Convexity subarachnoid haemorrhage (cSAH) is a rare type of spontaneous, non-traumatic, and nonaneurysmal SAH characterised by blood collections in one or more cortical sulci in the convexity of the brain; the aetiology varies. We report a clinical case series of 3 patients with cSAH associated with probable cerebral amyloid angiopathy (CAA) who presented with focal sensory seizures and responded well to corticosteroid treatment.
PatientsCase 1 was a 67-year-old man reporting right-sided paroxysmal sensory episodes with Jacksonian progression, cheiro-oral symptoms, and motor dysphasia. Case 2 was a 79-year-old man reporting left-sided paroxysmal episodes with cheiro-oral signs and dysarthria. Case 3 was a 71-year-old woman also reporting recurrent left cheiro-oral signs and dysarthria. None of the patients had headache or clinical dementia. Aneurysms were ruled out using MR angiography.
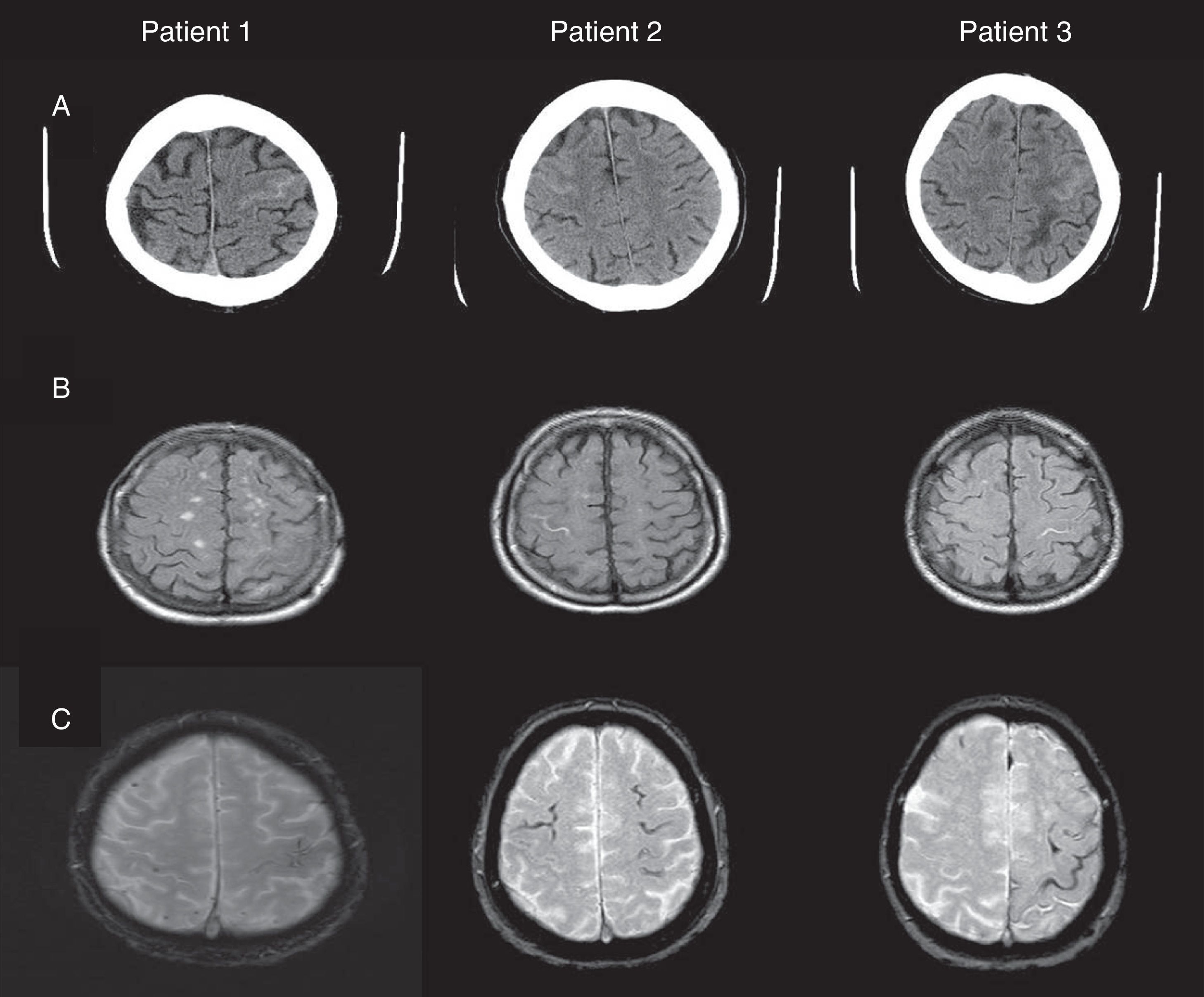
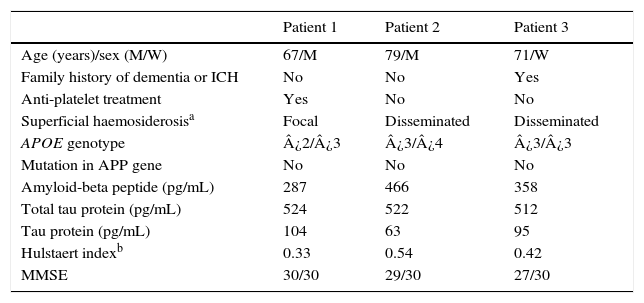
ResultsBrain CT scan detected an isolated hyperintensity in a sulcus of the frontal convexity; brain gradient echo T2-weighted MRI sequences showed meningeal haemosiderosis and microbleeds. However, no atrophy was identified in medial temporal lobes including the hippocampal formation. All patients had low levels of beta-amyloid in CSF, low values on the Hulstaert index and high levels of phosphorylated tau protein. Patients were initially treated with prednisone and levetiracetam, but symptoms recurred in 2 patients after prednisone was discontinued.
ConclusionsWe present a series of 3 patients with cSAH associated with CAA, characterised by a stereotypical syndrome responding well to corticoid treatment; there were no cases of headache or clinical dementia.
