Heart valve disease and coronary heart disease are very prevalent in the general population and often coincide in the same patient. Cardiac computed tomography (CT) makes it possible to noninvasively rule out coronary disease before valve surgery and to potentially avoid invasive heart catheterization in 66–75% of patients. The same imaging test provides abundant anatomic and functional information that complements the information from echocardiography, making it possible to characterize the etiology of the valve disease and its repercussions on the heart and aorta, as well as to quantify the severity of disease affecting the valves of the left side of the heart.
In this article, we describe the anatomy of the heart valves and the technical requisites of cardiac CT for the study of the valves. We go on to explore the usefulness of CT in the preoperative study of the coronary arteries and in the morphological and functional characterization of valve disease, with special emphasize on the valves of the left side of the heart.
La patología valvular cardiaca y la enfermedad coronaria son muy prevalentes en la población general y frecuentemente coinciden en el mismo paciente. La TC cardiaca permite descartar la enfermedad coronaria antes de la cirugía valvular de forma no invasiva y evitar potencialmente un 66-75% de las coronariografías invasivas. La misma prueba aporta abundante información anatómica y funcional complementaria a la ecocardiografía, que permite caracterizar la etiología de las valvulopatías, así como su repercusión en el corazón y la aorta y, en el caso de las válvulas izquierdas, cuantificar su gravedad.
En este trabajo se describe la anatomía valvular cardiaca y los requisitos técnicos de la TC cardiaca para el estudio valvular, para profundizar posteriormente en el valor de la coronariografía prequirúrgica y la caracterización morfo-funcional de la patología valvular mediante TC, haciendo hincapié en las válvulas izquierdas.
Valvular heart disease, both congenital and acquired, is a common heart condition with an estimated prevalence of 2.5% in the general population in the United States. Close clinical follow-up is required and very often patients with valvular heart disease end up requiring surgery.1 Valve surgery represents approximately 20% of all cardiac procedures, with an increase of 4–7% per year. Repeat surgery accounts for one third of procedures.
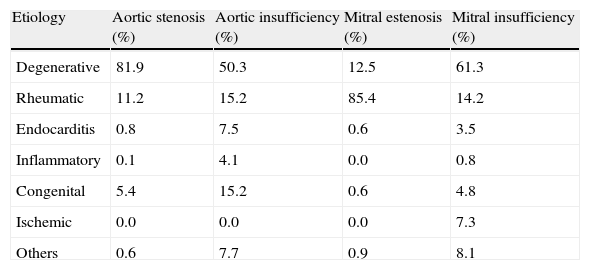
Degenerative disease is responsible for 63% of cases of valvular disease, with patients >65 years having higher incidence. Valvular disease reaches pandemic levels in developed countries due to long life expectancy.2 In these patients, degenerative aortic valve stenosis is the most common valvular disease. Sclerosis and calcification of aortic leaflets are accompanied by a progressive reduction in the aortic valve area, eventually leading to clinically significant stenosis. In our setting, the progressive increase of degenerative valvular diseases has been accompanied by a decrease in rheumatic heart disease incidence, which currently accounts for approximately 22% of all valvular diseases.2 Prevalence of rheumatic heart disease is higher among the elderly and immigrants from developing countries, where it is the most common cause of valvular disease. The pathologic spectrum also includes infective (endocarditis), congenital, inflammatory, radiation- and drug-induced valvular diseases2 (Table 1).
Etiology of left valvular diseases according to the Euro Heart Survey.
