Benign anorectal disease comprises a broad group of processes with very diverse origins; these processes may be congenital or acquired as well as inflammatory or tumor related. However, benign anorectal disease has received less attention in the scientific literature than malignant disease. In this second part of this image-based review of benign anorectal disease, we describe the most common inflammatory and fistulous diseases, the postsurgical anatomy, and complications that can occur after surgical treatment or radiotherapy for anorectal disease.
La patología benigna anorrectal comprende una vasta cantidad de entidades de muy diversos orígenes, congénitas o adquiridas, inflamatorias o tumorales. Sin embargo, ha recibido menos atención en la bibliografía científica que el estudio de la patología tumoral maligna. En esta segunda entrega de la revisión basada en imágenes de la patología benigna anorrectal describimos la patología inflamatoria y fistulosa más frecuente, la anatomía posquirúrgica y las complicaciones secundarias al tratamiento quirúrgico o radioterápico de la patología anorrectal.
Moving forward with the MRI review of the benign anorectal (AR) pathology we will dedicate this review to inflammatory and post-surgical pathology. The right diagnosis and description of inflammatory pathology are key in surgical management and might lead to urgent changes in the therapeutical approach.
The goal of this review is to go over the main AR inflammatory entities especially fistulose pathology, remember the most common surgical methods in AR pathology, post-surgical anatomy and the most common post-surgical and post-actinic complications of the AR region.
Anorectal inflammatory pathologyFistulose pathology and sepsisMRI is the preferred modality1,2 due to its greater sensibility and specificity in the detection of fistulose trajectories,3 assessment of complications and finding the possible implication of other organs which makes it essential during the pre-surgical decision making process.4 Fistulas and fistulose trajectories are better marked out with 3.0T rather than 1.5T images given both the tissular and anatomic definition improve with field power.5
Perianal fistulas are usually idiopathic secondary to chronic infection of anal intramural crypts (critpoglandular hypothesis),6,7 but they can also be associated with bowel inflammatory disease (BIS) especially Crohn’ disease, tuberculosis, trauma, radiotherapy, neoplasms or pelvic infection.1 The high frequency of relapses can be attributed to the persistence of non removed-trajectories or abscesses.4
We need to remember that: MRI is way better than surgical exploration when it comes to explore trajectories and pre-surgical collections which in turn reduces the rate of relapses.7–9
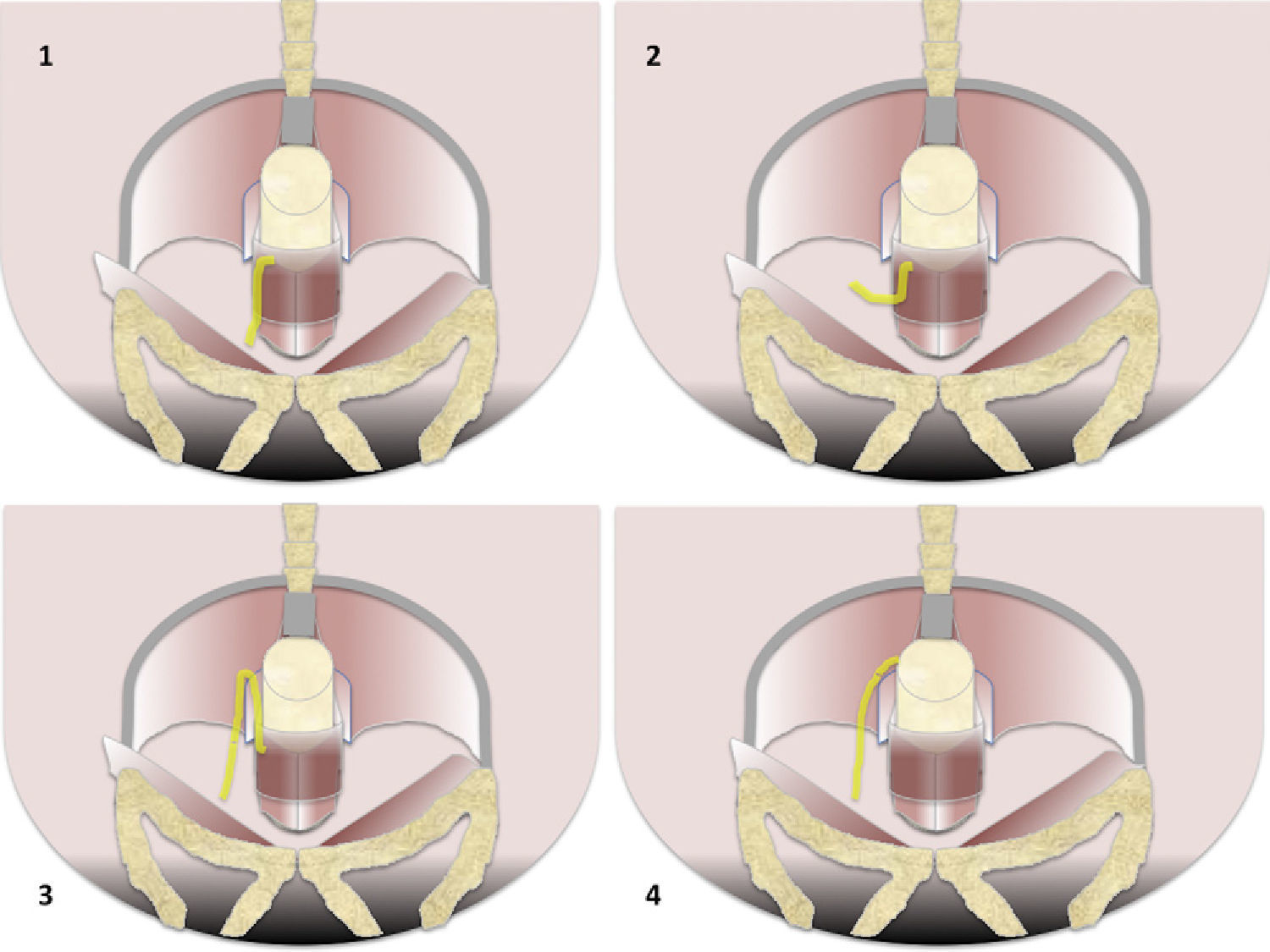
According to the trajectory fistulas can be divided into 4 types from greater to lower frequency: intersphyncteric, transphyncteric, suprasphyncteric and extrasphyncteric7 (Fig. 1).
Types of fistulas according to Parks’ classification. (1) Intersphincterian (45%). The trajectory of fistula is caudal along the interphincterian space toward the skin or perineum which is completely confined by the externa sphincter that it never gets to perforate. It does not touch the spaces of ischiorectal and ischioanal fossae. (2) Transphincterian (30%). It perfortes the external anal sphincter spreading toward the ischiorectal or ischioanal fossa and through them it can spread caudally toward the perineum or skin–previously toward the vagina or bladder or even cranially toward the elevator muscle of anus. (3) Suprasphincterian (20%). It dessicates the intersphincterian space toward the supraelevating space over the puborectalis muscle and perforates the elevator muscle of anus to reach the ischiorectal and ischioanal fossae and from that point toward the skin and the perineum. (4) Extrasphincterian (5%). It does not originate in the perianal region but it is produced by caudal extension of an infection focus of supraelevating space. The sphincterian space remains untouched. In one extrasphincterian fistula we will look for a pelvic purulent process caudally crossing across the elevator muscle of anus.
“Intersphyncteric” fistulas are 45–60% of fistulous trajectories.7,9,10 Intersphyncteric space is one anatomic barrier for the extension of ischiorrectal fossa. “Horseshoe”-shaped secondary trajectories can advance in the very Intersphyncteric space.1
“Transphyncteric” fistulas (20–30%)7,9,10 are those of a potentially higher risk of incontinence since they compromise both sphincters.1 They spread from ischiorectal to ischioanal spaces and they are usually accompanied by hyperemia, phlegmons or abscesses.1,2 “Suprasphyncterica” fistulas (5–20%) originate in the intersphyncteric space with an ascending trajectory spreading over the insertion of puborectalis muscles and the elevator muscle of anus, and perforate the elevator muscle of anus to reach the ischiorectal fossa.7,9,10 “Extrasphyncteric” fistulas (2–5%)7,9,10 do not compromise the intersphyncteric space7 suggestive that its origin is different possibly due to rectal or pelvic pathology – inflammatory or malignant.7,10
The commonly accepted surgical classification of perianal fistulas described by Parks et al.10 has been adapted to MRIs to become the classification of St James's University Hospital.1 In such classification fistulas are categorized into 5 different grades depending on whether trajectories are simple or complex, on the presence of abscesses, on the perforation of the external sphincter, and on the extension to supraelevating spaces. In grade 1 there is a simple intersphyncteric trajectory in the absence of abscesses, perforation of the external sphincter, or extension to supraelevating spaces (Fig. 2). In grade 2 there is a complex intersphyncteric trajectory or abscess-associated (Fig. 2). In grade 3 there is a transphyncteric or suprasphyncteric trajectory (perforating the external sphincter) with a simple trajectory in the absence of abscesses or extension over to supraelevating spaces (Fig. 3). In grade 4 there is a there is a complex transphyncteric or suprasphyncteric trajectory or abscess-associated (Fig. 4). Last but not least grade 5 consists of a cranially extended trajectory over to supraelevating spaces (Fig. 5).
We need to remember that: the radiological report needs to describe the affectation of the sphincterian complex, the point of origin, the type of main trajectory, the secondary trajectories, the abscesses, and the point exit of if exists, as well as the implication of the puborectalis muscles and the elevator muscle of anus.4,10 However on its own this is a limited classification because it is also necessary to describe the affectation of other pelvic organs (prostate, root of penis, urethra, and vagina) (Fig. 6), and perineal complications capable of modifying both treatment and prognosis (Fig. 7).