La hemorragia subaracnoidea de la convexidad cerebral (HSAc) consiste en la presencia de un sangrado espontáneo, no aneurismático ni traumático, localizado en los surcos de la convexidad cerebral, cuya etiología es muy variada. Presentamos una serie de 3 casos de HSAc con probable angiopatía amiloidea cerebral (AAC) con clínica sensitiva recurrente que respondió al tratamiento con corticoides.
PacientesCaso 1: varón de 67 años que presenta episodios paroxísticos sensitivos en el hemicuerpo derecho con progresión jacksoniana, episodios sensitivos queiroorales con disfasia motora. Caso 2: varón de 79 años, con trastorno paroxístico sensitivo-motor queirooral izquierdo y disartria. Caso 3: mujer de 71 años, con trastorno paroxístico sensitivo-motor queirooral izquierdo y disartria. Ningún paciente tuvo cefalea ni deterioro cognitivo. Se descartó la presencia de malformaciones aneurismáticas con una angio-RM cerebral.
ResultadosLa tomografía computarizada craneal mostró una hiperdensidad aislada en un surco de la convexidad frontal y la RM encefálica en la secuencia de eco-gradiente mostró depósitos de hemosiderina en dichos surcos y lesiones sugestivas de microsangrados. La RM no mostró atrofia de hipocampos ni temporal medial. En el líquido cefalorraquídeo todos los pacientes tuvieron un descenso del péptido beta-amiloide, valores bajos del índice de Hulstaert y aumento de la proteína tau fosforilada. Todos los pacientes se trataron inicialmente con prednisona y levetiracetam pero los síntomas recurrieron en dos pacientes tras la suspensión de la prednisona.
ConclusionesPresentamos a 3 pacientes con HSAc asociada a AAC, caracterizados por una clínica estereotipada, con ausencia de cefalea y de demencia clínica, con buena respuesta al tratamiento corticoideo.
