There is a common agreement on the existence of dysfunctional cortico-striatal–thalamus-cortical pathways in OCD. Despite this consensus, recent studies showed that brain regions other than the CSTC loops are needed to understand the complexity and diversity of cognitive and emotional deficits in OCD. This review presents examples of research using functional neuroimaging, reporting abnormal brain processes in OCD that may underlie specific cognitive/executive (inhibitory control, cognitive flexibility, working memory), and emotional impairments (fear/defensive, disgust, guilt, shame). Studies during resting state conditions show that OCD patients have alterations in connectivity not only within the CSTC pathways but also in more extended resting state networks, particularly the default mode network and the fronto-parietal network. Additionally, abnormalities in brain functioning have been found in several cognitive and emotionally task conditions, namely: inhibitory control (e.g., CSTC loops, fronto-parietal networks, anterior cingulate); cognitive flexibility (e.g., CSTC loops, extended temporal, parietal, and occipital regions); working memory (e.g., CSTC loops, frontal parietal networks, dorsal anterior cingulate); fear/defensive (e.g., amygdala, additional brain regions associated with perceptual – parietal, occipital – and higher level cognitive processing – prefrontal, temporal); disgust (e.g., insula); shame (e.g., decrease activity in middle frontal gyrus and increase in frontal, limbic, temporal regions); and guilt (e.g., decrease activity anterior cingulate and increase in frontal, limbic, temporal regions). These findings may contribute to the understanding of OCD as both an emotional (i.e., anxiety) and cognitive (i.e., executive control) disorder.
OCD is a disorder characterized by the presence of intrusive unwanted thoughts, images, ideas, urges involuntarily entering consciousness (obsessions), which the individual tries to neutralize by repetitive behaviors (e.g., checking) or mental actions (e.g., praying) (compulsions). OCD is probably one of the most disabling psychiatry disorders with a consistent cross cultural lifetime prevalence of about 2%, typical onset during adolescence and a female prevalent ratio of 1.5:1.0.1
Recently, there was a major shift in the classification of OCD in the Diagnostic and Statistical Manual of Mental Disorders – Fifth Edition (DSM-V).2 Classified in the previous editions as part of anxiety disorders, OCD is now integrated in a new cluster designated Obsessive–Compulsive and Related Disorders (OCRD) along with body dysmorphic disorder, hoarding disorder, trichotillomania, and excoriation disorder. Within the broader diagnostic cluster there was switch from anxiety as the core symptom to the emphasis on repetitive behaviors or repetitive mental actions.3 A similar approach has been undertaken by the working group for the eleventh edition of the International Classification of Diseases and Related Health Problems (ICD-11) with the proposal for the creation of a new category grouping all OCRD disorders (OCD, Body dysmorphic disorder, olfactory reference disorder, hypochondriasis, hoarding disorder, and body focused repetitive behaviors). This new diagnostic cluster is based on common features of intrusive thoughts and repetitive behaviors and grounded on evidence for the existence of shared pathophysiological and epidemiological features.4
The integration of OCD in the OCRD cluster, underscoring repetitive behaviors, fails, first, to recognize the continuity and the functional link between obsessions and compulsions3 and, second, OCD symptomatic heterogeneity which may be associated with distinct psychological and neurobiological mechanisms.5
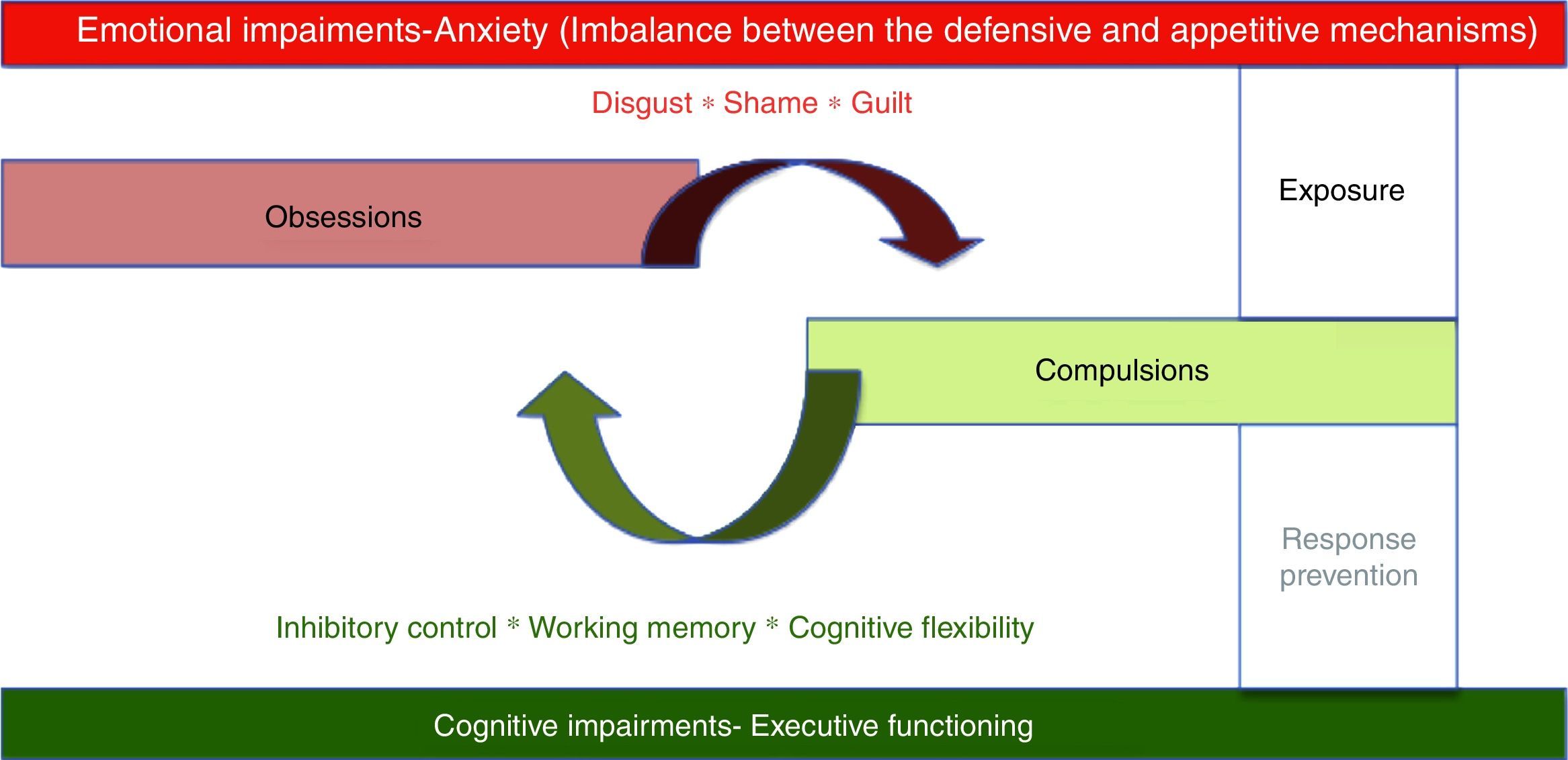
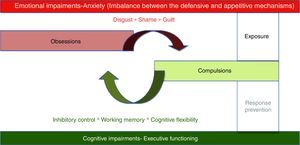
At the psychological level, OCD is thought to involve intrusive obsessions about eventual threats followed by repetitive compulsions or ritual aimed at neutralizing threats. Compulsions produced a temporary relieve from unpleasant maintaining the cycle by negative reinforcement.6 Two psychological mechanisms seem to be associated with this obsessive–compulsive cycle1: an emotional mechanism characterized by intense anxiety associated with intrusive thoughts of impending danger2; a cognitive mechanism exemplified by executive deficits (see Fig. 1).
The intense emotional arousal reported by patients has led to the classification of OCD as an anxiety disorder by the DSM-IV.7 The common core feature of this group of disorders was the presence of prevalent anxiety symptoms. However, anxiety is probably the expression of an affective-motivational imbalance between the defensive (i.e., fear response) and appetitive mechanisms (i.e., ingestion, copulation, and care giving responses)8 which will be responsible for a more extended dysregulation at higher level emotional processing as expressed in OCD propensity for disgust, shame, and guilt.9
There is abundant evidence on the existence of executive functioning deficits in OCD.10 Different aspects usually associated with executive functioning, are relevant for OCD: response inhibition, working memory and cognitive flexibility.11
First line psychotherapeutic treatment for OCD involves two strategies intended to deal with each of those mechanisms present in OCD – exposure and response/ritual prevention. While exposure involves confronting patients with symptom provoking stimuli with the objective of regulating emotional expression, response/ritual prevention is intended to enhance inhibitory control, promoting cognitive flexibility and improving working memory.12
Several distinct pathophysiological mechanisms have been associated with OCD at the immunological,13 neurochemical,14 and neuroanatomical levels.15 A variety of neuroanatomical models were proposed to explain the pathogenesis of OCD with a common agreement on the existence of dysfunctional cortico-striatal–thalamus-cortical (CSTC) circuits.6 Evidence for the involvement of the CSTC in self-regulation16 contributed to reframe OCD as a response inhibition disorder rather than an anxiety disorder.
