The circadian rhythm of sleep occurs in a cyclical 24-h pattern that is adjusted by the influence of several main synchronizers or “zeitgebers”. The most powerful synchronizer is the light–dark alternation, but also, socio-economic factors play a role, such as social and work relationships. Circadian rhythm regulation plays a crucial role in human health. This disruption of circadian rhythm can lead to increased incidence of diseases: diabetes, obesity, cancer, neurodegenerative diseases, increased risk of cardiovascular disease and stroke. Polygenic variations and environmental factors influence the circadian rhythm of each person. This is known as chronotype, which manifests itself as the degree of morning of evening preferences of each individual. There are indications to establish an association between individual chronotype preferences and the behavior of respiratory diseases.
El ritmo circadiano del sueño ocurre en un patrón cíclico de 24 horas que se ajusta por la influencia de varios sincronizadores principales o zeitgebers. El sincronizador más poderoso es la alternancia luz-oscuridad, además de los factores socioeconómicos, las relaciones sociales y las laborales. La regulación del ritmo circadiano juega un papel crucial en la salud humana. Esta interrupción del ritmo circadiano puede conducir a una mayor incidencia de enfermedades: diabetes, obesidad, cáncer, enfermedades neurodegenerativas, mayor riesgo de enfermedad cardiovascular e ictus.
Las variaciones poligénicas y los factores ambientales influyen en el ritmo circadiano de cada persona. Esto se conoce como cronotipo, que se manifiesta como el grado de preferencias matutinas o vespertinas de cada individuo. Existen indicios para establecer una asociación entre las preferencias individuales de cronotipo y el comportamiento de las enfermedades respiratorias.
Chronobiology is the science that studies biological rhythms. At first it was a term restricted to the field of botany, but, over the years, research was extended to animals and finally to humans. However, it was an astronomer, Jean Jacques d’Ortous de Mairan in 1729, who first spoke of the biological clock. He explained the endogenous rhythm of leaf movements in plants. Even in the absence of light, their own biological clock marked the movement of their leaves.
Later studies showed that not only plants had a biological clock but also animals and humans, which helps to prepare for the fluctuations of the day.1
Of vital importance are the discoveries about the genetics of the biological clock. In 1970 Seymour Benzer and Ronald Konopka, through studies in fruit flies, discovered that mutations in a gene, which they called the PERIOD gene, altered circadian rhythms. After that, thanks to the work of Jeffrey C. Hall, Michael Rosbash and Michael W. Young, the molecular basis for understanding the control of circadian rhythms was built. In 2017, they were awarded the Nobel Prize in medicine for their multiple investigations. This group of scientists discovered that the PERIOD gene encoded the PER protein. This protein accumulated in the cell during the night and was degraded during the day, so PER levels oscillated during the 24-h cycle in synchrony with the circadian rhythm. They hypothesized that the PER protein also blocked the activity of the PERIOD gene through a negative feedback loop, preventing its own synthesis.2–5
The PER activator was discovered in mammals and it was called CLOCK. This helped to understand that the biological clock was composed of activators that induce the expression of their own repressors, confirming a negative feedback loop that is highly conserved from flies to humans.6,7
Circadian sleep rhythmThe circadian rhythm of sleep occurs in a cyclical 24-h pattern that is adjusted by the influence of several main synchronizers or “zeitgebers”. The most powerful synchronizer is the light-dark alternation, but also, socio-economic factors play a role, such as social and work relationships.8
The central circadian clock that drives behavioral and mood rhythms in humans is located in the suprachiasmatic nucleus (SCN) which is formed by a group of neurons located near the midline above the optic chiasm, in the medial hypothalamus. It is important that circadian rhythm regulation plays a crucial role in human health.
Circadian disruption. Relationship of chronodisruption with diseasesCircadian disruption is a term that currently still lacks a clear definition. This disruption can occur at different levels, ranging from intrinsic changes at the molecular, cellular, tissue, organ or systemic level to mismatch between different levels of organization and/or with behavioral and environmental cycles.