| Etiology | Aortic stenosis (%) | Aortic insufficiency (%) | Mitral estenosis (%) | Mitral insufficiency (%) |
| Degenerative | 81.9 | 50.3 | 12.5 | 61.3 |
| Rheumatic | 11.2 | 15.2 | 85.4 | 14.2 |
| Endocarditis | 0.8 | 7.5 | 0.6 | 3.5 |
| Inflammatory | 0.1 | 4.1 | 0.0 | 0.8 |
| Congenital | 5.4 | 15.2 | 0.6 | 4.8 |
| Ischemic | 0.0 | 0.0 | 0.0 | 7.3 |
| Others | 0.6 | 7.7 | 0.9 | 8.1 |
Source: Modified from Iung et al.2
Overall prevalence of significant coronary disease in Mediterranean patients with valvular disease is estimated at 10–20%.1 Undiagnosed coronary disease is associated with a worse intra- and postoperative outcome and it represents a missed opportunity for performing coronary revascularization during the same surgical procedure. Spanish and European guidelines recommend ruling out coronary disease before heart surgery in patients with severe valvular disease, especially in patients with a previous history of the disease, clinical suspicion of myocardial ischemia, evidence of left ventricular systolic dysfunction, and as a rule in male patients >40 years and postmenopausal women, and in the presence of more than one coexiting risk factor for cardiovascular disease.1
In patients with valvular disease, coronary disease has particularities relevant for the imaging techniques. First, symptoms of ischemic heart disease are not specific of coronary disease1; second, functional tests (myocardial perfusion SPECT, stress echocardiography) are not able to distinguish it from other conditions that may cause similar symptoms.1 Last, risk factors for degenerative aortic stenosis are similar to those of atherosclerosis and coronary disease (age, male gender, smoking, arterial hypertension, hypercholesterolemia), unlike what happens in other etiologies.2
