
Edited by: Dr. Tomás Ripollés González - Servicio de Radiodiagnóstico, Hospital Universitari Doctor Peset, València, España
More infoAcute abdomen is a common reason for consultation in the emergency department. A broad spectrum of entities, including diverse diseases of the gastrointestinal tract, can cause acute abdomen. Although computed tomography is the technique most widely used to evaluate acute abdomen in the emergency department, abdominal ultrasound is often performed first and allows bowel disease to be suspected. This article describes the ultrasound features of diverse bowel diseases that can cause acute abdomen, such as acute diverticulitis, bowel obstruction, gastrointestinal perforation, bowel ischemia, intraabdominal fat necrosis, and miscellaneous processes such as endometriosis, foreign bodies, or vasculitis. Radiologists must be familiar with the different features of abnormal bowel that can be detected incidentally in patients without clinical suspicion of bowel disease. This article focuses on ultrasonographic signs of bowel disease; other articles in this series cover the ultrasonographic signs of acute appendicitis, inflammatory bowel disease, and infectious diseases.
El abdomen agudo es un motivo de consulta habitual en los servicios de urgencias. Sus causas son múltiples, y entre ellas se encuentran diversas enfermedades del tracto gastrointestinal. Si bien la tomografía computarizada es la técnica más aceptada, con frecuencia se lleva a cabo una ecografía abdominal inicialmente, mediante la cual es posible establecer la sospecha de patología intestinal. Este artículo describe las características ecográficas de diversos trastornos intestinales que cursan con abdomen agudo, como la diverticulitis aguda, la obstrucción intestinal, la perforación gastrointestinal, la isquemia intestinal, la necrosis aguda de la grasa intraabdominal y una miscelánea de procesos, como endometriosis, cuerpos extraños o vasculitis. Debemos conocer las diferentes apariencias ecográficas del intestino anormal, ya que pueden detectarse incidentalmente en pacientes en quienes no se sospecha clínicamente. La semiología ecográfica de la patología intestinal, así como la apendicitis aguda, la enfermedad inflamatoria intestinal o las enfermedades infecciosas, se tratan en otros artículos de la serie.
Patients with acute abdominal pain are a challenge for radiologists. In more than a third of cases, the cause is a severe acute bowel disease.1 Ultrasound is often used as an initial imaging tool for the diagnostic assessment of these patients and of others with nonspecific abdominal pain. Radiologists need to be familiar with the different ultrasound features of the normal and abnormal bowel,2 as abnormalities can be detected incidentally, even when bowel disease is not suspected.
Certain intestinal disorders associated with acute abdomen, such as acute appendicitis,3 inflammatory bowel disease and infectious diseases, are discussed in other articles in the series. This article addresses the other most important intestinal emergencies, such as diverticulitis, intestinal obstruction, gastrointestinal perforation, intestinal ischaemia, acute intra-abdominal fat necrosis and a variety of other processes, in order to facilitate detection and correct interpretation of the findings on ultrasound examination.
Acute diverticulitisLeft colon acute diverticulitisDiverticula of the left colon are herniations of the mucosa and submucosa; they are false diverticula, without a muscular layer.4 Muscle hypertrophy is the distinctive morphological finding and is visible on ultrasound in most cases. Diverticular disease mainly affects the sigmoid colon, due to the higher intraluminal pressure. The incidence is above 50% in the population over 60 and it increases with age. Undamaged diverticula are seen as small hyperechoic protrusions outside the intestinal wall and they usually contain air and produce reverberation artefact.5
Acute diverticulitis occurs due to inflammation of a diverticulum after it becomes occluded by faecal material. It is the most common cause of left lower quadrant pain. The diagnosis should be confirmed by imaging, as clinical assessment alone is associated with a high rate of incorrect diagnoses and it cannot reliably determine whether or not the diverticulitis is complicated. Ultrasound and computed tomography (CT) have shown similar efficacy for the diagnosis of sigmoid diverticulitis.6,7 One accepted strategy is to use ultrasound as the initial technique, and perform a computed tomography (CT) scan only in inconclusive cases or due to lack of experience.8,9
There are numerous classifications of acute diverticulitis of the left colon. The Hinchey classification,10 the most used in the last twenty or thirty years, depends on the surgical findings and distinguishes four levels of severity: (1) pericolic abscess; (2) pelvic, intra-abdominal or retroperitoneal abscess; (3) generalised purulent peritonitis; and (4) generalised faecal peritonitis.
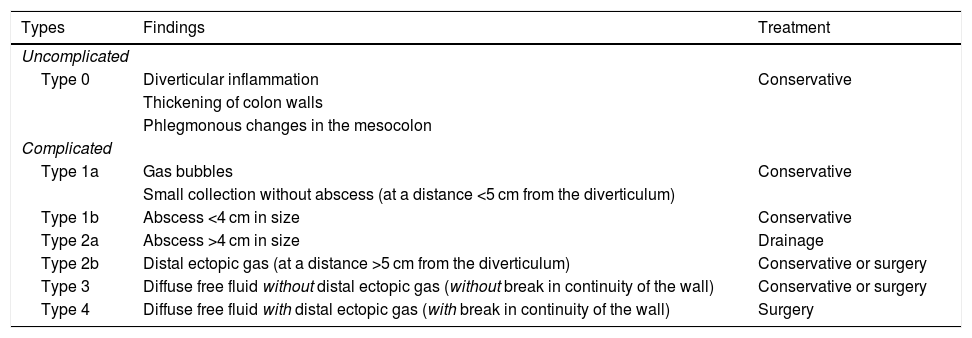
In 2016, the World Society of Emergency Surgery (WSES) group proposed a classification system based on the extent of the CT findings,11 easily applicable in practice and with recommendations on management; it divides acute diverticulitis into complicated and uncomplicated, depending on whether the process is limited to the wall of the colon or extends to the peritoneum, respectively (Table 1). The ultrasound findings of acute uncomplicated diverticulitis (Fig. 1A) are: (a) discrete concentric wall thickening (4–5 mm) in the affected colon segment; (b) inflamed diverticulum with little or no gas content, with a predominance of hypoechoic content; and (c) hyperechoic pericolic fat adjacent to the inflamed diverticulum.
World Society of Emergency Surgery Classification of acute left-sided colonic diverticulitis.11
| Types | Findings | Treatment |
|---|---|---|
| Uncomplicated | ||
| Type 0 | Diverticular inflammation | Conservative |
| Thickening of colon walls | ||
| Phlegmonous changes in the mesocolon | ||
| Complicated | ||
| Type 1a | Gas bubbles | Conservative |
| Small collection without abscess (at a distance <5 cm from the diverticulum) | ||
| Type 1b | Abscess <4 cm in size | Conservative |
| Type 2a | Abscess >4 cm in size | Drainage |
| Type 2b | Distal ectopic gas (at a distance >5 cm from the diverticulum) | Conservative or surgery |
| Type 3 | Diffuse free fluid without distal ectopic gas (without break in continuity of the wall) | Conservative or surgery |
| Type 4 | Diffuse free fluid with distal ectopic gas (with break in continuity of the wall) | Surgery |
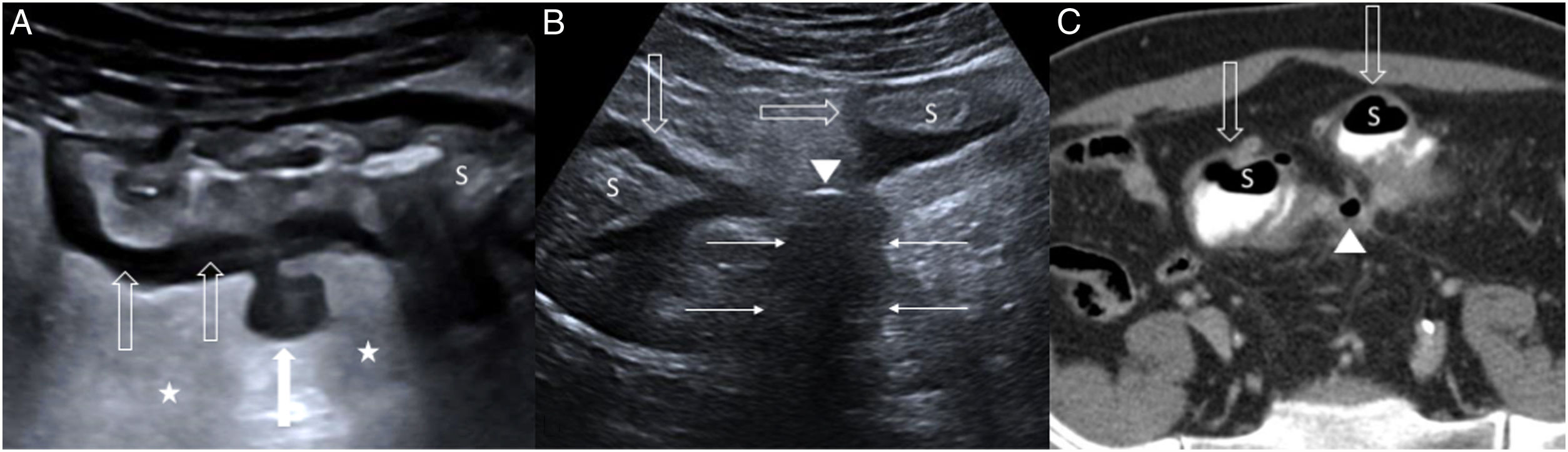
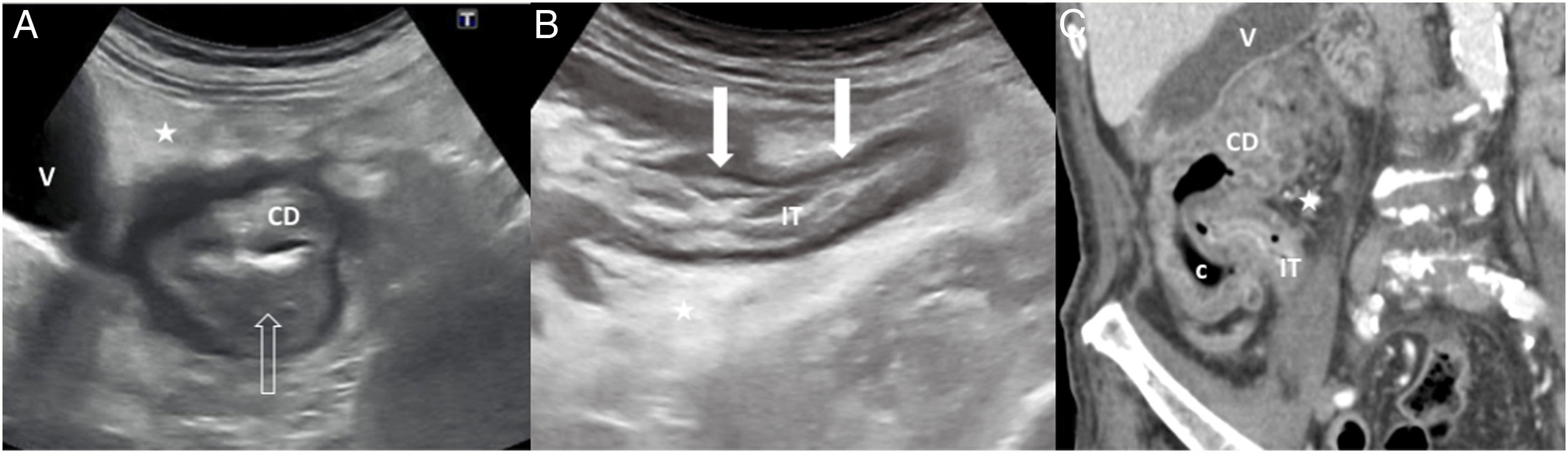
A) Uncomplicated acute colon diverticulitis (type 0) on ultrasound. Inflamed diverticulum with hypoechoic content (white arrow), concentric wall thickening (empty arrows) in the sigmoid colon (S) with preservation of the layered echotexture, and hyperechoic perisigmoid fat (stars) due to inflammation. B and C) Complicated acute colon diverticulitis (type 1 b) on ultrasound and computed tomography respectively. Hypoechoic fluid collection (only shown on ultrasound), less than 4 cm in size (thin arrows), next to the sigmoid colon (S), which has circumferential wall thickening (empty arrows); there is an extraluminal gas bubble (arrowhead) inside the fluid collection.
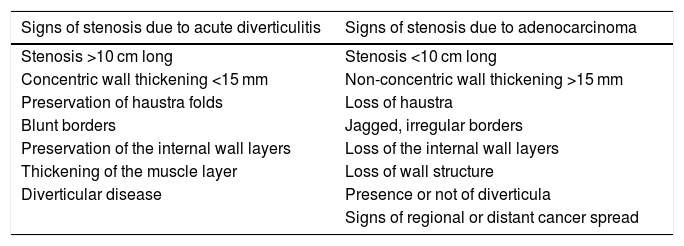
Typical complications are abscess formation, perforation, fistulas and strictures. Abscesses are seen as hypoechoic collections, sometimes with echogenic debris or gas bubbles inside (Fig. 1B and C). Contrast-enhanced ultrasound is useful for discriminating between phlegmon and abscess, as abscesses do not show internal enhancement, and for correctly delimiting their size.12 Fistulas are detected as linear hypoechoic band-shaped structures, with or without gas bubbles along their path. Gas in the bladder is an indirect sign of a sigmoid colon-to-bladder fistula. The classic sign of contained intestinal perforation is ectopic gas located in the mesentery or within a fluid collection. To distinguish an acute diverticulitis-related sigmoid colon stricture from one caused by perforated adenocarcinoma, we need to assess the signs listed in Table 2.13 Inflammatory signs, such as fat changes or collections, do not help with differentiation.
Ultrasound signs of sigmoid stenosis caused by acute diverticulitis and adenocarcinoma.13
| Signs of stenosis due to acute diverticulitis | Signs of stenosis due to adenocarcinoma |
|---|---|
| Stenosis >10 cm long | Stenosis <10 cm long |
| Concentric wall thickening <15 mm | Non-concentric wall thickening >15 mm |
| Preservation of haustra folds | Loss of haustra |
| Blunt borders | Jagged, irregular borders |
| Preservation of the internal wall layers | Loss of the internal wall layers |
| Thickening of the muscle layer | Loss of wall structure |
| Diverticular disease | Presence or not of diverticula |
| Signs of regional or distant cancer spread |
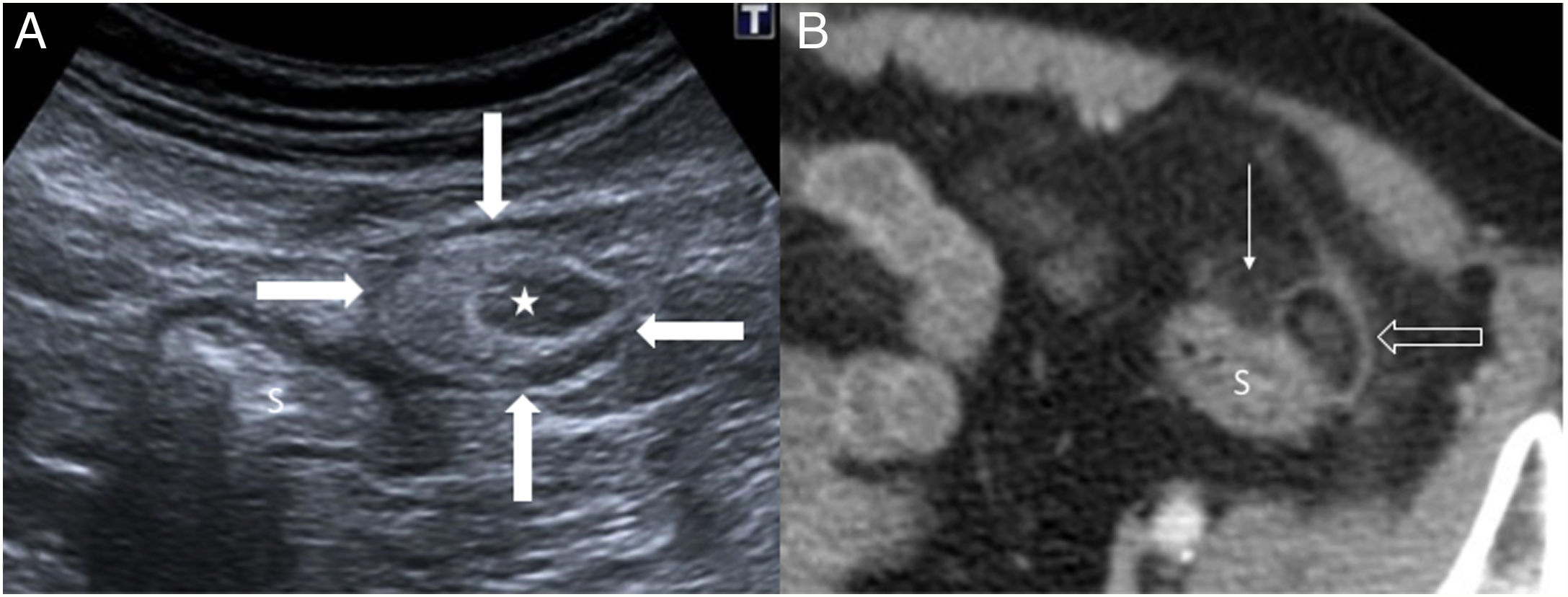
This is relatively uncommon (left:right - 15:1) and has several distinctive features. Diverticula in the caecum and ascending colon are usually congenital, single and true, as they contain all the wall layers. As they cause acute abdomen in the right iliac fossa, correct diagnosis is crucial to avoid unnecessary appendectomy. The ultrasound findings are similar to those of sigmoid-colon diverticulitis, although complications are very rare4,12 (Fig. 2A).
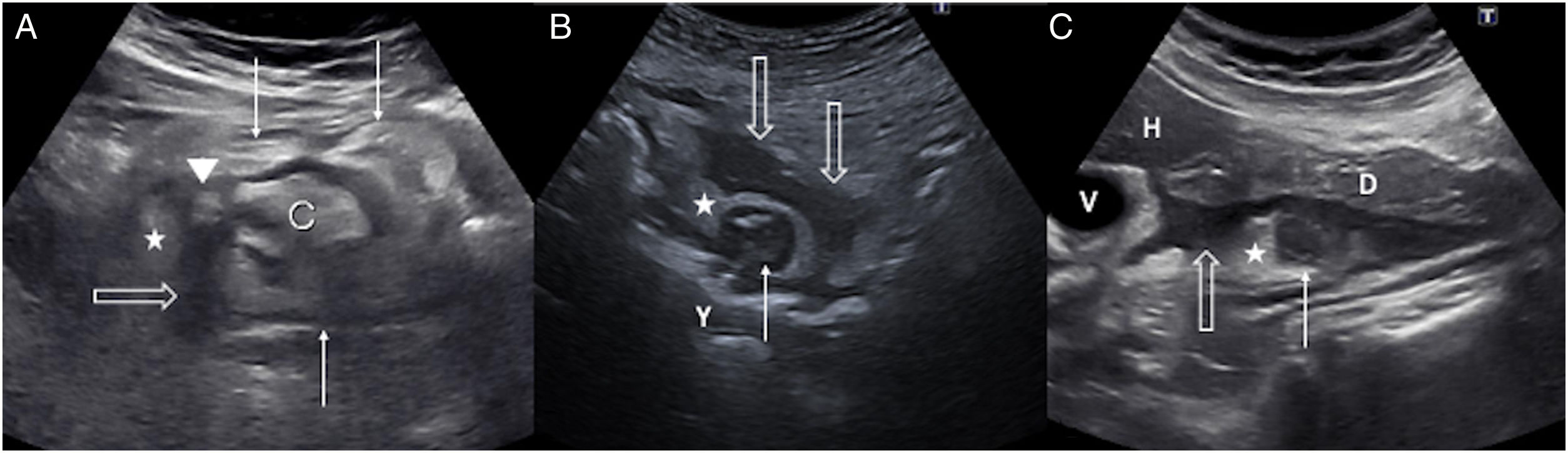
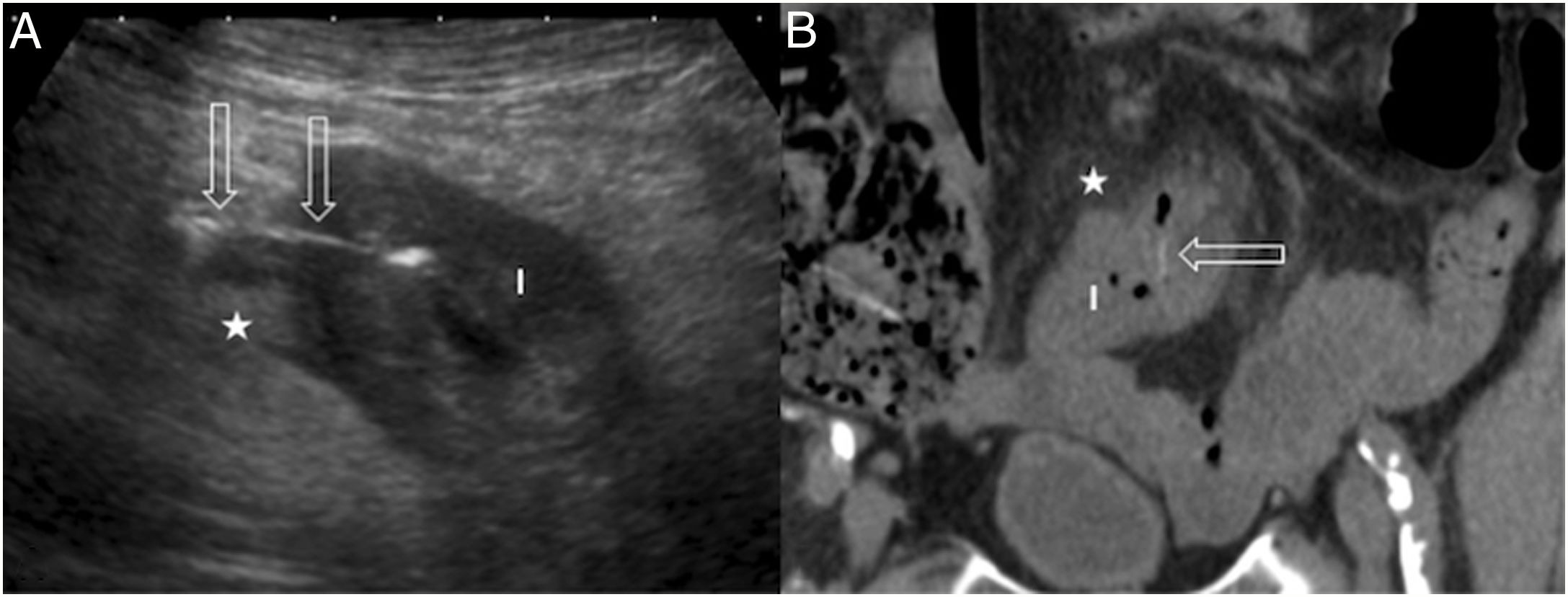
A) Acute diverticulitis in the caecum. Diverticulum (arrowhead) with a thickened wall on the lateral surface of the caecum (C). There is concentric wall thickening in the caecum (fine arrows), a peridiverticular laminar fluid collection (empty arrow) and phlegmonous changes in the surrounding fat (star). B) Acute diverticulitis in the jejunum. Diverticulum (thin arrow) with a thickened wall and hypoechoic centre close to a jejunal loop (J). Associated inflammatory changes in the surrounding fat (star) and a hypoechoic fluid collection (empty arrows). C) Acute duodenal diverticulitis. Inflamed diverticulum (thin arrow) with hypoechoic content attached to the posterior surface of the duodenum (D); hyperechoic adjacent fat due to phlegmonous changes (star) and hypoechoic fluid collection (empty arrow). H: left hepatic lobe; G: gallbladder.
Diverticula of the jejunum and ileum are false, without a muscular layer, and are located on the mesenteric border. The prevalence is 1–2%. The most common location is the proximal jejunum (75%), followed by the distal jejunum (20%) and the ileum (5%). They are usually larger in size and number in the proximal jejunum, tending to be smaller and fewer the more distal they are in the small intestine.14
On ultrasound, the inflamed diverticula are seen as irregular structures with a hyperechoic centre, in contact with the intestinal lumen (Fig. 2B). Phlegmonous changes in the surrounding fat are very often the first sign detected, associated with concentric thickening of the intestinal wall. Microperforation can appear as fluid collections with bubbles of ectopic gas inside.
Acute duodenal diverticulitisThe duodenum is the second most common site for diverticula to develop. The incidence is 22%.15 The diverticula predominate on the pancreatic border of the second and third parts of the duodenum.16 They are less likely to become inflamed than in the colon. Complicated duodenal diverticulitis is clinically nonspecific and can be confused with acute cholecystitis, acute pancreatitis, pancreatic cancer or peptic ulcer. The most difficult differential diagnosis to make is with perforated duodenal ulcer, although this tends to affect more proximal parts, close to the duodenal bulb.17
The ultrasound findings are diverticular inflammation, duodenal wall thickening, phlegmonous changes in the adjacent fat, free fluid in the lesser sac, and extraluminal gas in the case of perforation (Fig. 2C).
Gastrointestinal perforationThis is a complication which is generally acute and requires emergency surgery. Its causes include intestinal ischaemia, obstruction, diverticulitis, peptic ulcer, cancer, inflammatory bowel disease, trauma, iatrogenesis and foreign body.18,19 Sudden-onset abdominal pain is always a symptom, but the clinical signs can vary and lead to a late diagnosis.
CT is the technique of choice and detects the site of the perforation in 86% of cases.20 When symptoms are non-specific, it is common to initially perform an ultrasound, where the sensitivity for detecting pneumoperitoneum is higher than that of a plain abdominal X-ray.21 CT and ultrasound are appropriate for considering alternative diagnoses22 and assessing other findings in addition to the perforation, such as segmental parietal thickening, ileus, alteration of mesenteric fat, free fluid, abscess or fistula.19,20,22
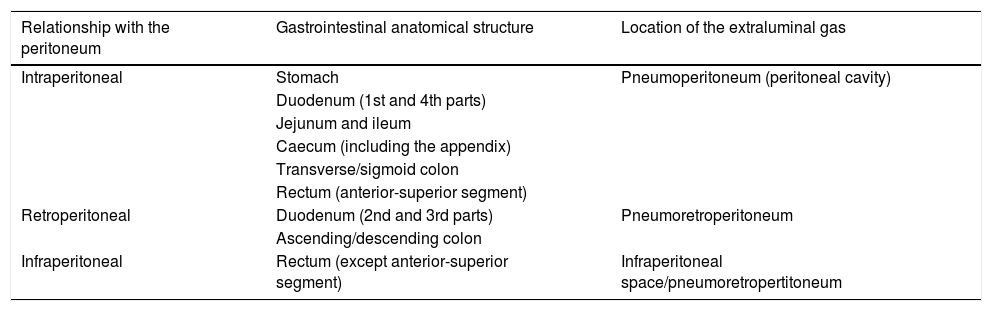
The site of the perforation determines the location of the extraluminal gas (Table 3). In general, gastroduodenal perforations cause pneumoperitoneum around the liver, duodenum and stomach.23 Less common, those that affect the retroperitoneal duodenum lead to pneumoretroperitoneum in the right anterior pararenal space.23,24 In the jejunum and ileum, perforations tend to generate very little free air in the inframesocolic space,19 while in the large intestine they lead to a variable amount of air, either in different locations or spread out.24
Relationship between gastrointestinal anatomical structures and the peritoneum, which determines the location of the extraluminal gas in the event of perforation.
| Relationship with the peritoneum | Gastrointestinal anatomical structure | Location of the extraluminal gas |
|---|---|---|
| Intraperitoneal | Stomach | Pneumoperitoneum (peritoneal cavity) |
| Duodenum (1st and 4th parts) | ||
| Jejunum and ileum | ||
| Caecum (including the appendix) | ||
| Transverse/sigmoid colon | ||
| Rectum (anterior-superior segment) | ||
| Retroperitoneal | Duodenum (2nd and 3rd parts) | Pneumoretroperitoneum |
| Ascending/descending colon | ||
| Infraperitoneal | Rectum (except anterior-superior segment) | Infraperitoneal space/pneumoretropertitoneum |
Convex and linear transducers of different frequencies help identify pneumoperitoneum on ultrasound.22 The examination should focus on the symptomatic area,18 the epigastrium and the right hypochondriac region after placing the patient in the left lateral decubitus position.21 Enhancement of the peritoneal stripe, which is normally a thin echogenic line, is considered a sensitive and specific sign of pneumoperitoneum25 (Fig. 3A and B). It may be accompanied by reverberation and “ring-down” artefacts, as occurs in hyperechoic foci, which indicate small extraluminal gas bubbles25 (Fig. 3C and D). Displacement of the air with the movements of the patient or by momentarily increasing the pressure with the transducer,26 even using its caudal part (“scissors manoeuvre”),27 confirms that it is intraperitoneal. False positives occur when intraluminal or lung-base air is misinterpreted as pneumoperitoneum (Fig. 3E and F). In some cases, echogenic or “dirty” free fluid is seen due to the spillage of gastric or enteric contents, which include food particles and air bubbles.28
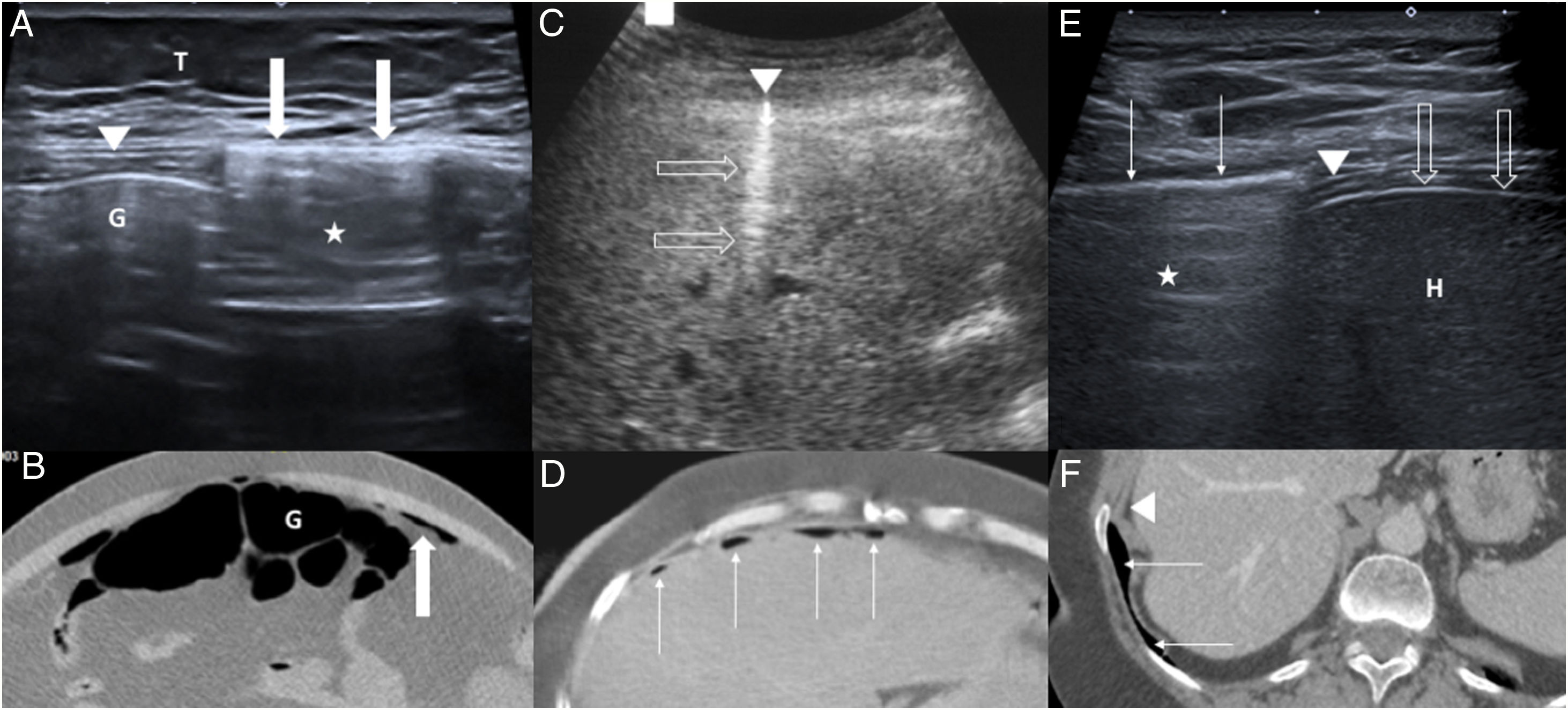
A and B) 45-year-old patient with pneumoperitoneum due to gastric perforation. Axial ultrasound (A) of the epigastrium showing the enhanced peritoneal stripe sign (arrows), associated with reverberation artefact (star). Intestinal gas (G) lies deep in relation to the normal adjacent peritoneal line (arrowhead). T: subcutaneous cellular tissue. Correlation with an axial computed tomography image without contrast (B): intraluminal (G) and extraluminal gas (arrow). C and D) 72-year-old patient with perforation of the duodenal bulb. Hyperechoic focus (arrowhead) with reverberation artefact (empty arrows) on the surface of the liver in axial ultrasound of the epigastrium (C), corresponding to a pneumoperitoneum bubble. Correlation with an axial computed tomography image with intravenous contrast (D): multiple foci of pneumoperitoneum (thin arrows) in the epigastric and right hypochondriac regions. E and F) 61-year-old woman with acute abdominal pain. Intercostal ultrasound of the right hypochondrium (E) in left lateral decubitus position, showing a hyperechoic line (thin arrows) with reverberation artefact (star) on the diaphragm (arrowhead), corresponding to pulmonary gas, which moves with breathing. L: liver; liver capsule (empty arrows). Correlation with an axial computed tomography image with intravenous contrast (F): pulmonary gas (fine arrows) and diaphragm (arrowhead).
Unlike pneumoperitoneum, pneumoretroperitoneum does not cause a displacement phenomenon22 and is characterised by the presence of gas around the duodenum and head of pancreas; plus the fact that the right kidney and the large abdominal vessels cannot be visualised, as they are obscured by the gas.19,29 An ultrasound diagnosis of pneumoperitoneum or pneumoretroperitoneum should be confirmed by CT.
Foreign bodiesAlthough the ingestion of foreign bodies is relatively common, most pass through the gastrointestinal tract without complications.18 Risk factors include alcoholism, false teeth, rapid food intake and mental retardation. Those that cause perforation are usually pointed (fish bones, chicken bones and toothpicks). Clinical manifestations include abdominal pain in different locations, nausea, vomiting, fever and peritonitis. As these are nonspecific symptoms, it is not uncommon for an ultrasound to be performed as first imaging technique. Many patients do not remember ingesting the foreign body and, in some cases, days or months go by before the onset of symptoms.
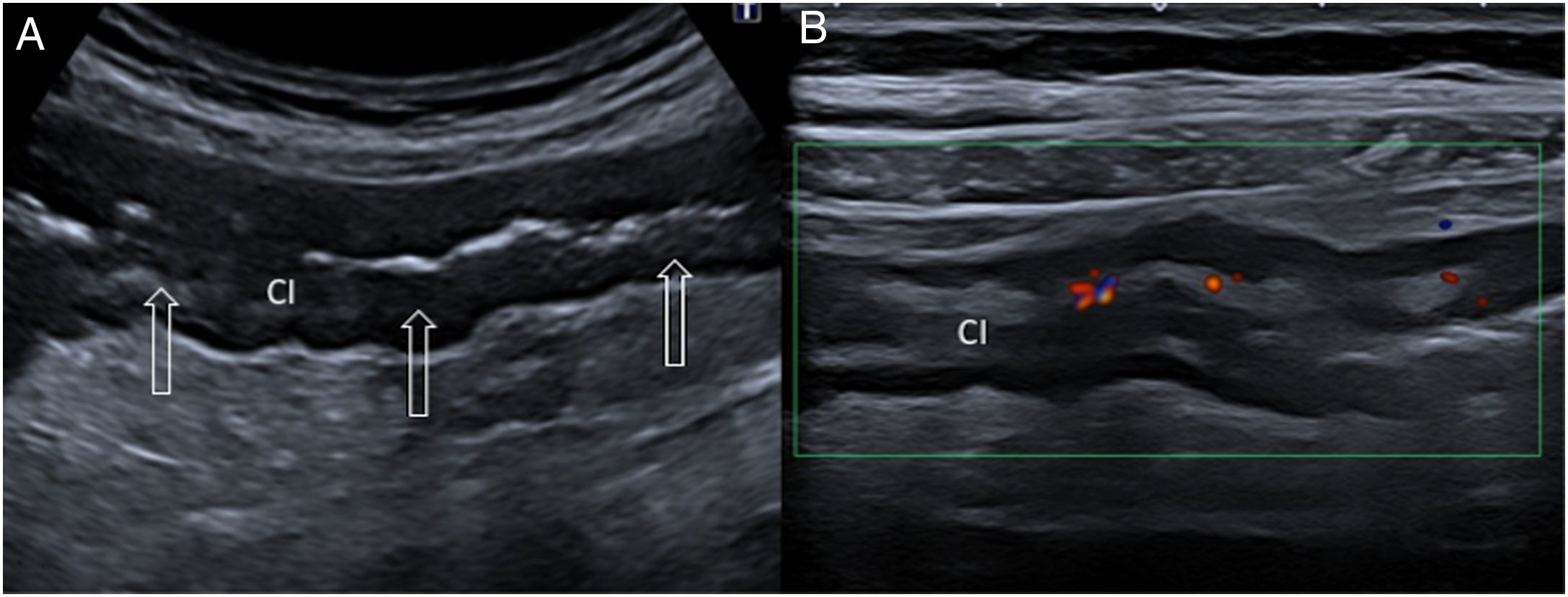
Once ultrasound signs related to perforation have been detected, such as fluid collections in the lamina propria and hyperechoic adjacent fat, with or without gas bubbles,30 the foreign body with the thickened bowel segment need to be identified, particularly in the aforementioned epidemiological contexts. This is typically seen in the longitudinal plane as a thin, straight echogenic line, with variable acoustic shadowing, and as a hyperechoic spot with sharp acoustic shadow in a cross-sectional view31 (Fig. 4).
90-year-old man with nonspecific lower abdominal pain. A) Ultrasound transverse view of the right inferior fossa showing a loop of bowel (B) with a thickened wall, increased echogenicity of the adjacent fat (star) and a linear hyperechoic image (empty arrows) going through the bowel wall to the mesocolon, corresponding to a fish bone. B) Correlation with a coronal non-contrast computed tomography image: bowel loop with thickened wall (B), hyperdensity of the adjacent fat due to inflammation (star) and hyperdense fish bone (hollow arrow).
Ultrasound is suitable for detecting foreign bodies made of wood (sensitivity 52-82%)32 or fish bones, which are difficult to recognise on CT due to their low density, and require careful interpretation of oblique multiplanar reconstructions.30,33
Ischaemic bowelAcute mesenteric ischaemiaAcute mesenteric ischaemia (AMI) is classically associated with sudden onset of abdominal pain, disproportionate to the physical examination.34 It is caused by arterial embolism, arterial thrombosis, venous thrombosis or non-occlusive mesenteric ischaemia; arterial aetiology is more common than venous.35 Urgent CT-angiogram is the method of choice. However, ultrasound is the first imaging test in some patients with nonspecific symptoms.34
Thickening of the intestinal wall is the most common ultrasound sign.35 Initially, there will be hyperperistalsis, followed by adynamic ileus, ascites and increased intraluminal secretions. However, these signs are nonspecific. Increased intraluminal air can make it difficult to assess the bowel.36 If the occlusion is close to the origin of the superior mesenteric artery, absence of flow can be confirmed by colour Doppler.37 If it progresses to a transmural intestinal infarction, there may be associated intestinal pneumatosis and portal-mesenteric venous gas.
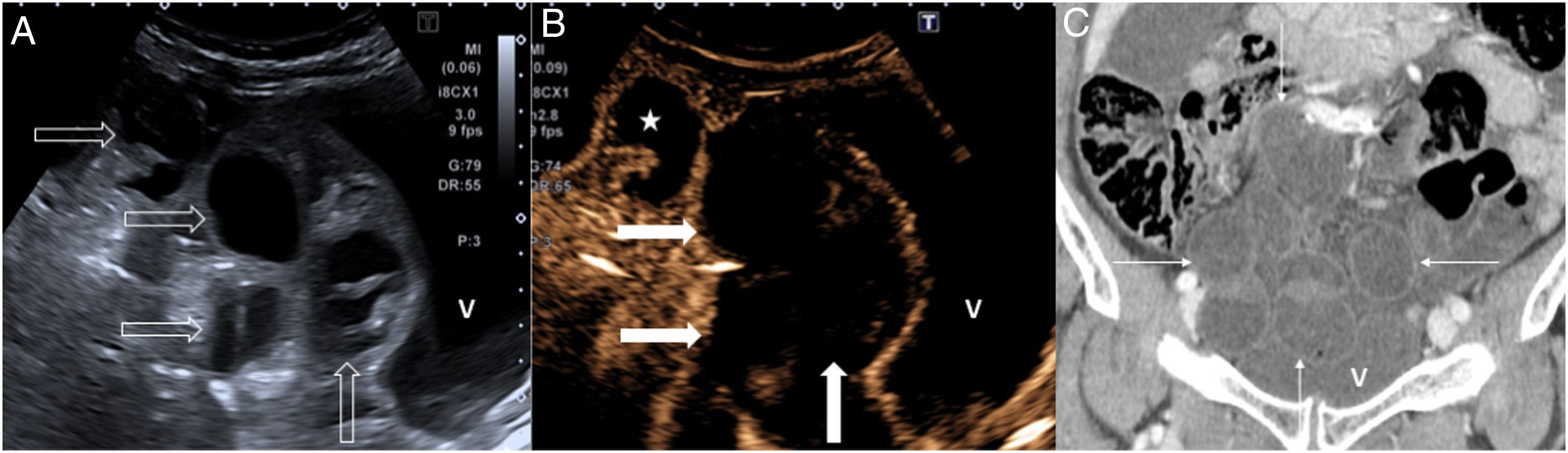
In expert hands, the use of contrast-enhanced ultrasound increases sensitivity for demonstrating hypoperfusion of the intestinal wall in AMI. It is indicated in patients with renal failure and it is also suitable for assessing loop perfusion in intestinal ischaemia caused by intestinal obstruction38,39 (Fig. 5).
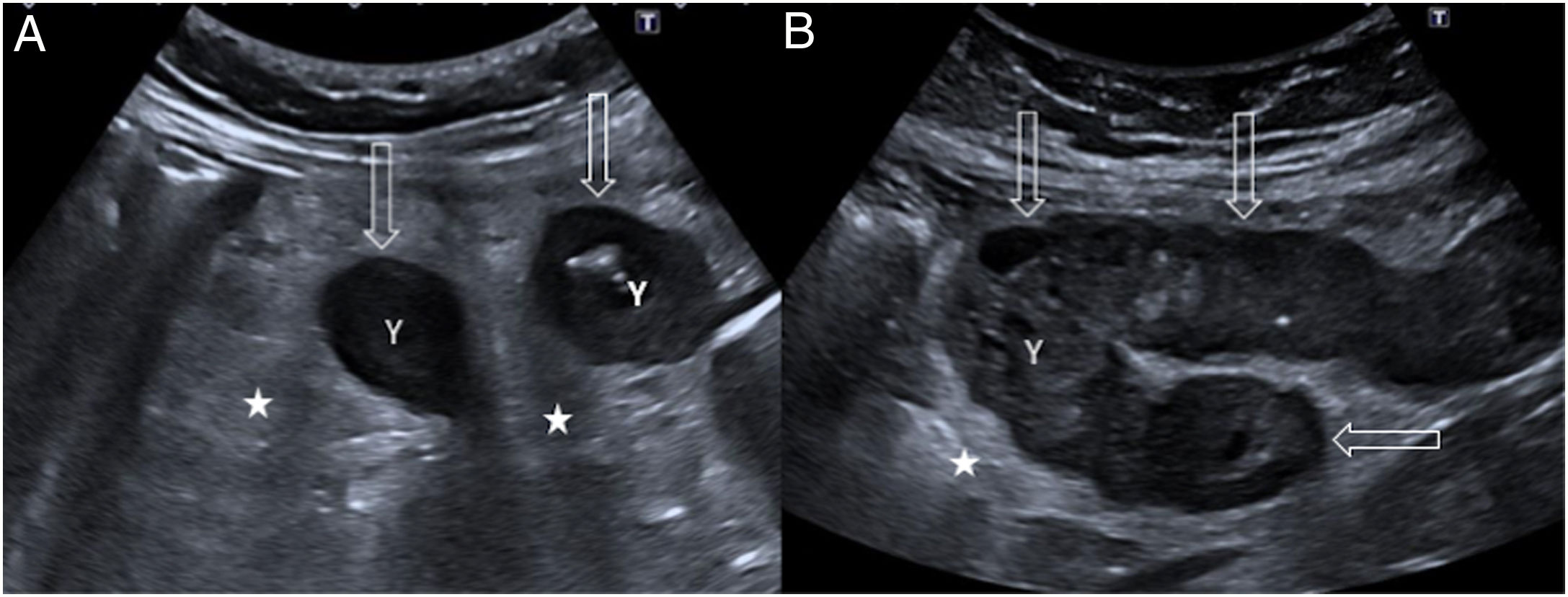
86-year-old woman with 24 hours of nonspecific abdominal pain. A) Longitudinal ultrasound image of the true pelvis showing multiple dilated bowel loops with a slightly thickened wall (empty arrows). B: bladder. B) Contrast-enhanced ultrasound shows absence of wall enhancement, involving several loops (arrows). Compare with the normal enhancement of another bowel loop (star). C) Coronal reconstruction of computed tomography with intravenous contrast showing dilation of the small intestine (fine arrows) in the hypogastrium and slight wall enhancement, in relation to intestinal occlusion due to a probable internal hernia. Surgery confirmed vascular compromise in several bowel loops due to a mesenteric flow obstruction; after adhesiolysis and rinsing with warm normal saline, a change in colour and peristalsis were observed in the entire portion of injured loops, so in the end it was decided against performing bowel resection.
This is the most common form of ischaemic bowel and the second most common cause of lower gastrointestinal bleeding. There are two forms: one is gangrenous (10–20%), with transmural necrosis and a high mortality rate; and the other is transient (80–90%), characterised by reversible lesions in the mucosa or submucosa, and is managed conservatively.40 Common symptoms are haematochezia, persistent diarrhoea and abdominal pain.40 Colonoscopy is the test of choice.
Non-occlusive ischaemic colitis (IC) generally affects segments of the colon, particularly the splenic flexure, descending colon and sigmoid colon. Ultrasound is useful for early detection.41 IC should be suspected in patients with symptoms and advanced age when the ultrasound shows thickening of a long segment of the colon (>10 cm), particularly on the left side, with little or no colour Doppler signal41,42 (Fig. 6). The positive predictive value of these findings for IC is 90%.43 In reversible cases, hyperaemia in the bowel wall due to reperfusion can be detected by colour Doppler. Altered perienteric fat has been associated with transmural necrosis.41,42 IC without transmural necrosis is self-limiting, with rapid recovery of the mucosal lesions. Lack of improvement in follow-up ultrasound suggests transmural necrosis, and requires additional radiological tests or endoscopy to confirm the diagnosis and rule out underlying cancer.41
72-year-old man with sudden-onset pain in left lower abdomen and rectal bleeding. A) Sagittal ultrasound of the left flank showing concentric thickening (empty arrows) of a long segment of the left colon (LC). B) Colour Doppler mode shows poor flow in the left colon (LC), which has thickened walls, but preserved layered structure. The ultrasound detection of thickening of a long segment of the left colon with poor colour Doppler flow in a patient with typical clinical signs has a very high positive predictive value for the diagnosis of ischaemic colitis.
The diagnostic performance of ultrasound and CT is similar in IC, except in the case of intestinal pneumatosis, a rare, late finding with a poor prognosis, which is difficult to detect with ultrasound.38,44
Intestinal obstructionThe most common causes of small bowel obstruction (SBO) are postoperative adhesions (80%), hernias, Crohn's disease, cancer and volvulus, whereas in the large intestine, cancer is the predominant cause.22 Other rare causes are endometriosis, foreign bodies, intussusception, gallstone ileus and bezoars. Patients present with abdominal pain, bloating, nausea and vomiting, although a subacute onset is possible in tumour-related strictures.22
Ultrasound is indicated as the initial technique when SBO is suspected.22 It has similar sensitivity (92%) and specificity (96%) to CT45 and is superior to plain X-ray.46 However, CT is more reliable for determining location and aetiology, primarily in internal hernias and volvulus, and complications.22
In the ultrasound examination the stomach and the different parts of the intestine must be assessed. Signs of intestinal obstruction include dilation of the intestinal loops proximal to the transition point (≥25 mm in diameter), collapse of the distal intestine, intraluminal fluid content, and slight thickening of the intestinal wall. The visualisation in real time of increased peristalsis in the initial phases (video in Additional material), with a back-and-forth movement (“to-and-fro motion”) is characteristic.22 As the process progresses, the decrease in or absence of peristalsis, combined with wall thickening and free fluid, are highly suspect of ischaemia or perforation.47 The Doppler mode with administration of contrast allow the viability of intestinal perfusion to be assessed39 (Fig. 5).
In contrast to mechanical obstruction, the prolonged absence of peristalsis in a fluid-filled intestinal segment is more indicative of adynamic ileus.22
EndometriosisEndometriosis is characterised by the presence of ectopic functional endometrial tissue outside the uterine cavity, in the form of ovarian endometriomas, peritoneal implants and deep pelvic endometriosis.48 Signs and symptoms include infertility and pelvic pain. The intestinal tract is the most common site of extragenital endometriosis (5–37% of cases),49 with descending order of frequency in the rectosigmoid region (65%), terminal ileum, caecum and appendix.50 Although uncommon, it can manifest as bowel stricture and obstruction. Definitive diagnosis requires histological confirmation.
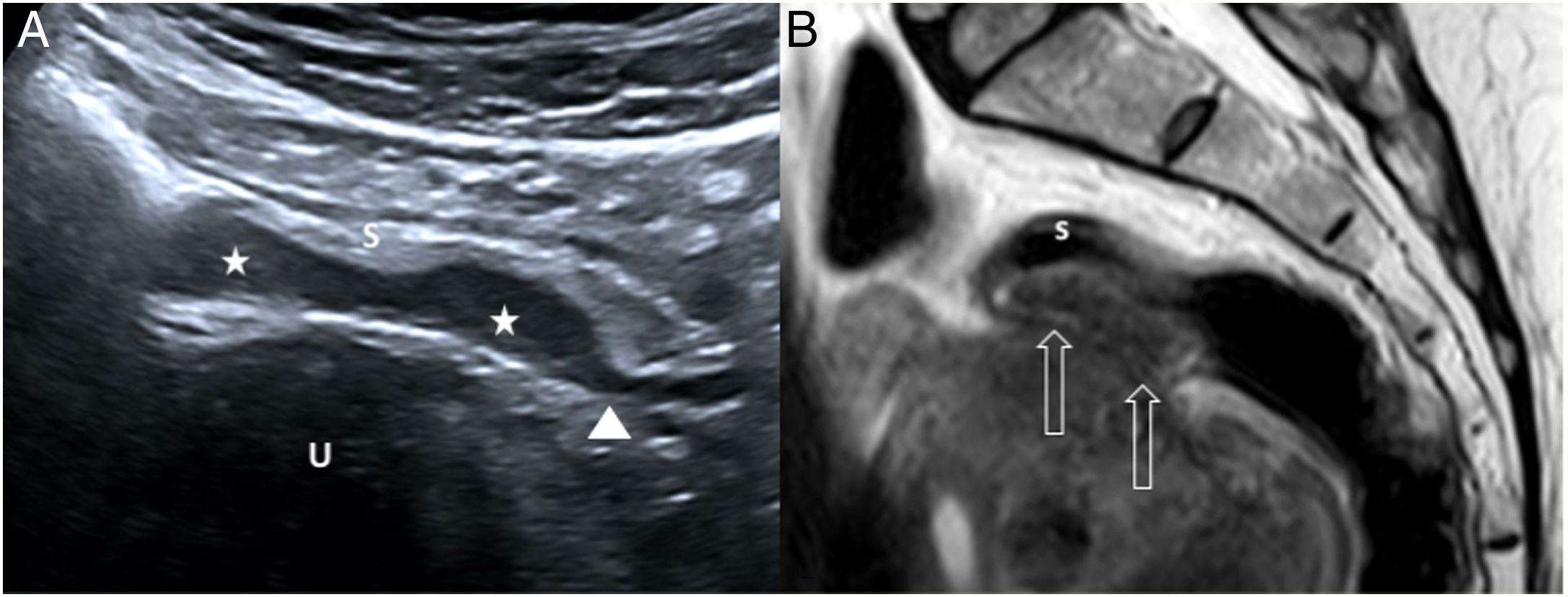
Endometrial implants are seen on ultrasound as hypoechoic fusiform masses in the serosa or muscularis propria; spicules at the ends of the thickened area make it resemble a comet with its tail49–52 (Fig. 7). Contrast-enhanced ultrasound reveals slight purely vascular vascularisation, reflecting their capacity for neovascularisation.53 Transvaginal examination helps detect foci of endometriosis in the rectum and at the rectosigmoid junction, adhesions between the uterus and rectosigmoid junction,51 and evidence of deep pain when pressing the lesions with the transducer. The differential diagnosis includes primary adenocarcinoma and peritoneal metastases of gastrointestinal or gynaecological origin48; in endometriosis, the submucosa and mucosa are usually intact, in contrast with what occurs in cancer.54
46-year-old woman with episodic abdominal pain along with stools containing mucus and blood in the last 6 months. A) Abdominal ultrasound shows two small hypoechoic masses (stars) adhered to the sigmoid serosa (S), pressing on the rest of the layers of the wall, suggestive of endometriosis with a “plaque-like” growth pattern. Note the comet-tail sign (arrowhead), continuing with the hypoechoic muscle layer. U: uterus. B) Sagittal pelvic magnetic resonance image (T2-weighted fast spin-echo [FSE] sequence) confirming a hypointense eccentric plaque lesion (empty arrows) in the anterior wall of the distal sigmoid colon. Pathology confirmed the diagnosis of intestinal endometriosis. s: sigmoid colon submucosal layer.
A hernia is the protrusion of the contents of the abdominal cavity through a congenital or acquired defect. Hernias are classified as external (inguinal, femoral, umbilical, epigastric, Spiegel or incisional) or internal, depending on whether the hole is located in the abdominal wall or in the peritoneum or mesentery respectively. Fat and bowel are the most common contents of a hernia. External hernias are very common and, in most cases, asymptomatic, although they can be complicated by intestinal obstruction, incarceration or strangulation. Internal hernias have a low incidence, but require emergency surgery as they usually involve strangulated SBO as a result of a closed loop obstruction.55
High-frequency linear transducers (≥10 MHz) are needed to study hernias of the abdominal wall. Knowledge of the anatomical landmarks and the usual location of the different hernias reduces false negatives. It is important to document the hernia in two orthogonal planes and carry out a dynamic study by applying Valsalva or compression manoeuvres.56 Visualisation in the sagittal plane helps avoid errors in inguinal hernias. Once the ultrasound diagnosis of hernia is confirmed, we need to record the size, the characteristics of its contents and whether or not it is reducible, and investigate possible signs of SBO, such as dilation of the proximal bowel or herniated loops, narrowing of both ends in the neck of the hernia, and normal or decreased lumen of the distal intestine.57 Additionally, wall thickening, hyperechoic fatty tissue, ascites, congestion of mesenteric vessels, and decreased wall vascularisation when using Doppler or intravenous contrast, are suggestive of ischaemia of the herniated bowel segment.57
Internal hernias can be challenging, both in clinical and diagnostic terms, as the symptoms may be nonspecific. CT is the technique of choice. Ultrasound can guide the diagnosis when there is a pattern of SBO accompanied by congestion and displacement of the mesenteric vessels, particularly in patients with no history of surgery or abdominal trauma.55
Acute focal intra-abdominal fat necrosisEpiploic appendagitisThe epiploic appendages are small structures made up of fat and blood vessels which protrude from the serosa of the colon into the peritoneal cavity. They are more common in the caecum and the left colon.58 Intraperitoneal fluid makes them easier to see with ultrasound.5 Their pedunculated shape and their mobility make them prone to thrombosis or to becoming twisted, which can then lead to haemorrhagic infarction. Patients present with acute abdominal pain, the location of which they can usually point to with a fingertip, associated with peritonism.59,60
An ultrasound scan concentrating on the area with the worst pain will show a solid hyperechoic, ovoid, non-compressible lesion, with a fine hypoechoic halo and hyperechoic adjacent fat44 (Fig. 8). The lesion is found next to the colon, where only 10% of cases have wall thickening,44 and is attached to the anterior abdominal wall, being easily recognisable watching the patient's respiratory movements.9 Doppler mode and contrast-enhanced ultrasound show absence of central flow and peripheral enhancement. Occasionally, a small hypoechoic central focus is identified, representing venous thrombosis.61,62
Epiploic appendagitis in a woman with sudden-onset left iliac fossa (LIF) pain. A) Axial ultrasound image showing an inflammatory oval-shaped mass with heterogeneous echogenicity (star) in the intraperitoneal fat, surrounded by a hypoechoic halo (arrows); in contact with the sigmoid colon (S), whose walls are of normal thickness. B) Axial computed tomography with intravenous contrast of the LIF shows that the lesion (empty arrow) has a fat density, a hyperdense central focus and a hyperdense ring; there is focal inflammation of the adjacent fatty tissue (thin arrow), but the wall of the sigmoid colon (S) is not thickened.
Primary omental infarction is caused by vascular compromise with venous stasis, congestion, oedema and haemorrhagic necrosis. It can also be caused by trauma, inflammation, cancer or recent abdominal surgery.44 It is more common in the area of the caecum or ascending colon due to the greater length and mobility of the omentum on that side. Like epiploic appendagitis, omental infarction is a self-limiting condition and management is conservative.
It is generally seen on ultrasound as a non-compressible hyperechoic mass of more than 5 cm in size,35 with poorly defined hypoechoic nodular areas, peripheral hyperaemia and no internal vascularisation.63
MiscellaneousGastrointestinal vasculitisDifferent types of vasculitis form a heterogeneous group of primary or secondary disorders (infection, autoimmune disease or neoplasm), in which vascular inflammation can be demonstrated, either histologically or by imaging.64 The group is further divided into small, medium or large-vessel, as well as variable-vessel and single-organ vasculitis.65 Vasculitis can affect different organs and systems, including the gastrointestinal tract, and this determines the symptoms.
Depending on the territory and size of the affected vessel, gastrointestinal problems may be from mild (paralytic ileus or submucosal oedema/bleeding) to severe (ischaemia, perforation or stricture), with either a focal or segmental pattern.66 Ultrasound findings are not specific to each type of vasculitis and depend on how the process progresses: thickening of the intestinal wall; hyperechoic mesenteric fat; free fluid; ulcers due to ischaemia; and intestinal perforation, stricture or obstruction.
The form with the highest rate of gastrointestinal involvement (50–75%) is IgA vasculitis (Henoch-Schönlein purpura) (Fig. 9), which affects small vessels and predominantly occurs in children.64 Clinical manifestations can involve the skin, kidneys, joints and gastrointestinal tract; the abdominal and joint symptoms may precede the purpuric rash.64 Areas of submucosal hyperechoic focal thickening due to intramural bleeding are characteristic on ultrasound. Intussusception is a major complication, but most paediatric cases resolve without further incident.67
Paediatric patient with pain in the mesogastrium, low-grade fever, vomiting, bloody diarrhoea and purplish erythematous rash on lower limbs. A and B) Axial and sagittal ultrasound respectively of the left flank, showing circumferential thickening of a long segment of jejunum (J) and hyperechoic adjacent mesenteric fat (stars) caused by inflammation, due to intestinal involvement associated with IgA vasculitis (Henoch-Schönlein purpura).
This can occur when either the small or large bowel is in the radiotherapy field. Acute side effects start early during treatment, while late side effects appear three months or more after completion68 and can be irreversible. The combination with chemotherapy increases the risk.
The acute form is characterised by diarrhoea, abdominal pain, nausea and vomiting. The main ultrasound finding is thickening of the bowel wall in the irradiated region, with preservation of the layered structure. Infectious gastroenteritis needs to be considered in the differential diagnosis.
Late toxicity, which includes malabsorption and diarrhoea, is more likely in patients who developed acute lesions. Ultrasound signs include segmental thickening of the bowel wall involving the submucosal layer, echogenic mesenteric fat changes, formation of abscesses or fistulas, and intestinal obstruction caused by strictures or adhesions.69
Neutropenic enterocolitisSerious complication of severe neutropenia caused by high doses of chemotherapy, particularly in patients with blood cancers. The signs are abdominal pain, mainly in the right iliac fossa, peritonism, fever and watery or bloody diarrhoea.70
Typical on ultrasound is significant thickening (up to 1 cm) of the bowel wall involving the submucosa layer, affecting the caecum and extending to the right colon and terminal ileum (Fig. 10). The vascularisation and the wall layers remain unaffected. Hyperechoic pericaecal fat and free fluid are signs of greater severity. Intraluminal fluid content and decreased peristalsis may be seen in the affected bowel segments.71 The condition can be complicated by intramural gas from superinfection or extraluminal gas from perforation or abscesses.
Neutropenic ileocolitis in a 79-year-old male with rectal bleeding, melaena and pain and guarding in the right flank. Leukaemia and a history of gastric adenocarcinoma. A) Axial ultrasound of the right flank showing severe circumferential thickening of the wall of the right colon (RC), predominantly submucosal (empty arrow), with preserved layering of the wall. There is increased echogenicity of pericolonic fat (star) due to inflammatory involvement. G: gallbladder. B) Axial ultrasound of the right flank, showing wall thickening (arrows) in the terminal ileum (TI), with preservation of the layering of the wall, associated with phlegmonous changes in the adjacent fatty tissue (star). C) Correlation with a coronal computed tomography image with intravenous contrast: severe circumferential thickening of the wall of the right colon (RC), caecum (C) and terminal ileum (TI), and hyperdensity of the adjacent fat due to inflammation. G: gallbladder.
Multisystemic disorder of bacterial origin, which presents with intestinal malabsorption. Whipple disease is caused by a type of bacterium called Tropheryma whipplei and belongs to the Actinomyces genus. It is considered a rare disease, more common between the ages of 40 and 50.72 It classically causes diarrhoea, weight loss and abdominal and joint pain. Although Whipple disease may be suspected from the clinical, laboratory, radiology and pathology data, definitive diagnosis requires detection of the germ by polymerase chain reaction (PCR).
Ultrasound helps identify thickening of the bowel wall and mucosal folds, especially in the jejunum, the normal or increased lumen of the small intestine, and rounded, hyperechoic mesenteric or retroperitoneal lymphadenopathy, with hypoechoic foci.73 However, these are nonspecific findings which can be seen in other malabsorption syndromes.
Non-steroidal anti-inflammatory drug-induced enteropathyLong-term use of non-steroidal anti-inflammatory drugs (NSAIDs) causes both acute and chronic adverse effects in the small and large bowel, although most cases are subclinical. One distinctive feature is the formation of diaphragms: circumferential, scarring lesions which cause strictures in the bowel loops.74
The radiological signs have been described mainly in CT, as multiple, short bowel strictures, focal wall thickening with hyper-enhancement and dilation of the bowel,74 which can progress to bowel obstruction or perforation. Isolated cases have been published in which ultrasound revealed ileocolic wall thickening, increased wall vascularisation, reactive mesenteric lymphadenopathy and free fluid.75
As the differential diagnosis is with Crohn’s disease, we need to investigate any history of NSAID use. Other drugs (acetylsalicylic acid, chemotherapy or immunotherapy) are also known causes of gastrointestinal toxicity.
Authorship- 1
Responsible for the integrity of the study: TRG.
- 2
Study conception: TRG.
- 3
Study design: JVR.
- 4
Data acquisition: JVR, TRG, GMB, JAMB, TS.
- 5
Analysis and interpretation of the data: JVR, TRG, GMB, JAMB, TS.
- 6
Statistical processing: not applicable.
- 7
Literature search: JVR, TRG, GMB, JAMB, TS.
- 8
Drafting of the manuscript: JVR, TRG, GMB, JAMB.
- 9
Critical review of the manuscript with relevant intellectual contributions: TRG, JVR, GMB, JAMB, TS.
- 10
Approval of the final version: JVR, TRG, GMB, JAMB, TS.
The authors declare that they have no conflicts of interest.
Please cite this article as: Vizuete del Río J, Martín Benítez G, Ripollés González T, Merino Bonilla JA, San-Miguel T, Ecografía intestinal y abdomen agudo: más allá de la apendicitis aguda, Radiología. 2021;63:193–205.










![46-year-old woman with episodic abdominal pain along with stools containing mucus and blood in the last 6 months. A) Abdominal ultrasound shows two small hypoechoic masses (stars) adhered to the sigmoid serosa (S), pressing on the rest of the layers of the wall, suggestive of endometriosis with a “plaque-like” growth pattern. Note the comet-tail sign (arrowhead), continuing with the hypoechoic muscle layer. U: uterus. B) Sagittal pelvic magnetic resonance image (T2-weighted fast spin-echo [FSE] sequence) confirming a hypointense eccentric plaque lesion (empty arrows) in the anterior wall of the distal sigmoid colon. Pathology confirmed the diagnosis of intestinal endometriosis. s: sigmoid colon submucosal layer. 46-year-old woman with episodic abdominal pain along with stools containing mucus and blood in the last 6 months. A) Abdominal ultrasound shows two small hypoechoic masses (stars) adhered to the sigmoid serosa (S), pressing on the rest of the layers of the wall, suggestive of endometriosis with a “plaque-like” growth pattern. Note the comet-tail sign (arrowhead), continuing with the hypoechoic muscle layer. U: uterus. B) Sagittal pelvic magnetic resonance image (T2-weighted fast spin-echo [FSE] sequence) confirming a hypointense eccentric plaque lesion (empty arrows) in the anterior wall of the distal sigmoid colon. Pathology confirmed the diagnosis of intestinal endometriosis. s: sigmoid colon submucosal layer.](https://static.elsevier.es/multimedia/21735107/0000006300000002/v1_202103070728/S2173510721000392/v1_202103070728/en/main.assets/thumbnail/gr7.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)