Despite this consensus on the role of frontal–subcortical loops in OCD, recent studies found evidence for the existence of functional impairments in more extended brain networks.17 In what follows, we will report examples of research showing the existence of abnormal brain processes in OCD that may underlie specific cognitive/executive (inhibitory control, cognitive flexibility, working memory), and emotional impairments (fear/defensive, disgust, guilt, shame). We begin by presenting the evolution in OCD brain functioning research from PET and SPECT studies to more recent symptom provocation fMRI studies. Then we discuss the results of current research on alterations on brain's functional connectivity in OCD using resting state paradigms. This will be followed by an illustration of studies on functional brain alterations during the processing of cognitive/executive (working memory, cognitive flexibility, and inhibitory control), and emotional tasks (fear/defensive, disgust, guilt, shame).
The evolution of brain functional studies with OCDThe first functional studies in OCD, using positron emission tomography (PET) to study the brain's level of glucose metabolism, reported increased metabolism in regions associated with the CSTC loops, such as: orbitofrontal cortex, caudate, thalamus and putamen.18
Likewise, initial studies with symptom provocation paradigms, still with rudimentary fMRI methods, showed that several regions were associated with OCD symptoms, namely the lateral frontal cortex, medial orbital gyrus, anterior cingulate, temporal cortex, insular cortex, amygdala and caudate, putamen and globus pallidus.19
In one of the first narrative reviews of brain functional studies in OCD, Saxena et al.20 confirmed an hyperactivation of CSTC regions such as such orbitofrontal cortex, caudate nucleus, thalamus and anterior cingulate. However, the same authors, a few years later, alert for the fact that, despite evidence for hyperactive CSTC regions, several inconsistencies remained in findings across studies.21
The first extended quantitative meta-analysis on functional neuroimaging studies, was published only in 2004,22 including controlled studies done with PET (positron emission tomography) and SPECT (single-photon emission computed tomography) between 1987 and 2003. Again, the authors confirmed increased metabolism in the orbitofrontal cortex, caudate nucleus and thalamus, pointing out to alterations in circuitry associated with the regulation of cognitive/executive functions such as working memory, cognitive flexibility and inhibitory control.
Later, Rotge et al.23 did a quantitative voxel based meta-analysis, this time of fMRI (functional magnetic resonance imaging) and PET studies with symptom provocation paradigms. The eight studies included in this meta-analysis found, once more, support for the hyperactivation of the CSTC circuits. However, the authors reported also data on the activation of other regions, namely the left superior temporal gyrus, precuneus and dorsolateral prefrontal cortex. This last data is suggestive of altered functioning in other psychological processes, such as memory monitoring (superior temporal gyrus), mental visualization and visual-spatial memory (precuneus).
In a narrative analysis by Del Casale et al.,24 most of the results reported Rotge et al.23 were confirmed showing, however, an even more complex picture involving, not only the CSTC pathways, but also parietal, temporal, hippocampal and even cerebellar regions. Consistent with these findings, recent studies showed that OCD patients tend to normalize their brain activity after successful therapy, namely by reducing the activation in the caudate, orbitofrontal and cingulated regions, but also the thalamus, temporal and occipital cortices.25
As suggested by Mataix-Cols,26 some inconsistency in findings may be due to the variability in responses from patients with different subtypes of OCD to distinct symptom provocation paradigms. In order to test this hypothesis, the authors studied if provoking different symptoms (i.e., contamination/washing; aggressive/checking; hording and aversive control) would be responsible for distinct patterns of brain functioning, in a sample of multi symptomatic OCD patients and healthy controls. As hypothesized, the study found that different types of symptomatic provocation were responsible for specific patterns of brain activation. For example, in the washing symptom provocation condition, in a OCD a pattern of increased activations was observed in the bilateral anterior cingulate and orbitofrontal gyri, left middle temporal gyrus, right subgenual anterior cingulate gyrus, left middle frontal gyrus, right caudate, and left dorsal anterior cingulate gyrus. For the checking provocation, increased activations were found for the OCD group in bilateral brainstem nuclei, right putamen/globus pallidus, right thalamus, inferior frontal gyrus, dorsal anterior cingulate, medial/superior frontal, middle/medial frontal, and precentral gyri. Finally, for hoarding, greater activations in OCD patients were evident in the left precentral/superior frontal, fusiform, and right orbitofrontal gyri. Additionally, significant positive correlations were reported between symptomatic characteristics and brain responses to the specific paradigm of symptom provocation.
More recently, Murayama et al.27 used a symptom provocation task to differentiate brain activation between OCD checkers, washers and healthy controls. The study found that, while checkers have a substantially decreased activation (when compared with normal controls) in the left caudate and left anterior cingulate, washers showed an increased activation in more extended regions such as right cerebellum, right posterior cingulate cortex, right medial frontal gyrus, left middle temporal gyrus, and left inferior occipital gyrus. Significant positive correlations were found between severity scores and activations in the left anterior cingulate for checkers and the right orbitofrontal cortex for washers. Again, different brain regions seemed to be associated with different symptomatic profiles in OCD. The correlation data showed that washers seem to have an increased brain response in regions of the CSTC predominantly associated with response inhibition, while checkers show a contrasting activation in areas related with emotional processing and regulation.
In sum, as we increase the level of methodological sophistication in brain functional studies, a more complex pattern of abnormalities emerges, reporting the involvement not only the on typical CSTC loops, but also more extended brain regions. As suggested previously, it is possible that these widespread functional alterations are related to cognitive and emotional impairments specific of different OCD subtypes.
Resting state brain connectivity in OCDMore recently, researchers began looking at functional connectivity in OCD using resting state studies.28 Resting-state functional studies analyze the temporal co-activation of the different brain regions during a resting fMRI acquisition in order to extrapolate the degree of functional connectivity among brain regions. Most often these studies looked at functional connectivity by analyzing temporal synchronization between different brain regions using low frequency fluctuations in the BOLD signal.
Initial functional resting state fMRI studies in OCD confirmed connectivity alterations on the CSTC pathways (see Table 1). For example, Harrison et al.,29 in one of the first resting state studies, testing the hypothesis of abnormal functional connectivity in the CSTC loops in OCD, found an interesting dissociation between the ventral and the dorsal loops, with increased connectivity between ventral caudate and the orbitofrontal cortex contrasting with a decreased connectivity between dorsal caudate/putamen and the lateral prefrontal cortex. This connectivity imbalance in regions associated with distinct CSTC loops confirms increased connectivity in loops related to emotional regulation and inhibitory control (ventral loop) and decreased connectivity in pathways more commonly associated with working memory and cognitive flexibility (dorsal loop). More recently, Chen et al.39 reported a decreased connectivity within the CSTC (dorso-medial prefrontal cortex-thalamus-caudate) along with an increased connectivity between the caudate and regions outside the CSTC (e.g., superior and middle temporal gyrus, middle and inferior occipital gyrus, lingual gyrus, calcarine sulcus, postcentral gyrus, and supplementary motor area). Alteration of connectivity in this specific CSTC loop may be, as remarked by the authors, associated with cognitive and behavioral regulation impairments in OCD while alterations of connectivity with temporal, parietal and occipital regions are possibly related to impairments in memory monitoring, visual spatial and sensory-motor processing. Connectivity abnormalities were further confirmed in a study by Sakai et al.30 observing in OCD an increased connectivity between ventral striate (accumbens and ventral caudate) and several brain regions (e.g., orbital gyrus, rectal gyrus, ventral medial prefrontal cortex, dorsal lateral prefrontal cortex, superior frontal gyrus). A more recent study by Kang et al.31 confirmed that ventral loop CSTC regions abnormally activated in OCD during an inhibition response task showed an increased connectivity in a rest condition. Abe et al.37 attempted to clarify the effective connectivity (i.e., the influence of one region over another) between orbitofrontal cortex and the ventral striatum using a Granger causality analysis. A situation of hyper-influence of the orbitofrontal cortex over the ventral striate was observed in OCD patients, without mediation of any other striate region. Given that we are in presence of a limbic loop, the hyper-influence of the orbitofrontal cortex may be responsible for emotional dysregulation in OCD and related, as suggested by the authors, with impairments in weighting reward and punishment contingencies (i.e., appetitive versus defensive emotional motivational systems).