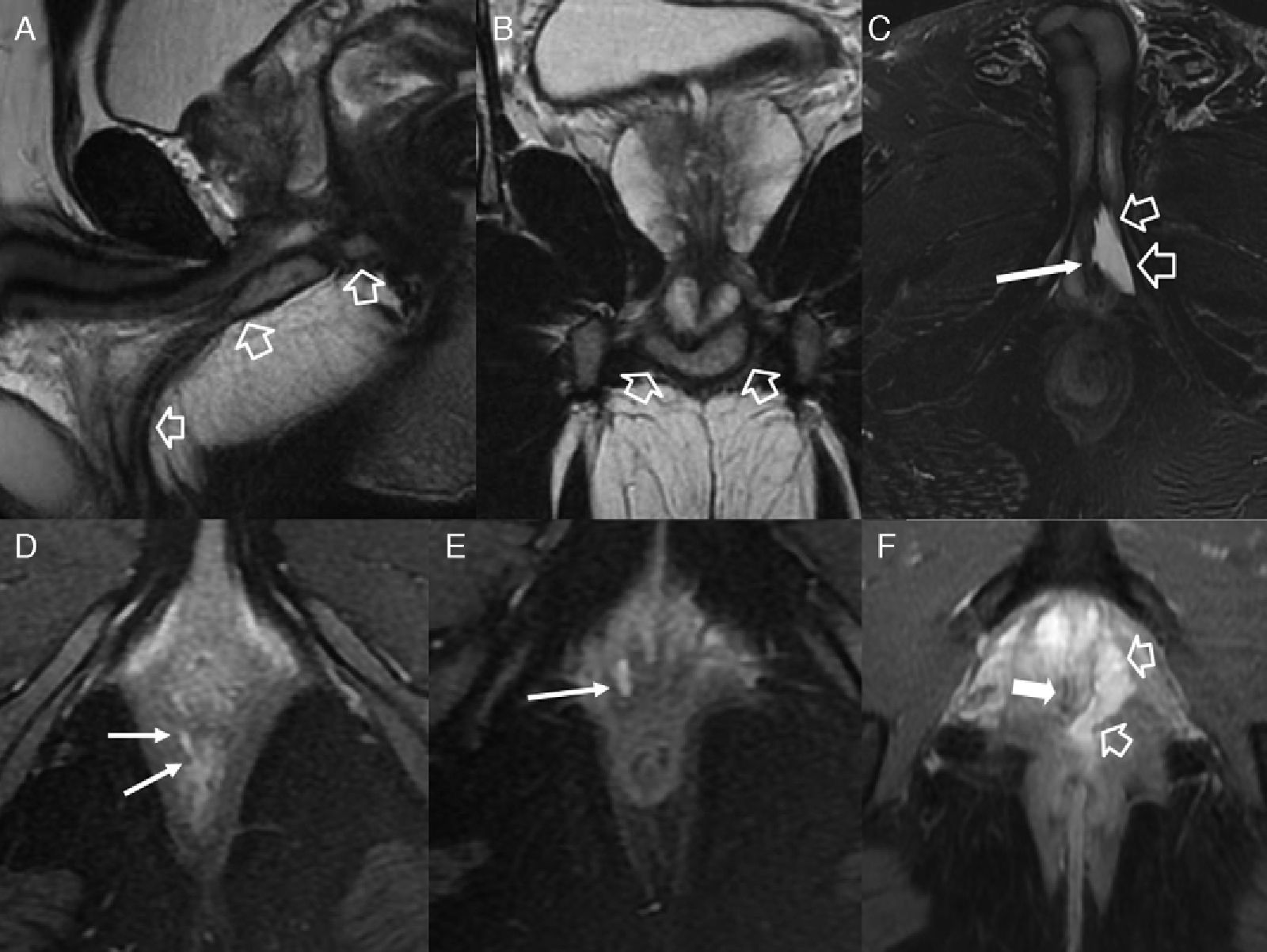
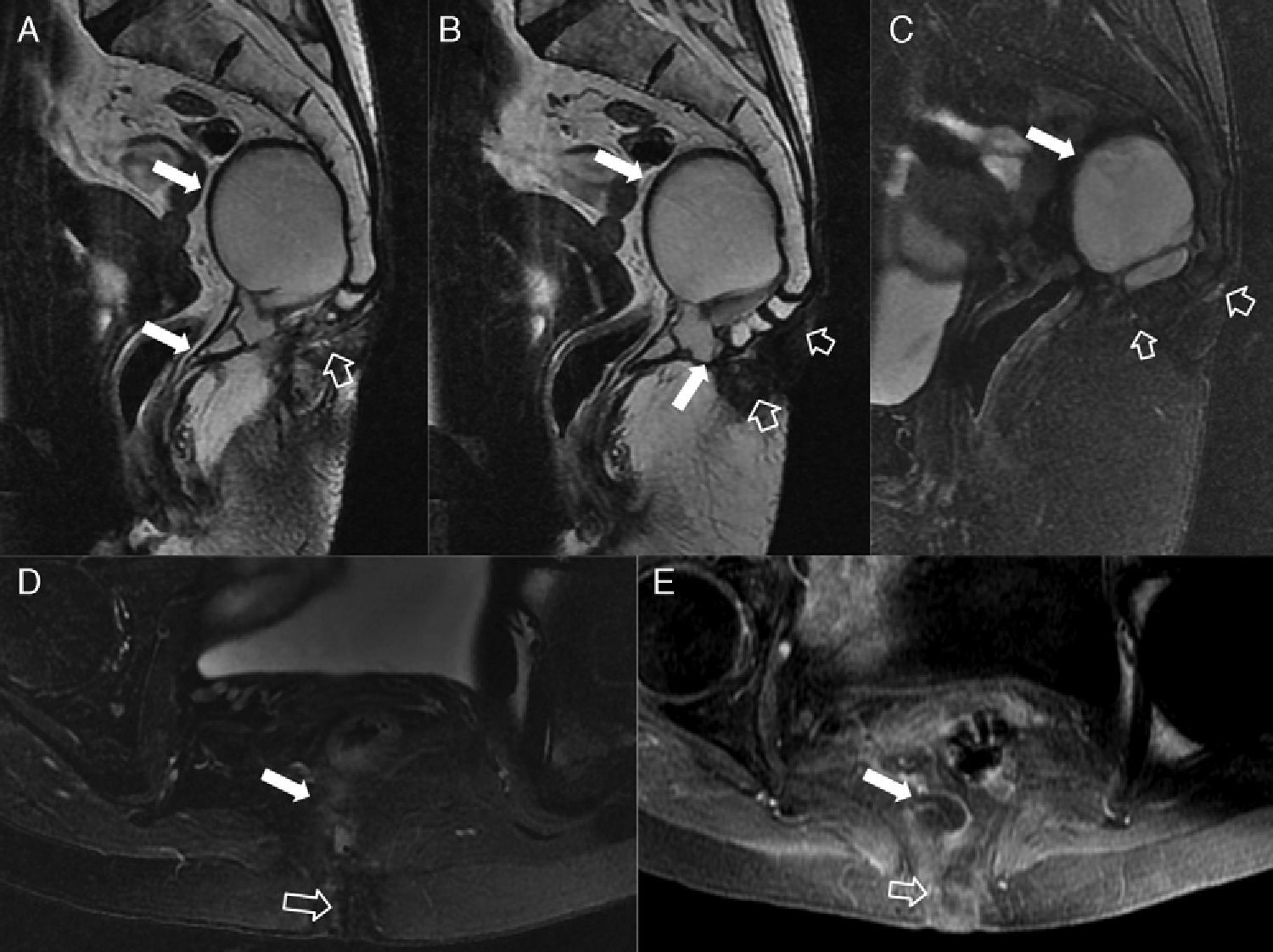
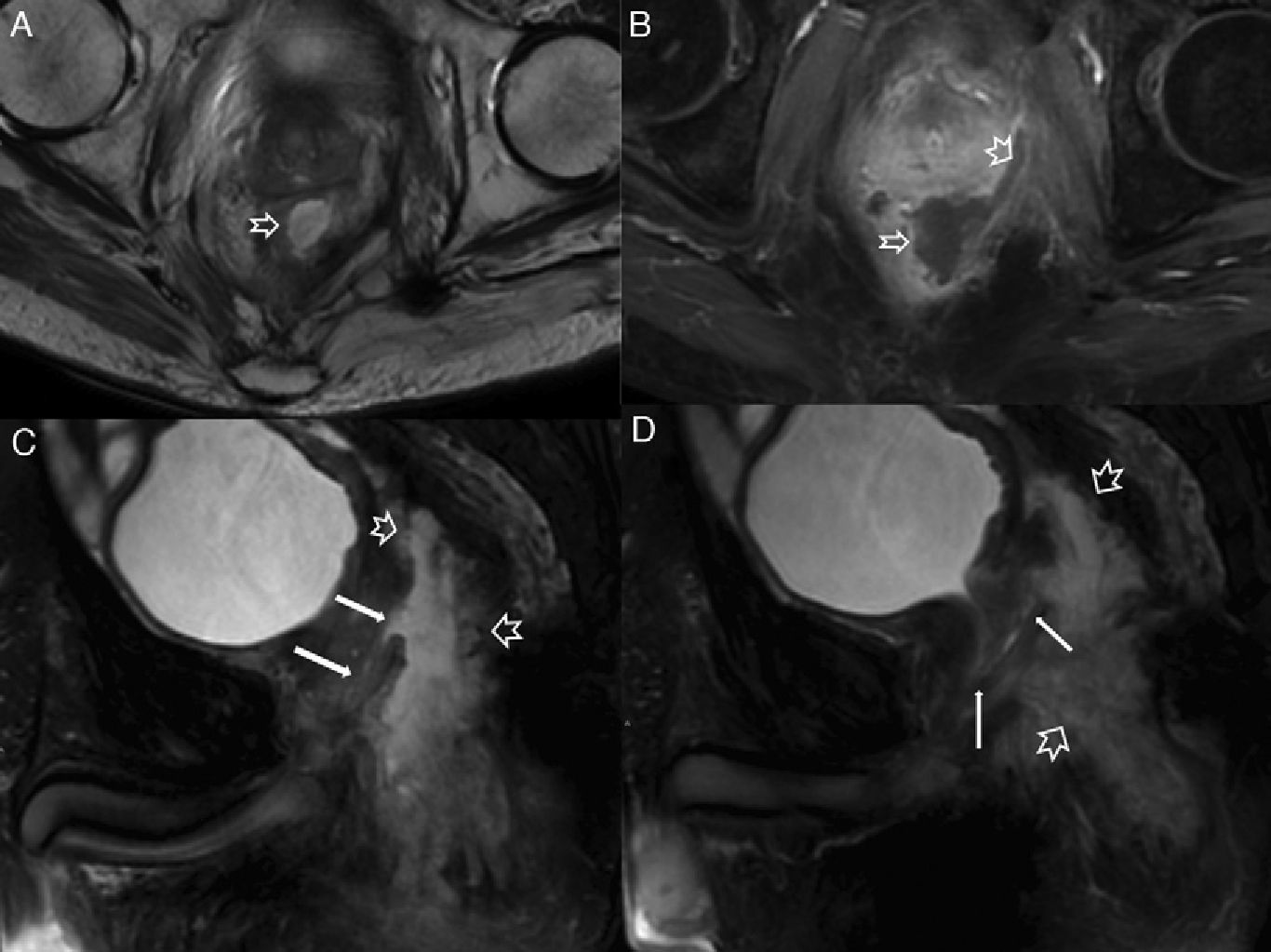
Fistulous affectation to other pelvic structures. (A) T2-weighted sagittal image. (B) T2-weighted coronal images where the hollow arrow shows one abscessed fistulous trajectory spreading toward the root of the penis (with horseshow-live secondary trajectory in B) and the scrotal cavity. (C) T2-weighted axial images with suppression of fat in other patient with abscessed fistulous trajectory (hollow arrows) in the root of the penis opening toward the penile urethral (thin solid arrow). (D and E) T1-weighted axial images with suppression of fat after the administration of IV contrast where thin solid arrows show rectovaginal one fistulous trajectory. (F) T2-weighted axial images with suppression of fat with hollow arrows showing one perianal fistulous abscessed intraprostatic trajectory. The solid arrow shows the position of the unaffected prostatic urethra.