Due to its high prevalence in today's society, circadian disruption has a great impact on public health and is associated with multiple diseases.
Different studies show that at the cardiovascular level, the risk of hypertension and left ventricular hypertrophy is increased. There is an elevation of inflammatory markers (C-reactive protein, interleukin-6, resistin, tumor necrosis factor-α) that lead to endothelial dysfunction, as well as a decrease in vasodilator substances (nitric oxide and prostaglandins) and an increase in prothrombotic factors (plasminogen activator inhibitor).9–11
On the one hand, circadian disruption alters glucose tolerance due to a reduction in insulin sensitivity and insulin secretion,12 which increases the risk of developing diabetes.13 On the other hand, it also increases the risk of overweight, obesity and osteoporosis.14,15
Moreover, in neurological diseases, circadian disruption is associated with cerebrovascular disease, epilepsy, migraines, multiple sclerosis and various neurodegenerative diseases.16,17
Finally, in neoplastic diseases, it has been shown that the desynchronization of circadian control in DNA replication, transcription and cell metabolism can contribute to both the onset and progression of cancer. It has been described in breast, prostate, colorectal, lung, hepatic and T-cell lymphoma cancers.18
Definition of circadian chronotypes. Association of chronotypes in general pathologyPolygenic variations and environmental factors influence the circadian rhythm of each person. This contributes to the characteristic known as chronotype, which manifests itself as the degree of morning or evening preference of each individual.
The study of the differences in rhythmic variations has made it possible to establish three different circadian chronotypes: eveningness, morningness and intermediate.19
The eveningness chronotype differs from the early chronotype or morningness in its melatonin profile. Melatonin is a hormone produced by the pineal gland that influences behavior and physiology through its rhythmic synthesis. Melatonin is the best predictor of sleep onset. It behaves as an endogenous rhythm synchronizer and promotes sleep through vasodilatory effects that produce a decrease in core temperature.
The main differences of each chronotype are described below:
- •
Morningness, early chronotype or “morninglarks”: corresponds to 25% of the population, and refers to people whose cognitive functions are maximal in the morning and start to decrease in the afternoon, which leads them to go to bed early and get up early.
- •
Eveningness or “night owl” chronotype: it also corresponds to 25% of the population. Their cognitive functions are maximal in the afternoon-evening, but they need to prolong their rest until well into the morning.
- •
Intermediate chronotype: corresponds to the remaining 50% of the population, and includes people who do not show a clear preference for the morning or the afternoon, but are in the middle of the two previous chronotypes.
For research purposes, scientists have developed several questionnaires that categorize subjects by morningness versus eveningness tendencies. Two of the most popular questionnaires are the Morning-Eveningness Questionnaire (MEQ) and the Munich ChronoType Questionnaire (MCTQ).20
It is very important to know the chronotype, not only to assign a work shift appropriate to the worker's capabilities, but also for the proper diagnosis of pathologies associated with the alteration of the circadian rhythm, known as chronopathologies.
The association between circadian rhythm and certain diseases has helped to understand that rhythmic body function is a health characteristic and that disruption of circadian rhythm can lead to increased incidence of disease.
Although future studies with longitudinal designs are needed, the results obtained emphasize that the evening type could be a risk factor for the development of psychological problems and mental disorders, whereas the morning type could be considered a protective factor.
Association of respiratory pathology with chronotypesBronchial asthmaBronchial asthma is a chronic inflammatory disease of the airways that presents with bronchial hyperresponsiveness and variable airflow obstruction.