Cerebral subarachnoid haemorrhage (cSAH) refers to subarachnoid bleeding in a sulcus (or several adjacent sulci) of the convexity of the brain that does not extend to the interhemispheric fissures, basal cisterns, or ventricles.1–5 Different authors have stated that age at presentation of haemorrhage is related to the multiple aetiologies of cSAH: cerebral vasoconstriction syndromes are more frequent among patients younger than 60 whereas cerebral amyloid angiopathy (CAA) is the main cause of bleeding in patients aged 60 and older.1–5 The clinical presentation of cSAH is also linked to aetiology: patients with cerebral vasospasm usually experience thunderclap headache whereas those with CAA more frequently experience transient episodes of TIA-like or aura-like focal neurological dysfunction.1–6
We present a series of 3 patients with cSAH associated with probable CAA and assess the potential role of the amyloid-beta and tau protein pathways in the pathogenesis of the disease using CSF tests. Our patients displayed typical neurological symptoms, which initially responded very well to corticosteroids. However, final outcomes were poor due to their episodes of recurrent intracranial bleeding.
PatientsCase 1: 67-year-old man with a history of hypercholesterolaemia, diagnosed with TIA in 2005 and treated with an antiplatelet drug (clopidogrel). He was seen by the neurology department due to multiple episodes of neurological dysfunction: a tingling sensation in the mouth area affecting the right side of the tongue, as well as in the fingertips of the right hand and spreading towards the forearm or shoulder. Tingling was accompanied by episodes of speech impairment lasting 10 to 15minutes and presenting up to 8 times per day. On some occasions, sensory symptoms extended over the right side of his ribcage and down to his right thigh. A neurological examination revealed no headache, no alterations in the level of consciousness, and no meningeal signs. A cranial CT scan disclosed a hyperdense area in a sulcus of the left frontal lobe; a brain gradient-echo MRI sequence revealed superficial haemosiderosis. Diffusion MRI ruled out acute ischaemia and MRI angiography ruled out aneurysms. No epileptiform activity was found on EEG. We started treatment with intravenous levetiracetam and dexamethasone; paroxysmal events resolved between 24 and 48hours. Discontinuing dexamethasone led to recurrence of the events. Symptoms remained despite treatment with oxcarbazepine and zonisamide and were only controlled when prednisone was added, after which we were able to withdraw antiepileptic drugs. At 4 months of follow-up, our patient developed sensory symptoms in the left side of his face and his left forearm; these symptoms were linked to a new cSAH located in a sulcus of the right frontal lobe.
Case 2: a 79-year-old man with a history of dyslipidaemia. He had experienced symptoms suggestive of TIA (dysarthria and sensory alterations in the right limbs) in 2013 but received no antiplatelet treatment during his hospital stay. In June 2014, he visited our hospital due to sudden episodes of paraesthesia in the left hand and subsequently on the left side of the tongue, with difficulty articulating words; episodes lasted 2 to 3minutes and occurred 3 times per day. Our patient had no headache or nuchal rigidity. A cranial CT scan revealed a hyperdense area in a right frontal sulcus suggestive of SAH; a gradient-echo MRI sequence revealed haemosiderosis in frontal sulci bilaterally. Diffusion MRI and MRI angiography ruled out acute ischaemia and aneurysms, respectively. Transient clinical symptoms resolved with levetiracetam and dexamethasone. However, paroxysmal sensory disturbances reappeared 48hours after corticosteroids were discontinued despite treatment with levetiracetam. As in the previous case, symptoms abated with oral prednisone.
Case 3: a 71-year-old woman with a family history of neuropsychiatric disorders (her sister had dementia, her brother had died of a cerebral haemorrhage, and her father had an unspecified psychiatric disorder). She was examined due to a 2-week history of dysarthria, difficulty holding objects, and recurrent episodes of paraesthesia on the right side of her face and her right hand and forearm. She experienced no headache and displayed no meningeal signs or alterations in level of consciousness. A cranial CT scan displayed a focal hyperdense area with marked leukoaraiosis in a frontal sulcus of the left hemisphere; a gradient-echo MRI sequence revealed haemosiderosis in sulci of the left parietal lobe and both frontal lobes. Diffusion-weighted MRI sequences ruled out acute small-vessel ischaemia and MRI angiography ruled out aneurysms. As in the previous cases, symptoms responded to levetiracetam and dexamethasone. Our patient received prednisone dosed at 30mg/day for 2 months on an outpatient basis. A brain MRI scan conducted after treatment revealed no changes in the white matter lesions. Six months later, our patient was admitted due to a left frontotemporal haemorrhage. She experienced a right parietal haemorrhage during hospitalisation which resulted in death.
