Respiratory diseases and cardiovascular diseases (CVDs) have high prevalence and share common risk factors. In some respiratory diseases such as sleep apnoea and COPD, the evidence of their negative impact on the prognosis of CVDs seems clear. However, in other diseases it is less evident whether there is any direct relationship. With this in mind, our objective was to provide information that may be helpful to better understand the relationship between respiratory pathology and CVDs.
There are different reasons for this relationship, such as shared risk factors, common pathophysiological mechanisms, side effects of treatment and the direct effect in the heart and great vessels of respiratory diseases.
Indeed, aging and smoking are risk factors for CVDs and also for respiratory diseases such as obstructive sleep apnea (OSA), COPD and interstitial lung diseases (ILD).
Furthermore, there are common pathophysiological mechanisms that affect both respiratory diseases and CVDs, such as accelerated atherosclerosis, microvascular dysfunction, endothelial dysfunction, inflammation, hypoxemia and oxidative stress.
Besides that, it is well known that lung cancer, sarcoidosis and amyloidosis may directly affect the heart and great vessels.
Finally, side effects of drugs for respiratory diseases and the discontinuation of treatments that are necessary for CVDs, such as β-blockers and aspirin, may have a deleterious impact on the cardiovascular system.
In conclusion, the coexistence of respiratory diseases and CVDs is very common. It makes modifying diagnostic and therapeutic management necessary and is also a relevant prognostic factor.
Las enfermedades respiratorias y cardiovasculares (ECVs) tienen una alta prevalencia y comparten factores de riesgo comunes. En algunas enfermedades respiratorias como la apnea del sueño y la EPOC, la evidencia de su impacto negativo en el pronóstico de las ECVs parece clara. Sin embargo, en otras enfermedades es menos evidente si existe alguna relación directa. Considerando esto, nuestro objetivo fue aportar información que pueda ser útil para comprender mejor la relación entre la patología respiratoria y las ECVs.
Existen diferentes razones para esta relación, como factores de riesgo compartidos, mecanismos fisiopatológicos comunes, efectos secundarios del tratamiento y el efecto directo en el corazón y los grandes vasos de las enfermedades respiratorias.
De hecho, el envejecimiento y el tabaquismo son factores de riesgo para las ECVs y también para las enfermedades respiratorias como la apnea obstructiva del sueño (AOS), la EPOC y las enfermedades pulmonares intersticiales (EPID).
Además, existen mecanismos fisiopatológicos comunes que afectan tanto a las enfermedades respiratorias como a las ECVs, como la aterosclerosis acelerada, la disfunción microvascular, la disfunción endotelial, la inflamación, la hipoxemia y el estrés oxidativo. Además, es bien conocido que el cáncer de pulmón, la sarcoidosis y la amiloidosis pueden afectar directamente al corazón y a los grandes vasos.
Finalmente, los efectos secundarios de los fármacos para enfermedades respiratorias y la suspensión de tratamientos necesarios para las ECVs, como los β-bloqueantes y la aspirina, pueden tener un impacto deletéreo sobre el sistema cardiovascular.
En conclusión, la coexistencia de enfermedades respiratorias y ECVs es muy frecuente, lo que obliga a modificar el manejo diagnóstico y terapéutico y es, además, un factor pronóstico relevante.
Respiratory and cardiovascular diseases (CVDs) often coexist in the same patient. This seems reasonable given the high prevalence of both and since they share common risk factors such as smoking and exposure to environmental pollutants.1–6 Other causes of these pathologies could be the cardiovascular side effects of the treatments used in pulmonology, insufficient therapeutic management of cardiovascular pathology in patients with respiratory comorbidity and shared pathophysiological mechanisms for heart and lung-related diseases.7–11
In respiratory diseases such as sleep apnoea and COPD, the evidence of their negative impact on the prognosis of CVDs seems clear, while in others, such as asthma and pneumonia, the evidence is rather weak.12–18 The objective of this review is to provide information that may be helpful to better understand the relationship between respiratory pathology and CVDs.
Obstructive sleep apnoea (OSA)Obstructive sleep apnoea (OSA) is a highly prevalent disorder characterized by repeated episodes of upper airway obstruction during sleep that lead to intermittent hypoxia and sleep fragmentation. Recent findings have suggested that 13% of men and 6% of women in the general population, which covers about 425 million adults worldwide, have moderate-to-severe OSA, defined as an apnoea-hypopnea index (AHI)≥15/h.19,20
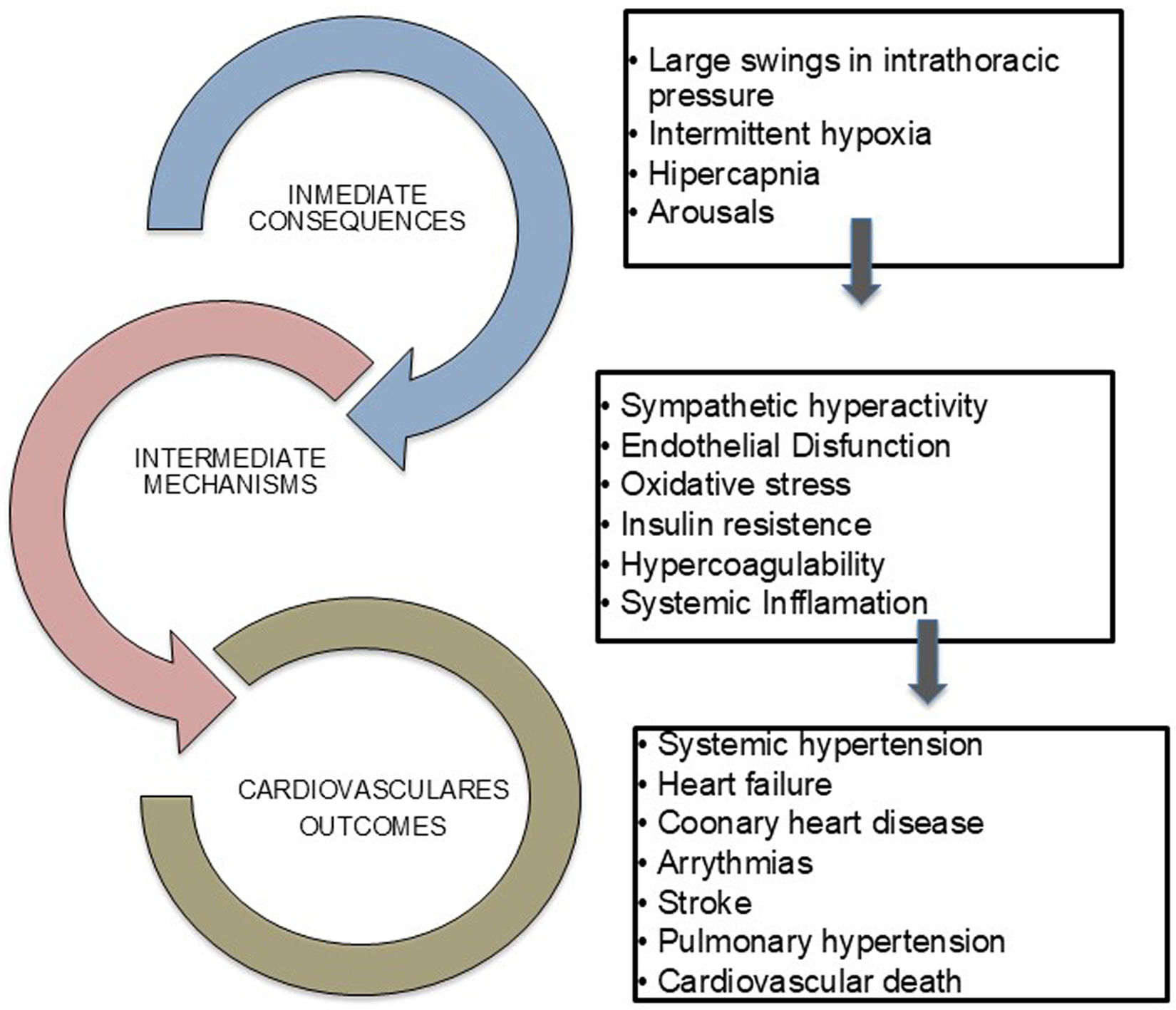
Mechanisms linking OSA with CVDsThe pathophysiological mechanisms by which OSA contributes to CVDs are multifactorial. Repeated episodes of upper airway obstruction during sleep trigger direct and intermediate mechanisms that may lead to CVDs, including large swings in negative intrathoracic pressure, repeated arousals from sleep with sleep fragmentation, and episodes of hypoxia-reoxygenation, which provoke intermittent hypoxia, inflammation, oxidative stress, metabolic abnormalities, endothelial dysfunction and increased sympathetic activity (Fig. 1).12,21,22
Cardiovascular consequences of OSASystemic hypertension (HTN)There is a close association between OSA and HTN. About 30% of patients suffering from HTN have OSA, while 50% of OSA patients have HTN.23,24 Moreover, OSA is present in 80% of patients with resistant hypertension (RH) and is the most recognizable cause of RH in about two-thirds of these patients.25,26 Amongst the aforementioned mechanisms, increased sympathetic activity seems to play a major role in the association between OSA and HTN.21
Large prospective population- and clinical-based cohorts have shown that OSA is an independent risk factor for incident HTN, with a dose-response relationship. Patients with an AHI>15 are two to three times more at risk of developing incident HTN than those without OSA.27 The sympathetic activation associated with obstructive events during sleep implies a nocturnal predominance of HTN and increases the risk of a non-dipping blood pressure pattern developing in OSA patients.28
This evidence has led the scientific community to include OSA as one common identifiable cause of HTN and to alert patients with RH and snoring to be screened for OSA.29,30
Other CVDsOSA has also been associated with stroke, coronary heart disease (CHD), arrhythmias and cardiovascular mortality.