The nocturnal worsening of asthma has been recognized historically by a 5th century Roman physician, Caelius Aurelianus, who noted: “Heavy, wheezy breathing, which the Greeks call ASTHMA, this disease is a burden that worsens in the winter and at night more than during theday”.21 Clinically, asthma exacerbations occur most frequently, in the early morning hours, around 4:00a.m. Approximately, 70% of the deaths related to this disease occurred between 12:00a.m. and 06:00a.m.22,23
There is circadian variability of the airways: decreased airway caliber and higher levels of inflammation at night, a situation that can exacerbate asthma. It has also been shown that peak expiratory flow (PEF) shows greater variability at night in both healthy and asthmatic patients. In addition, Bonnet et al.24 revealed circadian variation in airway responsiveness, with maximal responsiveness to methacholine and histamine at 3:00am and 4:00am. When transbronchial biopsies were performed in patients with/without asthma at 4:00pm (when lung function is optimal) and 4:00am (when airflow limitation is maximal), tissue biopsies from nocturnal patients with asthma had pronounced circadian variation in eosinophil numbers: with significantly higher eosinophil numbers at 4:00am than at 4:00pm.25
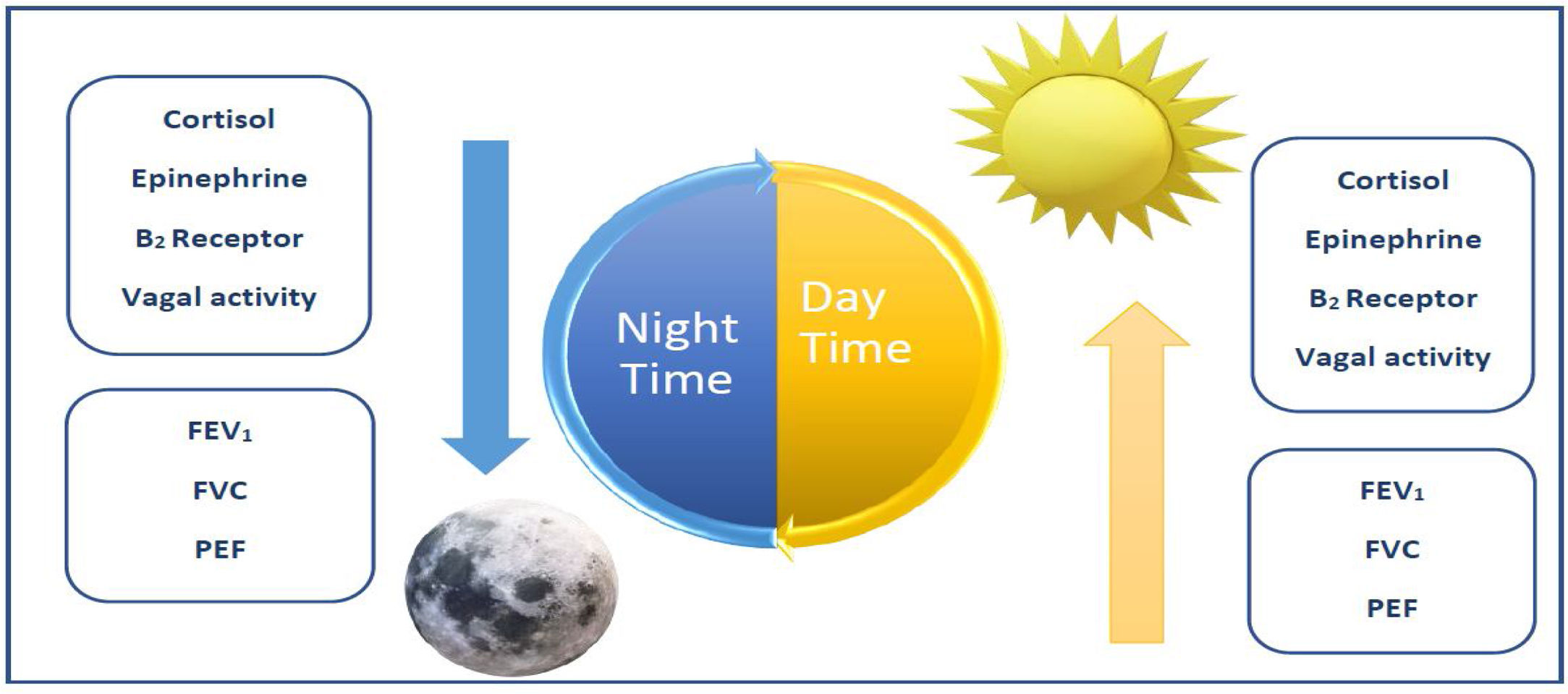
There are a variety of neuroendocrine and airway dynamic factors that are associated with nocturnal exacerbations of asthma (Fig. 1).26
Diurnal representation of neuroendocrine factors and airway dynamics associated with nocturnal exacerbations of asthma.26FEV1: forced expiratory volume in 1 second. FVC: forced vital capacity. PEF: peak expiratory flow.