Studies on resting state brain connectivity in OCD.
| Study Ref. | Method | Participants | Findings for OCD |
|---|---|---|---|
| Harrison et al.29 | Resting state connectivity in the CSTC loop with four striatal seeds (dorsal caudate, ventral caudate/nucleus accumbens, dorsal putamen, ventral putamen) | 21 OCD; 21 matched healthy controls | Increased connectivity between ventral striate and the orbitofrontal cortex ((i.e., ventral caudate/nucleus accumbens and the medial orbitofrontal, anterior frontal, rostral anterior cingulate, and parahippocampal regions); decreased connectivity was observed between dorsal striate and the lateral prefrontal cortex (putamen with the frontal operculum/inferior frontal cortex, and right dorsal caudate nucleus with the right dorsolateral prefrontal cortex) |
| Sakai et al.30 | Resting state connectivity in the CSTC loop with three striatal seeds (dorsal caudate, accumbens and ventral caudate) | 20 unmedicated OCD; 23 matched healthy controls | Increased connectivity between ventral striate and several brain regions (e.g., orbital gyrus, rectal gyrus, ventral medial prefrontal cortex, dorsal lateral prefrontal cortex, superior frontal gyrus) |
| Kang et al.31 | Resting state connectivity using as seeds fronto-striatal regions showing abnormal activation during a previous inhibition response task | 18 unmedicated OCD; 18 matched healthy controls | Increased connectivity between the caudate and middle cingulate cortex and precentral gyrus |
| Beucke et al.32 | Resting state connectivity exploring local and distant connectivity alterations in several brain hubs | 23 unmedicated OCD; 23 medicated OCD; two matched samples (23 each) of healthy controls | Increased distant connectivity for unmedicated OCD in orbitofrontal cortex and subthalamic nucleus with regions outside the CSTC along with increased local connectivity in the orbitofrontal cortex and putamen; medicated OCD, when compared with unmedicated, had a decrease of connectivity in the ventral striatum |
| Hou et al.33 | Resting state connectivity at the regional level using amplitude of low frequency activity (ALFF) | 23 OCD (18 unmedicated); 23 healthy matched controls | Increased connectivity in the orbitofrontal cortex (bilateral) and the anterior cingulate; decreased connectivity in bilateral parietal cortex and the cerebellum |
| Hou et al.34 | Resting state connectivity using whole-brain graph analysis | 39 OCD (33 unmedicated); 40 healthy matched controls; 20 healthy first degree relatives | Increased connectivity in the CSTC loops, contrasting with a decreased connectivity in the occipital cortex, temporal and cerebellum regions; OCD and healthy relatives shared similar patterns of increased connectivity in the bilateral caudate, left orbitofrontal cortex and left middle temporal gyrus |
| Posner et al.35 | Resting state connectivity with 12 (6 each hemisphere) striate regions of interest: limbic CSTC loop (i.e., ventral striate seeds) cognitive loop (dorsal caudate and ventral rostral putamen seeds) and sensorimotor loop (dorsal caudal putamen and the dorsal rostral putamen seeds) | 23 unmedicated OCD; 20 healthy matched controls | Decreased functional connectivity in the limbic loop (left inferior ventral striate with the left anterior cingulate, left medial orbitofrontal cortex, left superior ventral striate with body of the left caudate nucleus); decreased connectivity in the sensory loop between the left dorsal caudal putamen and the left supplementary motor area; an increased connectivity in the cognitive loop between the right dorsal caudate with the anterior prefrontal cortex and inferior parietal lobule |
| Anticevic et al.36 | Resting state data driven global brain connectivity | 27 OCD (13 unmedicated); 66 healthy matched controls | Decreased restricted global connectivity in OCD patients in several regions of the prefrontal cortex (bilateral inferior frontal gyrus, medial frontal gyrus, left precentral gyrus, and right superior frontal gyrus); for the whole brain, an increase of global connectivity in the cerebellum and right putamen and a decrease in the left inferior frontal gyrus, the left middle frontal gyrus and the left precentral gyrus; dissociation between decreased global connectivity in the ventral striate and an increased global connectivity in the dorsal striate |
| Ping et al.36 | Resting state regional connectivity (regional synchronization) and inter-regional connectivity | 20 OCD; 20 healthy matched controls | Increased synchronized regional activity in the right orbitofrontal cortex, bilateral insula and cerebellum; decreased activity in the ventral anterior cingulate cortex, caudate and inferior occipital cortex; inter-regionally, an increased functional connectivity was observed between the orbitofrontal cortex and ventral anterior cingulate |
| Abe et al.37 | Resting state effective connectivity between orbitofrontal cortex and the ventral striatum using a Granger causality analysis | 37 medication free OCD; 38 healthy matched controls | Hyper-influence of the orbitofrontal cortex over the ventral striate was observed in OCD patients, without mediation of any other striate region. |
| Tian et al.38 | Resting state connectivity graph based voxel-wise whole brain hub analysis | 29 unmedicated OCD; 29 healthy matched controls | Increased functional connectivity was observed in regions associated with the CSTC circuits (e.g., anterior cingulate; orbitofrontal cortex; striate), and tempo-parietal-occipital regions (e.g., sensorimotor cortex; superior and middle temporal cortex; supramarginal gyrus, cuneus, calcarine sulcus, middle occipital cortex) and the cerebellum. |
| Chen et al.39 | Resting state connectivity with left caudate as the seed region | 29 medication free OCD; 29 healthy matched controls | Decreased connectivity within the CSTC (dorso-medial prefrontal cortex-thalamus-caudate) along with an increased connectivity between the caudate and regions outside the CSTC (e.g., superior and middle temporal gyrus, middle and inferior occipital gyrus, lingual gyrus, calcarine sulcus, postcentral gyrus, and supplementary motor area). |
| Jhung et al.40 | Resting state connectivity using as seeds the dorsal caudate, the ventral caudate/nucleus accumbens, dorsal caudal putamen, and ventral rostral putamen | 26 OCD (13 with contamination/washing symptoms); 18 healthy matched controls | Alterations of connectivity shared by the two OCD samples in the regions of dorsal striatum and temporal cortex; increased connectivity in CSTC between the ventral striate and the insula, for the OCD patients with contamination/washing symptoms. |
| Jang et al.45 | Resting state connectivity using as seed region the posterior cingulate | 22 unmedicated OCD; 22 matched healthy controls | Decreased connectivity between several regions of DMN (e.g., right anterior cingulate cortex, middle frontal gyrus, putamen) and the posterior cingulate. |
| Cheng et al.47 | Resting state connectivity using fractional amplitude of the low frequency spontaneous brain activity (fALFF) with the anterior and posterior cingulate regions as seeds | 15 unmedicated OCD; 15 matched healthy controls | Increase fALFF between anterior cingulate, midcingulate, brainstem and cerebellum; a decrease of fALFF between the posterior cingulate, inferior parietal lobe, middle frontal cortex and precentral cortex |
| Peng et al.48 | Resting state connectivity in the DMN using posterior cingulate as seed regions | 15 OCD; 15 siblings; 28 matched healthy controls | Decreased functional connectivity of the DMN posterior cingulate node for both the OCD and unaffected siblings; OCD patients had increased connectivity in several fronto-temporal-parietal regions (e.g., inferior frontal lobe, insula, superior parietal cortex and superior temporal cortex). |
| Beucke et al.49 | Resting state connectivity in the DMN using as seeds all the 11 nodes of the DMN (midline core; dorsal medial prefrontal cortex self system; medial temporal lobe memory system) | 46 OCD; 46 matched healthy controls | In the midline core, a decreased connectivity between the posterior cingulate and medial prefrontal cortex/anterior cingulate and the right posterior cingulate but with an increased connectivity with fusiform gyrus; regarding the dorsal medial prefrontal cortex self-subsystem, a decreased connectivity between the lateral temporal cortex and the dorsal medial prefrontal cortex; the seed in the temporal pole had reduced connectivity with the anterior cingulate cortex and anterior medial prefrontal cortex; an increased connectivity between dorsal medial prefrontal cortex and the insula, as well as between the temporal pole and superior parietal lobe and precuneus; For the medial temporal lobe memory subsystem, a decreased connectivity between the parahippocampal cortex and the superior parietal lobe/precuneus; increased connectivity was identified between posterior inferior parietal lobe and lingual gyrus, the retrosplenial cortex and the inferior frontal gyrus/insula as well as between the hippocampal formation and the superior temporal gyrus; an increased connectivity between nodes of the dorsal medial prefrontal cortex subsystem and regions associated with salience and dorsal attention networks. |
| Zhang et al.50 | Resting state connectivity in the researched the connectivity in the top-down control network using a graph analysis. | 18 OCD; 16 matched healthy controls | Increased connectivity among several regions (e.g., cingulate, frontal cortex, precuneus, thalamus); decreased of connectivity in regions outside the control network (e.g., posterior temporal, fusiform gyrus); abnormalities in small world organization in the control network (i.e., high clustering and short paths facilitating segregation and integration in the transfer of information). |