The prevalence of OSA can be from 50% to as much as 75% amongst patients with stroke while those with recurrent stroke have a higher OSA prevalence than those with a first episode (74% vs. 57%).31,32 A meta-analysis including 8435 participants found an independent association between OSA and incident stroke (odds ratio 2.24; 95% confidence interval: 1.57–3.19), as well as a dose–response relationship which per 10-unit increase in the AHI, increases the odds of having a stroke by 36%.33 This evidence has led the American Heart Association to include OSA as a potential risk factor for stroke in their Guideline for the Primary Prevention of Stroke.34
Unlike stroke, OSA's association with CHD is more controversial. Patients with the former have shown evidence of increased arterial stiffness, early atherosclerosis, coronary artery calcification and increased coronary plaque vulnerability, which may be affected by intermittent hypoxia, oxidative stress and inflammation.35,36 Although some retrospective and cross-sectional studies have observed an increased risk of CHD amongst OSA patients,37,38 a meta-analysis did not find any association between OSA and incident CHD (odds ratio 1.56, confidence interval 95%: 0.83–2.91).33
However, OSA may be responsible for an increased risk of major adverse cardiovascular events after percutaneous coronary intervention.39
As regards arrhythmias, OSA is associated with a wide spectrum of cardiac rhythm disturbances and, especially, with atrial fibrillation (AF).21,40 The prevalence of OSA in patients with AF ranges between 21% and 80%.41 OSA has been reported to be an independent risk factor for AF in patients with no other underlying cardiac disorders.42,43 Different studies have found that severe OSA is associated with a lower response rate to antiarrhythmic drugs than those without OSA, and meta-analyses have shown that patients with OSA have a 31% greater AF recurrence rate after pulmonary vein isolation than those without it.44 Although sudden nocturnal cardiac death was 2.57 times more likely to occur in OSA patients than in the general population, at present there is not enough evidence to support OSA playing a role in ventricular and lethal arrhythmias.45
OSA is common in heart failure (HF), with prevalence rates of 50%-75%.46 Patients with HF are also at increased risk of central sleep apnoea (CSA). Most studies involving patients with HF have reported roughly equal proportions of OSA and CSA.12,21 Sleep apnoea is independently associated with an increased risk of adverse outcomes, including HF-related symptom progression, hospitalization and mortality.22
Cardiovascular mortalityData from prospective population-based and clinical cohorts have consistently shown an independent association between severe OSA (AHI≥30) and cardiovascular mortality.47–49 This association has been observed in middle-aged men, as well as in women and the elderly.50–52 Nevertheless, it has not been demonstrated for mild-to-moderate OSA.47–52
Systemic diseases with pulmonary involvementAutoimmune rheumatic diseases are characterized by multi-organ damage related to systemic inflammation and immune dysregulation. The most common diseases with pulmonary involvement are rheumatoid arthritis (RA), systemic sclerosis (SS), systemic lupus erythematosus (SLE) and systemic inflammatory vasculitis and myositis.53–55 In addition to pulmonary involvement, cardiovascular involvement is relevant, as it leads to an increased risk of morbidity and mortality. The mechanisms involved are derived from accelerated atherosclerosis and myocardial microvascular dysfunction.
Rheumatoid arthritisRA patients have a twofold increased risk of developing and dying from HF, and cardiovascular events following acute myocardial infarctions (MIs) and an increased risk of cerebrovascular disease.56,57 Furthermore, myocardial microvascular involvement is the main cardiovascular risk factor in patients with SS.53,56 Cardiac involvement is a major cause of morbidity and mortality in SLE patients, particularly in women.53,56–58
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitisAnti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis mainly includes granulomatosis with polyangiitis (GP) and microscopic polyangiitis (MP). Although data on cardiovascular risk is scarce, cardiac involvement is probably linked to coronary microvascular dysfunction.53,59 In the case of GP, electrocardiographic and echocardiographic abnormalities are present in up to 46% of patients, with an increased risk of cardiovascular mortality at follow-up.59
SarcoidosisThe actual prevalence of cardiac sarcoidosis is unknown and, like renal sarcoidosis, is very rare. A higher incidence has been reported before the age of 40 than those aged 40 and above and it is up to 40 times more common in black patients. It may occur in isolation, affecting only the heart in up to 25% of cases, and only 5% of all the sufferers have wide-ranging symptoms like palpitations, presyncope, HF and sudden death.60 The myocardium is most frequently affected, producing arrhythmias, tachyarrhythmias and cardiomyopathy. Atrioventricular block is usually the most common initial manifestation whereas infiltration of the valvular papillary muscles is rare. An endomyocardial biopsy is usually not necessary for diagnosis, but instead clinical suspicion can be integrated with cardiac magnetic resonance imaging (CMRI) or FDG-PET findings.61
Anti-glomerular basement membrane diseaseAnti-glomerular basement membrane disease leads to chronic renal failure, which results in an increased likelihood of cardiovascular death. Urinary albumin excretion is a risk marker for both kidney disease and CVDs. When chronic renal failure occurs, hypertension, dyslipidaemia and diabetes mellitus are major risk factors for endothelial dysfunction, inflammation, oxidative stress and accelerated atherosclerosis.62–66
Eosinophilic lung diseasesAnother large group that may carry cardiovascular risks are those with eosinophilic lung diseases (ELDs), which may come in the form of pulmonary symptoms, radiological changes and elevated levels of eosinophils in sputum and lung tissue. All are rare and there is a large overlap in clinical features, with a wide range of severity. ELDs can be classified into primary disorders, whereas secondary ones include allergic processes, drugs, toxins, infections, malignancies and autoimmune diseases.67 Eosinophils can cause tissue damage and cardiac involvement from coronary vasoconstriction, resulting in unstable angina, MIs, ventricular arrhythmias and/or sudden death. Other forms of aggression are caused by mast cell infiltration.68 Thus, Kounis syndrome is described in allergic MIs, provoked by coronary vasospasms triggered by vasoactive substances released by mast cells. In this respect, the prognosis may be better because the cause of vasospasm is transient.69
Certain triggers, such as non-steroidal anti-inflammatory drugs in patients with Aspirin-Exacerbated Respiratory Disease (AERD) can trigger MIs, even in parasitic infestations. In other patients, eosinophilia may be due to myeloproliferative disease and cytotoxic treatments. Eosinophilic myocarditis without peripheral eosinophilia has also been reported.
Allergic granulomatosis with polyangiitis is a form of small vessel vasculitis characterized by asthma, inflammation, granuloma formation in the respiratory tract and eosinophilia, which can affect the cardiac, gastrointestinal, renal and nervous systems as well as the joints. Cardiac involvement is a rare but severe manifestation, responsible for 50% of deaths.70
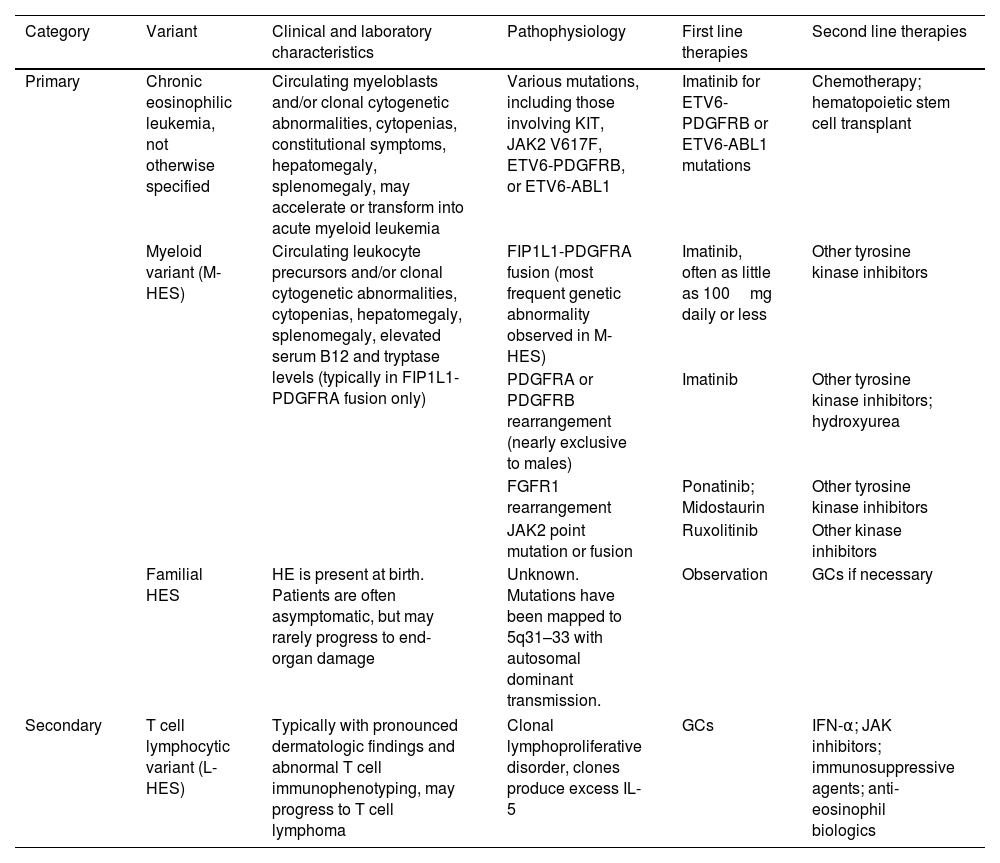
In hypereosinophilic syndrome (HES), cardiac involvement is present in up to 58% of sufferers and is the main cause of morbidity and mortality, affecting endocardial tissue and progressing in three stages: acute necrosis, intermediate thrombosis and fibrosis.71Table 1 shows the subtypes of HES and appropriate therapy considerations.72
Subtypes of hypereosinophilic syndromes and treatment.