Other relevant studies, such as that of Chang HH et al.,26 evaluated whether there is an association between chronotypes and bronchial asthma, using logistic regression. They divided the sample into two groups: one of adolescents diagnosed with asthma and the other without respiratory pathology. The results included that the group of asthmatic adolescents slept less (≤5h: 24.3% vs. 23.2%) than the group of healthy adolescents. This study showed that the evening chronotype was significantly associated with a higher frequency of asthmatic adolescents (OR: 1.05; 95% CI 1.01–1.09) compared to intermediate chronotypes.
Finally, another similar study carried out by Haldar P et al.27 found in their cohort of patients with respiratory symptoms, that adolescents with an eveningness chronotype had more respiratory symptoms than those of the morningness type. This effect was also observed, to a lesser degree, among intermediate types. This association was not modified by the presence of potential interpersonal, environmental or genetic factors.
Obstructive sleep apnea (OSA)Another association studied is that between the different chronotypes and the prevalence of sleep disorders, especially the most frequent, obstructive sleep apnea (OSA).
Kim LJ et al.28 evaluated the prevalence of different chronotypes in a representative sample of residents of the city of São Paulo and investigated their effect on the severity of this pathology. The authors found a possible protective effect of an intermediate chronotype for OSA, more marked in overweight older people. However, the highest apnoea–hypopnoea index (AHI) assessed by nocturnal polysomnography appeared in the morning and evening chronotypes, although when stratified by age, body mass index (BMI) and gender, no effect on OSA severity was observed.
However, previously Lucassen et al.29 had shown that the prevalence of OSA in individuals with “night owl” chronotypes was twice as high as in morning-type individuals, regardless of BMI and neck circumference. In addition, they demonstrated how this chronotype is associated with changes in eating behavior, higher resting heart rate and lower HDL-C levels with a tendency toward higher BMI. In addition, there was an increase in stress hormones, with 20–30% higher 24-h urinary adrenaline levels and higher morning plasma ACTH levels observed. All this indicates an activation of the adrenal sympathetic system, which predicts higher cardiovascular morbidity.
