Anomalous intracranial vessels are not uncommon, and this finding is not always associated with arteriovenous malformations. Other conditions such as anomalous connections between arteries or phlebitc patterns can also present as vessels with abnormal intracranial locations. Noninvasive diagnosis is important to determine whether to do more invasive tests such as cerebral digital subtraction angiography or to estimate the risk of bleeding in arteriovenous malformations and therefore to evaluate the need for endovascular/surgical treatment. In this paper, we present an algorithm for the differential diagnosis of anomalous intracranial vessels according to their location (intra/extra-axial) and function (whether the vessels are arterialized). Moreover, we analyze the important points of the angioarchitecture of the principal arteriovenous malformations with risk of intracranial bleeding, such as pial arteriovenous malformations and dural fistulas.
La detección de vasos anómalos a nivel intracraneal no es un hallazgo infrecuente y no siempre está asociada a la presencia de malformaciones arteriovenosas. Otras entidades como las conexiones arterioarteriales o un patrón flebítico también pueden presentarse como unos vasos en localización intracraneal anómala. El diagnóstico mediante pruebas no invasivas es importante para determinar la necesidad de realizar pruebas más cruentas como una angiografía cerebral por sustracción digital o para estimar el riesgo de sangrado en malformaciones arteriovenosas y, por tanto, evaluar la necesidad de tratamiento endovascular/quirúrgico. En este manuscrito presentamos un algoritmo de diagnóstico diferencial de la presencia de vasos anómalos intracraneales de acuerdo con su localización (intra/extraaxiales) y su funcionalidad (arterialización o no de dichos vasos). Asimismo, analizaremos los puntos importantes de la angioarquitectura de las principales malformaciones arteriovenosas con riesgo de sangrado intracraneal, como son las malformaciones arteriovenosas piales y las fístulas durales.
Anomalous intracranial vessels are not an uncommon finding, and are not always associated with arteriovenous malformations (AVMs). Other entities such as arterioarterial connections or a phlebitic pattern may also present as vessels in an anomalous intracranial location.
Diagnosis using non-invasive tests is important to determine the need for more invasive tests such as cerebral digital subtraction angiography (DSA), or to estimate the risk of bleeding in arteriovenous malformations and therefore assess the need for endovascular/surgical treatment. To stratify bleeding risk, it is necessary to study the angioarchitecture of both pial AVMs and dural arteriovenous fistulas (dAVFs).1–3
This manuscript presents an algorithm for the differential diagnosis of anomalous intracranial vessels according to their location (intra-axial or extra-axial) and “functionality” (arterialisation or non-arterialisation of said vessels). It will also analyse the key aspects of the angioarchitecture of the main AVMs that carry a risk of intracranial bleeding, such as pial AVMs and dural fistulas.
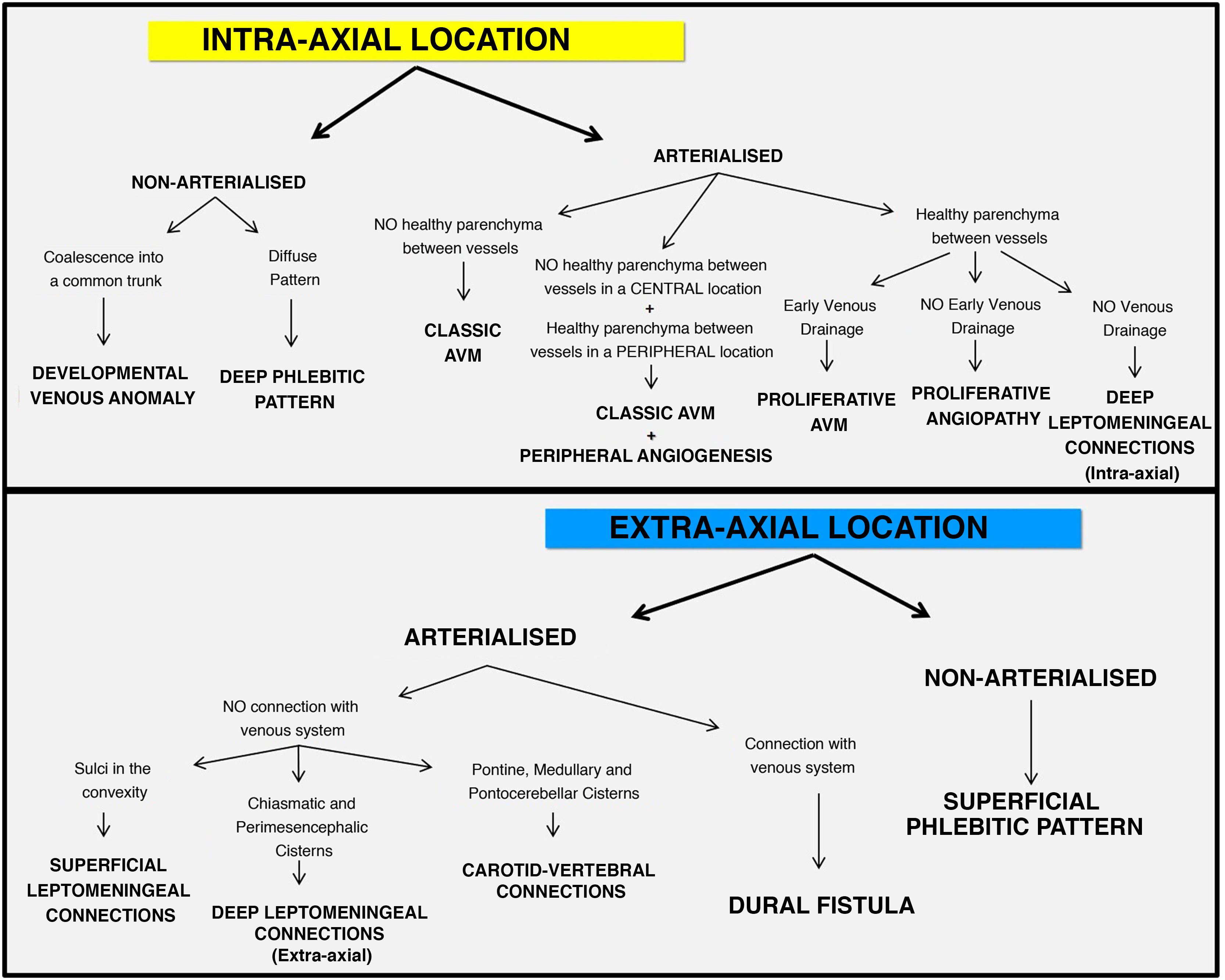
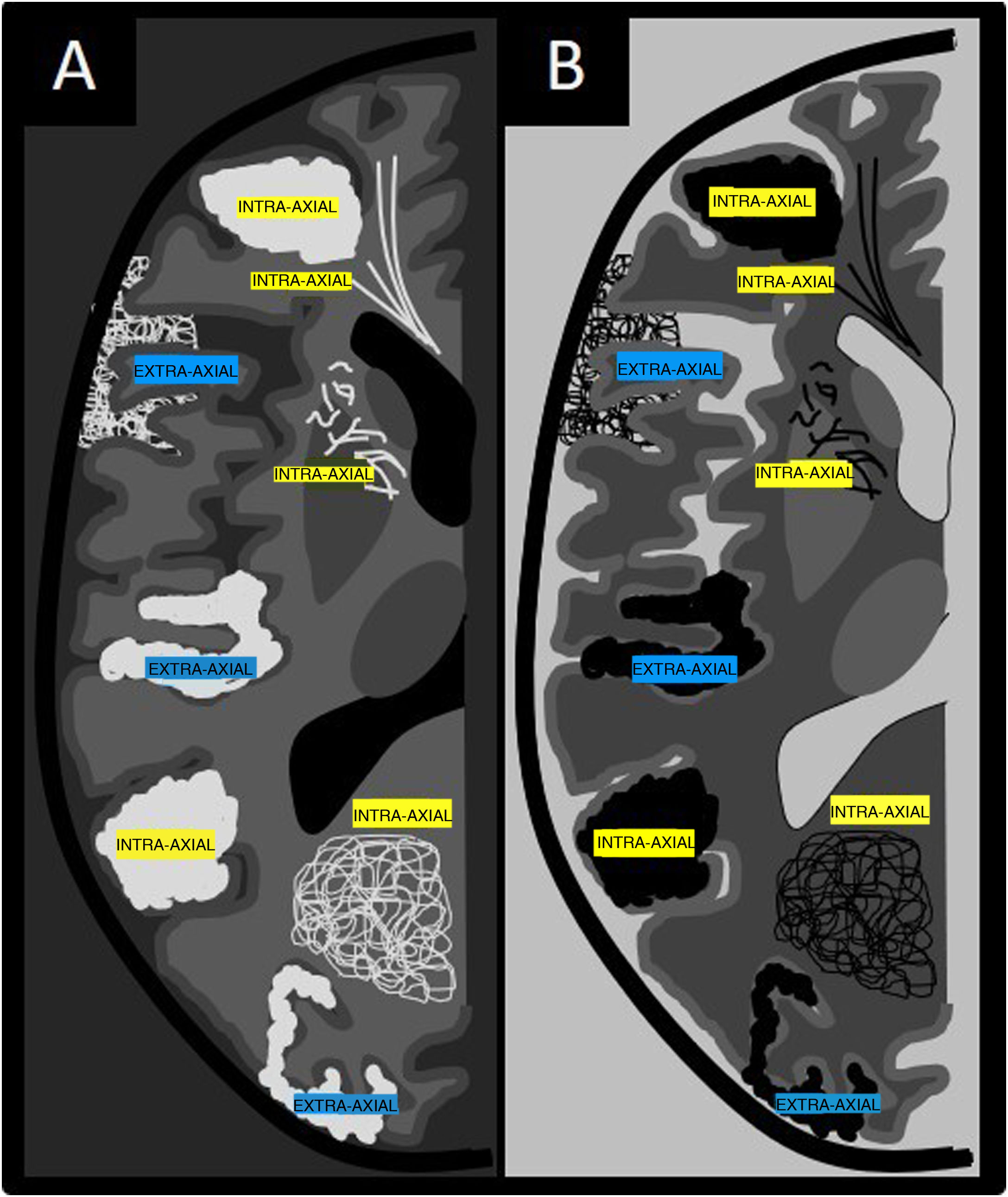
Introduction to the algorithm for diagnosis of vessels in an anomalous intracranial locationThe algorithm for diagnosis of vessels in an anomalous intracranial location is based on two important concepts: location and functionality (Fig. 1). As in characterisation of an intracranial tumour, the first step in characterising vessels in an anomalous location is to determine the compartment in which they are located, i.e. whether they are intra-axial (intraparenchymal) findings or extra-axial (extraparenchymal) findings (Fig. 2).4 This can be done using contrast-enhanced computed tomography (CT) imaging or volumetric magnetic resonance imaging (MRI) with or without contrast. The latter is the gold standard for determining the location of anomalous vessels.