| Category | Variant | Clinical and laboratory characteristics | Pathophysiology | First line therapies | Second line therapies |
|---|---|---|---|---|---|
| Primary | Chronic eosinophilic leukemia, not otherwise specified | Circulating myeloblasts and/or clonal cytogenetic abnormalities, cytopenias, constitutional symptoms, hepatomegaly, splenomegaly, may accelerate or transform into acute myeloid leukemia | Various mutations, including those involving KIT, JAK2 V617F, ETV6-PDGFRB, or ETV6-ABL1 | Imatinib for ETV6-PDGFRB or ETV6-ABL1 mutations | Chemotherapy; hematopoietic stem cell transplant |
| Myeloid variant (M-HES) | Circulating leukocyte precursors and/or clonal cytogenetic abnormalities, cytopenias, hepatomegaly, splenomegaly, elevated serum B12 and tryptase levels (typically in FIP1L1-PDGFRA fusion only) | FIP1L1-PDGFRA fusion (most frequent genetic abnormality observed in M-HES) | Imatinib, often as little as 100mg daily or less | Other tyrosine kinase inhibitors | |
| PDGFRA or PDGFRB rearrangement (nearly exclusive to males) | Imatinib | Other tyrosine kinase inhibitors; hydroxyurea | |||
| FGFR1 rearrangement | Ponatinib; Midostaurin | Other tyrosine kinase inhibitors | |||
| JAK2 point mutation or fusion | Ruxolitinib | Other kinase inhibitors | |||
| Familial HES | HE is present at birth. Patients are often asymptomatic, but may rarely progress to end-organ damage | Unknown. Mutations have been mapped to 5q31–33 with autosomal dominant transmission. | Observation | GCs if necessary | |
| Secondary | T cell lymphocytic variant (L-HES) | Typically with pronounced dermatologic findings and abnormal T cell immunophenotyping, may progress to T cell lymphoma | Clonal lymphoproliferative disorder, clones produce excess IL-5 | GCs | IFN-α; JAK inhibitors; immunosuppressive agents; anti-eosinophil biologics |
HES: hypereosinophilic syndromes; PDGFRB: platelet-derived growth factor receptor beta; FGFR1: fibroblast growth factor receptor 1; GC: glucocorticoid; IFN-α: interferon alpha; JAK2: Janus kinase 2; FIP1L1: Fip1-like1; PDGFRA: platelet-derived growth factor receptor alpha; L-HES: lymphocytic variant HES; IL-5: interleukin-5.
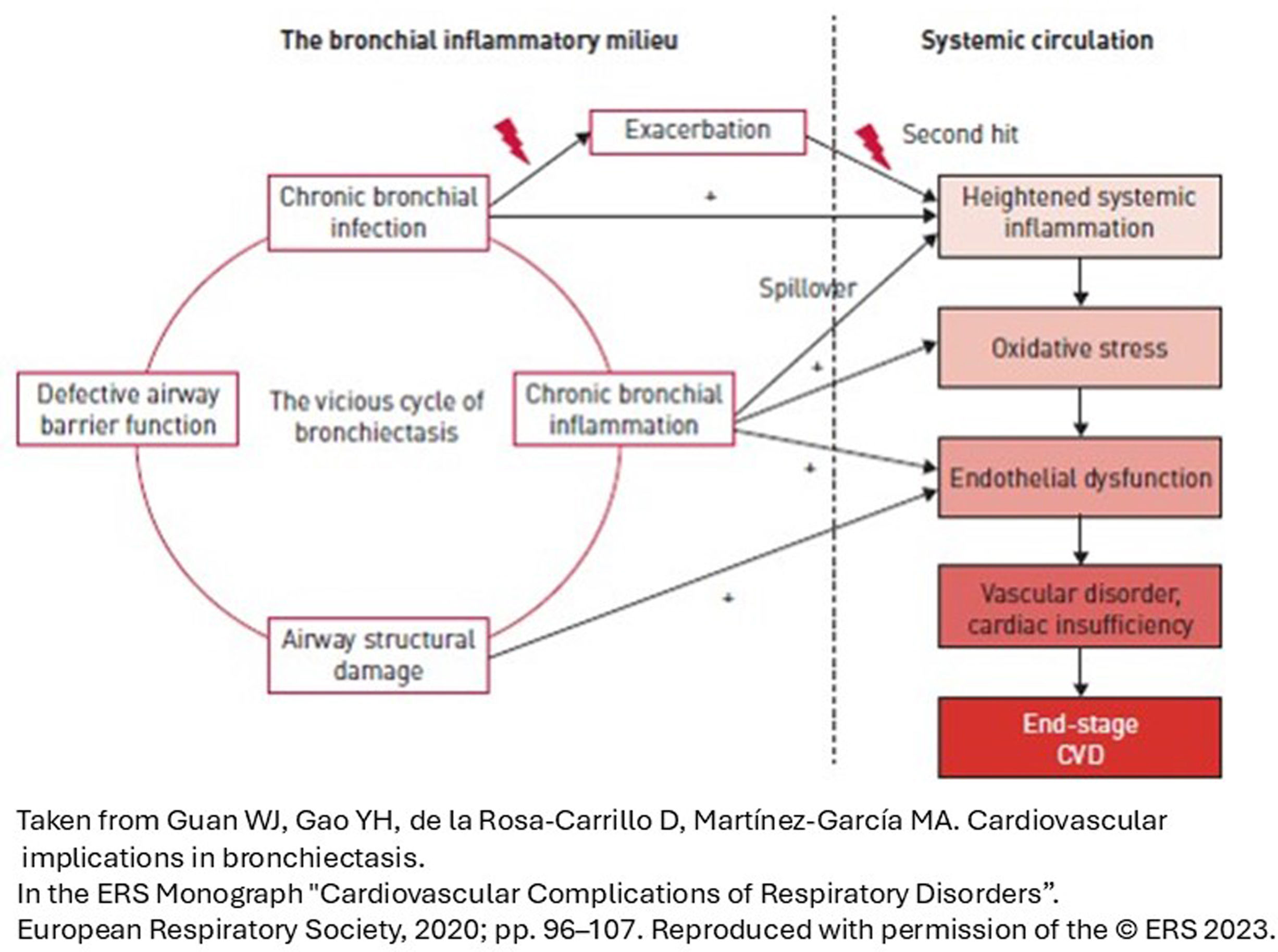
Bronchiectasis has emerged as a disease characterized by chronic airway and systemic inflammation,73 which could lead to atherosclerosis and CVDs.74–76 An increase in arterial stiffness,77 cardiac dysfunction,78 cerebrovascular accidents,79 and, in general, a high prevalence of CVDs80–82 have been reported in these patients. The aim of the Bronchiectasis Aetiology Comorbidity Index (BACI)83 was to assess comorbidities and aetiologies significantly associated with high mortality risk. A high prevalence of CVDs like arterial hypertension (30%), MI (12%), HF (12%), AF (10%) and pulmonary arterial hypertension (PAH) (8%) were observed.
EpidemiologyPatients with bronchiectasis are three times more at risk of suffering a cardiac ischemic event and a cerebral one is five times more likely to affect them than the general population.79 In addition, cardiovascular comorbidities are the most important independent risk factor associated with bronchiectasis exacerbations, with the presence of P. aeruginosa and s bronchiectasis severity trailing behind.84 HF is one of the main risk factors for hospitalization, together with ischemic heart disease (IHD) and the severity of bronchiectasis.85 PAH is present in 33–75% of patients with bronchiectasis,86,87 so it could be considered both as a marker of severity and an independent predictor of mortality.86 Ultimately, all these CVDs may contribute to increased morbidity and mortality.88 In the bronchiectasis severity index (BSI) score validation study, 26% of deaths were due to myocardial infarction, heart failure and stroke.89
BiomarkersAlthough the severity of bronchiectasis may be an independent risk for CVDs, specific biomarkers are being tested to detect patients with bronchiectasis and a high risk of cardiovascular mortality. Elevated levels of desmosine, a product of elastin degradation, were associated with increased mortality, both overall and from cardiovascular causes.90 Chest computerized tomography (CT) scans could also be considered a biomarker for CVDs, since coronary artery calcification has been associated with increased mortality (being 5 times higher than when there is no calcification).91
PathophysiologyThe pathophysiological link between CVDs and bronchiectasis is not completely known, although it has been associated with airway neutrophilic inflammation and its systemic inflammatory repercussions,92,93 which are more relevant in patients with severe bronchiectasis, COPD and/or chronic bronchial infection.94 All this favors atherogenesis, the increase in intima-media thickness, carotid plaque and arterial stiffness. These consequences are accentuated during exacerbations, which increase local and systemic inflammation.95 Therefore, in bronchiectasis, the risk of contracting CVDs is calculated by following a double-hit model: the baseline risk increases during exacerbations (Fig. 2).96 However, in some patients, the association between CVDs and bronchiectasis may be due to shared risk factors: smoking, connective tissue disease or comorbidities such as COPD.