| Hou et al.51 | Resting state connectivity in the DMN and the corticostriatal Network using as seeds: left caudate, left thalamus and posterior cingulate cortex (increased gray matter), medial orbitofrontal cortex, left anterior cingulate, and left inferior frontal gyrus (decreased gray matter). | 33 unmedicated OCD; 33 matched healthy controls | Increased connectivity in the corticostriatal network (e.g., increased connectivity between the left caudate and bilateral orbitofrontal cortex, right caudate, bilateral putamen, left inferior frontal gyrus, left thalamus) and the DMN (e.g., increased connectivity between the posterior cingulate and right medial frontal gyrus, bilateral middle temporal gyrus). |
| Stern et al.52 | Resting state connectivity in the DMN and the Fronto-Parietal Network | 30 OCD (17 unmedicated; 13 medicated); 17 unmedicated healthy controls; 15 medicated patient controls | Increased between some frontal-parietal seeds and regions within the DMN (e.g., For example, the anterior insula had an increased connectivity with posterior cingulate cortex/precuneus, parahippocampus, left posterior inferior parietal lobe, and dorso-medial prefrontal cortex). |
| Göttlich et al.53 | Resting state connectivity in several resting state networks using graph analysis | 17 unmedicated OCD; 19 matched healthy controls | Decreased functional connectivity not only within the limbic network but also between limbic network and the basal ganglia network, default mode network, and executive/attention network; increased connectivity was observed within the executive/attention network. |
The dissociation between different CSTC loops was further confirmed in a study by Posner et al.,35 in which a decreased functional connectivity was found in the limbic loop (left inferior ventral striate with the left anterior cingulate, left medial orbitofrontal cortex, left superior ventral striate with body of the left caudate nucleus) and the sensory motor loop (between the left dorsal caudal putamen and the left supplementary motor area), contrasting with increased connectivity in the cognitive loop (between the right dorsal caudate with the anterior prefrontal cortex and inferior parietal lobule). Again, this study confirms a connectivity imbalance between different CSTC loops even though not in the same direction as those reported in early studies.29 Anticevic et al.,36 this time using data driven approach (data driven global brain connectivity), and consistent with Posner et al.,35 found a pattern of dissociation between decreased connectivity in the ventral striate (i.e., accumbens – emotional regulation) and an increased global connectivity in the dorsal striate (i.e. putamen, caudate, thalamus – cognitive-executive functioning). For the whole brain, an increase of global connectivity was found for the OCD group in the cerebellum and right putamen and a decrease in the left inferior frontal gyrus, the left middle frontal gyrus and the left precentral gyrus. The inconsistency in connectivity findings between the ventral and dorsal CSTC loops may be associated with confounding factor associated with different OCD subtypes. For example, Jhung et al.40 compared functional connectivity in a sample of OCD patients with predominant contamination/washing symptoms, OCD patients without contamination/washing symptoms and healthy controls. The study confirmed common alterations of connectivity shared by the two OCD samples in the regions of dorsal striatum and temporal cortex. However, the authors also reported an increased connectivity in the limbic component of the CSTC between the ventral striate and the insula (both for the resting and symptom provocation conditions), specifically for the OCD patients with contamination/washing symptoms.
Resting state studies without a predefinition of specific seed regions confirmed alterations of connectivity beyond the CSTC loops. For example, Beucke et al.32 reported increased distant connectivity, for unmedicated OCD, in orbitofrontal cortex and subthalamic nucleus with regions outside the CSTC (precentral and superior temporal regions) along with increased local connectivity in the orbitofrontal cortex and putamen. A consistent finding was reported by Hou et al.33 and Ping et al.,36 confirming increased connectivity in the orbitofrontal cortex and the anterior cingulate along, in Hou et al.,33 a decreased connectivity in bilateral parietal cortex and the cerebellum, suggesting abnormal connectivity in regions associated with, namely, visual spatial processing. Hou et al.34 later confirmed increased connectivity for OCD patients, in the CSTC loops, contrasting with a decreased connectivity in posterior brain regions such as occipital cortex, temporal and cerebellum regions. Interesting to note that OCD and healthy relatives were shared similar patterns of increased connectivity in the bilateral caudate, left orbitofrontal cortex and left middle temporal gyrus.
Contrasting with Hou et al.34 findings, Tian et al.38 while confirming increased functional connectivity in regions associated with the CSTC circuits (e.g., anterior cingulate; orbitofrontal cortex; striate) showed also evidence for an increased connectivity in several tempo-parietal-occipital and cerebellum regions.
Other authors have looked at connectivity in regions belonging to several resting state networks.41 Among these networks, the Default Mode Network (DMN) has been extensively studied.42 The DMN is a network activated during a rest condition, connecting medial prefrontal cortex with the posterior cingulate extending to the precuneus, and regions of the parietal cortex.43 Besides these regions, there are extended DMN subsystems with core nodes on the dorsal lateral prefrontal cortex and medial temporal lobe.44 For example, Jang et al.45 found a decreased in connectivity between several regions of DMN (e.g., right anterior cingulate cortex, middle frontal gyrus, putamen) and the posterior cingulate. It is important to note that while both anterior and posterior regions of the DMN are associated with self-referential processes, the medial prefrontal cortex node seems to have an important role in social cognitive self-relevant tasks (e.g., theory of mind) while the posterior cingulate is associated with self-related processes (e.g., episodic memory).46 Therefore, decreased of connectivity with posterior regions may suggest impairments in self-related processes.
In order to study both the anterior and posterior connections of the cingulate cortex, Cheng et al.47 used, this time, as seeds the anterior and posterior cingulate regions. While posterior cingulate is a core DMN regions associated with self-related processing (e.g., episodic memory), the anterior cingulate is related with cognitive and emotional regulation. A dissociation pattern was found in OCD, between increased functional connectivity in anterior cingulate (between anterior cingulate, midcingulate, brainstem and cerebellum) and a decreased functional connectivity in the posterior cingulate (between the posterior cingulate, inferior parietal lobe, middle frontal cortex and precentral cortex). Contrasting correlations were also reported on the different networks associated with each of these cingulate regions. While for the DMN a significant negative correlation was found between symptom severity and functional connectivity from the posterior cingulate; in the self-referential network (thought to overlap with anterior nodes of the DMN) a positive correlation was observed between symptom severity and functional connectivity from the anterior cingulate. Interesting to note that Peng et al.48 found that abnormal DMN connectivity was observed not only in OCD patients, but also in a sample of unaffected siblings (i.e., decreased functional connectivity of the DMN posterior cingulate node). Additionally, OCD patients had increased connectivity in several fronto-temporal-parietal regions (e.g., inferior frontal lobe, insula, superior parietal cortex and superior temporal cortex).
Beucke et al.49 found also different patterns of connectivity associated with three DMN subsystems (midline core; dorsal medial prefrontal cortex self system; medial temporal lobe memory system). In the midline core, a decreased connectivity was found for the OCD patients between the posterior cingulate and medial prefrontal cortex/anterior cingulate and the right posterior cingulate but with an increased connectivity with fusiform gyrus. Regarding the dorsal medial prefrontal cortex self-subsystem, there was a decreased connectivity between the seed in the lateral temporal cortex and the dorsal medial prefrontal cortex. The seed in the temporal pole had reduced connectivity with the anterior cingulate cortex and anterior medial prefrontal cortex. On the contrary, an increased connectivity was observed between dorsal medial prefrontal cortex and the insula, as well as between the temporal pole and superior parietal lobe and precuneus. For the medial temporal lobe memory subsystem, a decreased connectivity was evident between the parahippocampal cortex and the superior parietal lobe/precuneus. Finally, increased connectivity was identified between posterior inferior parietal lobe and lingual gyrus, the retrosplenial cortex and the inferior frontal gyrus/insula as well as between the hippocampal formation and the superior temporal gyrus. The authors reported also an increased connectivity between nodes of the dorsal medial prefrontal cortex subsystem and regions associated with salience and dorsal attention networks (e.g., anterior insula; superior parietal lobule). Once again, the decreased connectivity in the anterior nodes of the DMN (dorsal medial prefrontal cortex subsystem) may be suggestive of abnormalities in self-related processes associated with social-cognitive tasks.