Chronic Obstructive Pulmonary DiseaseChronic Obstructive Pulmonary Disease (COPD) is characterized by irreversible airflow obstruction caused by significant exposure to noxious particles or gases, and is one of the three leading causes of death worldwide.30
COPD patients present a circadian variability of symptoms and lung function that varies throughout the day, with the worst times being early in the morning and late at night, and this is accentuated when lung function is impaired, affecting the quality of sleep.31–33
The mechanisms that generate this variability of symptoms are: the increase of the nocturnal parasympathetic system, the reduction of bronchial caliber and the increase of mucus production as well as its retention.34
Furthermore, we know that pulmonary function in COPD is also affected by circadian rhythms, with an increase in airway resistance both at night and in the early morning hours, with a circadian variability of Forced expiratory volume in 1 second (FEV1) with a peak at 4:00pm and a fall around 4:00am.35,36
Acute and chronic exposure to tobacco smoke (TS) causes circadian disruption at both the SCN and pulmonary levels. It affects the expression of major CLOCK genes, altering protein production (reduces BMAL1, REV-ERBα, PER2 and elevates RORα) and transcript levels of CLOCK molecular targets. All this leads to an increase in the inflammatory response and oxidative stress, in addition to alterations in the quality and duration of sleep in COPD patients.37–41
Both REV-ERBs and RORs have been shown to play a key role in the pathogenesis of COPD. Suppression of Rev-erbα, would increase pulmonary inflammation as well as mesenchymal epithelial transition in COPD.38–41 Secondly, overexpression of Rorα would increase cellular destruction leading to increased emphysema in response to TS.41–43
In smokers or COPD patients, a decrease of BMAL1 has been observed due to a reduction of SIRT1,44 an enzyme in charge of BMAL1 and PER2 acetylation, which plays a fundamental role in circadian variation.45–47 The reduction of SIRT1 activity by TS has not only been associated with circadian disruption,46 but also with telomere shortening and inflammasenescence in COPD patients,47,48 leading to increased exacerbations and complications, as well as increased disease progression.
Lung cancerCircadian disruption is related to tumorogenesis and tumor progression, since cell cycle regulation, metabolism, apoptosis and DNA repair follow circadian rhythms. In humans it has been shown that thanks to the circadian clock genes (BMAL1/CLOCK, Period 1 and Period 2) an adequate rhythm of proliferation and DNA damage repair is maintained.33
Nocturnal compared to diurnal chronotypes have been shown to be a risk for the development of lung cancer.34
This is probably due to the circadian disruption that these life habits would generate, not being the only possible cause of circadian disruption, as we have seen previously. Through the use of animal models, the effect that the alteration of circadian rhythms has on tumor growth has been demonstrated, finding a decreased expression of certain circadian clock genes in tumor cells. These alterations would cause a decrease in survival, stimulate tumor growth and progression and are risk factors for the development of lung cancer.35,36
One of the key circadian clock genes in cancer is BMAL1, due to its involvement in apoptosis, response to DNA damage, regulation of homeostasis and cell cycle progression.37
On the one hand, down-regulation of Bmal1 promotes tumor growth by decreasing P53 expression,38 interfering with cell cycle arrest when DNA damage is provoked by cellular stress stimuli.39 On the other hand, it has also been related to the reduction of sensitivity to antineoplastic drugs, such as irinotecan and oxaliplatin.37
An important player in tumorogenesis is the MYC oncogene, since its activation promotes genomic instability and tumor development. In circadian disruption, an absence of ATM gene activation has been observed, allowing MYC activation.33
The expression of PER2, a master clock gene, has been shown to promote the expression of anti-oncogenes such as BAX, P53 and P21, as well as to inhibit the expression of pro-oncogenes: vascular endothelial growth factor, CD44 and c-Myc.40
The degree of tumor differentiation in lung cancer is also related to alterations in certain circadian genes, such that overexpression of TIMELESS or reduction of PER1, PER2, PER3, DEC1, have been related to a low degree of differentiation.41,42
Regarding the TNM stage in lung cancer, it has been seen that a lower expression of PER1, PER2, PER3, CRY 2 and DEC1 was related to an advanced TNM stage, indicating that these genes are involved in growth and progression.42–44
Prognosis and overall survival in lung cancer are also influenced by circadian clock genes, and some studies have linked worse prognosis and decreased survival to overexpression of certain genes (TIMELESS, NPAS2 and DEC2) or deletions of PER1, PER2 and per3.42,44,45
Our experience in the sleep unit: association of chronotypes with Chronic Obstructive Pulmonary Disease and asthmaChronotype determination may have many external influences and interindividual variabilities, related to age, sex, environmental, social and even disease-related situations. Therefore, more studies are needed to explore these relationships and to genetically analyze biological clock polymorphisms in order to help understand this association.