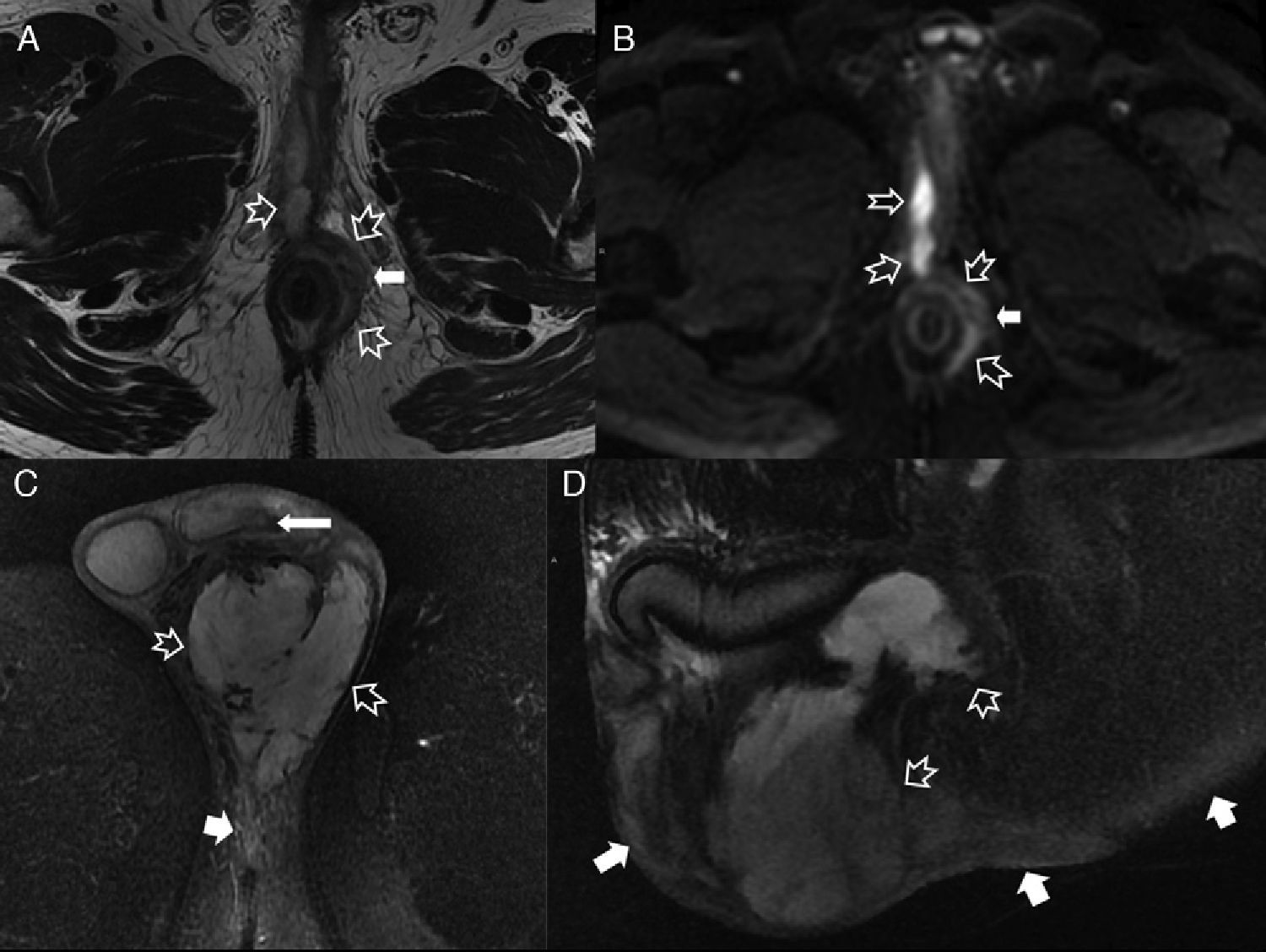
Septic perineal complications. (A) T2-weighted axial images. (B) T2 diffusion weighted axial images (B 600) of a patient showing a complex perianal fistula with horseshow-like intersphincterian trajectory that later perforates the external sphincter toward the left ischioanal fossa causing an abscess that spreads toward the root of the penis and to the right cavernous body of penis (hollow arrows). Notice the extense inflammatory affectation of the left puborectalis muscle and external sphincter due to serious myositis (solid arrows). (C) T2-weighted axial images with suppression of fat. (D) T2-weighted sagittal images with suppression of another patient showing large left scrotal abscess (hollow arrows) leaving the peritesticular tunica albuginea untouched (thin solid arrow in