More recently, Zhu et al.54 studied the alterations of connectivity in the salience network (i.e., dorsal anterior cingulate and bilateral insular region). The salience network, partially overlapping with the DMN, is also thought to play an important role in the processing of personal relevant stimuli. Connectivity indexes in bilateral insula were positively correlated with OCD severity (e.g., YBOCS, compulsive scores). Worth reminding that the insula has an important role in interoceptive awareness and emotional processing. Additionally, as we will be detailing later on, the insula plays a core role in the emotion of disgust, prevalent in OCD.
Other resting state networks have been studied in OCD patients. For example, Zhang et al.50 confirmed alteration of brain connectivity in the top-down control network, as revealed by increased connectivity among several regions (e.g., cingulate, frontal cortex, precuneus, thalamus). Additionally, a decrease of connectivity was also observed in regions outside the top-down control network (e.g., posterior temporal, fusiform gyrus). Finally, abnormalities in small world organization in the control network were present in OCD (i.e., high clustering and short paths facilitating segregation and integration in the transfer of information). These findings are consistent with data referred above on the hyperactivation of the CSTC loops.
Alterations of both the DMN and the Corticostriatal Network, were later confirmed in a functional resting state study by Hou et al.51 using as seeds regions identified as having morphometric abnormalities in a previous study (i.e., left caudate, left thalamus and posterior cingulate cortex, medial orbitofrontal cortex, left anterior cingulate, and left inferior frontal gyrus). Particularly evident was a pattern of increased connectivity in the corticostriatal network (e.g., increased connectivity between the left caudate and bilateral orbitofrontal cortex, right caudate, bilateral putamen, left inferior frontal gyrus, left thalamus) and the DMN (e.g., increased connectivity between the posterior cingulate and right medial frontal gyrus, bilateral middle temporal gyrus). The connectivity within the corticostriatal network was positively correlated with severity scores. While the finding of increased connectivity in the corticostriatal network is consistent with previous studies, the finding of increased activity in the DMN contradicts data reported by Cheng et al.47 and Jang et al.45
Stern et al.52 moved one step further by doing an analysis of DMN and the Fronto-Parietal Network (involved in attention and executive processes). An increased connectivity was found in OCD patients, when compared with healthy controls, between some frontal-parietal seeds and regions within the DMN. For example, the anterior insula had an increased connectivity with posterior cingulate cortex/precuneus, parahippocampus, left posterior inferior parietal lobe, and dorso-medial prefrontal cortex. These data suggest that different resting state networks may be affected in OCD, contributing, jointly or independently, to the diversity of psychological impairments. Additionally, a pattern of decreased connectivity was also found within DMN nodes, confirming this time the results from Jang et al.45 and Chang et al.47
Göttlich et al.53 extended this data by showing altered functional connectivity between different brain network systems in a OCD. They observed a decreased functional connectivity not only within the limbic network but also between limbic network and the basal ganglia network, default mode network, and executive/attention network. Contrastingly, an increased connectivity was observed within the executive/attention network.
Different methods in image acquisition and analysis are possible responsible for inconsistencies in the directions of differences in connectivity findings. However, building in some consistent findings on alterations in functional connectivity, we may draw some preliminary conclusions1: There is evidence for alterations in connectivity in regions associated with the CSTC pathways2; A dissociation/imbalance between patterns of connectivity in the dorsal versus ventral components of the CSTC loops have been reported in several studies3; an increased number of studies have been pointing out to altered connectivity between the CSTC and regions outside the CSTC4; there is also growing evidence for altered connectivity in widespread brain regions outside the CSTC5; abnormal connectivity within different nodes of the DMN (e.g., midline core, dorsal medial prefrontal cortex core, medial temporal lobe memory system) has also been repeatedly demonstrated6; other resting state networks were also shown to have abnormal patterns of functional connectivity (e.g., top-down control network, salience network, or between regions of the frontal-parietal network and the DMN).
Having discussed the major results for the research using task-negative paradigms (i.e., resting state) we will move now to an analyses of the major findings of functional neuroimaging research using task-positive paradigms in order to tackle brain correlates of major cognitive/executive and emotional impairments in OCD (see Tables 2 and 3).
Studies on brain correlates of cognitive/executive impairments in OCD.
| Study Ref. | Method | Participants | Findings for OCD |
|---|---|---|---|
| Page et al.57 | Brain activation in three inhibitory control tasks: Go/No-go task, Stroop task and switch task | 10 unmedicated OCD; 11 matched healthy controls | Decreased activation in frontal-striatal networks (i.e., orbitofrontal, dorsolateral prefrontal, striatal and thalamic regions) both in Go/No-Go and switch tasks; decreased activation in temporal-parietal regions in the Stroop task; Go/No-Go task was associated with an increased activation in the posterior cingulate and temporal lobe; Stroop task was associated with increased activity in left cerebellum and posterior cingulate. |
| Maltby et al.58 | Brain activation in one inhibitory control tasks: Go/No-go task | 11 OCD; 11 matched healthy controls | Action-monitoring processes associated with errors, as well as correct responses in high conflict trials, were associated with an hyperactive response of the anterior cingulate, involving also other regions of the frontal striatal network (e.g., orbitofrontal cortex, basal ganglia and thalamus). |
| Fitzgerald et al.59 | Brain activation in one inhibitory control tasks: Go/No-go task | 8 OCD; 11 matched healthy controls | Increased activation of the dorsal anterior cingulate. |
| Morein-Zamir et al.60 | Brain activation in a combined Go/No-Go and shifting task | 19 OCD; 19 matched healthy controls | For the Go/No-Go task an increased activity in the caudate, cuneus and precentral gyrus and a decreased activity in the shifting task in widespread frontal, temporal, parietal, occipital and subcortical regions. |
| Gu et al.64 | Brain activation in task-switching | 21 OCD; 21 matched healthy controls | Decreased activation on several prefrontal and subcortical regions (e.g., dorsal lateral prefrontal cortex, anterior cingulate cortex, ventral medial prefrontal cortex, right orbitofrontal cortex and caudate nucleus). |
| Remijnse et al.65 | Brain activation in task-switching | 18 unmedicated OCD; 19 unmedicated MDD; 29 matched healthy controls | Decreased brain activity in prefrontal regions for OCD and major depressive disorder; increased activation in the putamen was found for the OCD patients when compared with major depression or healthy controls along with increased activation of the anterior cingulate in the OCD when compared with the healthy control group. |
| Van Der Wee et al.66 | Brain activation on a spatial n-back task with four levels of increasing difficulty (i.e., 0-back, 1-back, 2-back, 3-back) | 15 unmedicated OCD; 15 matched healthy controls | Increased activity in the anterior cingulate, independently of the load level. |
| van der Wee et al.67 | Brain activation on a spatial n-back task with four levels of increasing difficulty (i.e., 0-back, 1-back, 2-back, 3-back) | 14 OCD (7 treatment responders) | Increase in performance only in responders and performance was associated with changes in brain activity in the working memory network (increased activity with increasing task difficulty in medial frontal, anterior cingulate, and the dorsolateral prefrontal and parietal cortex). |
| Henseler et al.68 | Brain activation in three different spatial and verbal working memory tasks (phonological – articulatory or nonarticulatory; and visuospatial). | 11 OCD; 11 matched healthy controls | Both groups activated similar task specific brain regions (e.g., verbal articulatory working memory – left precentral gyrus, left inferior frontal gyrus, bilateral inferior frontal sulcus, bilateral fronto-opercular cortex adjacent to the anterior insula, left intraparietal cortex and cerebellum; visuospatial working memory – bilateral posterior superior frontal sulcus; intraparietal sulcus, superior parietal and occipital cortices, precentral gyrus, insula, right middle frontal gyrus, right posterior inferiortemporal gyrus and cerebellum). OCD patients showed a significant increase on the activity of some of these brain regions. |
| Nakao et al.69 | Brain activation on a spatial n-back task with two levels of increasing difficulty (i.e., 0-back, 2-back) | 40 OCD; 25 matched healthy controls | Increased activation in the right dorsolateral prefrontal cortex, left superior temporal gyrus, left insula, and cuneus. |
| Koch et al.70 | Brain activation on a visual-spatial n-back task with three levels of increasing difficulty (i.e., x-baseline back, 1-back, 2-back, 3-back) | 21 OCD (19 unmedicated); 21 matched healthy controls | There was an increase of activation for OCD in the dorsal anterior cingulate from a 1 back task to a 2 back task but with a significantly drop of activation in the 3 back task; healthy controls have a linear increase of activation as the task evolves from 1 back to 3 back. |
| De Vries et al.71 | Brain activation on a verbal n-back task with three levels of increasing difficulty (i.e., 0-baseline back, 1-back, 2-back, 3-back) | 43 unmedicated OCD; 17 unaffected siblings; 37 matched healthy controls | OCD and unaffected siblings had an increase of activation from N1 to N2 and no increase from N2 to N3; while healthy controls showed an increase across all three working memory loads. |
| Diwadkar et al.72 | Modulation of cortical and subcortical regions by the dorsal anterior cingulate cortex during a verbal n-back task (0-back, 1-back, 2-back). | 18 OCD; 27 matched healthy controls | Increased activation of the frontal and parietal regions for both working memory loads; increased modulation of the frontal, parietal, and striatal regions, by the dorsal anterior cingulate for lower memory loads. |
Studies on task positive emotional processing in OCD.