Fig. 1 shows our patients’ cranial CT images and FLAIR and gradient-echo MRI sequences (T2*). Table 1 summarises the main findings from complementary tests: CSF, apolipoprotein E (APOE) genotype, mutations in the gene coding for amyloid precursor protein (APP), and cognitive impairment (Mini-Mental State Examination scores).
(A) Cranial CT: hyperdense areas in a sulcus of the frontal convexity corresponding to subarachnoid bleeding (left frontal lobe in patients 1 and 3, and right frontal lobe in patient 2). (B) FLAIR MRI: intrasulcal hyperintensity in the area with subarachnoid bleeding as displayed by CT imaging. (C) Gradient-echo MRI revealing microbleeds and superficial haemosiderosis.
Clinical variables and analysis results.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age (years)/sex (M/W) | 67/M | 79/M | 71/W |
| Family history of dementia or ICH | No | No | Yes |
| Anti-platelet treatment | Yes | No | No |
| Superficial haemosiderosisa | Focal | Disseminated | Disseminated |
| APOE genotype | ¿2/¿3 | ¿3/¿4 | ¿3/¿3 |
| Mutation in APP gene | No | No | No |
| Amyloid-beta peptide (pg/mL) | 287 | 466 | 358 |
| Total tau protein (pg/mL) | 524 | 522 | 512 |
| Tau protein (pg/mL) | 104 | 63 | 95 |
| Hulstaert indexb | 0.33 | 0.54 | 0.42 |
| MMSE | 30/30 | 29/30 | 27/30 |
Cerebral subarachnoid haemorrhage is a rare type of intracranial haemorrhage. However, it has important prognostic implications: although cSAH had initially been thought to have a better prognosis than aneurysmal SAH,1 some recent studies do not confirm this hypothesis.3,4 One of our patients experienced recurrent cSAH in the contralateral frontal lobe (associated with sensory alterations in the contralateral side of the body), while another presented 2 additional foci of intraparenchymal haemorrhage that resulted in death. Likewise, the fact that ischaemic stroke is more prevalent among patients with CAA provides further evidence that cSAH is an indicator of poor long-term prognosis.4
It seems clear that the different aetiologies of cSAH depend on age. These haemorrhages may be due to multiple causes in patients younger than 60, but the most common are reversible cerebral vasoconstriction syndrome2 and bilateral carotid artery stenosis.7 In contrast, the most frequent aetiology in patients older than 60 is CAA.2–4 From a clinical viewpoint, headache (thunderclap headache) may guide the aetiological diagnosis of cSAH: presence of headache is extremely frequent in patients with cerebral vasospasm whereas patients with CAA do not exhibit this symptom.5 CAA-related cSAH is a rare form of cerebrovascular disease; in fact, this aetiology was not listed by one extensive registry of acute cerebrovascular diseases present in Spain.8
Our patients displayed typical clinical symptoms, which were sudden, predominantly sensory, and affecting the mouth area. Their symptoms initially pointed to recurrent TIA, migraine aura, or focal seizures. One of the patients exhibited left-sided sensory disturbances which displayed a Jacksonian march pattern. Differential diagnosis must include pure sensory syndrome secondary to a small-vessel ischaemic vascular lesion; none of our 3 patients showed diffusion restriction indicating lacunar lesions in the internal capsule or thalamus on diffusion-weighted MRI sequences.9 Several authors have attributed presence of transient focal neurological deficits to a mechanism of cortical spreading depression, as occurs in migraine aura.2,4,6 One of our patients underwent an EEG, which detected no epileptiform activity, as other authors have reported.2,3 The patients were initially treated with levetiracetam and dexamethasone, which achieved symptom resolution; however, focal symptoms reappeared in 2 of them after corticosteroids were discontinued and resolved once more when the corticosteroids were resumed. These findings point to an irritative-inflammatory process originating in the area of the cerebral cortex in contact with the subarachnoid bleed rather than to a TIA or focal epileptic seizures. Likewise, recurrent focal episodes in patients with CAA may be linked to a high risk of intracranial haemorrhage. Clinicians must therefore suspect underlying CAA in patients with no cardiovascular risk factors displaying these symptoms and perform a gradient-echo MRI scan (T2*) to detect the superficial haemosiderosis and microbleeds typical of CAA. These measures will prevent unnecessary use of antiplatelet drugs, which may promote intracranial bleeding.6