Invasive coronary angiography (cardiac catheterization) is the technique of choice to rule out coronary disease before valve surgery. Cardiac computed tomography (CCT) has proven to accurately rule out significant coronary disease in selected populations.3,4 Replacemen of catheterization by non-invasive coronary angiography with CCT can reduce morbidity and mortality providing a similar diagnostic accuracy. In addition, given the low overall prevalence of the disease in this group of patients, only a few of them will need additional catheterization to confirm, grade or treat the coronary disease, which can eventually reduce costs.
Transthoracic and transesophageal echocardiographies are the techniques of choice for non-invasive assessment of heart valves, obviating the need for hemodynamic assessment by cardiac catheterization. If standard techniques prove insufficient, current guidelines suggest that CCT is an appropriate technique for the assessment of both native and prosthetic valve dysfunction.4,5
In this article, we review the anatomy of the heart valves and the technical requisites for their CT evaluation. In addition, we examine in depth the usefulness of preoperative coronary angiography and the morphological and functional characteristics of valvular disease at CCT, with special emphasis on left heart valves.
Anatomy of the cardiac valves at computed tomographyThe semilunar valves have three leaflets and lie between the ventricles and the major mediastinal arteries (the aortic valve between the left ventricle and the aorta, and the pulmonary valve between the right ventricle and the pulmonary trunk). The atrioventricular valves are, on the right side, the tricuspid valve with three leaflets and, on the left side, the mitral valve that contains two leaflets.
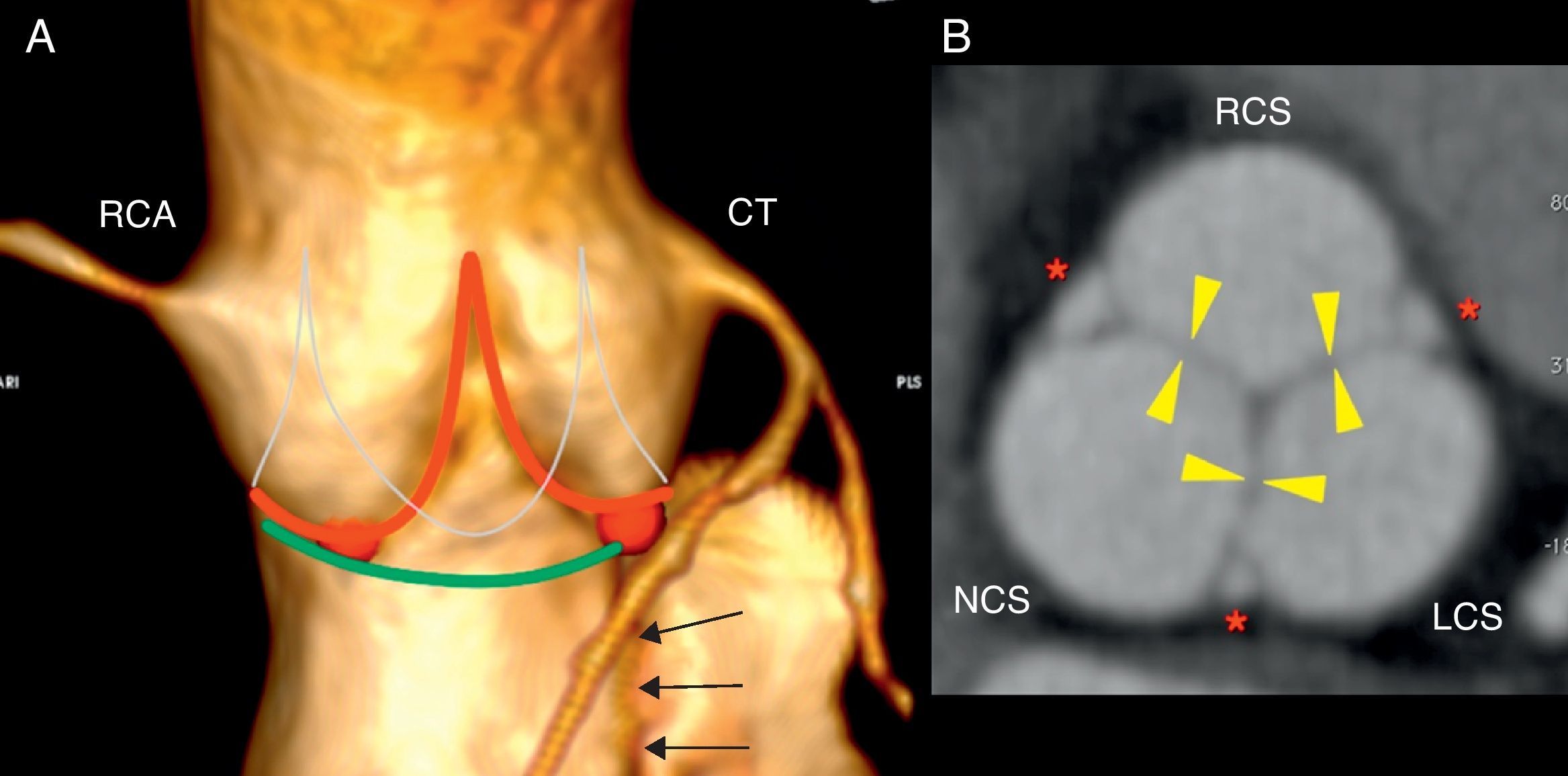
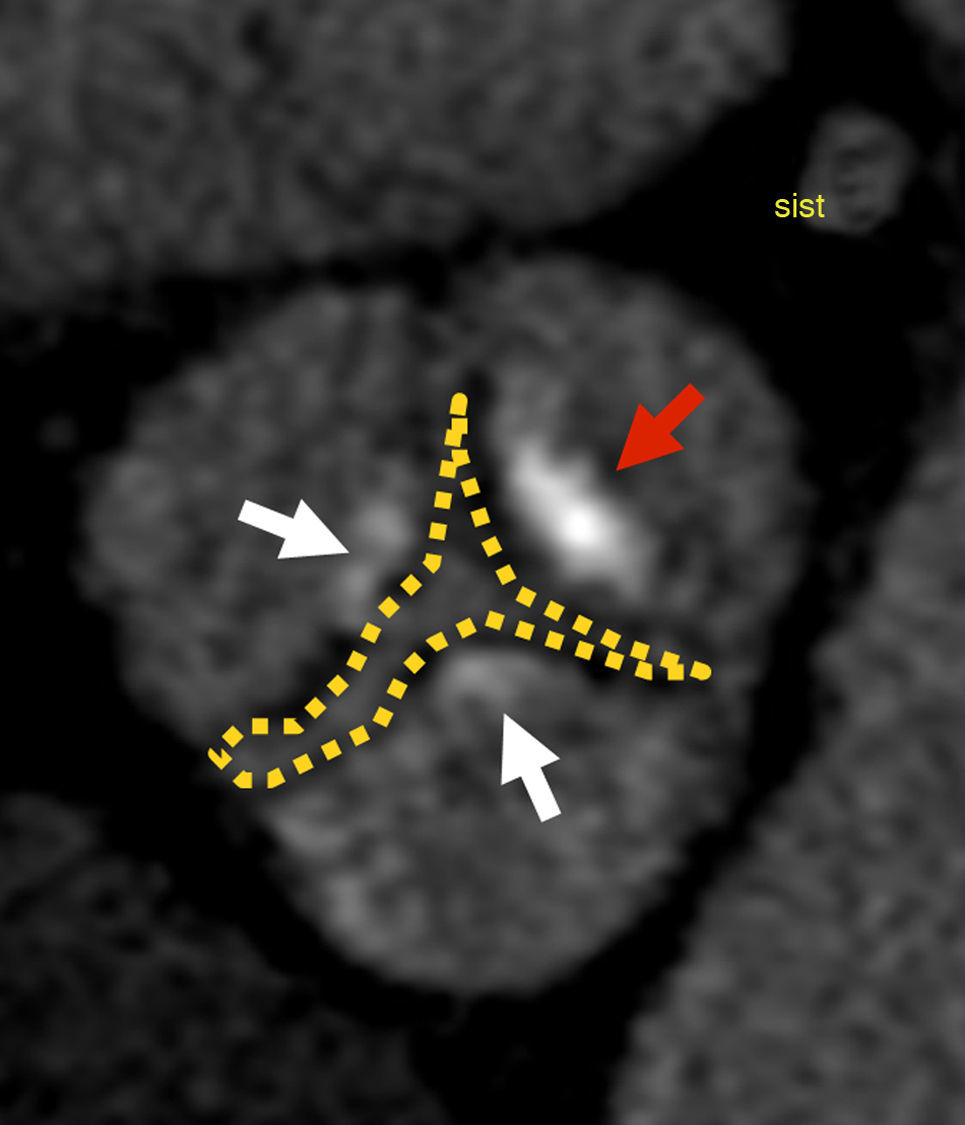
Aortic valveThe three leaflets of the aortic valve are thin and redundant and the line defined by their attachment points at the aortic wall is a 3-pronged crown (Fig. 1). The virtual ring formed by joining the basal attachment of the leaflets defines the aortic surgical annulus.5,6 The superior part of each leaflet attachment is parallel to that of the adjacent leaflet, forming three commissures (the crown's prongs), which joined by a virtual ring define the sinotubular junction. During ventricular diastole, the center of the ventricular surfaces of the leaflets comes together as the valve closes, whereas during ventricular systole the normal aortic valve area is 2.5–4cm2.5
