The measurement of fractional exhaled nitric oxide (FeNO) has gained widespread use in asthma clinical practice as an indicator of Type-2 airway inflammation.1,2 It is also utilized in epidemiological studies on environmental pollution and respiratory health,3–7 such as the ASTHMA-FENOP study, which examines the oxidative potential of particulate matter (PM) from air pollution and its association with airway inflammation.8 However, determining FeNO levels presents difficulties and limitations and different variables prior to the FeNO test have been associated with intrasubject variations in FeNO levels.9,10 On the other hand, epidemiological studies take into account lag time between airborne exposure and the outcome, with a variety of lag periods usually ranging from lag 0 to lag 3.11,12 In our ASTHMA-FENOP study,8 each volunteer was provided with a personal sampler to measure the oxidative potential of their inhaled PM upon arrival on the first day (visit 1), after signing the informed consent. Since FeNO is a noninvasive determination, for the first 41 asthmatic patients we protocoled three repeated FeNO measurements over three consecutive days, with the first FeNO measurement after 24h of wearing the personal sampler, at Visit 2 (lag 0); and the rest every 24h thereafter (lag 1, Visit 3; lag 2, Visit 4). As a specific objective, we aimed to determine the stability of FeNO levels in this group of asthmatic adults (25 women and 16 men) over these three consecutive days.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of Cantabria (CEIC) (internal code 2020.475), and the ethics committee of the University of Cantabria (CEPI) (internal code: 16.2021). All personal data were anonymized.
The overall mean age was 53.07±17.6 years in a range between 18 and 80 years. Patients were in stable condition, since the absence of exacerbations in the 4 weeks prior to recruitment was an inclusion criterion. The mean score in Asthma Control Test (ACT) was 22.12±3.86 points with 81% having their asthma controlled (≥20 points). Adherence to inhaled maintenance therapy according to Test of Adherence to Inhalers (TAI 10 items) was good in the majority of patients (76%). Most of the sample (57%) was in GINA stage 4 (medium dose maintenance of ICS-LABAs) and 19% were on GEMA stage 6 (with biologic treatment).
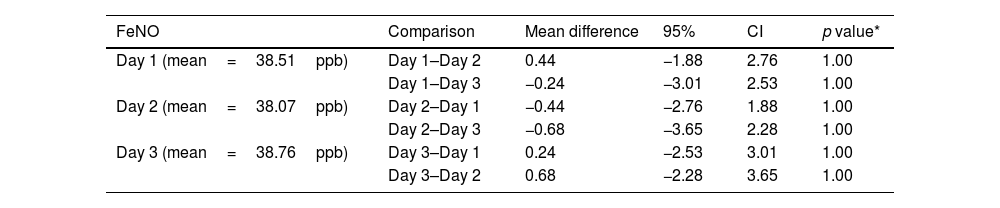
All single-breath, online measurements of FeNO were carried out using the same device (FeNO NIOX VERO® FeNO meter) and by the same person (JA) in the Pneumology Service of Hospital Universitario Marqués de Valdecilla (HUMV), according to the standardized procedures recommended by the American Thoracic Society, the European and the Spanish Respiratory Societies; and ensured that spirometry, physical exercise and the ingestion of certain foods or beverages were not allowed 1 hour prior to each of the determinations.9,10 FeNO mean levels at visit 2, 3 and 4 were 38.51±26.30, 38.07±28.0 and 38.76±26.33 parts per billion (ppb) respectively. 76.2% of volunteers had FeNO levels≥20ppb in at least one of their three consecutive FeNO determinations.
The results of the three determinations of FeNO levels were compared by studying their correlations and by using a repeated measures ANOVA, once the assumptions of sphericity (Mauchly's test of sphericity) was verified: X2(2)=3.093, p=0.213. The correlations between the three determinations were very high, with rho Spearman's coefficients>0.95. The repeated measures ANOVA results also support that FeNO levels were not affected by the day on which the determination was made, suggesting a high stability: F(2.0; 80)=0.205, p=0.815; Partial Eta squared=0.005 interpreted as <0.5% of the variability of the FeNO levels was due to the day it was made, with >99.5% of the results being similar. When comparing specifically pairs of days (day 1 with days 2 and 3; day 2 with days 1 and 3; day 3 with days 1 and 2) the results were even more similar (p=1.00 in all comparisons) with mean differences ranging from 0.244ppb to 0.683ppb (Table 1). Therefore, the mean differences for each pair of days comparison were less than 1ppb, without translational clinical implications. FeNO levels were similarly stable in the 8 asthmatic patients on biological treatment, and when we excluded these patients in a sensitivity analysis, results did not change either. Our results are supported by a recently published post hoc analysis from two studies in which FeNO levels (determined at weeks 2, 12 and 52) stayed stable throughout the 52 week study period for both children aged 6–11 years (VOYAGE study, n=135) and adolescents ≥12 years and adult patients (QUEST study, n=638), regardless of exacerbation events.13
FeNO mean differences for each of the three consecutive days.
| FeNO | Comparison | Mean difference | 95% | CI | p value* |
|---|---|---|---|---|---|
| Day 1 (mean=38.51ppb) | Day 1–Day 2 | 0.44 | −1.88 | 2.76 | 1.00 |
| Day 1–Day 3 | −0.24 | −3.01 | 2.53 | 1.00 | |
| Day 2 (mean=38.07ppb) | Day 2–Day 1 | −0.44 | −2.76 | 1.88 | 1.00 |
| Day 2–Day 3 | −0.68 | −3.65 | 2.28 | 1.00 | |
| Day 3 (mean=38.76ppb) | Day 3–Day 1 | 0.24 | −2.53 | 3.01 | 1.00 |
| Day 3–Day 2 | 0.68 | −2.28 | 3.65 | 1.00 |
What lag time to choose between exposure and the outcome is an interesting question in epidemiological studies, so prior to conducting our study, we reviewed the main systematic reviews and meta-analysis of time series studies on airborne exposure and asthma emergency room visits or hospital admissions12,14 as well as primary studies with personal samplers focusing on asthma with FeNO as outcome.6 No specific lag time clearly stands out, making it unclear whether today's high PM exposure causes emergency department visits or hospital admissions in asthmatic patients on the same day, the next day, or two or more days after high PM exposures. With regard to FeNO levels, despite our limited sample size, our results indicate a high stability, so we decided to simplify the protocol to a single FeNO determination (lag 1) for the remaining volunteers in our study.
As a conclusion, FENO levels were very stable, supporting that their repetition would not be necessary, at least within the same week, neither in clinical practice nor in epidemiological studies such as our ASTHMA-FENOP study. Further studies with a large sample size must corroborate our results.
FundingThis work was supported by the Spanish Society of Pneumology (SEPAR N° 1383/23; N° 1616/24) and the Spanish Ministry of Science and Innovation (Project PID2020-114787RBI00, funded by MCIN/AEI/10.13039/501100011033 and “ERDF A way of making Europe”).
Authors’ contributionsAll authors have contributed intellectually to the paper, meet the conditions of authorship, and have approved the final version.
Conflicts of interestThe authors declare not to have any conflicts of interest that may be considered to influence directly or indirectly the content of the manuscript.
We thank all the ASTHMA-FENOP study collaborators (the lists can be found below).
ASTHMA-FENOP study group: José Manuel Cifrian Martinez, Juan Agüero Calvo, Beatriz Abascal Bolado, Carlos Antonio Amado Diago, Juan José Ruiz Cubillán, Andrea Exposito Monar, Laura Ruiz Azcona, Esther Barreiro Portela, Adriana Núñez Robainas.