| Study Ref. | Method | Participants | Findings for OCD |
|---|---|---|---|
| Cannistraro et al.74 | Amygdala response to fear faces, when contrasted with happy and neutral faces | 10 OCD; 10 matched healthy controls | Lower levels of activity in the amygdala all the faces; no significant differences were found between OCD and controls in amygdala activation for fear faces. |
| Via et al.75 | Brain activation during an emotional face matching paradigm | 67 OCD; 67matched healthy controls | Increased amygdala for the fear faces, extending to other brain regions (e.g., secondary visual cortex, right anterior insula, premotor cortex, right orbitofrontal cortex and right middle temporal gyrus). |
| Simon et al.76 | Brain activation during the presentation OCD symptom relevant, aversive and neutral pictures | 21 unmedicated OCD; 21matched healthy controls | The presentation of the symptom relevant stimuli, when compared with neutral pictures, produced a significant increased activation in the following regions: amygdala, thalamus, caudate nucleus, globus pallidus, subthalamic nucleus, orbitofrontal cortex, anterior insula and parahippocampal gyri; when comparing OCD-relevant stimuli with the aversive and neutral stimuli, OCD patients showed increased activations in the thalamus, caudate nucleus, subthalamic nucleus, anterior insula, parahippocampal gyrus, middle temporal gyrus and posterior cingulate cortex/precuneus. |
| Gonçalves et al.8 | Brain activation during the presentation of to basic emotional pictures, with different levels of arousal (high arousal and low arousal), intended to trigger the defensive (unpleasant stimuli) and appetitive (pleasant stimuli) systems | 15 OCD; 12 matched healthy controls | Respond to unpleasant stimuli by recruiting a distributed brain network, involving frontal (e.g., left inferior frontal gyrus – pars triagularis; right inferior frontal gyrus – pars opercularis, left medial orbitofrontal gyrus, left pre central gyrus), temporal (e.g., right superior temporal gyrus, right middle temporal gyrus), parietal (e.g., right postcentral gyrus) and subcortical nuclei (e.g., bilateral putamen). Contrastingly, the presentation of pleasant pictures was responsible for a decreased brain activity in a major posterior visual processing region (i.e., superior occipital gyrus). |
| Shapira et al.78 | Brain activation during the presentation disgust inducing, threat inducing and neutral pictures | 8 OCD (contamination symptoms); 8 matched healthy controls | Increased activation, particularly in the right insula but also in the left parahippocampal region, left inferior frontal gyrus, left posterior cingulated gyrus, and left inferior occipital gyrus for the disgust inducing stimuli. |
| Schienle et al.79 | Brain activation during the presentation generally disgust, generally fear-inducing, affectively neutral, and OCD symptom-relevant pictures | 10 OCD; 10 matched healthy controls | Symptomatic relevant pictures were associated with increased activations in several brain areas, mostly from the fronto-striato-thalamo-parietal network in the OCD group (i.e., bilateral prefrontal cortex, left insula, right supramarginal gyrus, left caudate nucleus and right thalamus); increased activation of the insula for the generally disgusting and fear-inducing stimuli. |
| Hennig-Fast et al.83 | Brain activation to neutral, shame and guilt inducing scenarios | 20 OCD; 20 matched healthy controls | In the shame condition (compared with neutral), there was an increased activation of several limbic (e.g., uncus, parahippocampal gyrus), temporal (e.g. middle temporal gyrus), midbrain (e.g. red nucleus) and sublobar regions (e.g., hypothalamus), along with decreased activation in the middle frontal gyrus and inferior parietal lobe; for the guilt conditions (compared with the neutral), there was increased activations in widespread frontal (e.g., superior frontal gyrus, precentral gyrus), limbic (e.g., cingulate gyrus), posterior lobe (e.g. cerebellar tonsils), and temporal regions (e.g., superior temporal gyrus), along with decreases of activation in the anterior cingulate. |
| Basile et al.85 | Brain activation in response to response to deontological guilt, altruistic guilt and two other control emotions (anger and sadness) | 13 OCD; 19 matched healthy controls | Decreased activation in the anterior cingulate independent of the type of guilt being deontological or altruistic; a decreased activation was evident for the deontological guilt for the anterior cingulate, insula and precuneus when compared with control stimuli; no significant differences in activations between altruistic guilt and sad or anger stimuli. |
OCD patients tend to perform poorly in distinct types of inhibitory control tasks,55 namely in action restrain (suppression of a prepotent response – e.g., Go/No Go tasks), action cancelation (inhibition of a response already initiated – e.g., stop-signal) and interference control (inhibit a competitive stimulus – e.g. Stroop task). Several studies showed abnormal functioning in the CSTC loops during different inhibitory control tasks.56
In order to explore the role of different brain regions in tasks requiring processes of inhibitory control, Page et al.57 tested OCD patients and healthy controls in three different experimental paradigms: an action restrain Go/No-go task, a motor interference control Stroop task, and a cognitive flexibility switch task. Evidence of dysfunctional patterns of activity was observed for OCD in anterior (frontal-striatal) and posterior brain regions. More specifically, a decreased in frontal-striatal networks (i.e., orbitofrontal, dorsolateral prefrontal, striatal and thalamic regions) was found for OCD, both in action restrain Go/No-Go (orbitofrontal loop) and cognitive flexibility switch tasks (dorsolateral loop). Most interestingly, in the interference control Stroop task, an underactivation of temporo-parietal areas was observed. Additionally, both inhibitory control tasks (Go/No-Go and Stroop) were associated with an increased activation in the posterior cingulate and temporal lobe (Go/No-go task) and in left cerebellum and posterior cingulate (Stroop task). In other words, while in action restrain there was a dissociation between an underactivation of regions associated with the CSTC loops and an overactivation on temporal and posterior cingulate regions; for the interference control task only the posterior brain regions showed increased activity (left cerebellum and posterior cingulate). The orbitofrontal-putamen-thalamus loop has been traditionally considered an inhibitory control loop and, in this study, seems to play a critical role in action restrain. The alterations observed for the cognitive switch task in the dorsal prefrontal cortex network may be related with the role played by this CSTC loop (dorsolateral prefrontal – caudate – thalamus) in cognitive flexibility. Finally, the activation of temporal cortex, posterior cingulate and the cerebellum in both inhibitory control tasks deserves a comment. The authors interpret the activation on this posterior regions as a compensation for the decreased of activity in more anterior regions during inhibitory control. Therefore, at this point, there is some ground to speculate that in inhibitory control tasks, the OCD patients tend to compensate for the underactivation of CSTC loops (action restrain) or temporal-parietal networks (interference control) by overactivating posterior brain regions such as the posterior cingulate and the cerebellum.
It is important to note that Page et al.57 selected for analysis only instances of successful inhibition. This may be the reason why the typical activation of the anterior cingulate was not found. As suggested by the authors, the hyperactivation of the anterior cingulate may be more associated with performance overmonitoring during error trials. This is what was found in a previous study by Maltby et al.58 during a Go/No Go task. The study found that action-monitoring processes associated with errors, as well as correct responses in high conflict trials, were associated with an hyperactive response of the anterior cingulate, involving also other regions of the frontal striatal network. Once again, the CSTC orbitofrontal cortex loop seemed to play a central role in inhibitory control (this time in response suppression). However, a second component of the CSTC system (anterior cingulate loop) thought to be more involved in emotional regulatory processes, seemed to be particularly associated with overmonitoring of unsuccessful or difficult trials. Consistent with this conclusion, Fitzgerald et al.59 reported an increased activation of the dorsal anterior cingulate in OCD patients, when compared with controls, in error trials, during an interference control task (i.e., flanker interference task).