C). Notice the extense inflammatory affectation of the subcutaneous cell tissue of the perineum (thick solid arrows). The patient underwent urgent surgery where an early Fournier’ gangrene that required the extensive debridement of the perineum was confirmed.
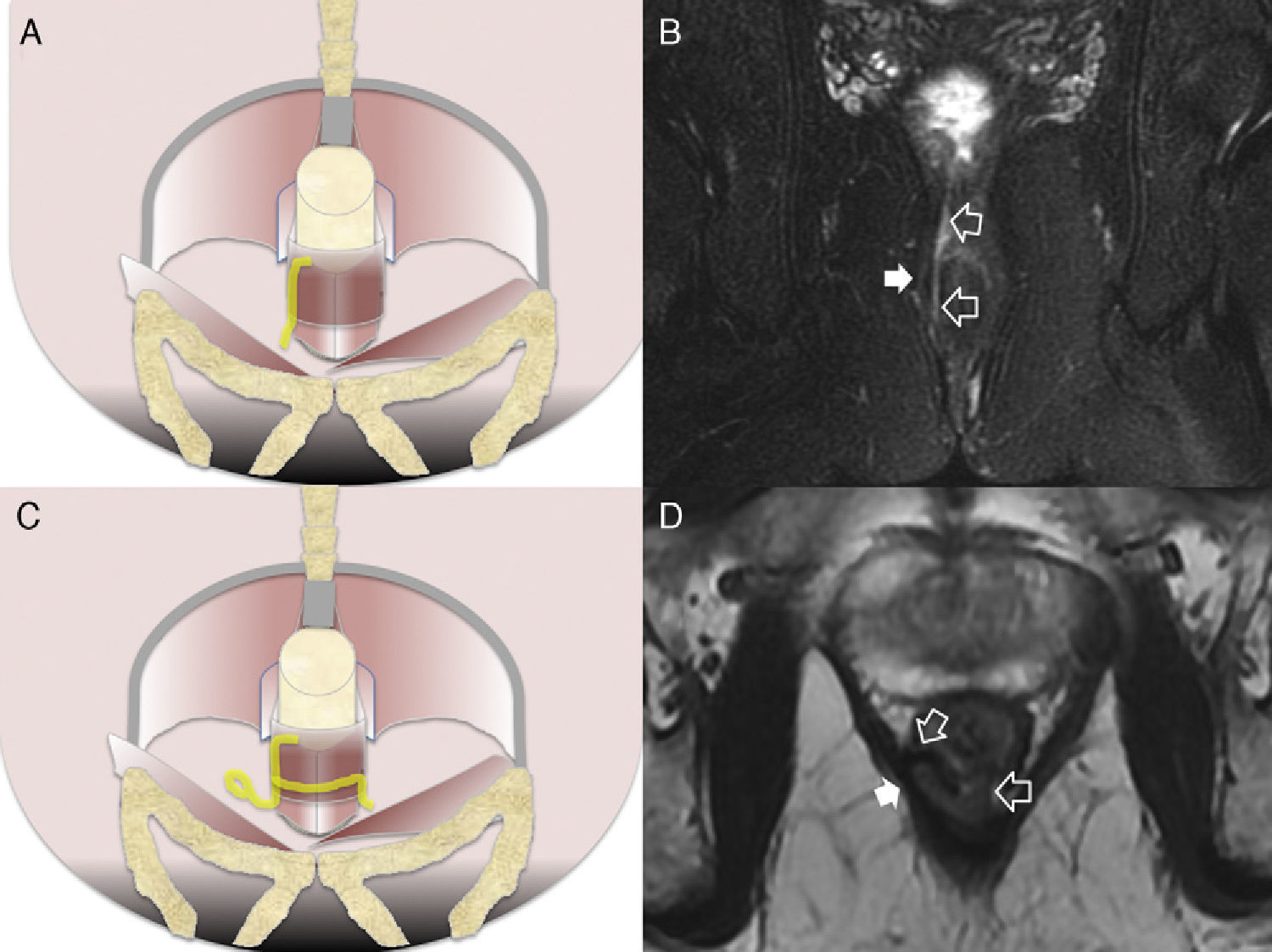
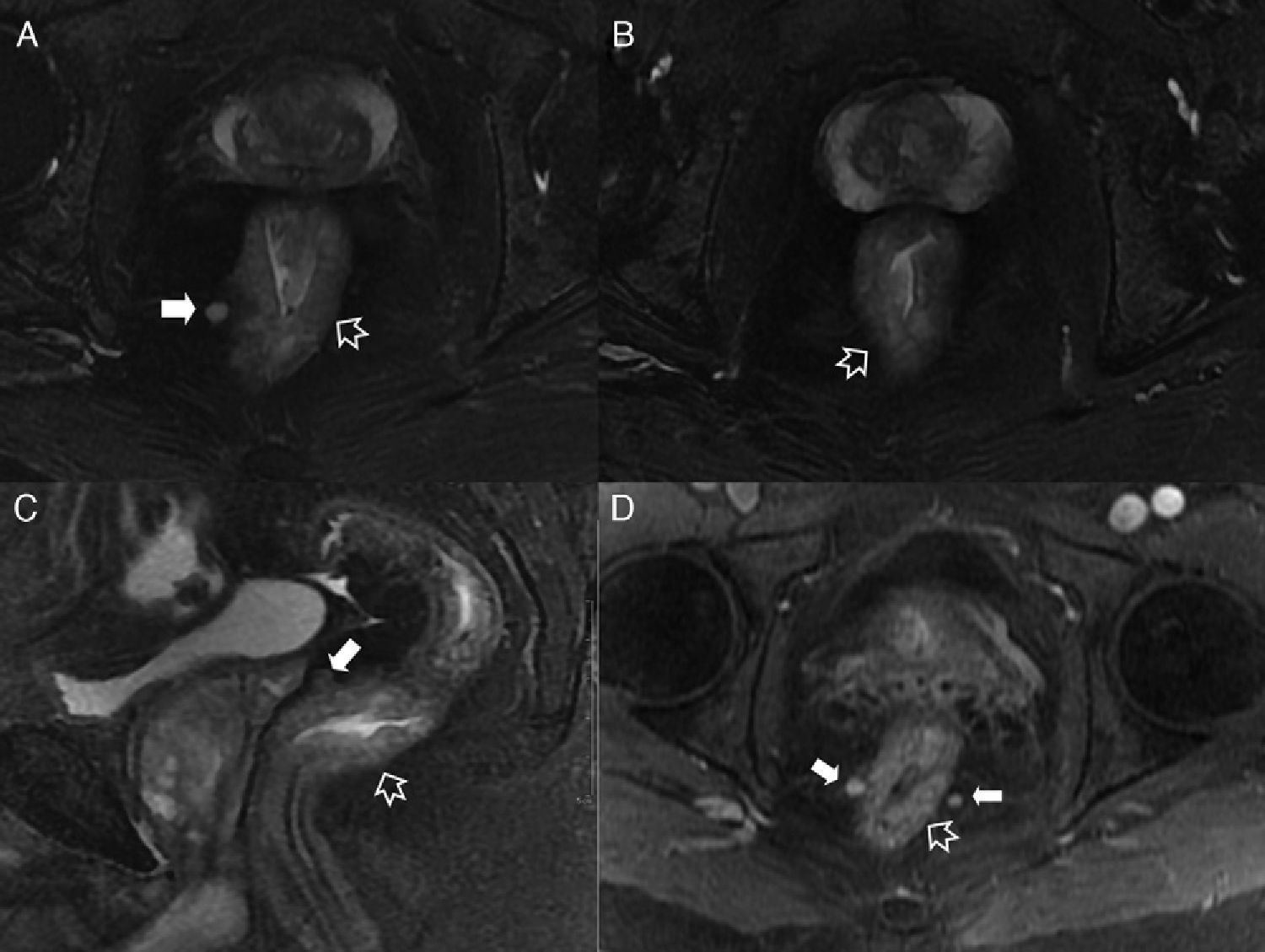
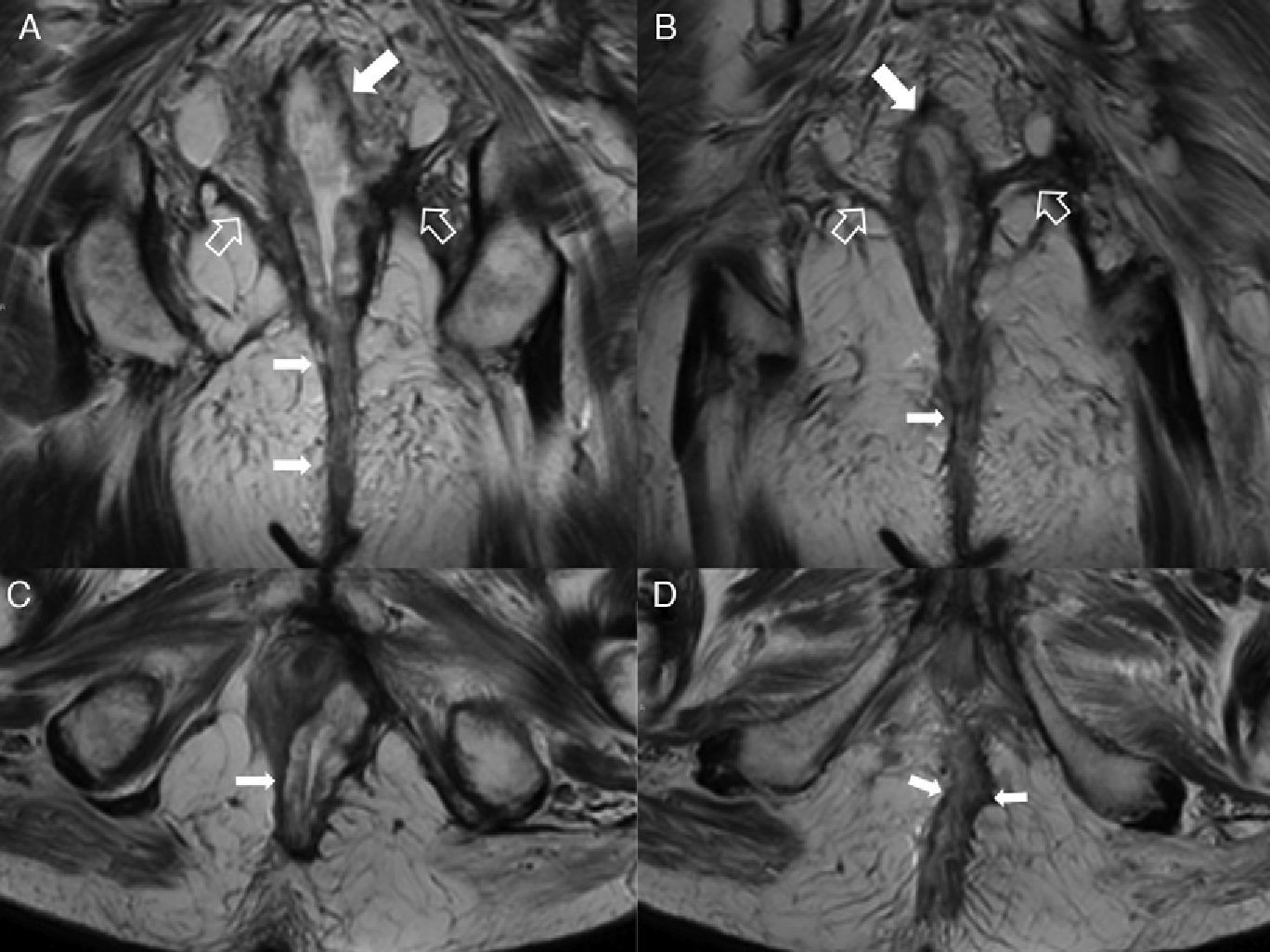
Intersphincterian fistulas. (A and C) Coronal-plane schemes of simple and complex intersphincterian fistulas, respectively. (B) T2-weighted coronal image with fat suppression showing one type 1 intersphincterian fistula (hollow arrows) keeping the external sphincter plane untouched (solid arrow). (D) T2-weighted axial image showing one type 2 horseshoe-like fistulous trajectory (hollow arrows) confined by the untouched external sphincter (solid arrow).
