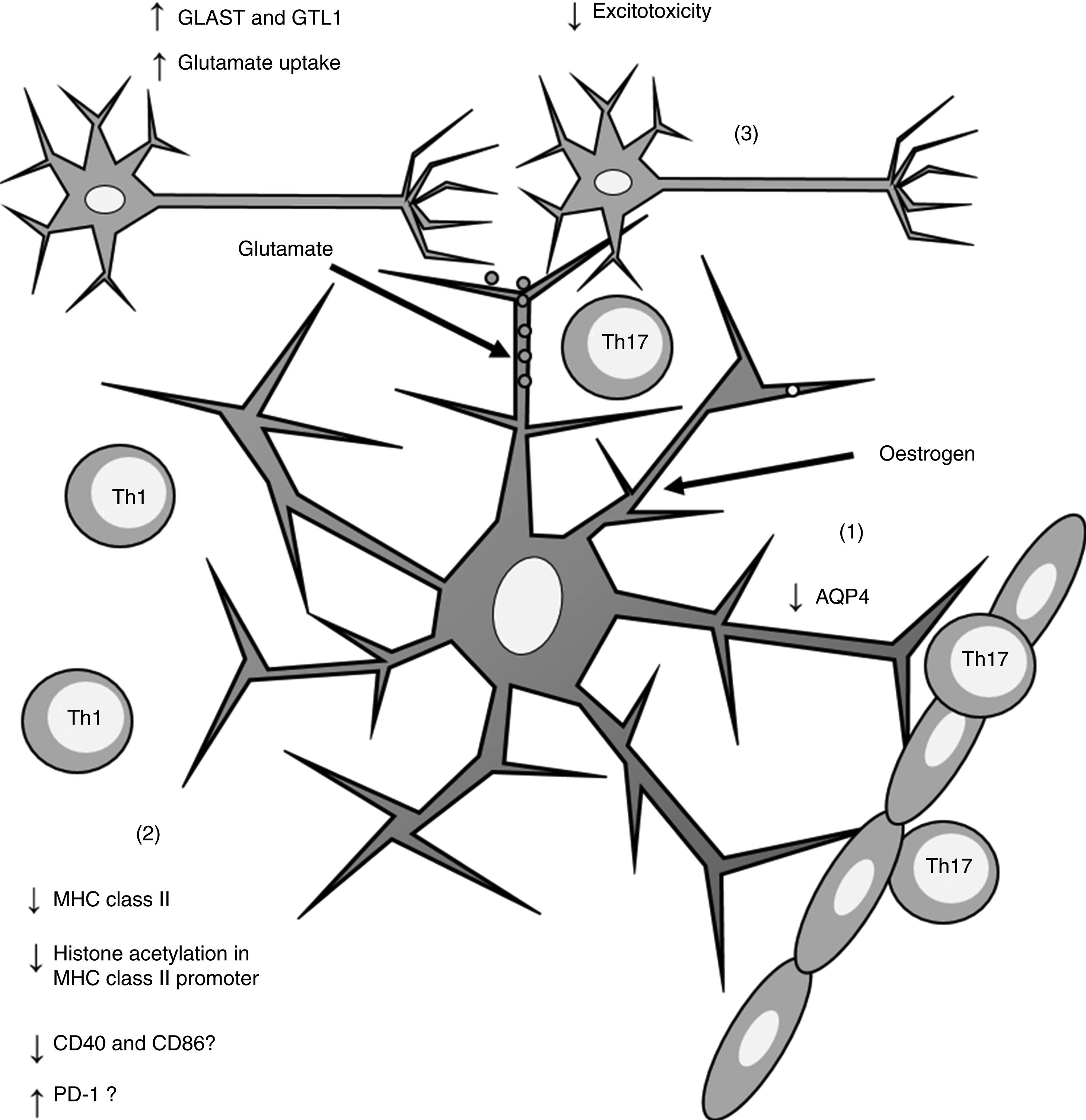
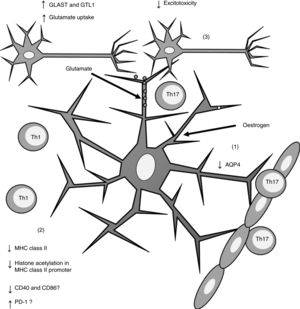
Furthermore, oestrogen has been found to have a neuroprotective effect, as it induces an anti-inflammatory response in astrocytes, decreasing the severity of EAE and MS66,67 through different mechanisms. One of these is the modulation of astrocytes’ activity as APCs. Adamski et al.68 showed that oestrogen decreases MHC class II expression in astrocytes through a mechanism independent of CIITA expression, attenuating histone acetylation in the MHC class II promoter (Fig. 2). Oestrogen has also been found to decrease the expression of cluster of differentiation (CD) 40 and CD86 in microglia69; in professional APCs (dendritic cells, macrophages, and B cells), it increases expression of programmed cell death protein 1.70 This suggests that oestrogen may also modulate the expression of co-stimulatory molecules in astrocytes (Fig. 2).
Effects of oestrogen on astrocytes. Oestrogen decreases AQP4 expression (1); decreases MHC class II expression, which in turn reduces antigen presentation (2); and increases the expression of GLAST and GTL1, which increases glutamate uptake in neuronal synapses and reduces excitotoxicity (3).
AQP4: aquaporin-4; CD40: cluster of differentiation 40; CD86: cluster of differentiation 86; GLAST: glutamate aspartate transporter; GTL1: glutamate transporter-1; MHC: major histocompatibility complex; PD-1: programmed cell death protein 1; Th1: T helper 1 cell; Th17: T helper 17 cell.