More recently, Morein-Zamir et al.60 looked at the differential mechanisms involved in either action retrain and cognitive flexibility in a combined Go/No-Go shifting task. For the action restrain task there was, for OCD patients, an increased activity in the caudate, cuneus and precentral gyrus. This overactivity contrasts with a decrease of activity for OCD patients in the shifting task in widespread frontal, temporal, parietal, occipital and subcortical regions. Particularly important for the purpose of the current discussion is the fact that the inhibitory control task was associated with impaired activations in regions belonging to CSTC loops as well as the frontal-parietal control network.
Summing up, there seems to exist evidence for abnormal patterns of brain functioning in OCD during inhibitory control tasks. Hyperactivation of the anterior cingulate cortex was found in high conflict paradigms where the patient is constantly monitoring for errors. On the contrary, when errors are excluded from the paradigm or the analysis, there is evidence of abnormal activity in the CSTC loops characterized either by an overactivation of the CSTC or, alternatively, an underactivation compensated with the activation of more posterior brain regions. There is also evidence that impairments in different types of inhibitory control tasks (restrain, cancelation, interference control) are associated with functional abnormalities in distinct brain regions.
Cognitive flexibilityThe lack of cognitive flexibility is another executive impairment often reported in OCD. As stated before, cognitive flexibility refers to the ability to change cognitive and behavior strategies in face of changing demands as assessed, either, in set shifting (applying new rules to solve the same task) or task switching paradigms (changing between tasks).61 OCD patients typically show poor performance in both, set shifting62 and task-switch situations.63 For instances, in the study of Page et al.57 reported above, an underactivation of the CSTC dorsal loop was present in the task switching condition. Morein-Zamir et al.,60 in the study discussed previously, extended these findings by showing a decrease of activity in frontal, temporal, parietal, occipital and subcortical regions for OCD patients during a shifting task.
Still with a different cognitive task, Gu et al.64 tested a task-switching paradigm (with task-repeat and task-switching conditions). As expected, OCD patients had significantly more errors in the task-switching condition. Brain activations for the switch minus repeat condition revealed a decreased activation in OCD patients, contrasted with controls, on several prefrontal and subcortical regions. Interestingly, both the dorsolateral and ventromedial/anterior cingulate loops of the CSTC seem to be underactivated during task switching. While the former has been more traditionally associated with cognitive flexibility, the second seems to play a key role in emotional regulation and may be responsible for conflict monitoring during a task switching condition.
In order to test how specific were these brain alterations for OCD, Remijnse et al.65 did a task switching study comparing OCD with patients diagnosed with major depressive disorder and a group of healthy controls. Consistent with results presented above, the authors observed a decreased brain activity in prefrontal regions for OCD but also for major depressive disorder. However, an increased activation in the putamen was found for the OCD patients when compared with major depression or healthy controls along with increased activation of the anterior cingulate in the OCD when compared with the healthy control group.
Concluding, studies on brain markers of cognitive flexibility impairments in OCD have consistently reported underactivation of regions of the CSTC involved in task-switching and set-shifting tasks. Additionally, other temporal, parietal and occipital regions were also found to have decreased activation during task switching conditions.
Working memoryWorking memory (i.e., capacity to hold and updating information online) has been repeatedly demonstrated to be significantly impaired in OCD patients.65 The first fMRI study of working memory processes in OCD was done by Van Der Wee et al.66 In this study, the authors compared OCD female patients and healthy matched controls, on a spatial n-back task with different levels of increasing difficulty (i.e., 0-back, 1-back, 2-back, 3-back). As expected, with increased difficulty more errors were evident in both groups. However, in the higher working memory load condition (3-back), OCD patients had significantly more errors than healthy controls. For both groups, the areas involved in the working memory task were similar (anterior cingulate cortex, dorsolateral prefrontal and premotor cortex). Worth pointing out that the anterior cingulate was more activated in OCD patients, independently of the load level. The authors conclude that the typical fronto-parietal working memory network does not seem to be impaired in OCD since that, independently of the memory load, similar types of activation were present in OCD and the control group. The increased activation of the anterior cingulate was interpreted as an expression of the conflict overmonitoring typical in OCD.
On a subsequent study, van der Wee et al.67 found that the deficits on a spatial working memory task were state (symptom) dependent. In this study the authors tested if spatial working memory deficits would improve, along with a decreased activation of the anterior cingulate cortex, after effective pharmacological treatment. From a final pool of 14 patients, seven responded to treatment. Bringing evidence to the state dependent hypothesis, only the responders had a significantly increase in performance on the working memory task and their performance was associated with changes in brain activity in the working memory network (medial frontal, anterior cingulate, and the dorsolateral prefrontal and parietal cortex).
From the studies reported above there is some indication that the deficits in working memory in OCD patients may be a question of the degree of activation in working memory brain regions shared by OCD and controls. To test further this hypothesis, Henseler et al.68 did a study in which they compared brain activations in OCD patients and matched healthy controls, this time using three different spatial and verbal working memory tasks (phonological – articulatory or nonarticulatory; and visuospatial). At the behavioral level, both groups perform equally well. Confirming previous studies, both groups activated similar task specific brain regions. However, OCD patients showed a significant increase on the activity of some of these brain regions suggesting the possibility of a compensation for underlying working memory deficits.
The fact that OCD patients, during working memory tasks, have different brain activity levels within similar brain regions was confirmed in a study by Nakao et al.69 showing greater activation in the right dorsolateral prefrontal cortex, left superior temporal gyrus, left insula, and cuneus.
Later, Koch et al.70 found that differences in brain activity between OCD and healthy controls was dependent on the working memory task load. While no significant differences were evident in terms of brain activation for low cognitive working memory loads, OCD patients and healthy controls have a different pattern of brain activations across load levels. In the dorsal anterior cingulate, there was an increase of activation in OCD patients from a 1 back task to a 2 back task but with a significantly drop of activation in the 3 back task. Contrastingly, healthy controls have a linear increase of activation as the task evolves from 1 back to 3 back. Given the role of the dorsal anterior cingulate in performance monitoring, these data suggests an interesting association between OCD impairments with high working memory loads and the correlative failure in brains systems associated with performance monitoring. De Vries et al.71 reported a similar pattern with the activation of the fronto-parietal network across working memory loads not only for OCD but also to unaffected siblings.
More recently Diwadkar et al.72 compared OCD and healthy controls analyzing the how the dorsal anterior cingulate cortex modulates the activity of other cortical and subcortical regions during a verbal n-back task. The authors found an increased activation of the frontal and parietal regions for both working memory loads. Additionally, there was an increased modulation of the frontal, parietal, and striatal regions, by the dorsal anterior cingulate for lower memory loads. This last finding seems consistent with the conclusion that performance monitoring by the dorsal anterior cingulate cortex seems to interact with CSTC loops responsible for working memory tasks, significantly impacting performance.
Concluding, there is now consistent evidence that OCD patients tend to decrease their working memory performance with increase task load. These impairments are accompanied by altered patterns of activity in frontal-subcortical and frontal-parietal networks (e.g., medial frontal, anterior cingulate, and the dorsolateral prefrontal and parietal cortex) and seem to be modulated by an increased activation of the dorsal anterior cingulate, most probably associated with performance overmonitoring.
Brain correlates of emotional processing in OCDFear/defensive systemAt the emotional level, the pathophysiology of OCD, along with other anxiety disorders, emphasizes the propensity for fear response and the correlative activation of the defensive system.73 One way of studying the brain mechanisms involved in fear processing is looking at the participant's response to human faces depicting emotions of fear. Using this paradigm, Cannistraro et al.,74 researched amygdala response in OCD patients, when compared with healthy controls, to fear faces, when contrasted with happy and neutral faces. No significant differences were found between OCD and controls in amygdala activation for fear faces. However, lower levels of activity in the amygdala were observed for all the faces, suggesting that the eventual abnormalities in amygdala processing are not emotionally specific (Table 3).