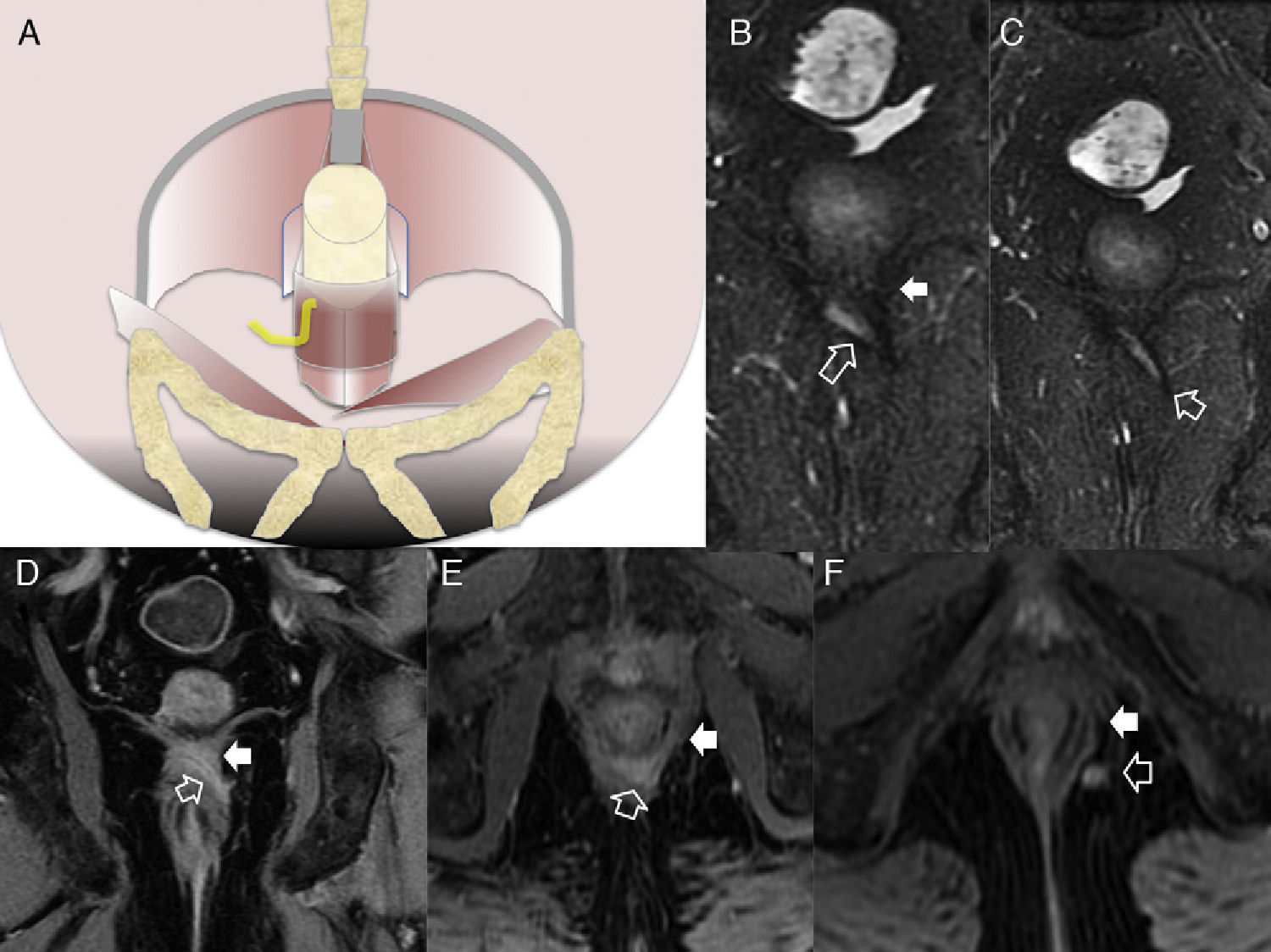
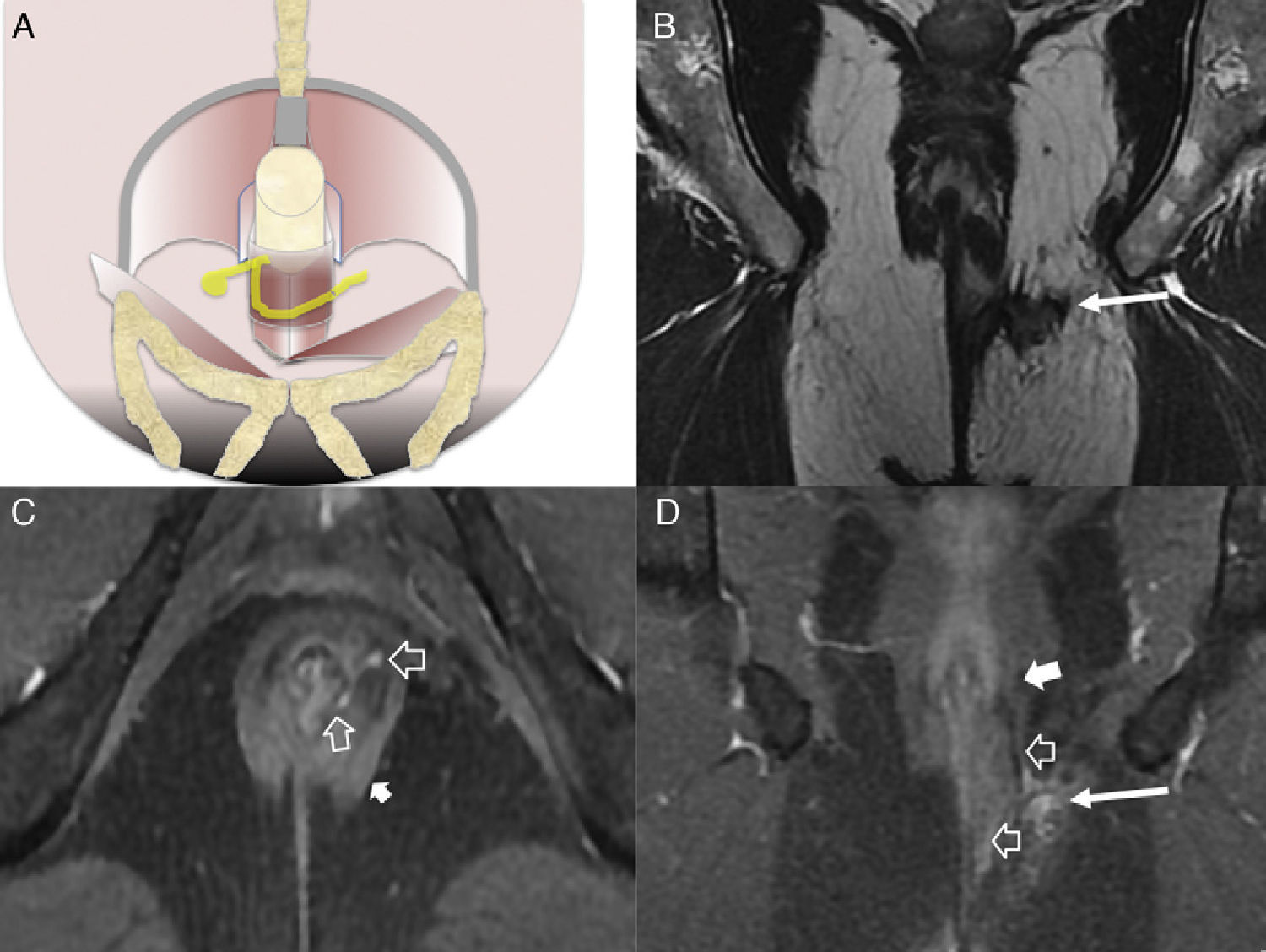
Type 3 fistula or transphincterian. (A) Coronal-plane scheme of fistula perforating both the internal and the external sphincters with a simple trajectory. (B and C) T2-weighted coronal images with suppression of fat. (D) T1-weighted coronal images with fat suppression after the administration of IV contrast. (E and F) T1-weighted axial images with fat suppression after the administration of IV contrast. Hollow arrows show the trajectory of one transphincterian fistula stemming from the position of 9 o’clock with one single descending lateral trajectory reaching into the left ischiotibial space while perforating the left external sphincter (solid arrows).
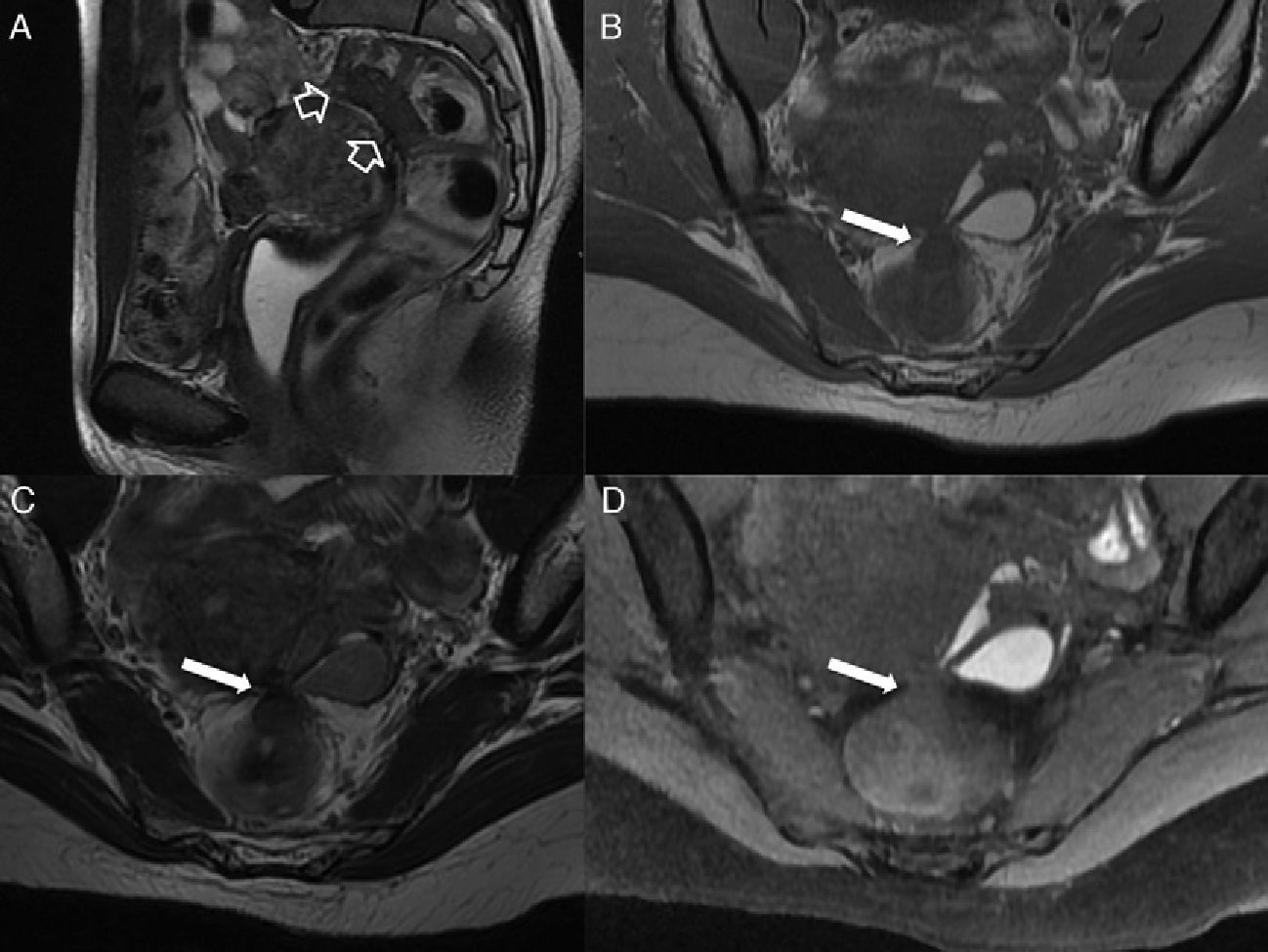
Type 4 or complex transphincterian fistula. (A) Coronal-plane scheme of one complex transphincterian fistula with an abscess in the right ischiorectal fossa and secondary trajectory. (B) T2-weighted coronal images. (C) T1-weighted axial images with fat suppression after the administration of IV contrast. (D) T1-weighted coronal images with fat suppression after the administration of IV contrast. One transphincterian fistula with one secondary trajectory can be identified (hollow arrows) while perforating the left external sphincter (thick solid arrows) and spreading toward the left ischioanal space where the abscess occurs (thin solid arrows).