As described by Charles Darwin, species have adapted over time: “It is not the strongest species that survives, nor the most intelligent, but the one that responds best to change”. Therefore, chronotype preferences can be a protective tendency or a risk factor.
The aim of our study was to evaluate the distribution of chronotypes, sleep quality and excessive daytime sleepiness in patients with COPD and asthma, and the relationship of chronotype to disease severity and control.
A descriptive cross-sectional study was carried out, including a cohort of patients with an established diagnosis of COPD and asthma, attended in the pulmonology department of the Hospital Universitario de Getafe, Madrid (Spain).
We collected sociodemographic variables, smoking history, severity and control of the disease, maintenance treatment, number of exacerbations in the last year, chronotype types measured by the Horne and Östberg questionnaire, sleep quality by the Pittsburg test and daytime hypersomnia by the Epworth test.
Regarding the results of our study, forty patients were included (20 COPD and 20 asthmatics) with a mean age of 63 years (44–86). We know that between 19 and 21 years of age, there is a preference for the night owl chronotype in women, but that after 50 years of age, this gender difference is equalized and both groups have a tendency toward early chronotypes.49 This is thought to be due to less social pressure, more regular light schedules, and a progressively less robust circadian system.19,49
Regarding gender, men were majority in the COPD sample (16), compared to the majority of women in asthmatic groups (18). Forty-seven percent were moderate early chronotype (11 COPD and 8 asthmatics) compared to 15% who were moderate eveningness chronotype (6 asthmatics and no COPD) and 30% intermediate (6 COPD and 6 asthmatics). Sleep quality was reported with a mean value of 6.55 on the Pittsburg scale (5.0 in COPD compared to 8.1 in asthma) and mean daytime sleepiness by Epworth with a mean of 4.55. Disease control was assessed by the COPD Assessment Test (CAT) with a mean value of 9.85 in COPD and 22.35 in Asthma Control Test (ACT) and 0.485 in Asthma Control Questionnaire (ACQ) in asthmatic patients. Regarding comorbidities, 72.5% were smokers (8 active and 21 ex-smokers).
Our study concludes that the moderate early chronotype is predominant in COPD patients, in contrast to asthmatic patients where the preference is more eveningness-intermediate. Sleep quality is reported to be better in COPD patients than in asthmatics, although no subgroup reported excessive daytime sleepiness. Morning chronotypes are related to better control of the underlying disease.
In this sense, our analysis found that early chronotypes showed better control of the underlying disease, especially in patients with COPD, in whom this chronotype predominated and who also reported a more satisfactory quality of sleep, compared to the group of asthmatic patients who had an eveningness preference in their chronotype.
Gender can also condition these results. In our sample, women were predominant in the asthma group, obtaining mainly early-intermediate chronotypes.
Knutson et al.50 reviewed a British cohort of more than 430,000 people followed up for 6.5 years to demonstrate how the eveningness chronotype was associated with higher mortality. Being a night owl was significantly associated with a higher prevalence of all comorbidities. Associations were strongest for psychological disorders, followed by diabetes, neurological disorders, gastrointestinal/abdominal disorders and respiratory disorders. Later chronotype was associated with a small increased risk of all-cause mortality. The risk of mortality at night may be due to behavioral, psychological and physiological risk factors, many of which may be attributable to chronic disruption between internal physiological synchronization and externally imposed work schedules and social activities.
These findings suggest the need to investigate possible interventions aimed at modifying circadian rhythms in individuals or allowing evening types to have greater work schedule flexibility.
There are indications to establish an association between individual chronotype preferences and the behavior of respiratory diseases. Some of these diseases are very prevalent and their better knowledge and control have a significant impact on patients.
Informed consentThe hospital ethics committee approved the research and since we have not worked directly with patients, we do not have informed consent.
FundingThis research has not received any specific grant from public, commercial, or non-profit sector agencies.
Authors’ contributionsAll authors have contributed to the preparation and writing of the manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest.