In Spain, approximately 28% of ischaemic strokes have an atherothrombotic cause, and most are due to carotid stenosis. Ultrasound is the most commonly used technique for diagnosing carotid stenosis. Changes in blood flow velocity at the point of maximum stenosis, together with haemodynamic changes in proximal regions (common carotid artery) and distal regions (poststenotic internal carotid, ophthalmic artery, and the circle of Willis), allow us to measure carotid stenosis precisely. This review explains the methodology to be followed when evaluating carotid stenosis ultrasonographically, according to the recommendations from the Spanish Society of Neurosonology (SONES). We review the findings that permit us to measure the degree of extracranial carotid stenosis using both carotid and transcranial ultrasound, with particular emphasis on the importance of assessing indirect signs.
En España se estima que aproximadamente un 28% de los ictus isquémicos son de etiología aterotrombótica y mayoritariamente se deben a una estenosis carotídea. La ultrasonografía es la técnica más habitual para el diagnóstico de la estenosis carotídea. Las alteraciones de la velocidad de flujo en el punto de máxima estenosis junto con los cambios hemodinámicos en regiones proximales (arteria carótida común) y distales (carótida interna postestenótica, arteria oftálmica y polígono de Willis) permiten cuantificar con precisión la estenosis carotídea. En esta revisión se detalla la metodología para la evaluación de la estenosis carotídea desde el punto de vista ultrasonográfico, siguiendo las recomendaciones de consenso establecidas por la Sociedad Española de Neurosonología (SONES). Se revisan los hallazgos que permiten cuantificar el grado de estenosis carotídea extracraneal utilizando tanto el estudio ultrasonográfico carotídeo como el transcraneal, con un especial énfasis en la importancia de la valoración de signos indirectos.
It is estimated that 28% of ischaemic strokes occurring in Spain are atherothrombotic in origin, and that most are caused by carotid stenosis.1 TIA precedes a disabling infarct in up to 43% of patients with a stroke of atherothrombotic origin.1 With this in mind, identifying and treating arterial stenosis correctly is a top priority. The risk of recurrence is particularly high in strokes with an atherothrombotic mechanism.2 The degree of stenosis of the internal carotid artery (ICA) is the most important indicator of risk of stroke, and the basis on which doctors decide if endarterectomy is needed.3–9