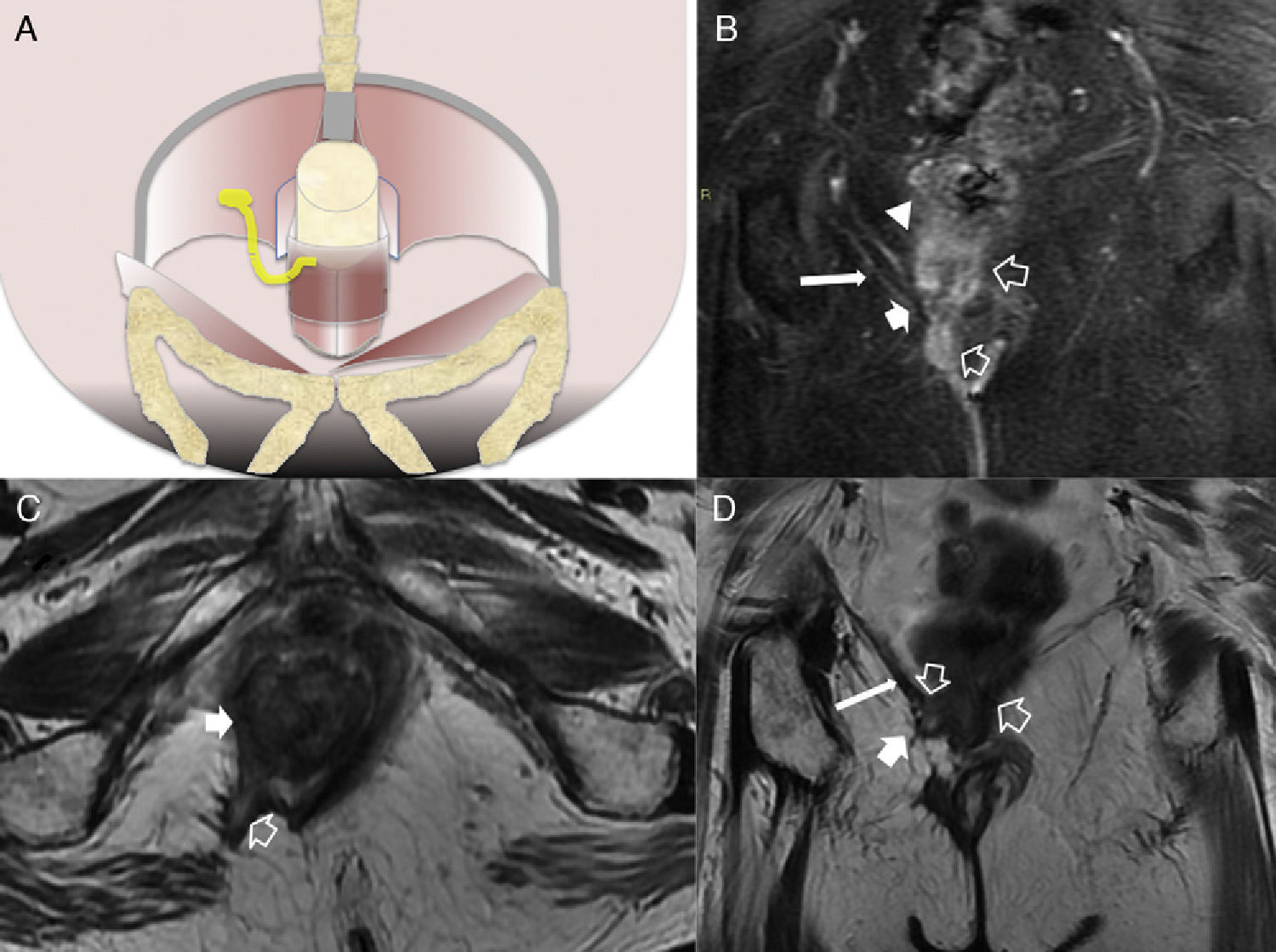
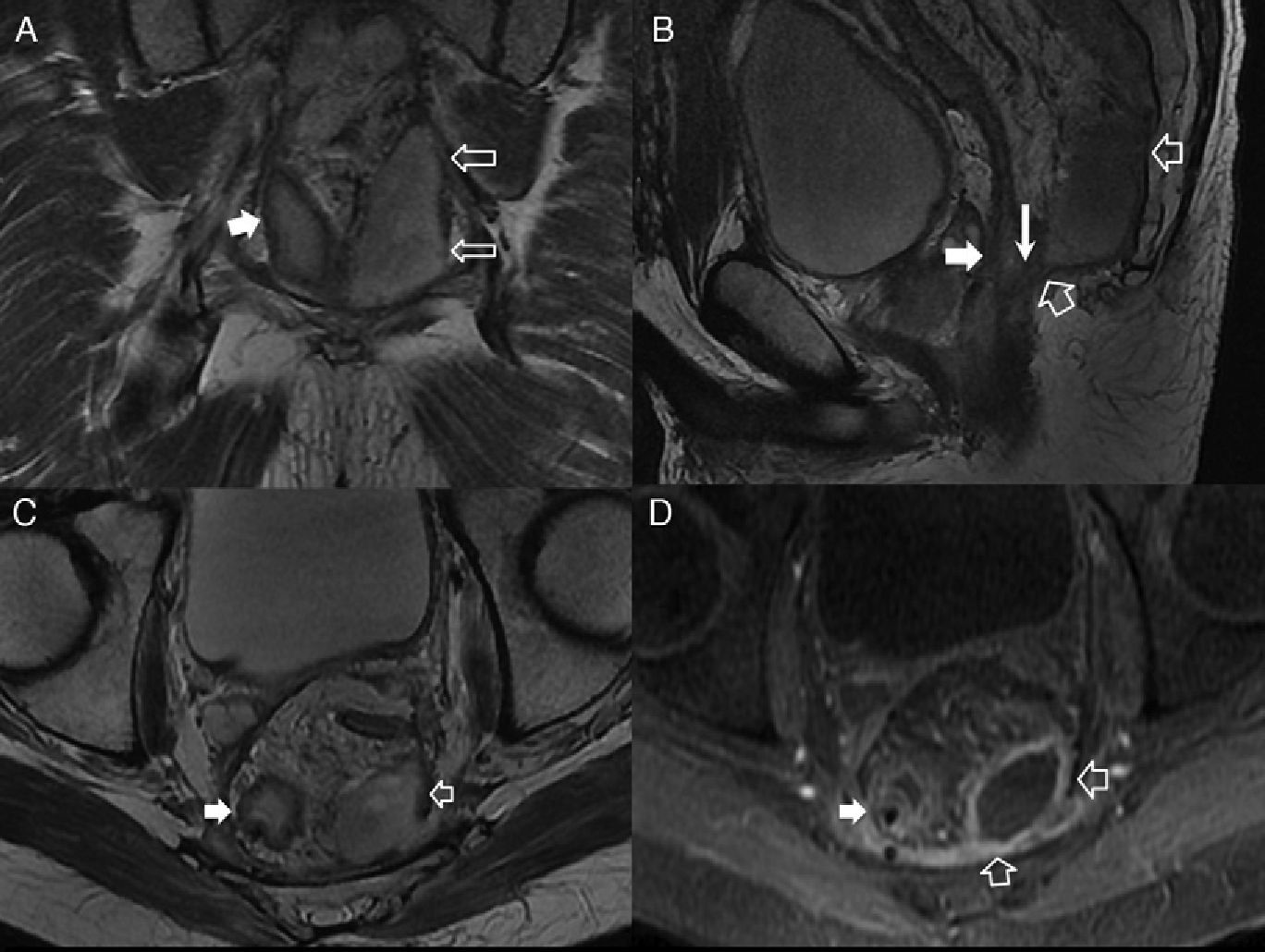
(A) Coronal scheme showing the supraelevating fistulous trajectory or type 5. (B) T2-weighted coronal image with suppression of fat. (C) T2-weighted axial image. (D) T2-weighted coronal image. The hollow arrows show one fistula of suprasphincterian trajectory while perforating the elevator muscle of right anus (thin solid arrows) and puborectal muscle (thick arrows). It would be a grade 5 fistula in Parks’ classification due to its extension–though minimal toward the right supraelevating space (white arrow head).
85–95% o fistulas have a simple trajectory.4 Complex trajectories or with abscesses associated are accompanied by a greater rate of relapse and surgical complications.4,7 One important complication is the loss of fecal continency. The lesion of the internal sphincter does not usually alter continency while the lesion of the external sphincter or puborectalis muscle is usually more problematic and the risk of incontinency is high.1,11,12 In some occasions doctors use a drainage seton (small medicalized drainage tubes) to leave the fistula opened for slow drainage thus reducing the risk of sepsis while delaying healing.13,14 Other feared complication is the development of extensive perineal septic processes that can usually lead to Fournier’ gangrene (Fig. 7).15
Pilonidal fistulasThey are a cutaneous fistulous defect of embrionary development in the cranial margin of the interglutea fold that can deeply extend to connect with raquideal conduit in many occasions. It can be associated with lumbosacral bone anomalies and intraraquideal injuries. Its extension toward the ischiorectal space16 has rarely been described and even one association to pathology to perianal fistula pathology or other presacral injuries17 (Fig. 8). It is easy to distinguish them from perianal fistulas because they do not usually affect the sphincterian complex.18
Pilondial fistulas. (A and B) T2-weighted sagittal images. (C) T2-weighted sagittal images with suppression of fat. (D) T2-weighted axial images with suppression. (E) T2-weighted axial images with suppression of fat after the administration of IV contrast. Pilonidal sinus with fistulous extension toward the right ischiorectal fossa (hollow arrows) and presacral space where one retrorectal cystic hamartoma (solid arrows) was confirmed during surgery.
The most common cause is ulcerative colitis. It starts in the rectal mucose and extends proximally toward the colon in a continuous way. MRIs show less acute parietal thickness and more symmetrical than in Chron’ disease (Fig. 9) with marked parietal enhancement.2 MRI shows T2-weighted signal hyperintensity in the wall with stratified enhancement after the administration of intravenous (IV) contrast.2 It can become complicated with sinus trajectories, fistulas and/or perianal abscesses even if perianal fistulous pathology is much more common in Chron’ disease.
Proctitis. (A and B) T2-weighted axial images with suppression of fat. (C) T2-weighted sagittal images with suppression of fat. (D) T1-weighted sagittal images with suppression of fat after the administration of IV contrast. Proctitis in one young patient with an active inflammatory episode of Chron’ disease. Inflammatory changes in rectum (hollow arrows) with irregular parietal thickening and transmural inflammation confirmed by the parietal signal hyperintensity on T2 sequeces and contrast uptake. Note the irregular mucosa through both the superficial and deep ulcers and the inflammatory mesorectal adenopathies too (solid arrows).
Rectal diverticula are rare.19,20 Most are diagnosed incidentally even though they can become swollen or cause discomfort when they are large.19,20
Anorectal endometriosisIt is a recurrent inflammatory process involving rectosigmoid colon and rectovaginal septum.21
Anorectal endometriosis often occurs in advanced cases and patients describe discomfort while defecating, subocclusive crisis, or dispareunia.21,24 The most common locations of bowel endometriosis are–in order: rectosigmoid,25 appendicle, cecal and terminal ileal.21
Endometrioisis can show different appearances in images. Endometriomas are cystic injuries with hematic, heterogeneous content, sometimes with hyperintense levels in both pulse sequences that remain hyperintense in T1-weighted sequences with fat suppression (necessary to distinguish them from cystic teratomae) and they can show internal signal degradation in T2 (shading) due to the component of chronic bleeding.21
We need to remember that: the deep pelvic endometriosis–infiltrating and solid (>5mm of subperitoneal invasion) is characterized by implants often hypointense on T2-weighted sequences and iso or hypointense on T1.21,25 Implants attach to serosa and profoundly invade the muscular layer stimulating the proliferation of flat muscle which might lead to stenosis due to parietal thickening and obstructive processes22,24,25 (Fig. 10). It rarely reaches the intestinal mucose or causes digestive bleedings. Glandular proliferation is possible as well and it is characterized by hyperintensity on T2.22 Less common is to see it present as a lineal fibrotic or hypointense spiculated nodule deposit retracting the affected intestinal loop.24 This type of presentation is often accompanied by episodes of intestinal subocclusion whose differential diagnosis are adhesions.24
Rectosigmoid infiltrating endometriosis. (A) T2-weighted sagittal images. (B) T1-weighted axial images. (C) T2-weighted axial images. (D) T1-weighted axial images with suppression of fat. Uterine torus (depression in the posterior side of the uterine body where uterosacral ligaments meet) is affected by deep endometriosis with one intermediate-low signal implant on T2-weighted sequences (solid arrows). The retraction of the rectosigmoid wall can be due to fibrotic adherences between the uterine torus and the rectosigmoid serosa or as it happens in this case to direct invasion of the muscular layer with fibromuscular proliferation (hollow arrows in A). Note the left adnexial endometrium.
One rare but typical location is rectovaginal septum.25 In these cases the distension of vaginal cavity with gel to improve the vaginal wall and the rectovaginal septum26 can be useful.
Postsurgical anatomy and complications secondary to the management of tumoral or benign anorectal pathologyMRIs are effective to detail post-surgical anatomy and study post-therapy complications of anorectal pathology.14 Getting to know the different surgical proceedings and manifestations on the MRI is key to be able to interpret the images. The most common post-surgical complications are: abscesses, fistulas, post-surgical or post-actinic fibrosis and tumor relapse–common to other proceeding as well.
Surgical proceedingsThe most common proceedings in the management of anorectal cancer are low anterior resection and abdominoperineal amputation.14 Both include total mesorectal excision. The sphincter is preserved with the low anterior resection proceeding while in the abdominoperineal amputation proceeding the sphincterian complex is completely resected including the elevator muscle of anus.14 In the abdominoperineal amputation caudal perineal closure is variable. A single closure or accompanied with muscular reinforcements of the gluteal muscularity or the rectus abdominis muscle14 (Taylor’ flaps) can be performed. Whenever possible the elevator muscle of anus should be preserved in an effort to preserve pelvic anatomy. Another option is closure with reinforcement through pediculated omentoplasty.14
In the lower anterior resection both the colorectal and the coloanal anastomoses can be performed with or without a colonic “J” pouch rectal reservoir in an effort to increase the volume of neorectum.14 It should not be taken for wound breakdown or anastomosis of post-surgical collection (Fig. 11). The alternative to the colonic “J” pouch rectal reservoir is coloplasty–a longitudinal colon incision through transversal suture as in strictureplasty proceedings.14
Anastomotic breakdown with abscess. (A) T2-weighted coronal images. (B) T2-weighted sagittal images. (C) T2-weighted axial images. (D) T1-weighted axial images with suppression of fat after the administration of IV contrast. The anastomotic leak can often found as in this case in one breakdown of the posterior appearance of anastomosis between two bowel segments at the reservoir along the line of suture (thin solid arrow). As in this case it can cause an infectious complication (pelvic abscess or fistula). The T1-weighted study with suppression of fat after the administration of IV contrast shows the abscess of the hypercaptation wall (hollow arrows) found on the left of coloanal anastomosis. The reservoir (thick solid arrows) finishes with metallic sutures and no hyper-enhancement after the administration of IV contrast.
Early complications are similar to other bowel surgical processes (suture breakdown, fistulas, and abscesses) (Fig. 12). Fistulas can affect the reservoir or any adjacent pelvic structure (vagina, bladder, urethra, perineum, Fig. 13). Phlegmons can be distinguished from abscesses through IV contrast.14 The most common late-onset complication is presacral fibrosis. It has a variable shape–often a flat angular concave homogeneous anterior margin different from tumor relapse which often presents as a nodular asymmetric irregular convex mass.14 Other late complications include fibrous stenosis in anastomosis (Fig. 14), the inflammation of the bowel reservoir in J or perineal hernia.14
Post-surgical abscess complicated with a fistula in the prostatic urethra in one 49 year old-patient with a history of radio-chemotherapy before abdominoperineal amputation. (A) T2-weighted axial images. (B) T1-weighted axial images with suppression of fat after the administration of IV contrast. (C and D) T2-weighted sagittal images with suppression of fat. Hollow arrows indicate one large abscess over the surgical bed of the abdominoperineal amputation. Solid arrows show the connection of fistula to the prostatic urethra.
Perineal fistula in a 65 year-old male patient after abdominoperineal amputation due to low rectal adenocarcinoma. (A and B) T2-weighted coronal images. (C and D) T2-weighted axial images. In this intervention the elevator muscle of anus was preserved (hollow arrows). One persistent perineal sinus (thin solid arrows) can be identified connecting the collection on surgical bed (thick solid arrows) to the skin along the surgical trajectory due to suture breakdown of the elevator muscle of anus. This is a rare complication consisting of a surgical wound at the trajectory of the abdominoperineal amputation that just will not close.
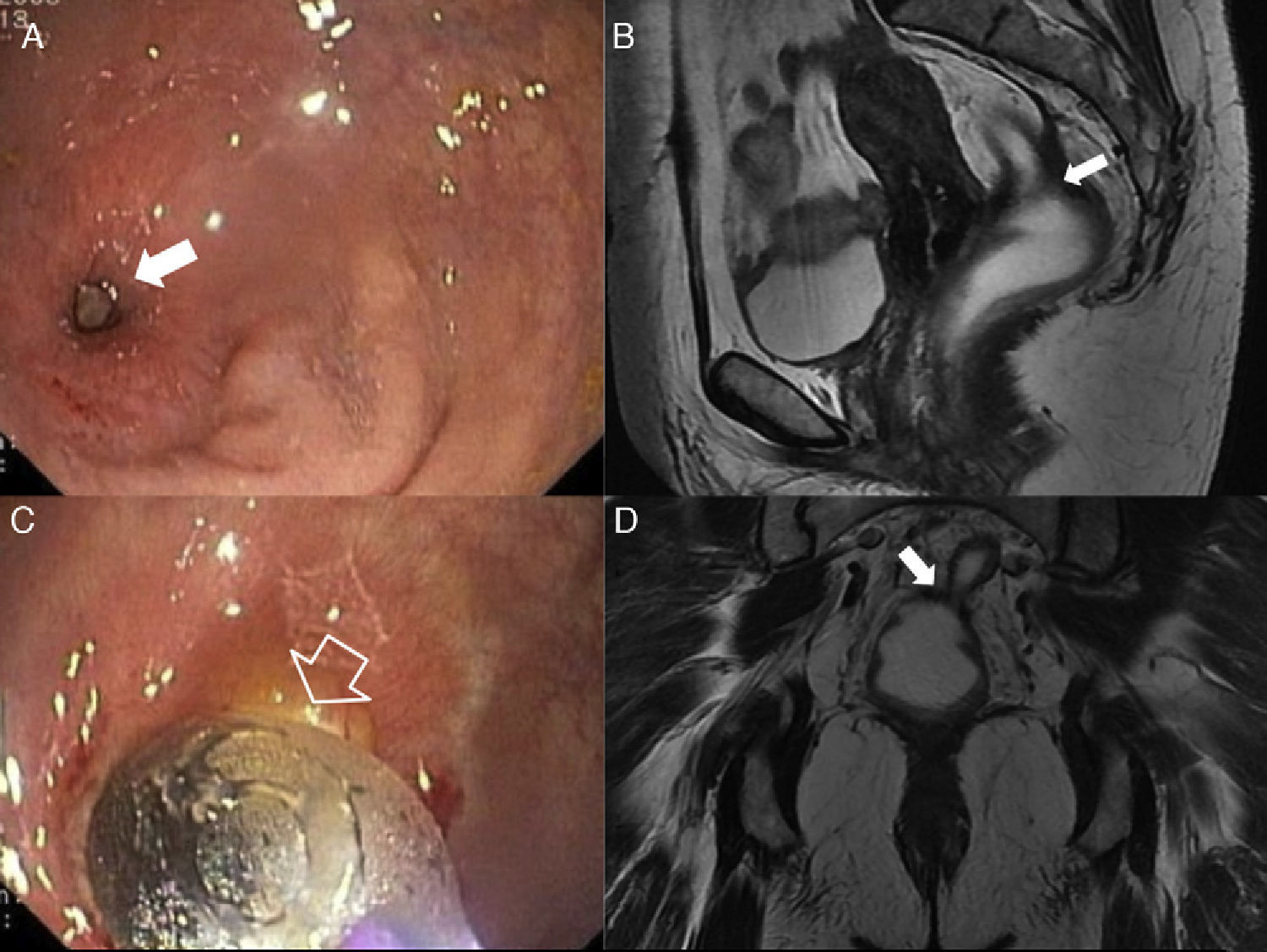
Post-surgical complications. Rectal stenosis. Rectal stenosis has been defined as the impossibility to advance with a 12mm-diameter sigmoidoscopy through a narrowed lumen. A benign stenosis after a low anterior resection is often the consequence of one anastomoric breakdown with subsequent fibrosis. (A and B) Endoscopic images of one benign rectal stenosis in a 55 year old-woman after low anterior resection surgery due to rectal cancer with colo-rectal anastomosis. (C) T2-weighted sagittal images. (D) T2-weighted coronal images after the administration of endorectal contrast showing the stenosis at the level of the colorectal anastomosis (solid arrows). The hollow arrow in B shows the catheter-balloon in the endoscopic proceeding of dilating the rectal stenosis (solid arrow in A).
Postradiotherapy complications can have an early onset like enteritis or acute actinic proctitis, or a late onset like fibrosis and pelvic structure retraction (Fig. 15).27 One diagnostic issue is being able to distinguish presacral fibrosis from tumor relapse.28 T2-weighted shape and intensity of signal have been proposed as the key elements to distinguish between the two14,28 yet the variability of presentation of both fibrosis (it can have a hyperintense inflammatory component on T2 even 12 months after radiotherapy) and tumor relapses makes them be not very useful.14
We need to remember that: the best options to suggest tumor relapse are dynamic study through IV contrast and the value of the diffusion ratio that is evident in diffusion sequences. Tumor injuries are characterized by contrast hyperuptake and diffusion restriction vs the late progressive enhancement and unrestriction of fibrotic tissue.28–30
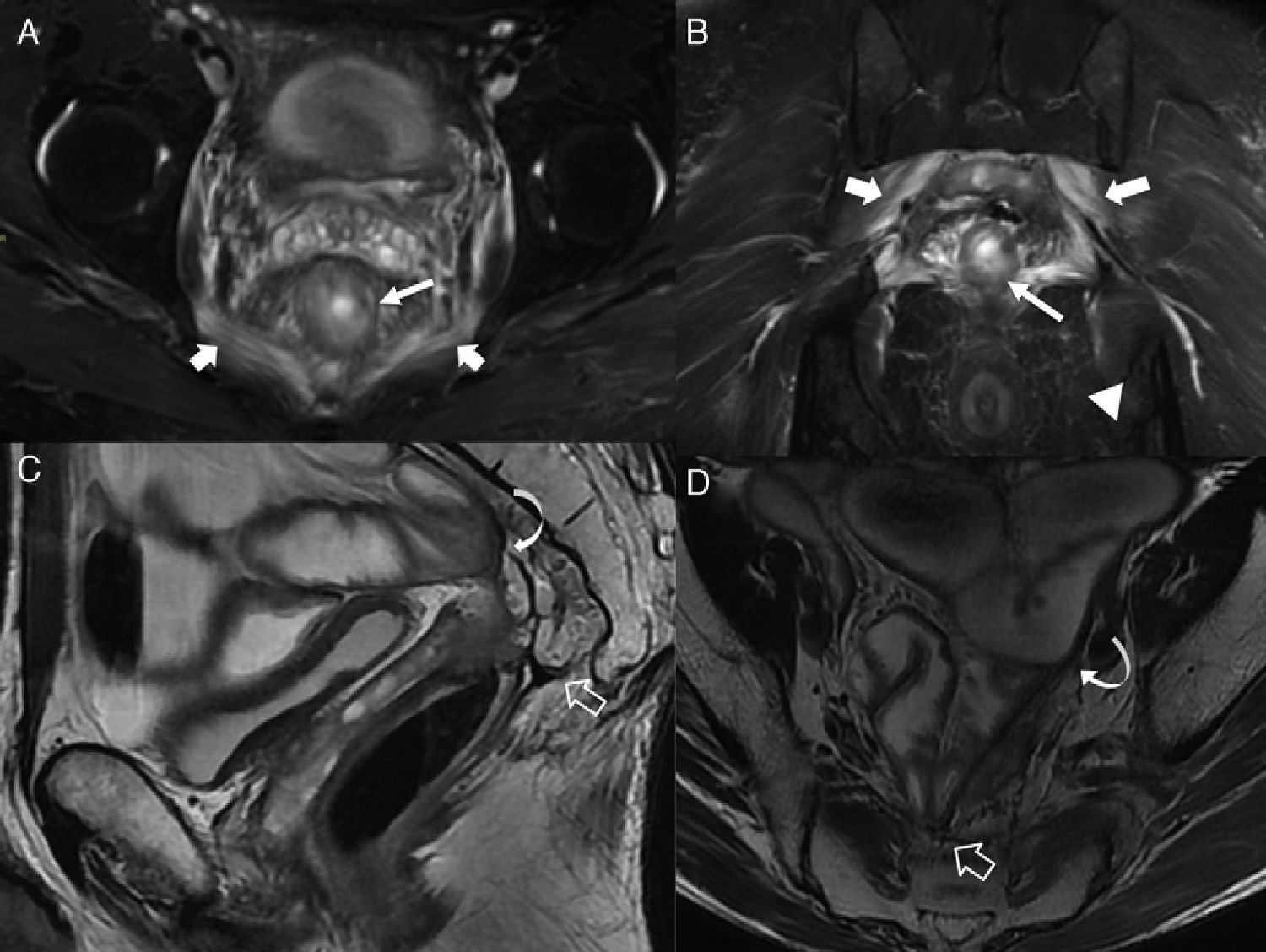
Early and late postactinic complications. (A) T2-weighted axial images with suppression of fat. (B) T2-weighted coronal images with suppression of fat in a male patient showing acute postactinic inflammatory chanes. Note both the thickness and hyperintensity of the wall of rectum (long thin arrows) due to acute proctitis. Similarly the postactinic myositis of pectineus muscles (thick arrows) and the inflammatory changes of ischiorectal fossae (arrow head in B) can be seen here. (C) T2-weighted sagittal images. (D) T2-weighted axial images of another patient with radiotherapic history due to rectal neoplasm and crisis of subocclusion. Presacral chronic fibrotic changes secondary to radiotherapy (hollow arrows) affecting both the colorectal anastomosis and the pelvic small intestine loops while causing one adherence syndrome with ileal distention (curved arrows).
Due to its high spatial and tissular resolution and non-invasive modality with no side effects high resolution–3.0T MRI are the main image modality to assess many perirectal and perianal processes. For surgeons MRIs are especially useful to be able to know the features of fistulas before the proceeding, define complications secondary to the management of tumor processes of rectum and anal canal, and distinguish them from prospective tumor relapses. We believe this is a useful review for the radiologist in an effort to get to know the main non-tumor processes affecting rectum and anus.
Ethical responsibilitiesProtection of humans and animalsAuthors confirm that no experiments have been done with humans or animals during this research.
Confidentiality of dataAuthors confirm that in this report there are no personal data from patients.
Right to privacy and informed consentAuthors confirm that in this report there are no personal data from patients.
Authors- 1
Manager of the integrity of the study: LHH, EAM and VMVF.
- 2
Original Idea of the Study: LHH, RCA and JCA.
- 3
Study Design: LHH, RCA, EAM and VMVF.
- 4
Data Mining: LHH, RCA, EAM, JCA and VMVF.
- 5
Data Analysis and Interpretation: NA.
- 6
Statistical Analysis: NA.
- 7
Reference Search: LHH, RCA, EAM, JCA and VMVF.
- 8
Writing: LHH, EAM and RCA.
- 9
Manuscript critical review with intellectually relevant contributions: LHH, RCA, EAM, JCA and VMVF.
- 10
Final Version Approval: LHH, RCA, EAM, JCA and VMVF.
Authors reported no conflicts of interests.
Please cite this article as: Herráiz Hidalgo L, Cano Alonso R, Carrascoso Arranz J, Alvarez Moreno E, Martínez de Vega Fernández V. La patología benigna de ano y recto con RM 3.0T. 2.a Parte: patología inflamatoria ano-rectal. Anatomía postquirúrgica y complicaciones postratamiento. Radiología. 2014;56:206–218.