Once the compartment in which the anomalous vessels are predominantly located has been identified, their functionality must be characterised. That is, it must be determined whether or not these anomalous vessels are arterialised. DSA is the gold standard, but it is an invasive test that should be reserved for uncertain cases and for characterisation of lesions with a high bleeding risk such as AVMs/dAVFs. The arterialisation of these anomalous vessels can also be assessed using CT or magnetic resonance (MR) angiography sequences.
To sum up, the intra-axial or extra-axial location of the anomalous vessels must be evaluated using CT or MRI morphology studies; the latter is the gold standard for morphology studies. In addition, the arterialisation of these vessels must be evaluated using CT angiography, MR angiography or DSA studies (DSA studies being the gold standard for functionality studies).5
Magnetic resonance imaging studyNext, the recommended MRI sequences in a protocol for evaluating intracranial vascular structures will be set out. Sequences for evaluating the morphology of the vascular structures can be distinguished from sequences for evaluating their functionality (arterialisation) based on their objective.
The recommended morphology sequences are:
- 1.
T1-weighted spin echo: the objective of this sequence is anatomical evaluation, in particular evaluation of the sellar region and the base of the brain.
- 2.
T2-weighted spin echo: this sequence is very important for analysing the cisterns and ventricular system and for delimiting extra-axial spaces. It is particularly useful in locating (intra-axial or extra-axial) signal voids in anomalous vessels.
- 3.
FLAIR: this is an important sequence for identifying areas of gliosis and/or vasogenic/cytotoxic oedema in the adjacent brain parenchyma as associated findings.
- 4.
Diffusion-weighted: this is performed to identify established acute or subacute ischaemia in the brain parenchyma.6
- 5.
T2-weighted gradient or magnetic susceptibility-weighted imaging (SWI): the main objective is not only to detect vascular structures, but also to identify blood and its by-products as well as calcifications in vascular structures. SWI also enables evaluation of prominent hypointense vessels, reported in relation to cortical venous reflux in dAVFs and as signs of venous hypertension in AVMs and dAVFs. It is reportedly useful in locating the site of the arteriovenous fistula in cerebral vascular malformations, which manifests as hyperintensity within the venous structures.7
- 6.
3D T1-weighted gradient + contrast: this is an optional sequence to better delimit vascular structures. It is important to note that visualisation of vascular structures in this sequence does not indicate arterialisation of these structures.
The most important and commonly used angiographic acquisition modality recommended is time-of-flight (TOF) intracranial cerebral angiography.8 It is mainly useful in identifying vascular structures with arterial flow and distinguishing them from those without arterial flow. It may be useful to perform contrast-enhanced MR angiography (CE-MRA) sequences to detect the nidus as well as the supplying arteries and drainage veins8,9
Algorithm for diagnosis of vessels in an anomalous intracranial location (Fig. 1)Intra-axialA) Arterialised
1. No healthy parenchyma between vessels: classic or pial AVM (Figs. 3 and 4)
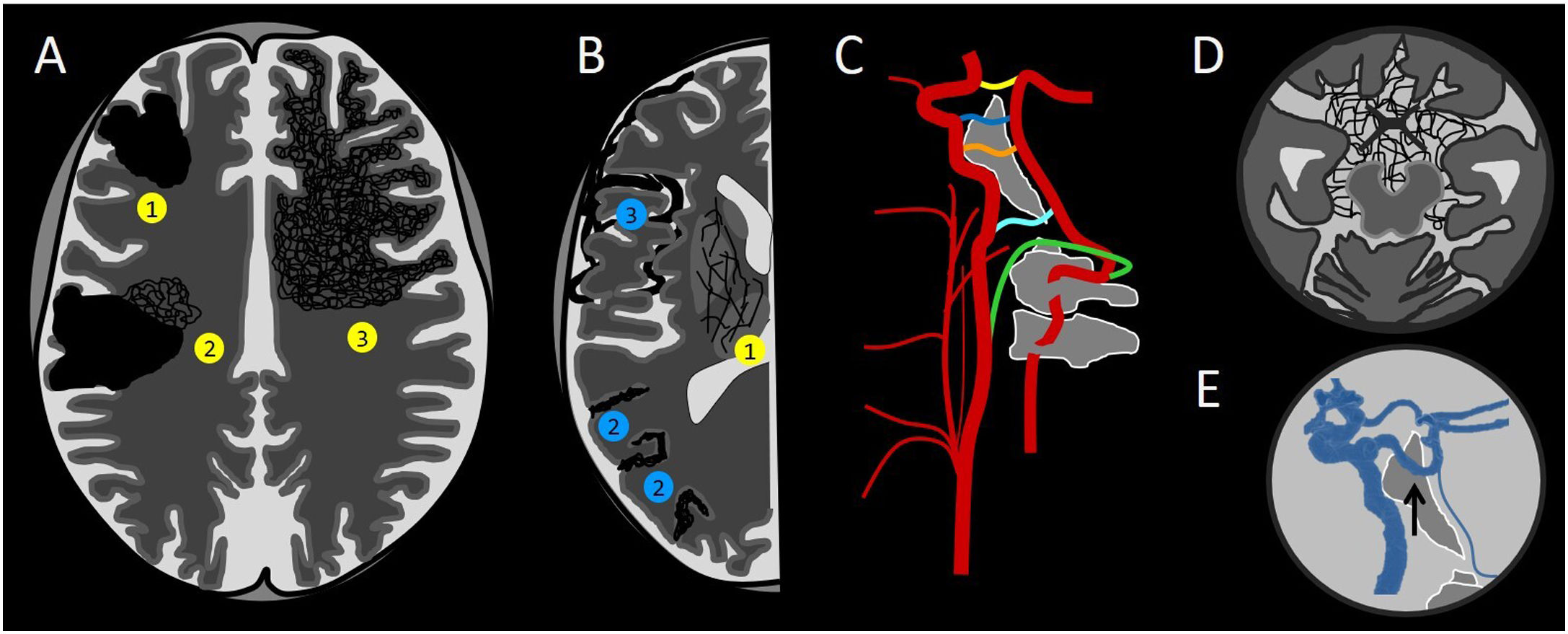
Diagram of arterialised anomalous intracranial vessels. The yellow circles correspond to intra-axial ones, and the blue circles correspond to extra-axial ones. (A) Classic arteriovenous malformation (AVM) (1), classic AVM with associated peripheral angiogenesis (2) and proliferative AVM/angiopathy depending on the calibre of the vessels and the arterialisation of the drainage vessels (3). (B) Intra-axial deep leptomeningeal connections (1), superficial leptomeningeal connections (2) and dural arteriovenous fistula (3). (C) Carotid-vertebral connections: posterior communicating artery (yellow), persistent trigeminal artery (blue), persistent otic artery (orange), persistent hypoglossal artery (light blue) and persistent proatlantal artery (green). (D) Extra-axial deep leptomeningeal connections. (E) Persistent trigeminal artery (arrow).
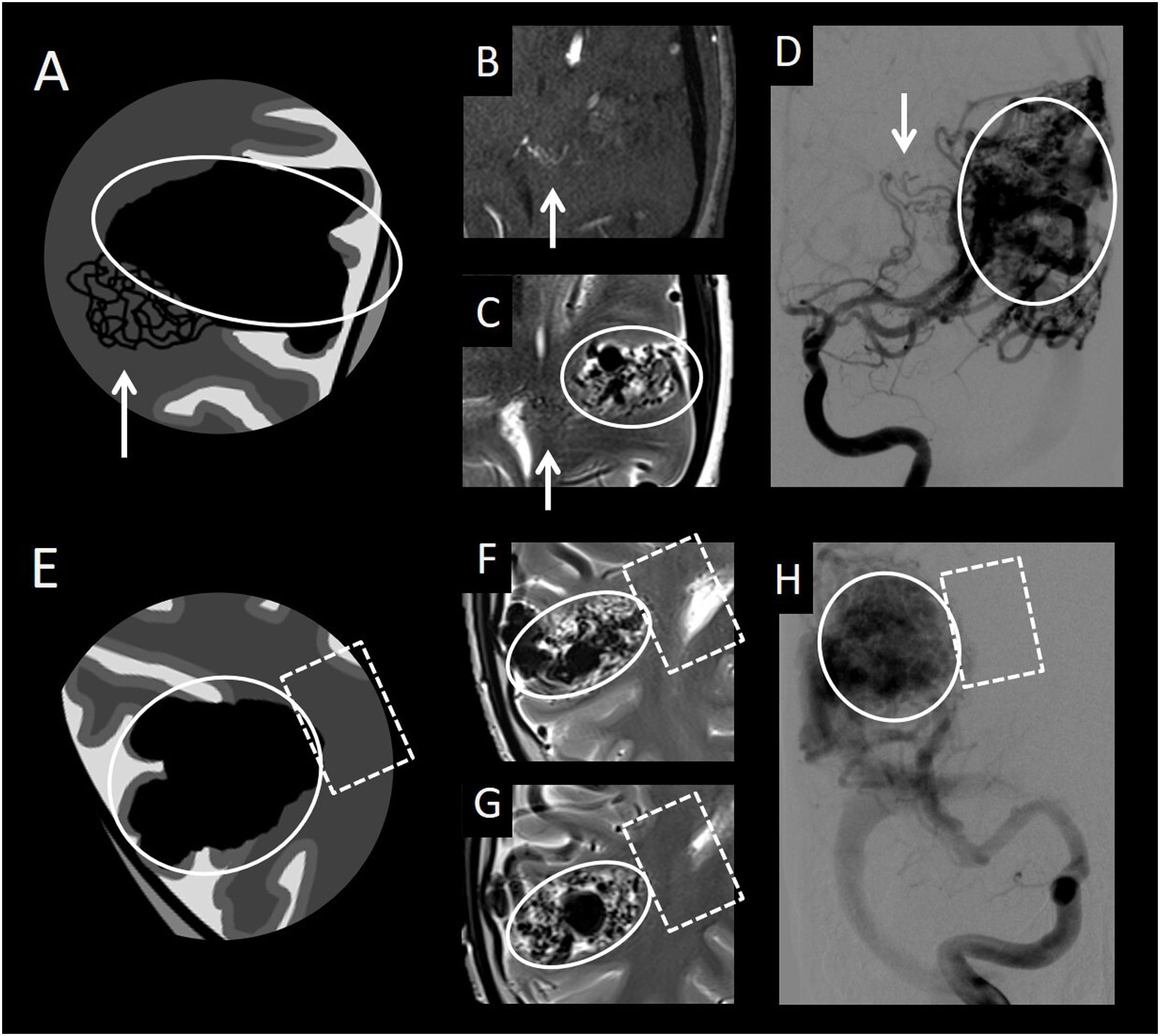
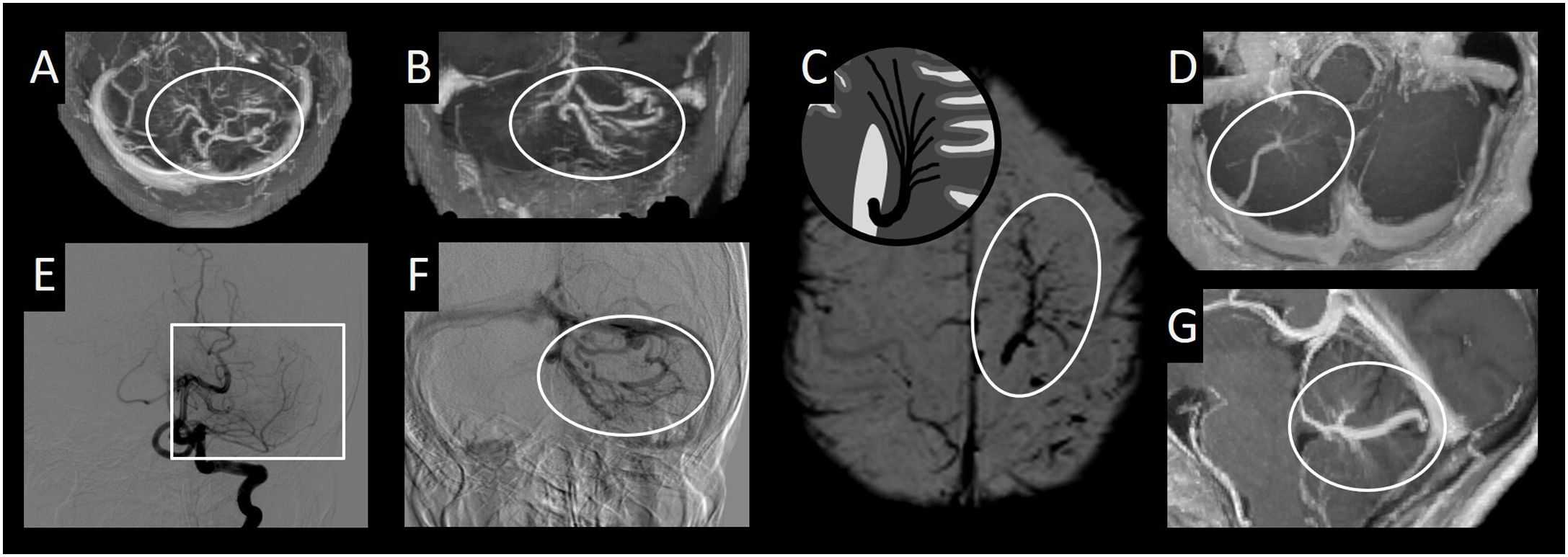
(A) Diagram of classic arteriovenous malformation (AVM) with peripheral angiogenesis. (B) Axial slices from an intracranial time-of-flight (TOF) angiography sequence. (C, F and G) Axial imaging from a T2-weighted sequence. (D) Frontal cerebral angiography projection with digital subtraction from the left internal carotid artery in the arterial phase. (E) Diagram of classic AVM. (H) Frontal cerebral angiography projection with digital subtraction from the right internal carotid artery in the arterial phase. This figure shows a classic AVM (compact nidus in circle) with peripheral angiogenesis (white arrow), distinguishing it from a classic AVM, with no associated anomalous vessels of this type (square with a dotted line), in which only the compact nidus (circle) is observed.
AVMs are anomalous connections between arteries and veins, with no normal capillary bed between them; in most cases, they are found to be hypertrophied. There are two subtypes: classic or pial AVM, and proliferative AVM, which will be explained later. Classic or pial AVM is characterised by a compact nidus of conglomerations of arterialised vessels, with no normal brain tissue between these vessels. DSA will show arterialisation of these anomalous vessels and their drainage veins; hence, in arterial phases, early venous drainage will be seen.9–11
NOTE: One characteristic that distinguishes AVMs from dAVFs is the location thereof. Classic AVMs are located in the brain parenchyma (intra-axial) and belong to the pial type; dAVFs, for their part, are located in the dura mater and therefore in an extra-axial location.
Cerebral AVMs are typically congenital, but usually do not cause symptoms until adulthood. The most common clinical sign is intraparenchymal haemorrhage, followed by focal neurological deficits, seizures and headache.10,11 The risk of bleeding of pial AVMs is 1%–2% per year, and the risk of rebleeding after a first episode of bleeding is 4%–5% per year.5
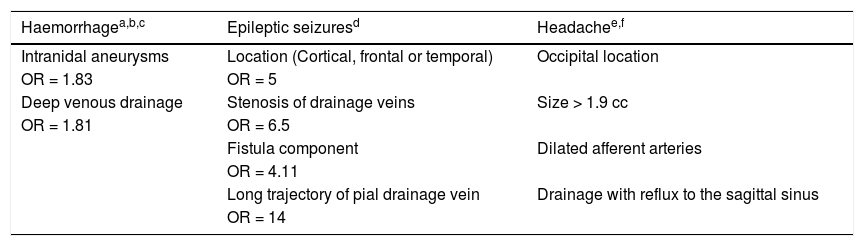
It is important to be aware of what a radiologist must report about classic or pial AVMs, especially in relation to their angioarchitecture, since these findings have implications for treatment decision-making and the patient’s prognosis (Tables 1 and 2).1,2,12–25
Angioarchitectural factors in classic or pial AVMs related to the form of presentation.
| Haemorrhagea,b,c | Epileptic seizuresd | Headachee,f |
|---|---|---|
| Intranidal aneurysms | Location (Cortical, frontal or temporal) | Occipital location |
| OR = 1.83 | OR = 5 | |
| Deep venous drainage | Stenosis of drainage veins | Size > 1.9 cc |
| OR = 1.81 | OR = 6.5 | |
| Fistula component | Dilated afferent arteries | |
| OR = 4.11 | ||
| Long trajectory of pial drainage vein | Drainage with reflux to the sagittal sinus | |
| OR = 14 |
da Costa L, et al. The natural history and predictive features of hemorrhage from brain arteriovenous malformations. Stroke. 2009;40:100–5.
Hofmeister C, et al. Demographic, morphological, and clinical characteristics of 1289 patients with brain arteriovenous malformation. Stroke. 2000;31:1307–10.
Pollock BE, et al. Factors that predict the bleeding risk of cerebral arteriovenous malformations. Stroke. 1996;27:1–6.
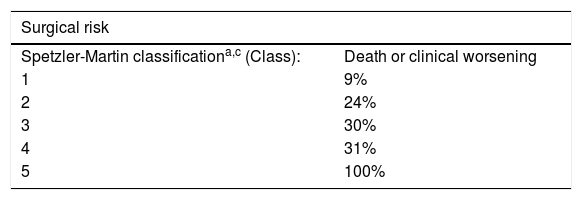
Angiographic factors in classic arteriovenous malformations related to the risk of complications of the treatment modalities.
| Surgical risk | |
|---|---|
| Spetzler-Martin classificationa,c (Class): | Death or clinical worsening |
| 1 | 9% |
| 2 | 24% |
| 3 | 30% |
| 4 | 31% |
| 5 | 100% |
| Endovascular riskd,e | Odds ratio — complications |
|---|---|
| Eloquent areab | 2.57 |
| Single deep venous drainage | 4.56 |
The class is calculated by adding up the points for the three factors: size (1 = less than 3 cm; 2 = 3−6 cm; 3 = more than 6 cm). Location in eloquent area (0 = no; 1 = yes) and venous drainage (0 = superficial; 1 = deep).
Eloquent area: sensorimotor area, language area, visual area, thalamus, hypothalamus, internal capsule, brainstem, cerebellar peduncles or deep cerebellar nuclei.
Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476–83.
Hartmann A, et al. Risk of endovascular treatment of brain arteriovenous malformations. Stroke. 2002;33:1816–20.
Pan J, et al. Angioarchitectural characteristics associated with complications of embolization in supratentorial brain arteriovenous malformation. AJNR Am J Neuroradiol. 2014;35:354–9.
The radiologist should attempt to determine whether an AVM has previously bled, because that is the most important risk factor in terms of likelihood of having another episode of bleeding.14 Gradient echo sequences (T2*) and SWI in MRI are especially sensitive for detecting stigmata of previous bleeding. In addition, when performing an angiographic study (CT, MRI or DSA), angioarchitectural factors associated with a haemorrhagic presentation must be detected.14,20Table 1 summarises the angioarchitectural factors related to the different forms of presentation.
Intranidal aneurysms are saccular dilations found within the tangled vessels; rupture thereof causes an intraparenchymal haemorrhage. They represent the most significant angiographic factor for prediction of both bleeding and rebleeding.20 It is important to distinguish them from flow aneurysms, which occur in the afferent arteries of the AVM at the circle of Willis; rupture of these aneurysms would cause a subarachnoid haemorrhage.
The venous drainage of the AVM may be single or multiple, and may occur through the superficial and/or deep system. Hence, good knowledge of venous anatomy is very important. Stenosis of the drainage veins is an important angioarchitectural finding in AVMs, as it is associated with a higher risk of bleeding due to an increase in the retrograde pressure of the malformation resulting from restriction of venous blood flow. Similarly, deep venous drainage is associated with a higher risk of bleeding, although this is probably due to its location, since a deep location also carries a higher risk of bleeding. Single deep venous drainage is associated with a higher risk of haemorrhagic presentation.12
Epileptic seizures can occur as a result of structural lesions (such as traces of haemosiderin in the subarachnoid space or a residual porencephalic cavity) or due to functional abnormalities (such as poor drainage of healthy brain parenchyma). Angiographic factors related to this form of clinical presentation are a cortical location (especially frontal or temporal) and venous overload, identified on angiography as drainage vein stenosis, a direct fistula or a pial drainage vein with a very long trajectory. This venous overload impedes drainage of healthy parenchyma; this may be one cause of epileptic seizures in the patient.13,21
Another possible form of presentation is headache, which is usually related to an occipital location of the lesion. A link between this clinical presentation and significant dural involvement, female sex and larger lesion size has also been reported.15
Regarding the treatment of classic or pial AVMs, there are three treatment modalities: endovascular, surgical and radiosurgical. In some cases, the therapeutic approach is a combination of these. Therefore, it is important for the radiology report to include angiographic factors with an impact on morbidity and mortality secondary to each type of treatment (Table 2). The Spetzler-Martin Grading Scale is a classification of surgical risk based on nidus size, type of venous drainage and AVM location in eloquent brain areas.19
It is important to clarify that, although single deep venous drainage is a risk factor in arterial endovascular treatment, in recent years, the venous endovascular approach has proven to be a valid option in the treatment of these AVMs.26,27 For now, in this type of intravenous endovascular treatment, there are no known angiographic factors related to the risk of complications.
2. No healthy parenchyma between vessels in a central location and healthy parenchyma between vessels in a peripheral location: classic AVM with angiogenesis (Figs. 3 and 4)
Classic AVMs can cause arterial steal phenomena in the adjacent brain tissue, and therefore ischaemic phenomena in this parenchyma. To compensate, the normal arteries that supply said tissue hypertrophy, yielding an image of an increase in the size and number of vessels in the parenchyma adjacent to the malformation.28
It is important to detect and delimit these areas where the vessels are found within healthy parenchyma, since they correspond to healthy brain tissue and should not be targeted in endovascular, surgical or radiosurgical treatment. Resection of this tissue or endovascular occlusion of these arteries can cause complications, with the onset of focal neurological deficits depending on the function of this area.
3. Healthy parenchyma between vessels
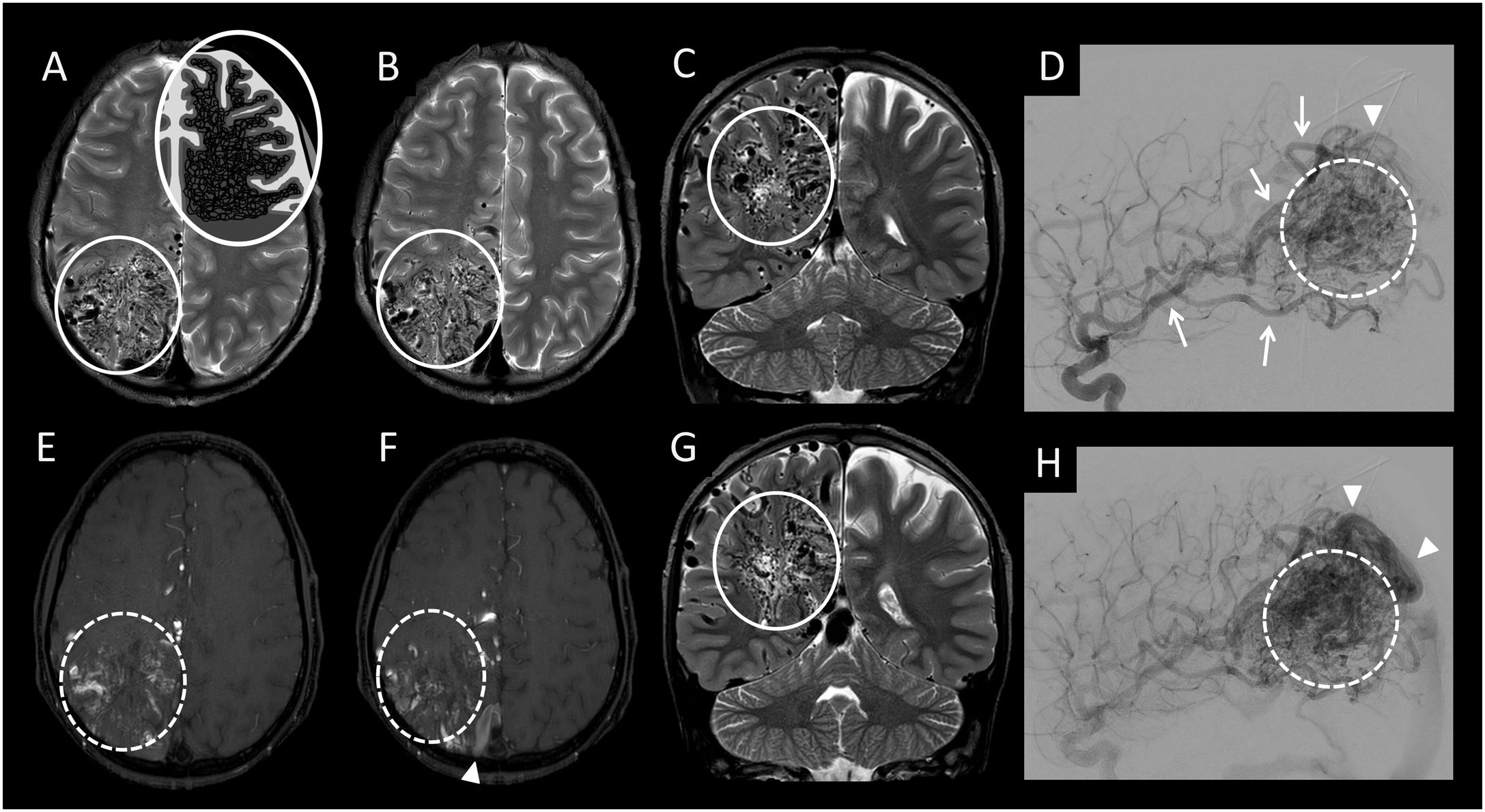
a) Proliferative AVM (Figs. 3 and 5):29,30 this entity is a subtype of arteriovenous malformation that, unlike classic AVM, has a diffuse malformation nidus with normal brain tissue between vascular structures. This is the main distinguishing feature, since, as in classic AVM, the arteries and veins involved in the malformation will be hypertrophied, and the malformation nidus and its drainage veins will be arterialised.
(A and B) Axial imaging from a T2-weighted sequence with a diagram of arteriovenous malformation (AVM)/proliferative angiopathy. (C and G) Coronal slices from a T2-weighted sequence. (E and F) Axial imaging from an intracranial time-of-flight (TOF) angiography sequence. (D and H) Lateral cerebral angiography projection with digital subtraction from the right internal carotid artery in the arterial phase. This figure shows a proliferative AVM featuring a diffuse malformation nidus with normal brain tissue between vascular structures (white circle with a solid line in A, B, C and G), showing hypertrophy of the arteries afferent to the malformation (white arrows in D) and arterialisation of both the nidus (white circle with a dotted line in E, F, D and H) and the drainage veins (white arrow tip in F, D and H).
b) Proliferative angiopathy (Fig. 3):29,30 cerebral proliferative angiopathy is a subtype of arteriovenous malformation characterised by being large in size (with a lobar, multilobar or even hemispheric distribution) and featuring, unlike classic pial AVM, normal brain tissue between vascular structures. In addition, a large increase in the size of the supplying arteries or the drainage veins is not usually seen, whereas in pial and proliferative AVMs, an increase in the size of both vascular structures is usually observed.
Cerebral perfusion studies have shown an increase in mean transit time in proliferative angiopathy, compared to AVMs (pial and proliferative), in which said brain circulation times are seen to be shortened, as a sign of the arteriovenous shunt that develops in the nidus.31 Therefore, on DSA of proliferative angiopathy, venous drainage of the malformation can be observed in normal or near-normal brain circulation times (non-arterialised), compared to AVMs (pial and proliferative), in which early venous drainage of the malformation (an arteriovenous shunt) tends to be seen. This lack of early venous drainage is an important distinguishing factor from a proliferative AVM.30
The clinical presentation of these malformations includes epileptic seizures, headaches and progressive neurological deficits; less commonly, they present with acute neurological deficits or intracranial haemorrhages.29
c) Deep intra-axial leptomeningeal connections (Figs. 3 and 6): occlusion or stenosis of the main intracranial arteries may be replaced with arterioarterial connections in different places: through the circle of Willis, between the external and internal carotid arteries or through the leptomeningeal connections.
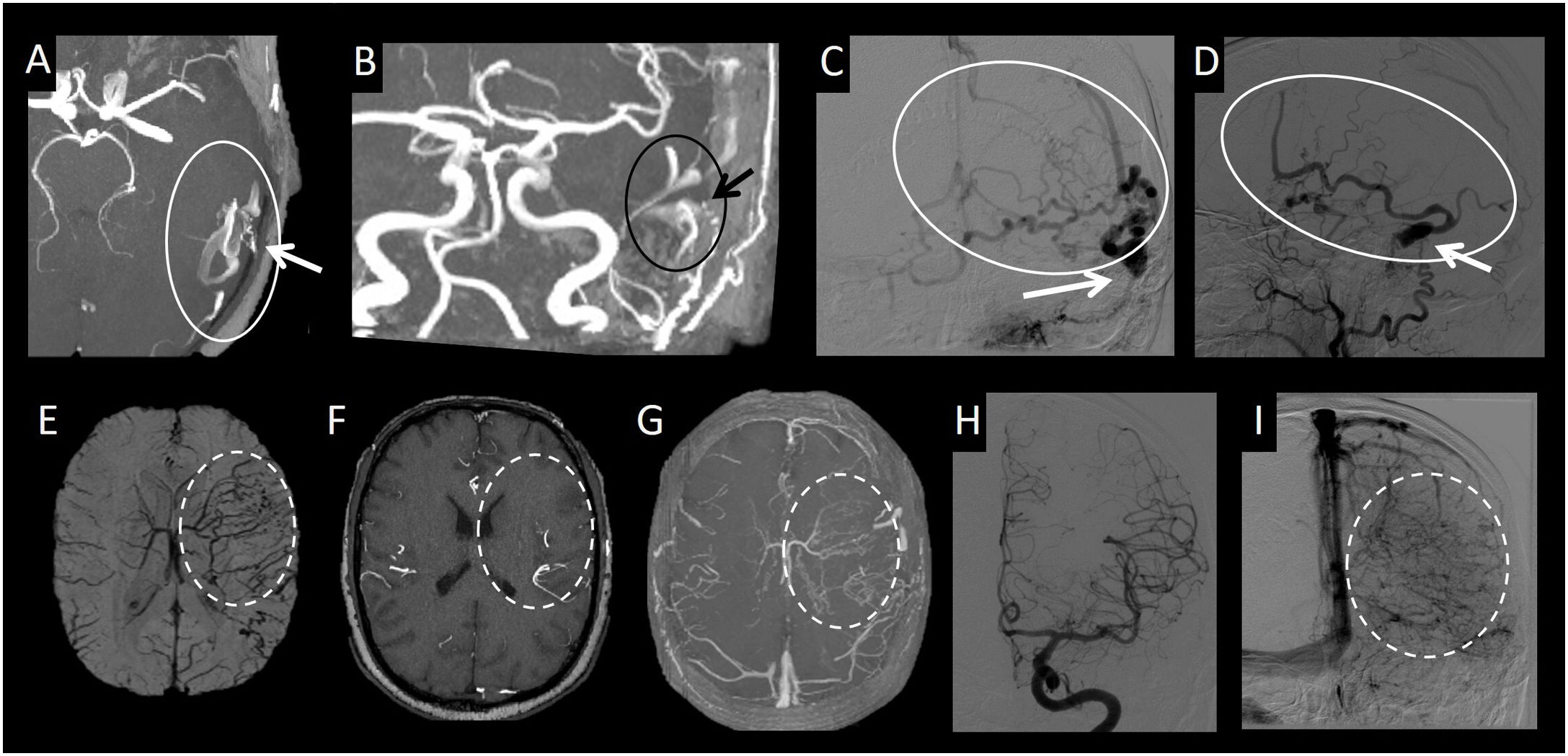
(A–C) Axial imaging from a T2-weighted sequence with a diagram of extra-axial (A) and intra-axial (B and C) deep leptomeningeal connections. (D and E) Frontal cerebral angiography projection with digital subtraction from the right and left internal carotid artery, respectively, in the arterial phase. (F–H) Axial imaging from an intracranial time-of-flight (TOF) angiography sequence. (I and J) Lateral cerebral angiography projection with digital subtraction from the left vertebral artery and the right internal carotid artery in the arterial phase, respectively. This figure depicts the deep leptomeningeal connections, both extra-axial (solid circle) and intra-axial (dotted circle), in a patient with moyamoya syndrome.
These leptomeningeal connections are classified as superficial or deep; the latter are located in the basal ganglia and thalamus (intra-axial) or in the chiasmatic and perimesencephalic cisterns (extra-axial).32
Moyamoya disease is a classic example of a condition in which this type of connection is typically seen. In it, the terminal segment of the internal carotid artery and/or the proximal segment of the middle cerebral artery is/are occluded. In this case, re-permeabilisation of the distal segments (distal M1 and M2s segments) can be caused by deep leptomeningeal connections, both intra-axial and extra-axial, yielding the characteristic appearance on angiography of a puff of smoke (or “moyamoya”, in Japanese).33,34
B. Non-arterialised
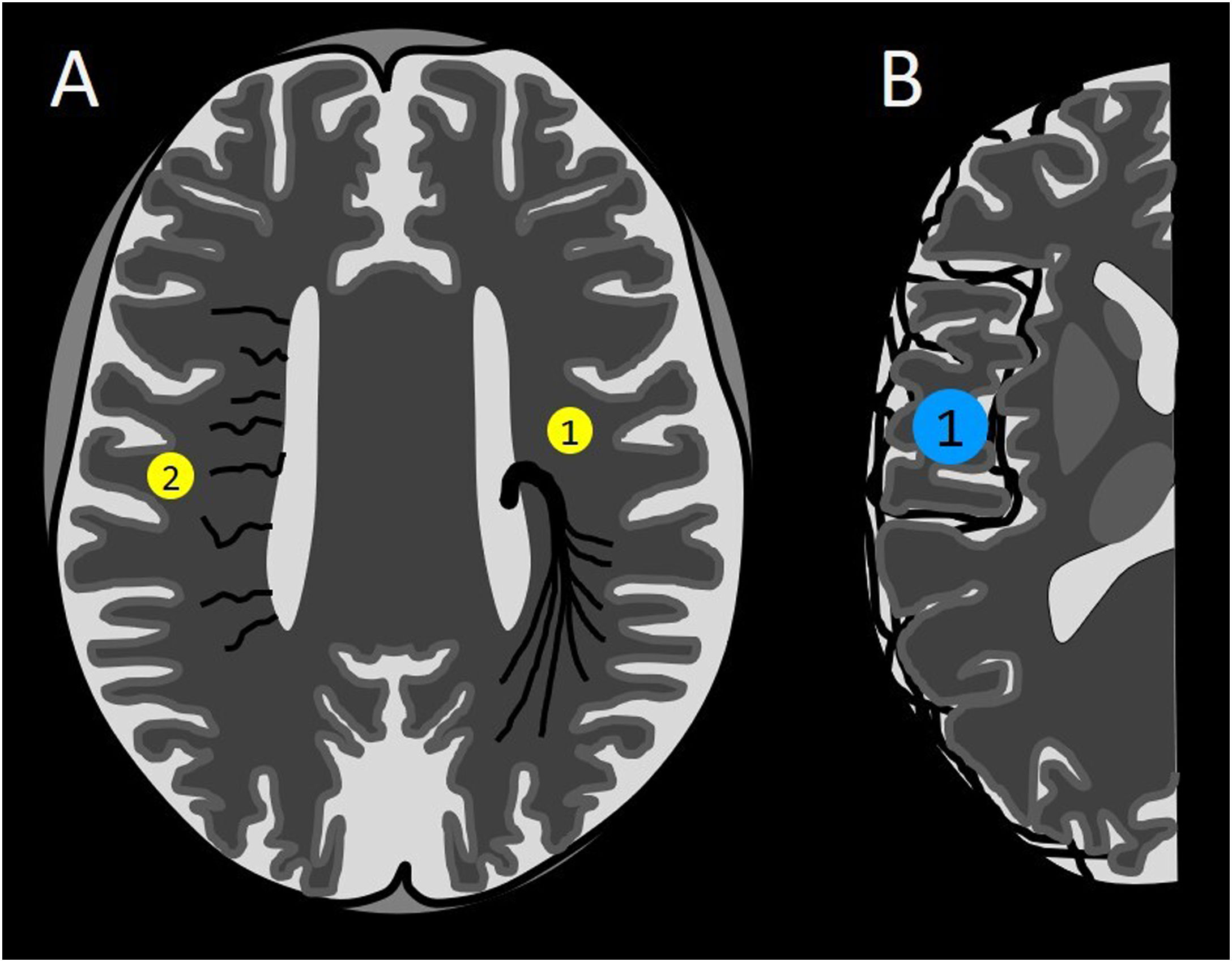
1. Coalescence into a common trunk: developmental venous anomaly (DVA) (Figs. 7 and 8)
Diagram of non-arterialised intracranial vessels in an anomalous location. The yellow circles correspond to intra-axial ones, and the blue circles correspond to extra-axial ones. (A) Developmental venous anomaly (1), deep phlebitic pattern (2). (B) Superficial phlebitic pattern (1).
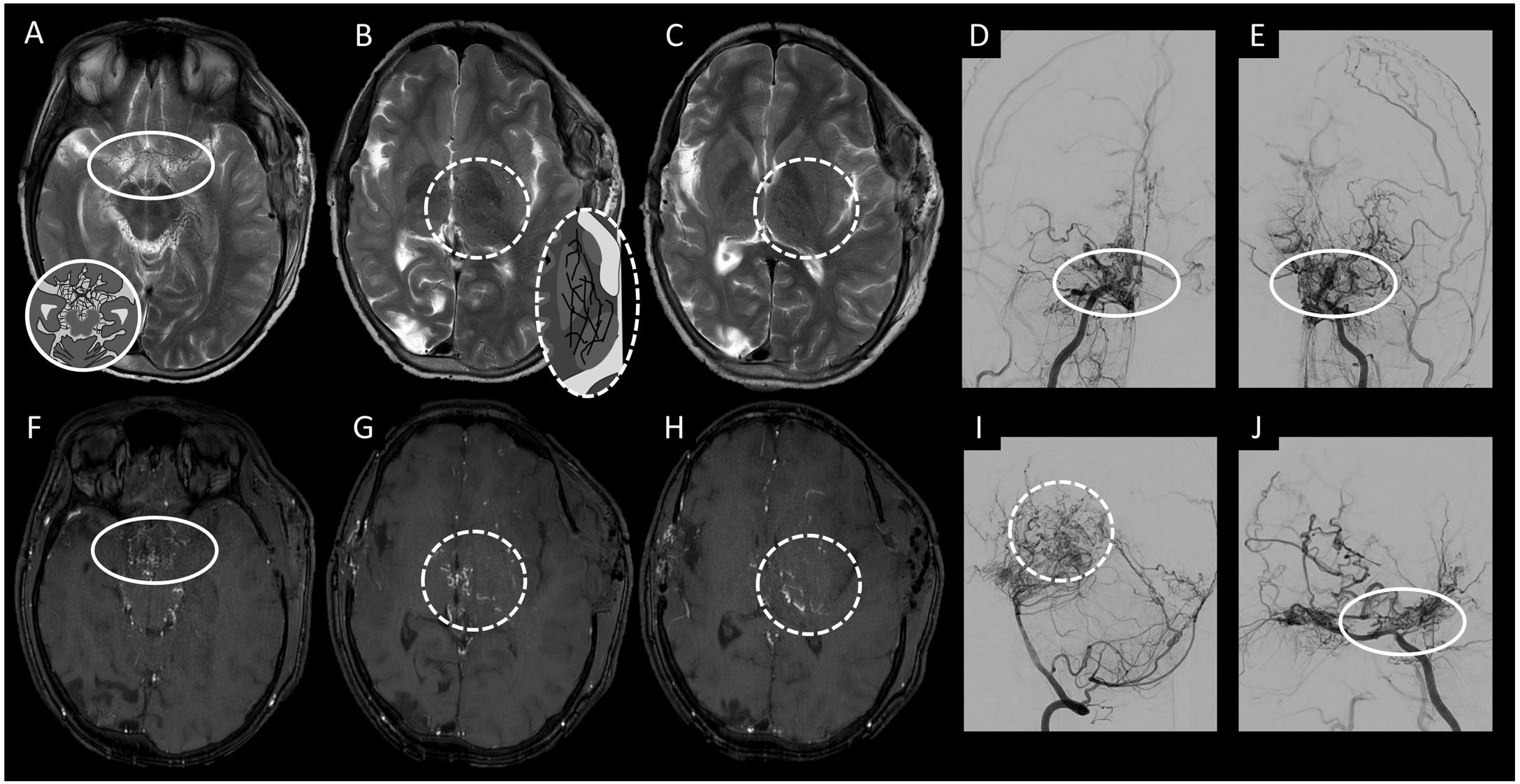
(A) Axial maximum intensity projection (MIP) reconstruction imaging acquired in a contrast-enhanced T1-weighted scan. (B) Coronal maximum intensity projection (MIP) reconstruction imaging acquired in a contrast-enhanced T1-weighted scan. (C) Diagram of a developmental venous anomaly on axial minimum intensity projection (MinIP) reconstruction imaging from a susceptibility-weighted imaging sequence. (D) Axial MIP reconstruction imaging acquired in a contrast-enhanced T1-weighted scan. (E) Frontal cerebral angiography projection with digital subtraction from the left vertebral artery in the arterial phase. (F) Frontal cerebral angiography projection with digital subtraction from the left vertebral artery in the venous phase. (G) Sagittal MIP reconstruction imaging acquired in a contrast-enhanced T1-weighted scan. This figure shows several anomalies of venous development (circle), with a characteristic jellyfish head-shaped appearance, that are not observed in the arterial phase (square).
Developmental venous anomalies are congenital vascular malformations that drain normal brain parenchyma in normal circulation times (i.e. are not arterialised). They are the most common type of vascular malformation, found in 2%–3% of the population and accounting for 60% of all vascular lesions.30,35,36
In this vascular malformation, dilated medullary veins drain centripetally and radially towards a common collector; these veins carry blood from healthy brain tissue to superficial subcortical veins or to deep pial veins.
Thus, they are normal venous structures that drain normal brain parenchyma, but they follow an anomalous trajectory. These lesions lack proliferative potential and rarely cause symptoms; hence, they are considered benign.35,37
2. Diffuse: deep phlebitic pattern (Figs. 7 and 9)
The venous drainage of the brain consists of a superficial system and a deep system. The superficial system is primarily made up of the venous sinuses and the cortical veins, and the deep venous system is composed of the internal cerebral veins and the straight sinus. These systems are interconnected through transmedullary veins located in the subcortical white matter.
(A) Axial MIP reconstruction imaging acquired in time-of-flight (TOF) angiography without contrast. (B) Coronal MIP reconstruction imaging acquired in TOF angiography without contrast. (C) Frontal cerebral angiography projection with digital subtraction from the left external carotid artery in the arterial phase. (D) Lateral cerebral angiography projection with digital subtraction from the left external carotid artery in the arterial phase. (E) Axial MinIP reconstruction imaging from a susceptibility-weighted imaging sequence. (F) Axial imaging acquired in TOF angiography without contrast. (G) Axial MIP reconstruction imaging acquired in a contrast-enhanced T1-weighted scan. (H) Frontal cerebral angiography projection with digital subtraction from the left internal carotid artery in the arterial phase. (I) Frontal cerebral angiography projection with digital subtraction from the left internal carotid artery in the venous phase. This figure shows a dural fistula from branches of the left external carotid artery to the left transverse sinus (arrow) with the arterialisation of the cortical veins on the left side (circles with solid black and white lines), visible on TOF sequences (A and B) and in the arterial phase of cerebral arteriography (C and D). The arterialisation of these cortical veins leads to poor venous drainage in the left cerebral hemisphere, with the appearance of a deep phlebitic pattern and hypertrophy of the transmedullary veins (circle with a dotted line). These anomalous vessels are not seen in arterial phases (F and H) but are seen in venous phases (I); hence, they are not arterialised.
Should one of these systems drain poorly (as in, for example, stenosis/occlusion of the transverse sinus), blood from the superficial region may drain, through the hypertrophy of the transmedullary veins (subcortical white matter), by way of the internal cerebral veins and the straight sinus. These connections can also drain the deep region in the event of stenosis/occlusion of the straight sinus by connecting with the cortical veins and draining through the venous sinuses. This is termed a deep phlebitic pattern.38,39
Hypertrophy of these transmedullary veins, in an attempt to redirect venous drainage, can be seen as anomalous intra-axial vessels (often in the subcortical white matter) that are not arterialised (i.e. that are seen to have circulation times typical of the venous system). In these cases, it is important to analyse the venous system in order to detect any stenosis/occlusion that will aid in making a correct aetiological diagnosis of these anomalous vessels.38,39
Extra-axialA) Arterialised
1. No connection with the venous system
a) Located in sulci of the convexity: superficial leptomeningeal connections (Fig. 3). Superficial leptomeningeal connections are those that develop between the cortical branches of the pial arteries in a supratentorial location (anterior cerebral artery [ACA], middle cerebral artery [MCA] and posterior cerebral artery [PCA]) and between the cerebellar arteries in a subtentorial location (superior cerebellar artery [SCA], anterior inferior cerebellar artery [AICA] and posterior inferior cerebellar artery [PICA]).32
Superficial leptomeningeal connections are the ones most commonly activated in acute occlusion of cerebral arteries; in acute ischaemic stroke, their function is to maintain cerebral perfusion and prevent necrosis of ischaemic tissue. These leptomeningeal connections are located on the pial surface of the brain, and therefore have an extra-axial location.32,40,41
They may also be activated in the event of chronic atheromatous phenomena, arterial steal phenomena in AVMs or other diseases leading to haemodynamic compromise.32,40
b) Located in chiasmatic and perimesencephalic cisterns: extra-axial deep leptomeningeal connections (Figs. 3 and 6). As seen in the previous sections, deep leptomeningeal connections may be in an extra-axial location in the chiasmatic and perimesencephalic cisterns, and usually occur as a result of stenosis/occlusion of the internal carotid artery or middle cerebral artery.33,34 It is important to determine whether these vessels in an anomalous location are connected to the arterial system, or instead to the venous system, in which case a dural fistula must be suspected.30
c) Located in pontine, medullary and pontocerebellar cisterns: carotid-vertebral connections (Figs. 3 and 10).42,43 During human embryonic development, arterial connections develop between the precursors of the internal carotid artery and the precursors of the basilar artery. These anastomoses are obliterated around the sixth week of embryogenesis. Only the posterior communicating artery persists into adulthood as a vessel connecting the anterior system to the posterior system.
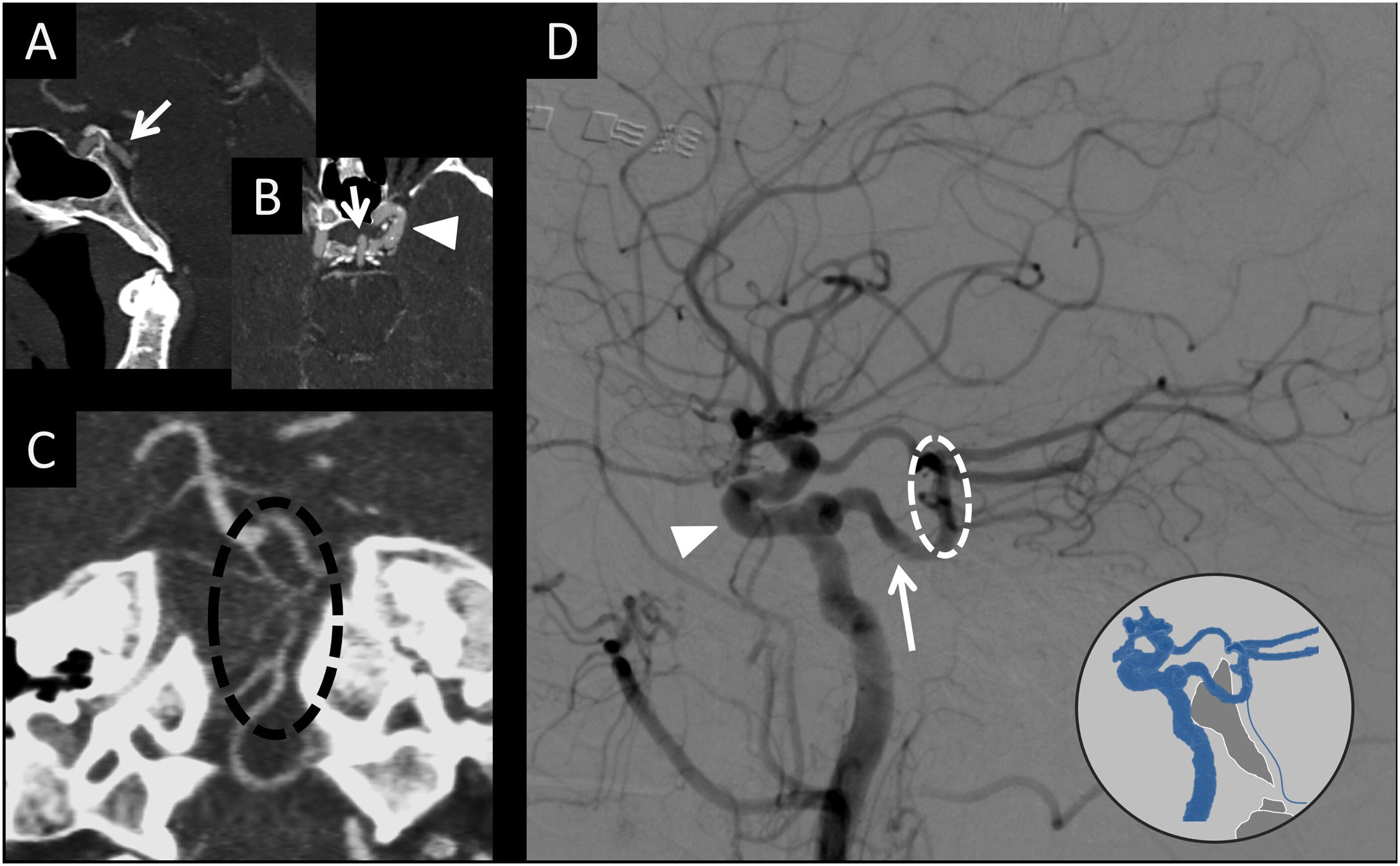
(A) CT angiography with sagittal MIP reconstruction. (B) CT angiography with axial MIP reconstruction. (C) CT angiography with coronal MIP reconstruction. (D) Brain arteriography with lateral digital subtraction with contrast injection from the left internal carotid artery in the arterial phase. This figure depicts a persistent trigeminal artery carotid-vertebral connection (white arrows in A, B and D) at the midline of the dorsum sellae (A and B) connecting the cavernous portion of the internal carotid artery (white arrow tip in B and D) with the middle third of the basilar artery (dotted white circle in D). As a result of the persistent trigeminal artery, the basilar artery proximal to the connection with the persistent trigeminal artery is hypoplastic (dotted black circle in C).
In some cases, these connections are not obliterated and persist into adulthood. There are four types of carotid-vertebral connections: persistent trigeminal, hypoglossal, otic and proatlantal arteries. All of the connections take the name of the cranial nerve that they accompany, with the exception of persistent proatlantal artery.
Persistent trigeminal artery is the most cephalic and the most common of all of them (85%) (Figs. 3 and 10). It originates at the junction between the petrous and cavernous portions of the internal carotid artery and follows a posterolateral course along with the trigeminal nerve or a medial course through the dorsum sellae to end in the basilar artery, between the superior cerebellar artery and the anterior inferior cerebellar artery. It is found in 0.02%–0.6% of cerebral angiographies and is associated with an increased prevalence of other vascular abnormalities, such as intracranial saccular aneurysms.43,44
Persistent otic artery arises from the internal carotid artery in the carotid canal, runs through the internal auditory meatus and joins the basilar artery also between the superior cerebellar artery and the anterior inferior cerebellar artery.
Persistent hypoglossal artery is the second most common type, after persistent trigeminal artery. It originates in the cervical segments of the internal carotid artery (usually, between C1 and C3), passes through the hypoglossal canal and ends in the inferior portion of the basilar artery. It is also associated with vascular anomalies such as brain aneurysms.
Finally, persistent proatlantal artery is the most caudal of all. It is divided into two types. The more common type originates in the internal carotid artery; the second type is much less common and originates in the external carotid artery. Regardless of its origin, the artery runs through the foramen magnum and ends its course in the vertebral artery. The ipsilateral vertebral artery is often hypoplastic.42
2. Connection with the venous system: dAVF (Figs. 3 and 9)
Dural fistulas are defined as anomalous arteriovenous connections on the surface of the dura mater; the arteries that supply them come from dural/meningeal branches, and they can drain through the venous sinuses, meningeal veins or cortical/pial veins.30 When the fistula drains into the cortical veins, these dilate and enhance in arterial phases on DSA and MR angiography.38,39 If the fistula drains into the venous sinuses (without arterialisation of the cortical veins) or the dural fistula is associated with thrombotic phenomena, the cortical veins may drain poorly, resulting in a superficial and/or deep phlebitic pattern. In the latter case, the dilated cortical veins will not enhance in the arterial phase.
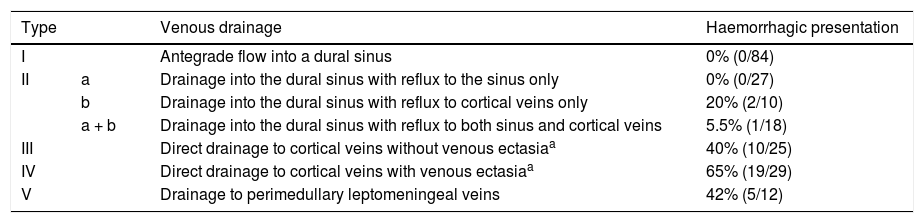
The most commonly and widely used classification of dural fistulas is that of Cognard et al.,3 which groups them according to their venous drainage pattern and correlates with their form of haemorrhagic presentation. Table 3 summarises the classification. It is important to make a distinction between the percentage of patients who present with an intracranial haemorrhage and the risk of bleeding from a dural fistula. The Cognard classification would not be appropriate for determining the risk of bleeding from a dural fistula, since it is derived from a cross-sectional study of patients who presented with neurological symptoms, and therefore bleeding risk cannot be inferred from it. The answer to this question of bleeding risk should come from a population study.
Haemorrhagic presentation of dural arteriovenous fistulas according to the Cognard et al. classificationb.
| Type | Venous drainage | Haemorrhagic presentation | |
|---|---|---|---|
| I | Antegrade flow into a dural sinus | 0% (0/84) | |
| II | a | Drainage into the dural sinus with reflux to the sinus only | 0% (0/27) |
| b | Drainage into the dural sinus with reflux to cortical veins only | 20% (2/10) | |
| a + b | Drainage into the dural sinus with reflux to both sinus and cortical veins | 5.5% (1/18) | |
| III | Direct drainage to cortical veins without venous ectasiaa | 40% (10/25) | |
| IV | Direct drainage to cortical veins with venous ectasiaa | 65% (19/29) | |
| V | Drainage to perimedullary leptomeningeal veins | 42% (5/12) |
dAVFs may present as focal neurological deficits, cranial nerve pair deficits, increased intracranial pressure, etc., depending on the location of the shunt, the venous drainage of the fistula and the characteristics of the venous drainage of the brain.45
Regarding the risk of bleeding and rebleeding of dural fistulas, few studies have referred to angioarchitecture or conducted long-term follow-up of untreated fistulas (the natural history of the disease). Two prospective cohort studies found a risk of bleeding from a dural fistula with cortical venous drainage of 8% per year46 and a risk of rebleeding of 35%.47 Cortical venous drainage in a dural fistula is the angiographic sign with the most significant implications for prognosis; for this reason, dural fistulas with this drainage pattern are called aggressive. The treatment of dural fistulas is debated, but all authors have agreed upon the indication for endovascular or surgical treatment in aggressive dural fistulas, i.e. ones with cortical venous drainage.
B) Non-arterialised: superficial phlebitic pattern (Fig. 7)
The deep phlebitic pattern, which results from stenosis/occlusion of the superficial venous system and an attempt to drain through the deep venous system, or vice versa, was reviewed earlier. The superficial phlebitic pattern, for its part, is usually caused by stenosis/occlusion of a superficial venous drainage sinus and an attempt to drain through the undamaged superficial system. One example is stenosis in the left transverse sinus, with hypertrophy of the cortical veins and the connection between them (in an extra-axial location) to facilitate blood drainage into the superior sagittal sinus.38,39
Therefore, hypertrophy and connections between the cortical veins will correspond to non-arterialised extra-axial anomalous vessels, i.e. vessels that drain blood in circulation times typical of the venous system.
ConclusionIn order to study the different types of anomalous location of intracranial vessels in adults, it is important to perform a morphological and functional study, which allows a more accurate differential diagnosis to be made in this group of findings, ranging from variants of normality to AVMs with a potential risk of intracranial bleeding. In the latter case, it is also essential to detect the angioarchitectural findings that are most closely related to the risk of bleeding.
Authorship- 1.
Responsible for study integrity: ALR, MP, JM, MW, OC.
- 2.
Study concept: ALR, MP, JM, MW, OC.
- 3.
Study design: ALR, MP, JM, MW, OC.
- 4.
Data collection: ALR, MP, JM, MW, OC.
- 5.
Data analysis and interpretation: ALR, MP, JM, MW, OC.
- 6.
Statistical processing: N/A.
- 7.
Literature search: ALR, MP, JM, MW, OC.
- 8.
Drafting of the manuscript: ALR, MP, JM, MW, OC.
- 9.
Critical review of the manuscript with intellectually significant contributions: ALR, MP, JM, MW, OC.
- 10.
Approval of the final version: ALR, MP, JM, MW, OC.
The authors declare that they have no conflicts of interest.
Please cite this article as: Porta M, Moreno J, Werner M, Chirife Ó, López-Rueda A. Vasos intracraneales en localización anómala en adultos. Radiología. 2022;64:41–53.