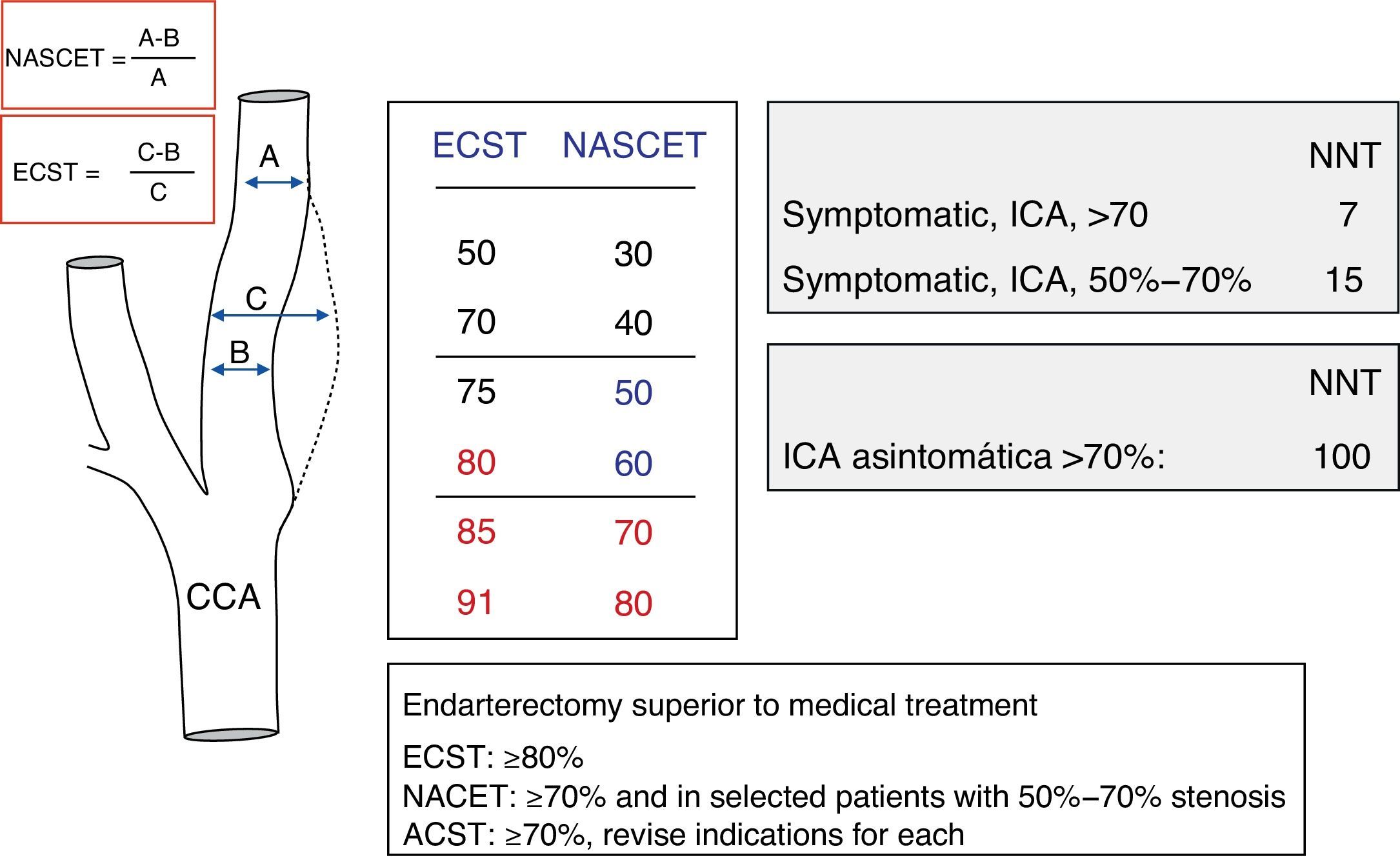
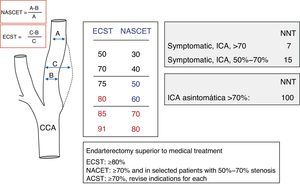
The NASCET3,4 and ECST5 studies showed that under certain conditions, carotid endarterectomy effectively prevented stroke recurrence in patients with symptomatic stenosis ≥70% who had experienced TIA or mild cerebrovascular event. The procedure was assigned a recommendation level of A since it decreased overall risk of new cerebrovascular events by about 50% compared to a group receiving medical treatment only.4,5 In cases of stenosis ≥70%, 24.4% of patients receiving medical treatment experienced recurrence during a 2-year follow up period. The recurrence rate was only 7.2% in patients who also underwent surgical treatment. Risk levels were higher for stenosis exceeding 90% (35% of all vascular events/year) than for stenosis in the 70% to 79% range or stenosis >99% (yearly risk of 11%).4 Although surgery may be beneficial for patients with symptomatic stenosis of 50% to 70% or asymptomatic stenosis, surgical treatment – whether endarterectomy or angioplasty with/without stenting – must be considered on a case-by-case basis. Recall that benefits are less marked in cases of moderate stenosis (50%–70%) and marginal in cases of asymptomatic stenosis.
Vascular imaging techniques used to diagnose carotid stenosis have advanced considerably in recent years, especially ultrasound imaging.10 Although different expert consensus groups have attempted to set down criteria for diagnosing and quantifying carotid stenosis using ultrasound,11–13 they do not agree on which haemodynamic parameters should be used. Likewise, the usefulness of performing transcranial Doppler or duplex sonography to assess distal effects of carotid stenosis is unclear.14 This review explains sonography methods used in evaluating carotid stenosis and follows the consensus recommendations issued by the Spanish Society of Neurosonology (SONES).
Measuring carotid stenosis using sonographyIn the NASCET3,4 and ECST5 studies, in which carotid endarterectomy is indicated for symptomatic patients, carotid stenosis is quantified using conventional angiography. Measurement methods differ, but they are comparable, and the results they produce may be considered complementary. For purposes of providing a haemodynamic assessment of carotid stenosis in this review, ultrasound results will be used and normalised according to the degree of stenosis measured in the angiography by following the method described in the NASCET study (Fig. 1).
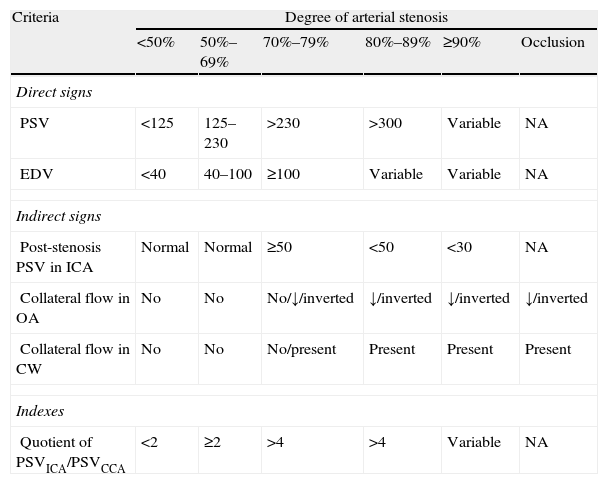
Parameters to be analysed in a carotid stenosis studyThe most frequently used parameters for measuring degree of stenosis are peak systolic velocity (PSV) and end-diastolic velocity (EDV). Changes in flow velocity at the point with the greatest arterial stenosis, known as direct signs, are most commonly used to measure the degree of stenosis. Indirect signs are the haemodynamic changes resulting from carotid stenosis that are observed in the common carotid artery (CCA), the post-stenotic extracranial segment of the ICA, or intracranial circulation. These changes indicate that haemodynamically significant stenosis or occlusion is present. Systolic and diastolic indexes, a series of indexes including both direct and indirect signs, are typically used to evaluate special situations. The parameters most frequently employed to assess carotid stenosis, according to validation studies published in the literature,15–19 are shown in Table 1.
Haemodynamic criteria for establishing the degree of carotid stenosis.
| Criteria | Degree of arterial stenosis | |||||
| <50% | 50%–69% | 70%–79% | 80%–89% | ≥90% | Occlusion | |
| Direct signs | ||||||
| PSV | <125 | 125–230 | >230 | >300 | Variable | NA |
| EDV | <40 | 40–100 | ≥100 | Variable | Variable | NA |
| Indirect signs | ||||||
| Post-stenosis PSV in ICA | Normal | Normal | ≥50 | <50 | <30 | NA |
| Collateral flow in OA | No | No | No/↓/inverted | ↓/inverted | ↓/inverted | ↓/inverted |
| Collateral flow in CW | No | No | No/present | Present | Present | Present |
| Indexes | ||||||
| Quotient of PSVICA/PSVCCA | <2 | ≥2 | >4 | >4 | Variable | NA |
CCA: common carotid artery; ICA: internal carotid artery; OA: ophthalmic artery; NA: not applicable; CW: circle of Willis; EDV: end-diastolic velocity; PSV: peak systolic velocity.
Arterial stenosis should preferably be assessed using colour duplex sonography to capture images of the arterial wall in longitudinal and transverse planes. Longitudinal images may be difficult to capture in some patients, and in such cases it may be useful to obtain a coronal projection of the artery by placing the transducer behind the sternocleidomastoid. Viewing the arterial wall is a means of measuring carotid intima-media thickness and determining if and where atheromatous plaque may be present.
Once it has been located, arterial stenosis is measured based on haemodynamic parameters (direct and indirect signs), and not according to the decrease in diameter or the area of the arterial lumen on a B-mode ultrasound. Measuring diameters or areas visible on ultrasound images taken with any mode is not an accepted method of calculating stenosis. Its sensitivity and specificity are considerably lower than those of digital subtraction angiography analysis of anatomical pathology specimens. As will be described shortly, colour duplex sonography (power Doppler) may help in specific cases of pre-occlusive stenosis.
When measuring PSV and EDV, the insonation angle should be as nearly parallel as possible to the direction of the blood flow. If necessary, the angle can be modified to obtain a peak velocity reading at the insonation point, but the angle should not be corrected by more than 60°. The entire stenotic area must be examined to pinpoint the location of the peak velocity corresponding to the area with the greatest stenosis.
Doctors must determine direct signs, i.e. PSV and EDV, at the point of maximum stenosis. PSV and EDV should always be measured for the CCA. Patients with suspected stenosis ≥50% should always undergo a blood flow assessment for the post-stenotic extracranial segment of the ICA, ophthalmic artery, and intracranial arteries.
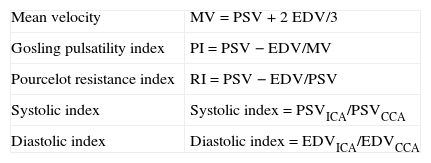
The systolic and diastolic indexes (Table 2) are of particular interest in patients presenting carotid stenosis in addition to stenosis or occlusion of the contralateral carotid artery; those with tandem lesions; and those with a hyperdynamic or hypodynamic circulatory state (for example, fever, anaemia, hypothyroidism, hyperthyroidism, bradycardia, etc.). In such situations, estimates of stenosis based on PSV and EDV alone may be erroneous.
Calculation of haemodynamic indexes most frequently used to measure carotid stenosis.
| Mean velocity | MV=PSV+2 EDV/3 |
| Gosling pulsatility index | PI=PSV−EDV/MV |
| Pourcelot resistance index | RI=PSV−EDV/PSV |
| Systolic index | Systolic index=PSVICA/PSVCCA |
| Diastolic index | Diastolic index=EDVICA/EDVCCA |
CCA: common carotid artery; ICA: internal carotid artery; PI: pulsatility index; RI: resistance index; MV: mean velocity; EDV: end-diastolic velocity; PSV: peak systolic velocity.
We recommend measuring stenosis using intervals rather than absolute values. Recommended intervals are as follows: <50%, 50%–69%, ≥70% (can be subdivided as 70%–79%, 80%–90%, and ≥90%) and occlusion.
Haemodynamic criteria for quantifying carotid stenosisThere is still debate over which haemodynamic parameter is the best for evaluating the degree of stenosis, but the most commonly used and reliable is probably PSV (Table 1). Nevertheless, in addition to elevated PSV, changes in flow in the post-stenotic extracranial segment of the ICA, the ophthalmic artery, and intracranial arteries also indicate stenosis ≥70%. Where blood flow is extremely reduced or absent (for example, occlusion caused by hypoechogenic intraluminal material, as occurs in arterial dissections or distal occlusions caused by embolic material), the carotid study may appear to be normal. Nevertheless, transcranial Doppler duplex imaging will reveal the presence of a significant degree of carotid stenosis.
We will now describe findings broken down by the degree of arterial stenosis.
Internal carotid stenosis of less than 50%Carotid stenosis of less than 50% has no haemodynamic repercussions, and therefore flow velocity measurements are normal in these cases.
Direct and indirect findings in the carotid arterySmall plaques may be apparent, but PSV is less than 125cm/s and EDV is less than 40cm/s for all the examined segments. The systolic index (PSV ICA/PSV CCA) in the internal carotid and common carotid arteries is less than 2. Plaques located in the carotid sinus and impeding normal dilation of the arterial lumen may cause the turbulent pattern typical at this level to be replaced by a laminar flow pattern with velocity in normal ranges.
Indirect findings in the transcranial Doppler/duplex studyStenosis <50% does not result in a decrease in cerebral flow. Studies of ophthalmic arteries and the transcranial Doppler/duplex study will therefore yield normal results.
Internal carotid artery stenosis of 50% to 69%Carotid stenosis exceeding 50% will begin to show haemodynamic repercussions. Flow velocity will increase at the point of maximum stenosis, and so will the risk of plaque rupture (vulnerable plaque, with a risk of distal embolisation or thrombosis and occlusion in situ).
Direct and indirect findings in the carotid arteryCarotid stenosis levels of 50% to 69% are associated with a discrete increase in flow velocity with a PSV≥cm/s (125–230cm/s) and an EDV of 40% to 100cm/s. Haemodynamic alterations are not typically observed in either the common carotid artery or the post-stenotic segment of the extracranial ICA when stenosis is between 50% and 69%. The systolic index (PSV ICA/PSV CCA) in the internal carotid and common carotid arteries is ≥2 (2–4). Some patients with degrees of stenosis approaching 69% may present a PSV somewhat higher than 230cm/s, even when indirect local and intracranial haemodynamic changes are absent.
Indirect findings in the transcranial Doppler/duplex studyGiven that stenosis in the 50%–69% range does not provoke a decrease in cerebral blood flow, transcranial Doppler or duplex ultrasound findings will be normal or nearly so, with symmetrical middle cerebral arteries (MCAs) and orthodromic anterior cerebral arteries (ACAs). Changes in blood flow in ophthalmic arteries are not typically observed.
Internal carotid artery stenosis of 70% to 79%The most clinically relevant cut-off point for stenosis is 70%, since this is usually the threshold at which revascularisation treatment is necessary. Measuring direct signs alone is not a reliable method of establishing that stenosis has reached the 70% cut-off point; evaluating indirect signs is also an important part of this process.13,20
Direct and indirect findings in the carotidWhile every laboratory must validate its own normal values, a PSV >230cm/s tends to correspond with stenosis>70%. The acoustic window of the Doppler wave form disappears or decreases and the end-diastolic velocity increases (≥100cm/s) to maintain a constant total cerebral blood flow. Indirect signs may include a decrease in flow velocity and a discrete increase in the pulsatility index (PI) in the ipsilateral CCA. It is also possible to detect changes in the post-stenotic segment of the ICA with blood flow velocities ≥50cm/s. The systolic index (PSV ICA/PSV CCA) in the internal carotid and common carotid arteries tends to be >4.
Indirect findings in the transcranial Doppler/duplex studyA 70% degree of stenosis is still associated with normal cerebral blood flow. Blood flow remains normal due to the increase in flow velocity in both systole and diastole, and transcranial ultrasonography studies should therefore yield normal results. The haemodynamic finding that can typically be detected in intracranial arteries is a discrete decrease in PI of the ipsilateral MCA compared to the normal MCA. This is due to the decrease in cerebral perfusion caused by ICA stenosis. Blood flow in the ophthalmic artery is usually normal. To summarise, a case of 70% stenosis (Table 1) may present changes in the distal haemodynamic pattern with null or inverted velocity in the ipsilateral ophthalmic artery and presence of collateral flow through the anterior or posterior communicating arteries (ACA or PCA) with stenosis ≥80%. This will be described in detail in a later section.
Severe internal carotid artery stenosis (80% to 90%)Direct and indirect findings in the carotidThe most relevant direct finding is aPSV of more than 300cm/s. In contrast, EDV is variable, and tends to decrease as the degree of stenosis increases. Indirect signs detectable in the ipsilateral CCA include reduction in both PSV and EDV, especially in the latter (CCA externalisation), which results in increased PI. Post-stenotic ICA flow disorders are also present, with PSV levels <50cm/s. On some occasions, due to the presence of major turbulence in the post-stenotic segment, a ragged waveform and a characteristic sound (shot noise) are detected. Images taken with colour Doppler mode showed the ‘confetti phenomenon’.
The systolic index (PSV ICA/PSV CCA) in the internal carotid and common carotid arteries is >4. Another parameter that can be used is the resistance index (RI) of the internal and common carotid arteries (RI ipsilateral CCA/RI contralateral CCA) where RI>0.15.
If the study of direct haemodynamic parameters is inconclusive, a diagnosis of stenosis ≥80% can be accepted based exclusively on indirect signs if at least 2 of the following criteria can be confirmed: IR>0.15, blood flow inversion in the ipsilateral ophthalmic artery, or inversion in the A1 segment of the ipsilateral ACA.21
Findings in the transcranial Doppler/duplex studyStenosis exceeding 80% is associated with a drop in cerebral perfusion pressure and overall ipsilateral cerebral blood flow. The MCA ipsilateral to stenosis will be dampened with a decrease in PI and a slow systolic rise (‘tent pattern’). Mean velocity and total cerebral blood flow in the MCA may or may not be normal, depending on the quality of collateral circulation through the ACA and PCA.
Blood flow in the ipsilateral ACA will be inverted, while flow in the contralateral ACA will be orthodromic and accelerated, typically with a normal or low PI (collateral circulation pattern with low resistance, similar to those in arteriovenous malformation). This is produced by collateral circulation through both the ACA and long circumferential arteries. Depending on the resistance in the ACA (which in turn results from its diameter and length, if the artery is not hypoplastic or absent), compensation will be more or less effective. A bruit can usually be detected at the level of the ACA, and it will be more pronounced in cases of greater insufficiency. The presence of marked arterial bruit, normally presenting in the form of low-frequency systolic turbulence and sometimes associated with a high-pitched diastolic murmur in medium frequencies, demonstrates poor collateral circulation. This reflects the ACA's inability to pass the flow volume required by the ipsilateral hemisphere through the carotid stenosis.
The PCA will also irrigate the hemisphere ipsilateral to the lesion. A functioning PCA will be associated with asymmetry at the level of right-left P1 due to increased flow in the P1 segment ipsilateral to stenosis. Finding increased flow in the ipsilateral P2 segment points to the presence of collateral circulation through the long circumferential arteries. As in the case of the ACA described above, a harsh or high-pitched murmur at the level of the ipsilateral PCA reflects PCA insufficiency and failure of collateral circulation in general. Findings from the ophthalmic artery will vary greatly, from reduced to null to inverted flow; this depends on the functioning of the ACA and PCA.
The contralateral MCA tends to present an increased flow pattern; collateral flow may also be observed in this case in the long circumferential arteries.
Internal carotid artery stenosis>90%Direct and indirect findings in the carotidIncreases in velocity in carotid stenosis are proportional to the degree of stenosis as long as stenosis remains below about 90%. Above that threshold for extremely pronounced carotid stenosis, resistance to blood flow is so high that PCV decreases at the point of stenosis and reaches a velocity of 0 in cases of arterial occlusion. It is obvious that in cases of pre-occlusive stenosis, changes in PCV have a lower diagnostic value and diagnosis will be based essentially on indirect signs. Diagnosis by means of indirect signs is usually a straightforward process, considering the major haemodynamic repercussions of the stenosis. Cerebral brain flow decreases distal to stenosis and resistance increases proximal to the point of stenosis.
The Doppler waveform will be ragged at the point of stenosis, with loss of acoustic window due to disappearance of the normal laminar flow.
A clear increase in PI in the common carotid artery is often found proximal to the stenosis. The PI quotient between the two common carotid arteries will be asymmetrical, and decreased flow volume in the ipsilateral common carotid is usually present. The segment of the ICA distal to stenosis will show a dampened pattern with PCV<30cm/s.
Colour mode is particularly useful in cases of pre-occlusive stenosis, which should be studied using longitudinal and axial slices and both flow-velocity mode and power Doppler imaging. We recommend using the lowest possible pulse repetition frequency since the purpose is to identify slow-moving flows that allow us to confirm ICA permeability. Power Doppler mode is especially useful in such cases; it is able to detect slow blood flow and it is more sensitive than colour mode. Power Doppler mode generates a signal according to the amplitude of the ultrasonic wave (as this is related to red blood cell mass, it has little to do with flow velocity). In contrast, standard colour mode bases the signal obtained on the frequency of the ultrasonic wave, and this frequency is very low when velocity approaches 0 or when flow inversion is present. It is used to detect any residual flow to determine if surgical treatment should be indicated. This will prevent false-positive diagnosis of carotid occlusion.22 Distinguishing between stenosis and occlusion is sometimes difficult, but extremely important from a clinical perspective.23
Indirect findings in the transcranial Doppler/duplex studyThe pattern is similar to that described for degrees of stenosis>80%.
Internal carotid artery occlusionThe comments and haemodynamic patterns given for stenosis>90% also apply to carotid occlusion. As stated above, indirect signs (which are very obvious in carotid occlusion) must be carefully evaluated so as not to erroneously interpret the Doppler/duplex study of supra-aortic trunks as normal.
A characteristic biphasic, short, and low-velocity pattern can be found immediately proximal to the point of occlusion on spectral Doppler or colour-mode sonography (colour image shows orthodromic and antidromic flows, red–blue, proximal to the occlusion). These findings are helpful although not very specific. In acute occlusions, which may be atherothrombotic or due to more infrequent causes (cardiac embolus impaction in proximal ICA, arterial dissection), B-mode images may be anechoic and the lumen may appear to be permeable. However, colour-mode or Doppler sonography will detect lack of flow. Hyperintense images at the carotid level, which suggest ICA occlusion in B-mode sonography, typically reflect chronic occlusions.
The challenge presented by a carotid occlusion is avoiding a false-positive diagnosis, which can occur with pre-occlusive stenosis (see preceding section: stenosis>90%). Contrast-enhanced ultrasound is to be considered in these cases. When carotid occlusion is diagnosed by ultrasonography, doctors should always consider using an additional non-invasive neuroimaging technique, such as contrast-enhanced MR angiography or CT angiography.
Internal carotid stenosis following endarterectomy or affecting a stentAbout 10% of patients whose carotid stenosis has been treated with endarterectomy or angioplasty/stenting will experience restenosis.24 Placement of a stent or arterial patch after endarterectomy will change carotid artery biomechanics. The degree of restenosis may be overestimated if it is calculated using the velocity criteria accepted for measuring untreated carotid stenosis. Several published studies confirm the validity of sonography studies for monitoring and diagnosing restenosis in carotid arteries treated with endarterectomy and angioplasty/stenting. They also validate velocity parameters in this disease.25,26 Commonly used velocity criteria will overestimate the degree of stenosis in treated patients. A good correlation may be achieved by applying a correction factor (increase velocity by about 20% to diagnose the same degree of stenosis). As a result, PCV for diagnosing carotid stenosis treated with endarterectomy or angioplasty/stenting will be between 200 and 300cm/s for stenosis of between 50% and 70%. In any case, regardless of the velocity reading, periodic sonography studies are recommended in order to assess the relevance of any progressive increases in velocity, and the magnitude of changes from post-intervention baseline values.
Common problems in interpreting and diagnosing carotid stenosisThe most common problem is derived from the extensive calcified plaques in the carotid sinus that create acoustic shadows. This prevents proper insonation of the point of greatest stenosis, meaning that the procedure will not deliver a quality image in colour B mode or detect flow using spectral Doppler. The problem is relevant in 2 situations: when carotid occlusion is suspected, and in symptomatic patients with significant degrees of stenosis ranging from 50% to 70%. In these cases, doctors should base diagnosis on both proximal and distal indirect signs, and especially on the collateral circulation findings and haemodynamic patterns in the transcranial Doppler/duplex study. The presence of indirect signs will allow us to confirm stenosis ≥70%, but we will not be able to determine whether or not an occlusion is present (which would rule out revascularisation). Normal results from a transcranial study of the ophthalmic artery and proximal haemodynamic patterns will rule out the presence of stenosis 70–80%. Nevertheless, as mentioned in preceding sections, this cannot rule out the possibility of stenosis in the 50–70% range. In an asymptomatic patient, this finding may not be important, since surgical treatment is unlikely to be performed if transcranial duplex study results are normal. This is not the case for symptomatic patients, who will require additional non-invasive tests. Taking axial slices from the CCA to the distal ICA often allows doctors to detect and measure any flow that may be present, even in pre-occlusive stenosis. The situation and approach are similar in patients with ‘hostile neck’ (anatomical features, high carotid bifurcations, scars, neck treated with radiation therapy, or postsurgical area just after endarterectomy).
Factors that can contribute to underestimating the degree of stenosis include advanced age, severe arterial rigidity with low compliance, and tandem lesions. Proximal CCA stenosis associated with ipsilateral ICA stenosis causes a drop in pressure and flow volume, which in turn elicits a decrease in PSV, EDV, and PI. Obtaining additional data, such as the haemodynamic pattern distal to the point of stenosis in the CCA (dampened) and the intracranial pattern (collateral circulation), will facilitate a correct diagnosis.
Factors that can contribute to overestimating stenosis include age (young patients) and hyperdynamic states that can elicit an increase in flow volume (low haematocrit, fistulas, or intracranial arteriovenous malformations). A very common situation involves single trunk disease. In such cases, patients undergo procedures to adjust the degree of stenosis in one carotid artery when the contralateral artery is occluded. As in the case described previously, a correct diagnosis can be delivered by using indexes, comparing right and left territories and the ipsilateral CCA, ICA, and vertebral arteries, and examining the transcranial duplex pattern.
ConclusionCollecting a single direct sonography parameter, such as PSV or EDV, is not sufficient to deliver a reliable diagnosis of the degree of carotid stenosis. Velocity overlap is a very important factor in the published studies and meta-analyses. Positive and negative predictive values for a specific degree of stenosis are between 85% and 95% when parameters are used alone (PSV, EDV, or indexes). Diagnosis should be based on both direct and direct signs, as recommended by the current consensus statement for every specific degree of stenosis.
The sonography study must be assessed completely. The evaluation is based on the spectral Doppler findings, with PSV as the main diagnostic parameter, but doctors should also consider other parameters such as the EDV or the systolic index (Tables 1 and 2). The process will also involve comparing the affected and contralateral territories and using information obtained from the transcranial Doppler or duplex study. Transcranial examination should always be performed for stenosis ≥50%. The study provides highly useful information when normal results are confirmed, and this is especially relevant for avoiding false negatives in supra-aortic trunk studies. Where blood flow is substantially reduced (pseudo-occlusive stenosis) or lacking (occlusion), results from the carotid study may appear normal. A transcranial duplex or Doppler scan will then let us determine whether the patient has major stenosis or an occlusion.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Serena J, Irimia P, Calleja S, Blanco M, Vivancos J, Ayo-Martín Ó, et al. Cuantificación ultrasonográfica de la estenosis carotídea: recomendaciones de la Sociedad Española de Neurosonología. Neurología. 2013;28:435–442.