TreatmentThere are only a few publications on how CVDs treatment may affect patients with bronchiectasis, so it is assumed that its adverse effects are similar to those that occur in patients with COPD. Thus, care should be taken with antihypertensives such as angiotensin-converting enzyme (ACE) inhibitors (which can cause coughs) and thiazides (where there is a risk of hypokalaemia, especially in patients taking -β-agonists or inhaled steroids).97 Loop diuretics make contracting metabolic alkalosis and secondary hypercapnia possible, especially for patients with severe functional respiratory impairment. As for -β-blockers, the risk of bronchospasm is low, although the use of cardioselective molecules is recommended.98 Furthermore, the potential risk of haemoptysis must be taken into account for patients who are receiving anticoagulant treatment, even with the new direct-acting anticoagulants, so the risks and benefits of this treatment should always be discussed with patients with a history of haemoptysis.99,100
COPDPatients with COPD are especially vulnerable to cardiovascular illnesses, since they have common risk factors such as smoking and a sedentary lifestyle.101
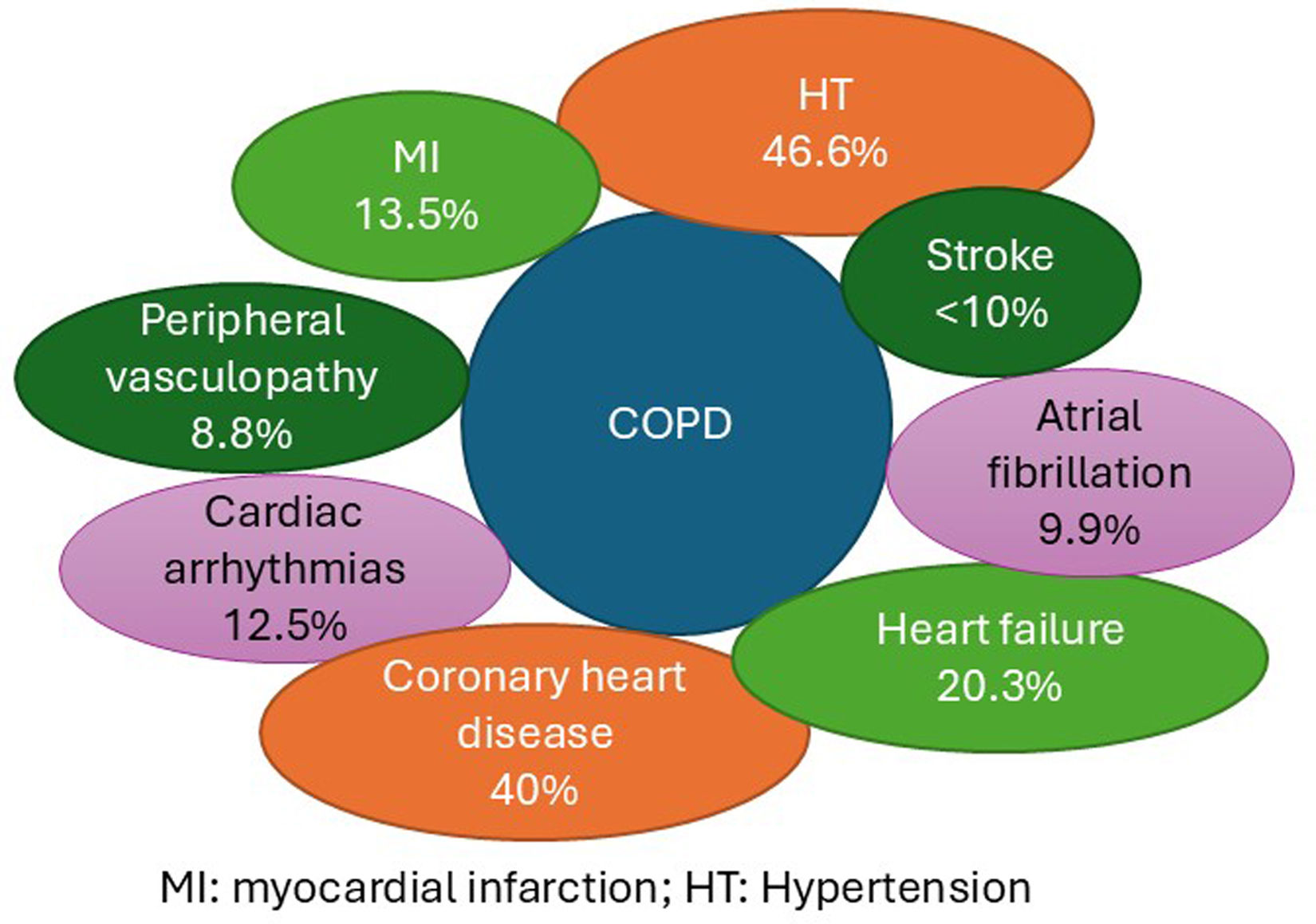
The estimated prevalence of pathology and cardiovascular risk factors for COPD can be seen in Fig. 3.13,102–104
PathophysiologyThere are many mechanisms that link COPD to CVDs, including pulmonary hyperinflation, hypoxemia, pulmonary hypertension, systemic inflammation, oxidative stress, exacerbations and genetic factors. Here are a few relevant aspects of the physiopathology of this connection:
Systemic inflammationFor patients with COPD and CVDs, the inflammation markers are higher than for those without CVDs.105 What is more, the coronary artery calcium score obtained from CT scans of COPD patients correlate with pulmonary and systemic inflammation markers.106
Pulmonary hyperinflationAirflow limitation causes air trapping and pulmonary hyperinflation, which puts pressure on the cardiovascular system, leading to left ventricular dysfunction and a reduction in the filling of the left ventricle and cardiac output.107–110
HypoxiaThis creates pulmonary vasoconstriction and vascular remodeling, resulting in right ventricular diastolic dysfunction. In addition, hypoxia can cause an increase in systemic inflammation, oxidative stress and molecular adhesion in endothelial cells, which contribute to the progression of arteriosclerosis and CVDs.110,111
COPD exacerbationsPatients that have shown a COPD exacerbation are at a greater risk of experiencing a cardiovascular event. The risk during or after the exacerbation may be related to the increase in systemic inflammation and dynamic hyperinflation.112
Pulmonary hypertensionHypoxia is the main mechanism which causes this condition, via pulmonary vasoconstriction and vascular remodeling; this leads to a thickening of the intima of the arterioles, increasing vascular resistance.110
Risk prediction models and the effects of the treatmentRisk prediction modelsIt is important to determine the cardiovascular risks of COPD patients ahead of their prognostic assessments and also to be able to prevent any side effects following treatment.
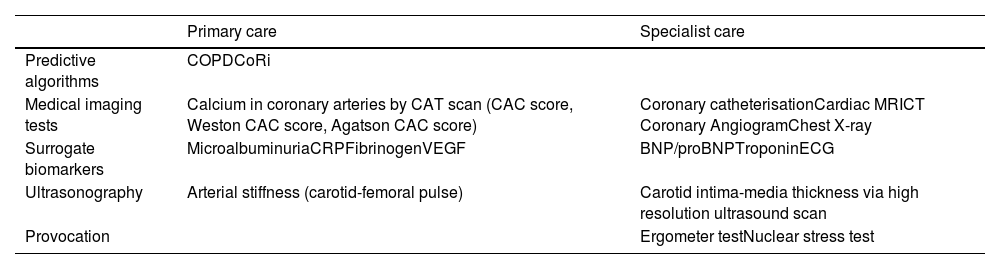
There are biomarkers of cardiovascular risk for COPD patients that can be applied to many levels of care (Table 2).
Predictive tests and cardiovascular risk biomarkers in patients with COPD.
| Primary care | Specialist care | |
|---|---|---|
| Predictive algorithms | COPDCoRi | |
| Medical imaging tests | Calcium in coronary arteries by CAT scan (CAC score, Weston CAC score, Agatson CAC score) | Coronary catheterisationCardiac MRICT Coronary AngiogramChest X-ray |
| Surrogate biomarkers | MicroalbuminuriaCRPFibrinogenVEGF | BNP/proBNPTroponinECG |
| Ultrasonography | Arterial stiffness (carotid-femoral pulse) | Carotid intima-media thickness via high resolution ultrasound scan |
| Provocation | Ergometer testNuclear stress test |
COPDCoRi: Chronic Obstructive Pulmonary Disease Coronaropathy Risk; CAC: coronary artery calcification; MRI: magnetic resonance imaging; CT: computed tomography; CRP: C reactive protein; VEGF: vascular endothelial growth factor; ECG: electrocardiogram.
The treatment recommended for COPD may have an effect on the cardiovascular system and vice versa.