Diagnosis of CAA-related intracranial haemorrhage is based on the Boston criteria,10 which show greater sensitivity in the presence of superficial haemosiderosis.11 Superficial haemosiderosis is found in 60.5% of the patients with CAA and microbleeds in 47.4%9; microbleeds have been associated with both risk and potential location of intraparenchymal haemorrhage.12 According to the modified Boston criteria, our patients may be classified as having probable SAH secondary to CAA. Furthermore, the study of biomarkers in CSF supports the diagnosis of probable CAA, since these patients frequently display lower levels of the amyloid-beta peptide.12,13 Although our patients showed decreased levels of amyloid-beta peptides and increased levels of tau protein, a biochemical profile similar to that in Alzheimer disease, none met clinical criteria for dementia at the time of the cSAH. However, given the specificity of these biomarkers for diagnosing Alzheimer disease, especially in association with APOE ¿4, we decided to monitor our patients to detect any incipient clinical symptoms of cognitive impairment.
Presence of ¿2 or ¿4 alleles of APOE entails an increased risk of lobar intracranial haemorrhages.14–16 Risk is greater for genotypes ¿4/¿4, ¿2/¿4, and ¿2/¿2; intermediate for ¿3/¿4 and ¿2/¿3, and low for ¿3/¿3.14 From a pathogenic viewpoint, ¿4 is associated with a dose-dependent increase in the amount of vascular amyloid, which results in increased amyloid-beta aggregation and impaired clearance of the peptide, whereas ¿2 is believed to promote damage to the vascular wall leading to haemorrhage.12 One of our patients, a carrier of the ¿2 allele, displayed recurrent cSAH. In contrast, the patient with genotype ¿3/¿3, who seemingly had a lower risk of bleeding, experienced additional lobar haemorrhages resulting in death. A recent study of anatomical pathology samples from patients with CAA with and without cerebral haemorrhage found no correlation between cerebral haemorrhage and small-vessel disease or microbleeds. Likewise, no statistically significant correlations were found between cerebral haemorrhage and presence of the ¿4 allele of APOE (which was found to have a protective effect). However, this study did find a significant association between disseminated superficial haemosiderosis and ¿2.16 Furthermore, anatomical pathology studies revealed senile plaques and neurofibrillary tangles in patients with CAA and no intracranial haemorrhage; ¿4 was expressed in 87% of these patients, which suggests that a different pathophysiological mechanism may be at work in this group. However, our patients displayed decreased levels of amyloid-beta and increased levels of tau protein, which suggests that both of these physiological pathways are involved in CAA pathogenesis in these cases.
The most frequent form of CAA is sporadic; familial forms of CAA, which are rarer, are associated with point mutations in the APP gene located on chromosome 21. However, no mutations in the APP gene have been linked to sporadic CAA; systematic genetic screening is therefore not recommended except for cases with a family history of intracranial haemorrhage or dementia.17,18 None of our patients had mutations in the APP gene, not even patient 3, whose family history included dementia and cerebral haemorrhage.
Our results cannot be extrapolated to the general population due to the small sample size. However, our findings suggest that CAA-related cSAH is not characterised by presence of headache or cognitive impairment in the form of dementia; rather, patients typically display episodes of paroxysmal sensory and/or motor alterations that respond to corticosteroid treatment. Long-term prognosis is poor due to the risk of additional cSAH or life-threatening intraparenchymal haemorrhages.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: García Estévez DA, García-Dorrego RM, Nieto-Baltar B, Marey-Garrido M, Hierro-Torner T. Hemorragia subaracnoidea espontánea de la convexidad cerebral: una serie clínica de 3 pacientes asociada con angiopatía amiloide cerebral. Neurología. 2017;32:213–218.