Astrocytes present close contact with the basal lamina of the BBB, since their processes line blood vessel walls.71 At this level, astrocytes are interconnected by gap junctions formed by connexin 43 and connexin 30.10 Brand-Schieber et al.72 observed decreased expression of connexin 43 in astrocytes from mice with EAE, which suggests loss of connectivity between astrocytes and increased BBB permeability. Furthermore, astrocytes express aquaporin-4,71 which has been shown to be overexpressed in active MS lesions73 (Fig. 1). In a more recent study of rats with post-traumatic brain injury conducted by Soltani et al.,74 oestrogen was found to decrease aquaporin-4 expression (Fig. 2), which may suggest a protective effect on the BBB in EAE and MS.
Furthermore, astrocytes can modulate BBB permeability through cytokine production. The combination of cytokines produced by astrocytes depends on their interactions with other cells and the stimuli received from other cytokines. Production of IL-1β, IL-6, and TNF-α by astrocytes increases BBB permeability, whereas production of TGF-β has the opposite effect.75 Furthermore, the BBB transports cytokines bidirectionally (blood to brain, brain to blood),76 which means that systemic overproduction of proinflammatory cytokines promotes inflammation associated with the underlying CNS disease.
Immune cells in the peripheral blood have limited access to the CNS. To enter the CNS, cells must migrate via diapedesis through the BBB to the perivascular space, and then to the brain parenchyma25; astrocytes are the first CNS cells to be activated by T cells, due to their direct contact with the BBB.77 IL-17 production by Th17 cells and subsequent Act1-mediated IL-17 signalling induce the production of chemokines that increase immune cell recruitment and diapedesis through the BBB.77,78 Thus, interaction between Th17 cells and astrocytes may trigger inflammation in MS due to astrocyte activation and production of soluble factors (chemokines and cytokines), which increase BBB permeability.
The role of astrocytes in the expression of the Th1/Th2/Th17 profileTh1 and Th17 cells are the CD4 + T cell lineages responsible for inflammation in MS, whereas Th2 and Treg cells limit inflammatory activity.79 Several studies have addressed the role of astrocytes on the modulation of the Th1/Th2/Th17 profile in the CNS. Astrocytes have been found to produce cytokines from the IL-12 family, such as IL-12,80,81 IL-23 (both p4080,81 and p19 subunits81), and IL-27 (both p28 and EBI3 subunits).82 In MS, production of these cytokines seems to determine the predominant profile over the course of the disease; this is addressed later in the review.
On the one hand, IL-12 and IL-23 production favours the presence of Th1 and Th17 cells, respectively.83 As both cytokines share the p40 subunit, previous studies have shown that IL-23 is essential in MS pathogenesis, since presence of IL-23-induced Th1784 in the absence of Th1 is sufficient to induce EAE in animals.85,86 Therefore, there is a correlation between presence of IL-23 and severity of disease induced by IL-17,84 which induced excitotoxicity and neuronal death due to glutamate accumulation in the synaptic region65,87 (Fig. 1). On the other hand, when IL-12 production is promoted, Th1 cells are the predominant population83; therefore, IFN-γ production inhibits differentiation into Th17 lineage cells.88 This suggests that IL-23 production by astrocytes promotes CNS inflammation through Th17-dependent mechanisms, and that IL-12 production partially promotes inflammation via Th1. However, increased IL-12 production suggests that the predominant population is Th1, which together with subsequent IFN-γ production regulates the activity of the Th17 population.
Regarding IL-17- mediated glutamate excitotoxicity, several studies have shown that oestrogen increases the expression of the glutamate aspartate transporter66,89–91 and glutamate transporter-1 in astrocytes.66,90–92 This suggests that oestrogen plays a neuroprotective role in astrocytes by increasing glutamate reuptake in the synaptic region,66,90 which may decrease neuronal death secondary to excessive glutamate activity (Fig. 2).
IL-27 is extensively produced by astrocytes and microglia in patients with MS, and its receptor is expressed by both T cells and astrocytes.82 IL-27 mainly has anti-inflammatory effects and has been found to limit the development of EAE in animals as it regulates Th1 and Th2 cell response through a decrease in IL-2 levels, which limits the proliferation of these populations.83 Furthermore, IL-27 decreases Th17 cell differentiation93,94 and, together with IL-6, may induce IL-10 production in Th1, Th2, and Th17 cells.95 This suggests that astrocytes modulate the inflammatory response in MS through various mechanisms, including IL-27 secretion by astrocytes and microglia, inducing a self-limiting process due to IL-10 production by different T cell populations.
ConclusionsAstrocytes play a major role in MS pathogenesis since they are actively involved in CNS inflammation and also limit inflammation through several mechanisms, including: 1) expression of TLRs, which enable astrocytes to act as immune cells; 2) expression of MHC class I and II molecules, as well as co-stimulatory molecules, which allows astrocytes to serve as professional APCs; 3) regulation of BBB permeability; and 4) production of IL-12, IL-23, and IL-27, which regulate the expression of Th1 and Th17 inflammatory profiles in the CNS. Furthermore, there is evidence suggesting that oestrogen has a neuroprotective effect on the mechanisms involving astrocytes. Future studies should address the role of astrocytes and modulation of their activity in CNS inflammatory processes with a view to finding new therapeutic targets for MS.
Conflicts of interestThe author has no conflicts of interest to declare.
The author wishes to thank the Mexican Institute of Social Security for his doctoral scholarship, and Dr Daniel Ortuño-Sahagún and Karla Itzel Padilla Chavoya for reviewing the manuscript.
Please cite this article as: Guerrero-García JJ. Participación de los astrocitos en la patogénesis de la esclerosis múltiple. Neurología. 2020;35:400–408.