Short-acting β-2 agonists (SABAs) are associated with the appearance of cardiac arrhythmias and should be used with caution when patients have underlying pathologies.113 Long-acting bronchodilators such as long-acting β2 agonists (LABAs) and long-acting muscarinic antagonists (LAMAs) have been linked to an increase in cardiovascular risk,114 but trials which were devised to assess whether this was the case (SUMMIT, TIOSPIR and UPLIFT)115–117 confirmed their safety. In the CLAIM study, the combination of indacaterol and glycopyrronium improved ventricular function, diastolic volume, and FEV1, compared with placebo.118
Besides this, treatment for cardiovascular pathology does not have deleterious effects on COPD. Clinical trials and metanalyses have demonstrated that the use of selective β-blockers on COPD patients does not have a significant effect on FEV1, neither on the β-agonist response nor on respiratory symptoms in patients who have not received this treatment.119 There is no evidence regarding the effects of antiaggregants, anticoagulants or other vasodilators.
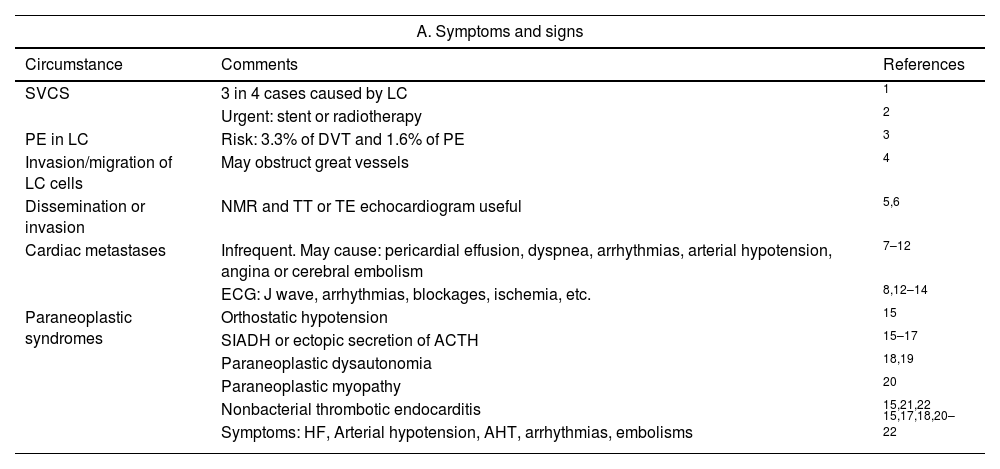
Lung cancerTable 3 shows the symptoms/signs of how the heart and great vessels are affected by lung cancer (LC)120–141 and the descriptors of this relationship in the TNM-staging system.142–145
Impact of lung cancer on heart and great vessels. Symptoms, signs and their classification in the TNM-staging system, 9th edition.
| A. Symptoms and signs | ||
|---|---|---|
| Circumstance | Comments | References |
| SVCS | 3 in 4 cases caused by LC | 1 |
| Urgent: stent or radiotherapy | 2 | |
| PE in LC | Risk: 3.3% of DVT and 1.6% of PE | 3 |
| Invasion/migration of LC cells | May obstruct great vessels | 4 |
| Dissemination or invasion | NMR and TT or TE echocardiogram useful | 5,6 |
| Cardiac metastases | Infrequent. May cause: pericardial effusion, dyspnea, arrhythmias, arterial hypotension, angina or cerebral embolism | 7–12 |
| ECG: J wave, arrhythmias, blockages, ischemia, etc. | 8,12–14 | |
| Paraneoplastic syndromes | Orthostatic hypotension | 15 |
| SIADH or ectopic secretion of ACTH | 15–17 | |
| Paraneoplastic dysautonomia | 18,19 | |
| Paraneoplastic myopathy | 20 | |
| Nonbacterial thrombotic endocarditis | 15,21,22 | |
| Symptoms: HF, Arterial hypotension, AHT, arrhythmias, embolisms | 15,17,18,20–22 | |
| B. Tnm-staging classification | ||
|---|---|---|
| Circumstance | Descriptor | References |
| Parietal pericardium involvement | T3 | 23–25 |
| Direct cardiac invasion | T4 | 23–25 |
| Great vessel invasion | T4 | 23–25 |
| Presence of pericardial nodules | M1a | 23,24,26 |
| Presence of pericardial effusion | Mia | 23,24,26 |
| Isolated cardiac metastasis | M1b | 23,24,26 |
| Multiple metastases just in the heart | M1c1 | 23,24,26 |
| Multiple metastases in the heart and elsewhere | M1c2 | 23,24,26 |
SVCS: superior vena cava syndrome; LC: lung cancer; DVT: deep vein thrombosis; PE: pulmonary embolism; NMR: nuclear magnetic resonance; TT: transthoracic; TE: transoesophageal. AHT: arterial hypertension; ECG: electrocardiogram; SIADH: syndrome of inappropriate antidiuretic hormone secretion; ACTH: adrenocorticotropic hormone; HF: heart failure.
Even though it is considered to be safe,146,147 it may cause tachycardia, arterial hypertension and ischemia, among other conditions, especially in the elderly and patients with severe heart disease.148,149 Therefore, it is always recommended to monitor the electrocardiography (ECG).146,147,150 Due to hypoxemia and adrenergic response, the fiberoptic bronchoscopy should be delayed for between 4 and 6 weeks for patients who have suffered MIs.146,147,151 Unstable angina is a contraindication and, as with arrhythmias and severe atrioventricular blocks, must be resolved beforehand.147,151
Heart complications and cardiovascular risk assessment for patients eligible for surgeryOld age and smoking induce cardiovascular comorbidity,152–155 which generally worsens the prognosis.153–155 In addition, after lung cancer (LC) surgery, between 1% and 17% of patients may experience complications,156 such as MIs and cardiac insufficiency (in ≤1%)157,158 and atrial fibrillation (5–25%),157,159 all of which can be prevented with treatment.160 Therefore, we need to assess the risk to heart disease patients by way of clinical factors and the Thoracic Revised Cardiac Risk Index.152
Surgery for carefully selected patients with non-small cell LC: As well as the situation where it coexists with IHD,158,161,162 it can also be seen to affect the heart and great vessels. 19.9% of patients in stage IIIA with cardiac invasion live for at least 5 years (T4)163 and 17–36% do so with superior vena cava invasion164–166 as well as 17–60% with aortic involvement.164,167
Cardiotoxicity following radiotherapy (RT) found in LCThere is a relationship between the dose administered and cardiotoxicity (e.g., MIs, unstable angina pectoris and arrhythmias), which may be due to vascular damage being induced.168–175 Previously being diagnosed with heart disease, receiving chemotherapy and old age are all risk factors.168,170,176 High RT doses shorten lifespans.170,171,177–180 It would be wise to plan RT administration with mitigation strategies, limiting regional doses and the impact on implantable cardiac devices as much as possible.170,171,179,181,182
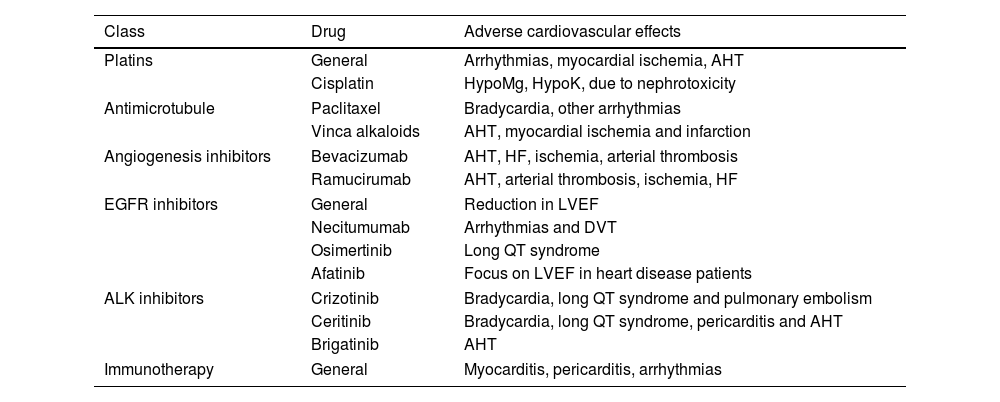
Cardiotoxicity following chemotherapy and new LC treatmentTreatments of Lung Cancer for which adverse cardiovascular effects are most frequent are shown in Table 4.183–190,173,191
Medication for the treatment of Lung Cancer for which adverse cardiovascular effects are most frequent.
| Class | Drug | Adverse cardiovascular effects |
|---|---|---|
| Platins | General | Arrhythmias, myocardial ischemia, AHT |
| Cisplatin | HypoMg, HypoK, due to nephrotoxicity | |
| Antimicrotubule | Paclitaxel | Bradycardia, other arrhythmias |
| Vinca alkaloids | AHT, myocardial ischemia and infarction | |
| Angiogenesis inhibitors | Bevacizumab | AHT, HF, ischemia, arterial thrombosis |
| Ramucirumab | AHT, arterial thrombosis, ischemia, HF | |
| EGFR inhibitors | General | Reduction in LVEF |
| Necitumumab | Arrhythmias and DVT | |
| Osimertinib | Long QT syndrome | |
| Afatinib | Focus on LVEF in heart disease patients | |
| ALK inhibitors | Crizotinib | Bradycardia, long QT syndrome and pulmonary embolism |
| Ceritinib | Bradycardia, long QT syndrome, pericarditis and AHT | |
| Brigatinib | AHT | |
| Immunotherapy | General | Myocarditis, pericarditis, arrhythmias |
AHT: arterial hypertension; HF: heart failure; LVEF: left ventricular ejection fraction; DVT: deep vein thrombosis.
Immunotherapy, mostly in combinations, may cause, after approximately 30 days, myocarditis (3.2/1000) or pericarditis (8.3/1000), with mortality at 30–50% and about 20%, respectively.184,187,188,192–194 Even survivors have been found to have a greater rate of cardiac pathology.195
For patients with heart disease, treatment and monitoring for early signs of illness (e.g., with markers and cardiac imaging) need to be adapted.183,185,190,173,196 When toxicity is suspected, suspending the therapy and using specific medication should be considered.182,185,192–195,197
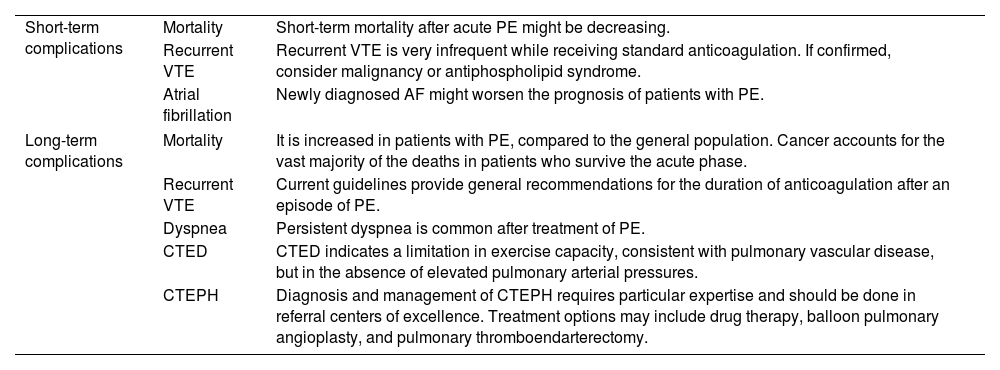
Pulmonary embolismPulmonary embolism (PE) is a common cause of vascular death, while the patients who survive this acute condition may experience long-term cardiovascular and non-cardiovascular complications (Table 5).198–200
Cardiovascular complications after acute symptomatic pulmonary embolism.
| Short-term complications | Mortality | Short-term mortality after acute PE might be decreasing. |
| Recurrent VTE | Recurrent VTE is very infrequent while receiving standard anticoagulation. If confirmed, consider malignancy or antiphospholipid syndrome. | |
| Atrial fibrillation | Newly diagnosed AF might worsen the prognosis of patients with PE. | |
| Long-term complications | Mortality | It is increased in patients with PE, compared to the general population. Cancer accounts for the vast majority of the deaths in patients who survive the acute phase. |
| Recurrent VTE | Current guidelines provide general recommendations for the duration of anticoagulation after an episode of PE. | |
| Dyspnea | Persistent dyspnea is common after treatment of PE. | |
| CTED | CTED indicates a limitation in exercise capacity, consistent with pulmonary vascular disease, but in the absence of elevated pulmonary arterial pressures. | |
| CTEPH | Diagnosis and management of CTEPH requires particular expertise and should be done in referral centers of excellence. Treatment options may include drug therapy, balloon pulmonary angioplasty, and pulmonary thromboendarterectomy. | |
PE, pulmonary embolism; VTE, venous thromboembolism; AF, atrial fibrillation; CTED, chronic thromboembolic disease; CTEPH, chronic thromboembolic pulmonary hypertension.
Over the past 4 decades, short-term mortality after acute symptomatic PE has been in decline.201,202 Researchers at the RIETE Registry have examined temporal trends in risk-adjusted rates of 30-day all-cause and PE-related mortality among 23,858 patients with PE.201 Adjusted rates of all-cause mortality significantly decreased from 6.6% in the period 2001–2005 to 4.9% in the period 2010–2013 (adjusted rate ratio per period, 0.84). Similarly, the rates of PE-related mortality decreased over time, from 3.3% in 2001–2005 to 1.8% in 2010–2013 (adjusted rate ratio per period, 0.73).
Early cardiopulmonary morbidity from acute PE includes recurrent PE and new-onset AF. Recurrent venous thromboembolism (VTE) while on anticoagulant therapy is unusual and should prompt the following: (1) a revaluation of whether VTE is actually recurrent; (2) an evaluation of compliance with anticoagulant therapy; (3) further checks for conditions such as underlying malignancies, antiphospholipid antibody syndrome, Behcet's disease and inflammatory bowel disease. AF may also occur soon after PE is diagnosed203 and the majority of studies and systematic reviews indicate an association between coexisting AF and adverse outcomes of PE.203,204
Long-term mortality and morbidityStudies have reported an increased risk of long-term mortality among PE patients compared to the general population. One study enrolled 866 patients with PE who were compared with 334 patients who had been suspected of having the condition but who were later discovered not to have it.205 Compared with patients without PE, those with unprovoked PE were 1.4 times more likely to be at risk of mortality whereas others with provoked PE were 2.9 times more at risk.
The risk of recurrent VTE increases after anticoagulant therapy is terminated.206,207 Guidelines recommend treatment with anticoagulation for no longer than 3 months in most patients with PE provoked by a major reversible risk factor.208 Patients whose PE is due to a persistent risk factor should receive indefinite anticoagulation, which should only be terminated when the risk factor is adequately managed.208 Clinical practice guidelines suggest indefinite anticoagulation for patients with unprovoked PE.209 For those who have contracted it because of a minor transient risk factor, the use of additional information, the assessment of the risk of bleeding and patient preferences may be helpful to decide upon the duration of the anticoagulant therapy.
Post-PE syndrome encompasses three groups of patients according to the following conditions: dyspnea with functional limitations without identifiable pulmonary vascular disease; chronic thromboembolic disease (CTED); chronic thromboembolic pulmonary hypertension (CTEPH). CTED describes a group of patients with mean pulmonary artery pressure (mPAP)<25mmHg at rest, who have persistent vascular obstructions, and an impaired response to exercise.210 Cardiopulmonary exercise testing demonstrates a limitation to exercise consistent with pulmonary vascular disease.211 CTEPH is defined by the presence of a mPAP>25mmHg at rest with pulmonary artery wedge pressure<15mmHg and mismatched perfusion defects during the ventilation/perfusion scan, despite having received at least 3 months of anticoagulation.210 All patients with CTEPH require lifelong anticoagulation. Endarterectomy is the treatment of choice for patients who are surgical candidates with operable disease. For those who are regarded as not being suitable for an operation or have residual PAH after endarterectomy, Riociguat and/or balloon pulmonary angioplasty may note an improvement in hemodynamics and quality of life.
In conclusion, cardiovascular complications are not infrequent after an episode of acute symptomatic PE. Follow-up algorithms are key to counsel patients accurately on treatment options and to inform them of the duration of their course and to detect complications early, thus increasing the chance that a timely intervention can occur if deemed appropriate.
Community-acquired pneumoniaCommunity-acquired pneumonia (CAP) is associated with significant cardiovascular morbidity and mortality.14 This happens both in the short and long term and is due to specific pathophysiological mechanisms.
Frequency and types of episodesThe most frequent cardiovascular events (CEs) are arrhythmias and heart failure, followed by ischemic heart disease.212 Up to 10% of patients show silent myocardial ischemia during CAP.213 Non-cardiological vascular events, such as cerebrovascular accidents and/or venous thromboembolic disease represent a lower percentage. The period of greatest risk for developing cardiovascular complications is in the short term, with an incidence between 10% and 26%.14,212,214 In the long term, the incidence of CEs is 6.6–9.3%.14,212 In summary, the cumulative incidence during the first year after CAP means that up to 1 in 5 patients develop a type of CE.14,212
Risk factors and pathophysiological mechanismsThe main risk factors are related to demographic data (aging, smoking), comorbidities (previous heart disease), severity and microbiology (i.e. Streptococcus pneumoniae).212,215 There are several pathophysiological mechanisms underlying cardiovascular risk.216
Platelet activation and neutrophil extracellular traps (NETs)Patients with high platelet activation frequently present acute MIs.217 NETs are networks released by neutrophils in response to stimuli such as infections, which are made up of DNA, histones and proteases capable of trapping bacteria.218,219 The interaction of neutrophils with bacteria, damaged endothelium and activated platelets favors the production of NETs.220 In turn, NETs increase platelet activation and induce the growth and progression of atheromatous plaque.221
Endothelial damagePneumonia produces endothelial dysfunction with NETs, which favor glycocalyx degradation, the disassembly of intercellular junctions, endothelial cell death, the growth of atheromatous plaques and the inducement of a procoagulant state.222 This endothelial damage is linked to cardiovascular risk in CAP and can be identified using biomarkers.223
Myocardial injuryMicroorganisms such as S. pneumoniae are associated with an increased risk of contracting a CE.224 Pneumococcus can invade the myocardium, forming inclusions inside it. After treatment, these inclusions are replaced by connective tissue, favoring arrhythmogenic risk.225 In addition, the release of pneumolysin promotes myocardial damage.226
Systemic inflammationThe inflammatory response can cause damage locally and in other distant organs. An initial rise in inflammatory markers and the persistence of them is associated with a poor response to treatment and death.227 This inflammation can cause endothelial dysfunction, the destabilization and rupture of atherosclerotic plaque and an increased risk of thrombogenesis.
Other mechanismsOther mechanisms include an imbalance between oxygen supply and demand and oxidative stress.
Therapeutic optionsFinally, there are preventive or therapeutic measures such as vaccinations,228 antiplatelets,229 statins230 and corticosteroids, as well as other more recent ones to treat for cardiovascular risk in CAP.231 These options could reduce the cardiovascular risk of pneumonia. However, to date, there is a lack of data to support a specific treatment.
Interstitial lung diseasesDiffuse interstitial lung diseases (ILDs) may have the following features: (a) fibrotic predominance, with idiopathic pulmonary fibrosis (IPF) as the paradigm and being therapeutically based on antifibrotics232–234; (b) inflammatory predominance, like in sarcoidosis, with an improvement after glucocorticoid treatment in most cases232; (c) any other well-defined pathogenic bases, such as proliferating cysts, which occur in deposits in the case of amyloidosis or may be hereditary or genetic, as can be seen in Hermansky–Pudlak syndrome.232 With fibrosing ILDs, especially IPF, cardiovascular comorbidity is the most common outcome (reaching 77.9%).235,236 These alterations may be linked to risk factors that are common to both types of illness, such as smoking or aging, but they could also be due to the unique modifications in the vascular bed caused by the increase in the extracellular matrix.233 Nevertheless, ILDs such as sarcoidosis may be associated with cardiovascular involvement,232 which in one way or another increases the risk of death.234
These illnesses include IHD, ventricular dysfunction, arterial hypertension, PE and PAH.235 For patients with arterial hypertension, it is important to control it and monitor them, intensifying antihypertensive treatment if necessary and avoiding the use of ACE inhibitors if coughing worsens.237,238 If dyspnea and hypoxemia increase, echocardiography and ECG are recommended, whereas if thoracic pain appears, serum enzymes of myocardial necrosis should be determined and exercise test to provoke ischemia should be run.237,238 In the event that patients with IPF or progressive pulmonary fibrosis (PPF) being treated with Nintedanib who suffer from acute MIs require antiaggregation and anticoagulation, the antifibrotics should be suspended temporarily until the acute event has been resolved.238 If stent placement is needed, the type of device can be chosen, such as the Biofreedom® stent, when double antiaggregation is required for a short period of time.238 In all other cardiovascular illnesses, it is advisable to use medication that does not interfere with antifibrotics.
PE and PAH are linked to fibrosing ILDs more often than in the general population.237,239 A PE diagnosis should be suspected if dyspnea suddenly becomes worse, especially in cases of previous immobilization or limitation of activity that may favor deep vein thrombosis (DVT), thoracic pain or haemoptysis, as well as newly appearing hypocapnia during gasometry.239 The clinical presentation of PE may seem identical to a severe exacerbation of an ILD, meaning that if there is a sudden increase in dyspnea and hypoxemia. A CT scan with contrast and D-dimer test is strongly recommended to rule out this possibility.239 The treatment is the same as for the general population. As for PAH, the prevalence is variable, although it tends to be more frequent in IPF and combined pulmonary fibrosis and emphysema syndrome.239 An echocardiogram is recommended as a screening method for patients whose desaturation on exertion has worsened or whose diffusing capacity for carbon monoxide (DLCO) deteriorated disproportionately when taking the drop in forced vital capacity (FVC) into account.240 Right cardiac catheterisation diagnoses PAH, its severity and prognostic factors.240 Unlike other respiratory diseases, group 3 PAH associated with ILD represents a comorbidity that lowers the chances of survival, regardless of the degree of severity.241 Treatment is assessed by a multidisciplinary team to decide upon the best therapeutical approach, taking into account the result of the clinical trials with pulmonary antihypertensives.242,243
Moreover, an ILD in systemic diseases, such as sarcoidosis and amyloidosis, may result in cardiomyopathy. By performing echocardiography and cardiac magnetic resonance imaging (MRI) scans, a diagnosis of cardiomyopathy can be made.244 Systemic sclerosis is frequently connected with PAH and ILDs, both forms of pulmonary involvement being the main causes of death.
AsthmaThe clinical evidence supporting the hypothesis that asthma increases the risk of various cardiovascular pathologies being contracted by patients is not unfounded. Late-onset asthma may also heighten the risk of CVDs occurring. Furthermore, recent evidence suggests that cigarette smoking is not unusual in adult-onset asthma and smokers with this respiratory condition have higher rates of cardiovascular comorbidities.245,246 Nevertheless, it has been suggested that asthma is a comorbidity rather than a cause of CVDs. In a retrospective study of the Spanish Society of Pneumology and Thoracic Surgery (SEPAR), among patients admitted to 13 Spanish hospitals, the most frequent causes of death in asthmatic patients were CVDs, making up 29.3% of the cases.247
Recent studies seem to reveal an asthmatic phenotype in which endothelial dysfunction phenomena are more likely to develop, leading to the triggering of cardiovascular episodes; this phenotype may affect female patients with late-onset asthma.9,15,248–260 A more detailed analysis indicates that female asthmatics with adult-onset asthma and patients with chronic rhinosinusitis, nasal polyposis and intolerance to nonsteroidal anti-inflammatory drugs are the patient groups at highest risk of developing CVDs. In this regard, in recent years studies have been published on the association of asthma with CVDs,9,15,250–260 some with retrospective data15,248–250 and others with prospective data, in both cases from a range of epidemiological cohorts.250–256 There is evidence that asthma exacerbations are associated with a significantly increased risk of CVDs (e.g., acute MIs and ischemic strokes), particularly in the first 1-week period after these exacerbations begin.261
Systemic inflammation is a potential link between asthma and CVDs. The release of cytokines is central to almost every stage of the immune response in asthma and the consequent systemic dysregulation of inflammatory homeostasis may explain why there could be a higher risk of developing CVDs. The presence of eosinophils and cysteinyl leukotrienes, strongly proinflammatory cytokines found in high concentrations in asthmatic bronchioles and also active in atherosclerotic plaque, may contribute to the development of arteriosclerosis and coronary vasospasm.262
Rich et al.263 observed that 36% of the patients who suffered from ischemia with no obstructive coronary arteries (INOCA) had a history of obstructive bronchial disease that, in most cases, corresponded to asthma. Histological studies of some deaths from IHD have revealed the existence of an inflammatory process characterized by eosinophils and mast cells infiltrating the adventitia and periadventitial tissue of the coronary vessels.264–266 Inflammation of the vascular adventitia may be responsible for there being fibromuscular hyperplasia in the coronary vessels, which in turn may help explain the exaggerated vasoconstrictor reaction to nonspecific stimuli. The potential role of eosinophilic inflammation in INOCA has also been observed in these patients due to eosinophil levels in their blood being high, the severity of the process directly correlating with the intensity of blood eosinophilia.267,268
A Canadian study claims that there is a relationship between asthma and IHD, since it was shown that treatment with inhaled glucocorticoids significantly reduced MI incidence in asthmatic patients.269
A recently identified association is the relationship between asthma and PE. According to a study published in the European Respiratory Journal,270 moderate or severe asthmatics are highly at risk of developing PE, although this association has not been observed for venous thrombosis of the lower limbs. Besides this, managing asthma with an excessive use of β2-agonists, the discontinuation of β-blockers and aspirin or other non-steroidal anti-inflammatory drugs in patients with aspirin-exacerbated respiratory disease may play a role in subsequent cardiovascular event risks. Corticosteroids and inhaled bronchodilators are independently associated with an increased risk of AF.271,272 In particular, high doses of β2-agonists for asthma makes the risk of arrhythmias occurring more likely. This probably also happens because the majority of patients with severe asthma have electrolyte imbalances, well-known for causing cardiac arrhythmia disturbances in patients with chronic stable asthma and in those who suffer asthma attacks. Excessive β2-agonist use is also associated with an increased risk of MIs, congestive heart failure, cardiac arrest and sudden cardiac death.272
Lung transplantationLung transplant recipients are high-risk patients for immediate or delayed cardiovascular complications, either due to pre-existing comorbidities, intrinsic surgical risks or following life-saving immunosuppression. Cardiovascular events account for the fourth leading cause of death within the first month (13.1% of deaths) and constitute 6.2% (8% in patients with COPD) of deaths at five years.273,274 Risk minimization strategies include controlling risk factors, early diagnosis and treating comorbidities suitably.
Pulmonary hypertension and transplantationPAH is usually identified in 30–60% of patients on the waiting list, generally those with moderate severity.275–279 Sufferers experience accelerated deterioration, an increased risk of mortality while on the waiting list275,278–281 and post-transplant morbidity, although there is no negative impact on early mortality.275,279 To detect PAH and its impact on the right ventricle, echocardiography is the preferred non-invasive screening test. Right heart catheterisation (RHC) is the gold standard for diagnosis. Theoretically, all patients on the waiting list, or, at the very least, those showing signs of PAH, should undergo RHC when clinical deterioration is not justified by the degree of hypoxemia or when clinical worsening is observed during their time on the waiting list.282
Global cardiovascular riskThe possibility of developing atherosclerotic vascular disease (ASCVD) during lung transplantation (LT) is calculated as the intrinsic procedural risk283 plus the presence of risk factors (RFs) in the recipient, as shown in Fig. 4. Not all RFs carry the same weight, nor do they necessarily involve silent pathologies. Diabetes mellitus (DM) is considered an ischemic equivalent, while arterial hypertension and hypercholesterolemia have a multiplicative effect when they coexist. The best strategy is always prevention, where actions are aligned with internationally recognized consensus guidelines.284 Secondly, a screening protocol for silent ASCVD should be implemented, for IHD and carotid disease at least, in which coronary angiography and Doppler ultrasound should be given to patients waiting to be added to the transplantation waiting list or, failing that, to those manifesting RFs. To assess the risk of ASCVD, the most applicable indices are the Framingham Equation285 and the American College of Cardiology (ACC)’s ASCVD Risk Estimator,286,287 a more up-to-date online risk calculator, the latter of which offers more dynamic assessments in that it evaluates its modification after therapeutic interventions. To evaluate the risk of a coronary event in the next ten years, a color chart is applied, and to assess the risk of intra- and postoperative cardiac complications, the Lee Index288,289 and the National Surgical Quality Improvement Program (NSQIP) Model290,291 are used. There is a discussion as to whether these clinical models are robust enough for cardiovascular risk prediction, as they could be complemented with new parameters such as coronary calcium detection, calcified plaque, intimal thickening or medial layer thickening in carotid arteries, genetic factors, serum biomarkers or subclinical atherosclerosis markers.292 Due to the complexity of detection, their uncertain effectiveness and the lack of comparative studies with current models, it is questionable whether they are applicable or not.
TreatmentTherapeutic strategies for risk control or minimization can be either pharmacological or non-pharmacological. Maintaining a healthy weight, avoiding a sedentary lifestyle, minimizing steroid dosage and controlling comorbidities all require healthy habits to be implemented and, in many cases, pharmacological treatment to be modified. If vascular obstructions requiring treatment are identified, it is preferable to treat them with balloon angioplasty and for uncovered stents to be inserted. Although these stents have a high likelihood of becoming obstructed, they only require one month of triple antiplatelet therapy and maintenance with aspirin, allowing patients to be included on the waiting list after thirty days. Drug-eluting stents are more effective than uncovered stents for controlling restenosis but require a longer anticoagulation period293 and any scheduled surgical intervention is discouraged until a minimum of 6 months has passed.283,294
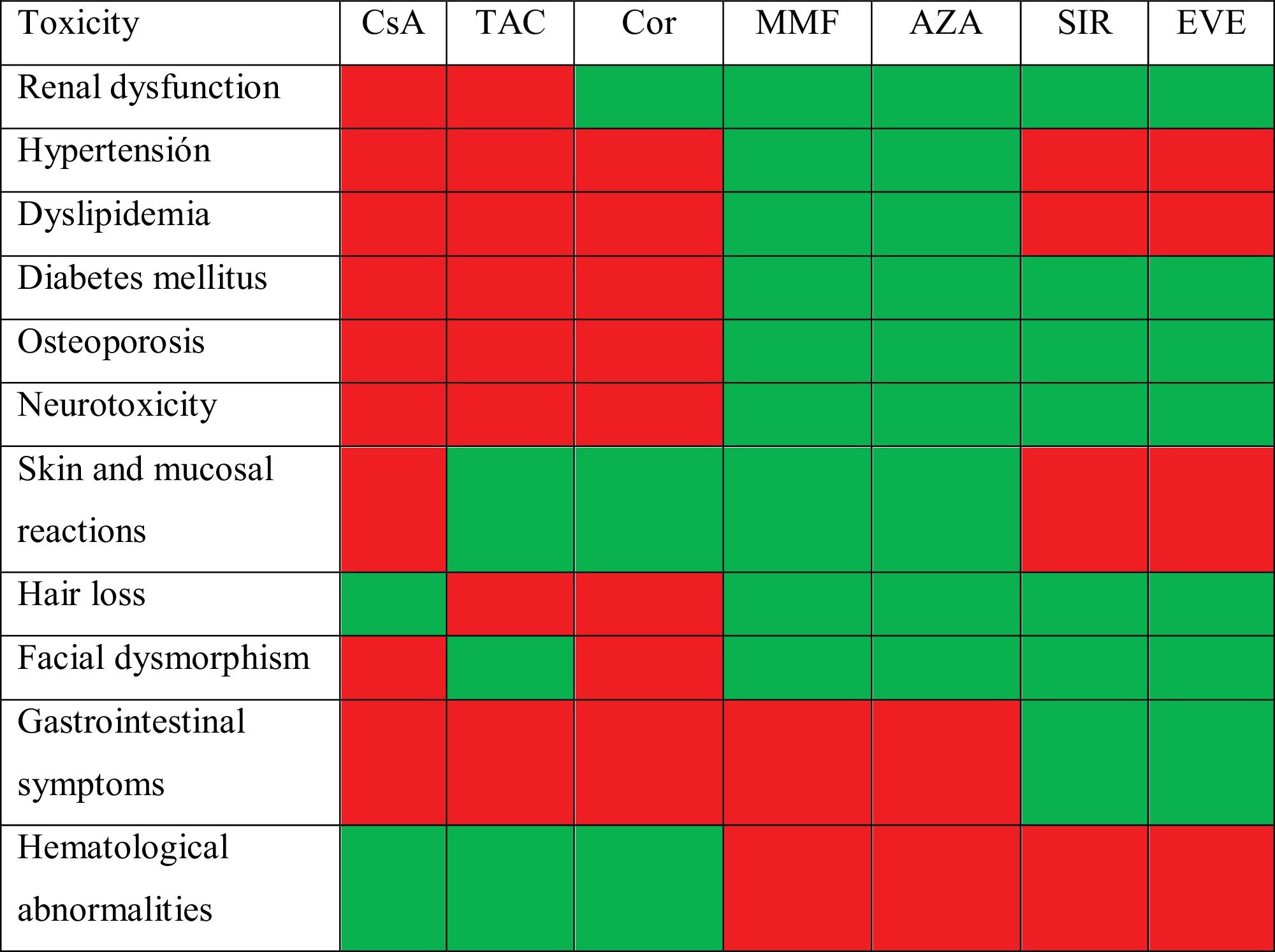
ImmunosuppressionEssential immunosuppression (IS) in lung transplantation aims to prevent graft rejection while maintaining sufficient immunity to guarantee response to infections, all with the lowest possible toxicity. These drugs have significant side effects, many of which are detrimental to the cardiovascular system,295 as listed in Table 6.
Arterial hypertensionIts prevalence exceeds 80% at 5 years post-LT.273 IS, including steroids, promotes its development, along with obesity, DM, renal insufficiency and advanced age.295 Aiming for blood pressure values below 130/80mmHg, or 120/80mmHg if microalbuminuria is detected, is advisable. The therapy of choice depends on the stage of development, side effects and drug interactions. Successful control requires a multidisciplinary approach that includes general measures, minimizing or changing IS and specific pharmacological therapy, with dihydropyridine calcium antagonists (amlodipine, nifedipine) being the first choice when there is little or no proteinuria.
Renal dysfunctionIts prevalence, considering chronic kidney disease with a glomerular filtration rate below 60ml/min, exceeds 50% at 5 years post-LT.273,295 Risk factors for its development include acute renal failure in the peri-transplant period, arterial hypertension, DM, elderly or cystic fibrosis (CF) recipients, cytomegalovirus (CMV) infection, the use of nephrotoxic drugs and the cumulative dose of calcineurin inhibitors.296 The solution does not always involve reducing or modifying immunosuppression, as that can compromise the viability of the lung graft. In many cases, referral to nephrology is necessary to establish an aetiological diagnosis, with renal biopsy sometimes required. Key factors in decision-making include reducing salt intake, controlling acidosis, managing RFs, minimizing potentially nephrotoxic IS and monitoring albuminuria.
DyslipidaemiaThis disorder is identified in over 50% of recipients at 5 years post-LT.273 IS, along with age, sedentary lifestyle and corticosteroid use are RFs for its development.295,297 The target values for lipid control are not clear and should be determined based on ASCVD risk. These patients’ lifestyles need to be changed and, if necessary, statins should be used at increasing doses. When dyslipidaemia is not controlled, ezetimibe and subcutaneous PCSK9 inhibitors, such as evolocumab and alirocumab, may be added.297 Rhabdomyolysis and renal failure should be monitored due to the interaction between statins and calcineurin inhibitors.
HyperglycaemiaThe onset of hyperglycaemia exceeds 20% in the first year of LT and 40% at 5 years,273 with an even higher percentage in CF recipients. RFs include CF itself, age, post-LT transient hyperglycaemia, episodes of rejection, prolonged corticosteroid use at high doses, hypomagnesemia, calcineurin inhibitors and a risk of hepatitis C and CMV infections.273,295,298 The target fasting glucose level should be below 140mg/dl under treatment. Alongside lifestyle modifications, biguanides (metformin) are the drugs of choice, except in cases of congestive heart failure or chronic renal failure, where SGLT-2 inhibitors (or glifozins) are preferred, or ASCVD, where GLP-1 agonists (or glutides) are recommended.298
ConclusionsThe coexistence of respiratory diseases and CVDs is very common, conditioned by both shared aetiological factors and the treatment administered or the direct effect of some diseases on others. This coexistence makes modifying diagnostic and therapeutic management necessary and is also a relevant prognostic factor.
FundingThere is not any external funding for this article.
Authors’ contributionAll authors have taken part in conceptualization, writing the original draft, revised the article critically and approved the version to be published.
The authors have accepted responsibility for the entire content of this manuscript and consented to its submission to the journal.
Conflicts of interestThe authors of the article “Respiratory pathology and cardiovascular diseases: A Scoping Review” do not have any conflict of interest related to the article.