Substantially different findings were reported in a more recent study by Via et al.75 using an emotional face matching paradigm in a sample of OCD patients and healthy controls. Both groups show an activation of the amygdala (along with visual cortex, fusiform gyrus, hippocampus, premotor cortex, lateral prefrontal cortex and orbitofrontal cortex) while matching fear faces. Increased amygdala activation was observed for the OCD patients for the fear faces, but extending to other brain regions as well. However, the amygdala activation was significantly associated with two classes of OCD symptoms: aggression/checking and sexual/religious. Additionally, severity of these symptoms was also a predictor of increased activation in additional brain regions such as the dorsal anterior cingulate (aggression/checking) and left premotor cortex (sexual/religious).
Using a symptom provocation paradigm, Simon et al.76 reported an increased amygdala activation across OCD symptom dimensions. Therefore, the generalization of amygdala activation across symptoms may be evident if one uses symptom related paradigms rather than just general aversive stimuli.
The studies on brain activations to fear in OCD patients are showing the increase not only on brain regions more typically associated with the arousal response (e.g., amygdala) but also other regions related with perceptual (e.g., visual cortex) and higher level cognitive processing (e.g., prefrontal cortex). This is consistent with a recent study from Gonçalves et al.8 researching brain responses in OCD, when compared with healthy controls, to basic emotional pictures, with different levels of arousal (high arousal and low arousal), intended to trigger the defensive (unpleasant stimuli) and appetitive (pleasant stimuli) systems. The results show that OCD patients tend to respond to unpleasant stimuli by recruiting a distributed brain network, involving temporal, parietal, and subcortical nuclei. Contrastingly, the presentation of pleasant pictures was responsible, in OCD, for a decreased brain activity in a major posterior visual processing region.
DisgustBerle et al.77 found that propensity for disgust was associated with all types of OCD symptoms with the exception of hoarding. A more recent study by Whitton et al.9 confirmed also an increase propensity for disgust while facing core disgust stimuli (i.e., body waste) in OCD patients when compared with both, other anxiety disorders (non OCD sample) and a healthy control sample.
Shapira et al.78 compared the effects of presenting disgust inducing, threat inducing and neutral pictures in OCD patients with contamination preoccupations when compared with healthy controls. While no significant differences were found in terms of brain activations for the threat inducing stimulus, the OCD patients revealed an increase activation, particularly in the right insula but also in the left parahippocampal region, left inferior frontal gyrus, left posterior cingulated gyrus, and left inferior occipital gyrus for the disgust inducing stimuli.
Later, Schienle et al.79 compared a OCD sample with a matched control group in four sets of emotional pictures: generally disgust, generally fear-inducing, affectively neutral, and OCD symptom-relevant pictures. Two types of results are worth mentioning here. First, as expected, the symptomatic relevant pictures were associated with increased activations in several brain areas, mostly from the fronto-striato-thalamo-parietal network in the OCD group. Second, and most important for the objectives of the present discussion, there was an increased activation of the insula for the generally disgusting and fear-inducing stimuli in OCD patients. In other words, while symptom relevant activations are consistent with data presented before, there seems to be a brain signature of increased insula activation for disgust stimuli helping understanding OCD's sensitivity and propensity for disgust.
Interestingly, a recent study using real time fMRI showed the efficacy of training OCD patients in downregulating anterior insula activation while facing disgusting stimuli with correlative improvement with symptoms of contamination obsessions and compulsive washing.80
ShameWetterneck et al.81 found that shame proneness was significantly associated with specific OCD symptom dimensions (harm and symmetry) but not with other dimensions (unacceptable thoughts and contamination). In a study comparing OCD with body dysmorphic disorder, social anxiety and a group of healthy controls, Clerkin et al.82 observed that OCD patients were particularly prone to feelings of shame associated with obsessions, while body dysmorphic disorder had more feelings of shame associated with the body and, to a less extent, generalized anxiety feelings of shame associated with performance.
Hennig-Fast et al.83 extended the study of emotional processing in OCD to include shame and guilt. In a study with OCD and health controls, the brain's response to neutral, shame and guilt inducing scenarios showed specific patterns of activations in the OCD group. In the shame condition (compared with neutral), OCD patients had an increased activation of several limbic, temporal, midbrain, and sublobar regions, along with decreased activation in the middle frontal gyrus and inferior parietal lobe. For the guilt conditions (compared with the neutral), OCD patients had increased activations again in widespread frontal, limbic, posterior lobe, and temporal, along with decreases of activation in the anterior cingulate.
GuiltAccording to Shapiro and Stewart,84 the presence of pathological guilt may be a core emotion mediating a variety of OCD obsessions (e.g., scrupulosity, aggressive, sexual, contamination) and an important motivational trigger for almost all types of compulsions (e.g., cleaning, checking, repeating, counting, ordering).
Basile et al.85 attempted to differentiate brain's response to deontological guilt (produced by transgression of a moral rule), altruistic guilt (compromising a personal altruistic goal) and two other control emotions (anger and sadness) in a sample of OCD patients and healthy controls. The authors confirmed a decreased activation in the anterior cingulate independent of the type of guilt being deontological or altruistic. When compared with the control stimuli, a decreased activation was evident for the deontological guilt for the anterior cingulate, insula and precuneus. No significant differences in activations were observed in OCD patients between altruistic guilt and sad or anger stimuli.
ConclusionsConcluding, functional brain studies confirmed alterations in several regions and networks, either during rest conditions as well as during cognitive/executive (working memory, cognitive flexibility, and inhibitory control), emotional tasks (fear/defensive, disgust, guilt, shame). More specifically, the following major conclusions can be drawn:
- (1)
OCD patients show alterations in connectivity in regions associated with the CSTC pathways, with evidence for a dissociation/imbalance between patterns of connectivity in the dorsal versus ventral CSTC loops.
- (2)
There is also evidence for connectivity abnormalities between CSTC loops and other brain regions.
- (3)
Different nodes of the DMN showed altered connectivity in OCD.
- (4)
There is evidence for altered connectivity in several resting state brain networks (e.g., top-down network, salience network, fronto-parietal network).
- (5)
Impaired inhibitory control in OCD has been associated with functional abnormalities in the CSTC loops, and fronto-parietal networks. Additionally, anterior cingulate seems to be particularly active in overmonitoring performance in these tasks.
- (6)
OCD's cognitive flexibility impairments are related with decreased activations not only in the OCD loops but also extended temporal, parietal and occipital regions.
- (7)
Working memory performance in OCD patients is affected by functional abnormalities in the CSTC and frontal parietal networks. Again, the dorsal anterior cingulate seems to modulate the activity the remaining brain regions during the course of working memory tasks.
- (8)
The predominance of fear/defensive affective motivational system is associated with increased amygdala responsiveness but also increased activity in additional brain regions associated with perceptual (e.g., parietal, occipital) and higher level cognitive processing (e.g. prefrontal, temporal).
- (9)
Increased activity in the insula seems to be a brain marker of increased OCD sensitivity to disgust, while propensity for shame and guilt may be attributed to an increase in widespread brain (e.g. frontal, limbic, temporal) and a deactivation in other regions such as the middle frontal gyrus and inferior parietal lobe (shame) and anterior cingulate (guilt).
The studies reported here bring evidence for regional and connectivity functional abnormalities evident during resting state and task conditions that may help understand OCD as both an emotional (i.e., anxiety) and cognitive (i.e. inhibitory control) disorder with a diversity of psychological and symptomatic expressions. Despite this evidence little is known about the specificity of the present results for OCD. It remains to be known if these findings are transdiagnostic to other psychiatry disorders (particularly within the OCRD and anxiety spectra). Additionally, distinct OCD subtypes (e.g., washers, hoarders, checkers) and endophenotypes (genetic risk versus environmental) were reported to be associated with specific brain activation patterns.86,87 Future studies should try to differentiate distinct OCD subtypes and endophenotypes as well as including as comparison groups from other psychiatry disorders within and outside the OCRD and anxiety spectra.
Conflicts of interestThe authors declare no conflicts of interest.
The first author was funded by the Brazilian National Counsel for Scientific and Technological Development (CNPq) as a Special Visiting Researcher of the Science Without Borders program (grant number: 401143/2014-7). This study was partially conducted at the Neuropsychophysiology Lab from the Psychology Research Centre (UID/PSI/01662/2013), University of Minho, and supported by the Portuguese Foundation for Science and Technology and the Portuguese Ministry of Science, Technology and Higher Education through national funds and co-financed by FEDER through COMPETE2020 under the PT2020 Partnership Agreement (POCI-01-0145- FEDER-007653). This work was also supported by the Portuguese Foundation for Science and Technology (FCT) and European Union (FSE-POPH) with two individual grants (SFRH/BPD/86041/2012 and SFRH/BPD/86027/2012).