Aortic valve anatomy. (A) Anterior projection volume reconstruction from cardiac computed tomography data shows the external appearance of the aortic root. The red and the thing white line represent the attachments of aortic valve leaflets and define the three-prong crown. The red points are the nadirs of the leaflets, which delimit the aortic surgical annulus (green). The right coronary sinus gives rise to the right coronary artery (RCA) and the left sinus give rise to the common trunk (CT). Continuity between the anterior mitral leaftlet and left coronary sinus (black arrows). (B) Planimetry of the aortic valve during diastole at sinus level shows valve closure with complete apposition of the leaflets (yellow arrowheads). Each bulge on the root correspond to one sinus: right (RCS), left (LCS), and non-coronary (NCS). The interleaflet triangles (red asteriks) are extensions of the left ventricle that extend cranially toward the commissures and delimited by the inferior aspect of the aortic leaflets laterally.
The aortic root comprises the structures located between the ring and the sinotubular junction. The spaces between the three bulges on the wall of the aortic root and their respective leaflets are the coronary sinuses.6 In the most common configuration, the right and left coronary sinuses give rise to the right coronary artery and the common trunk, respectively. The third is a non-coronary sinus, in a posterior location. The fibrous core of the aortic root extends into the anterior mitral leaflet.6
Imperfect bicuspid aortic valves are charaterized by congenital fusion, complete or incomplete, of one commissure known as “raphe”. The presence of two leaflets with rectilinear overlapping is pathognomonic of perfect bicuspid aortic valve. Both types of bicuspid valves are pathological and the opening creates a “fish-mouth” appearance.6
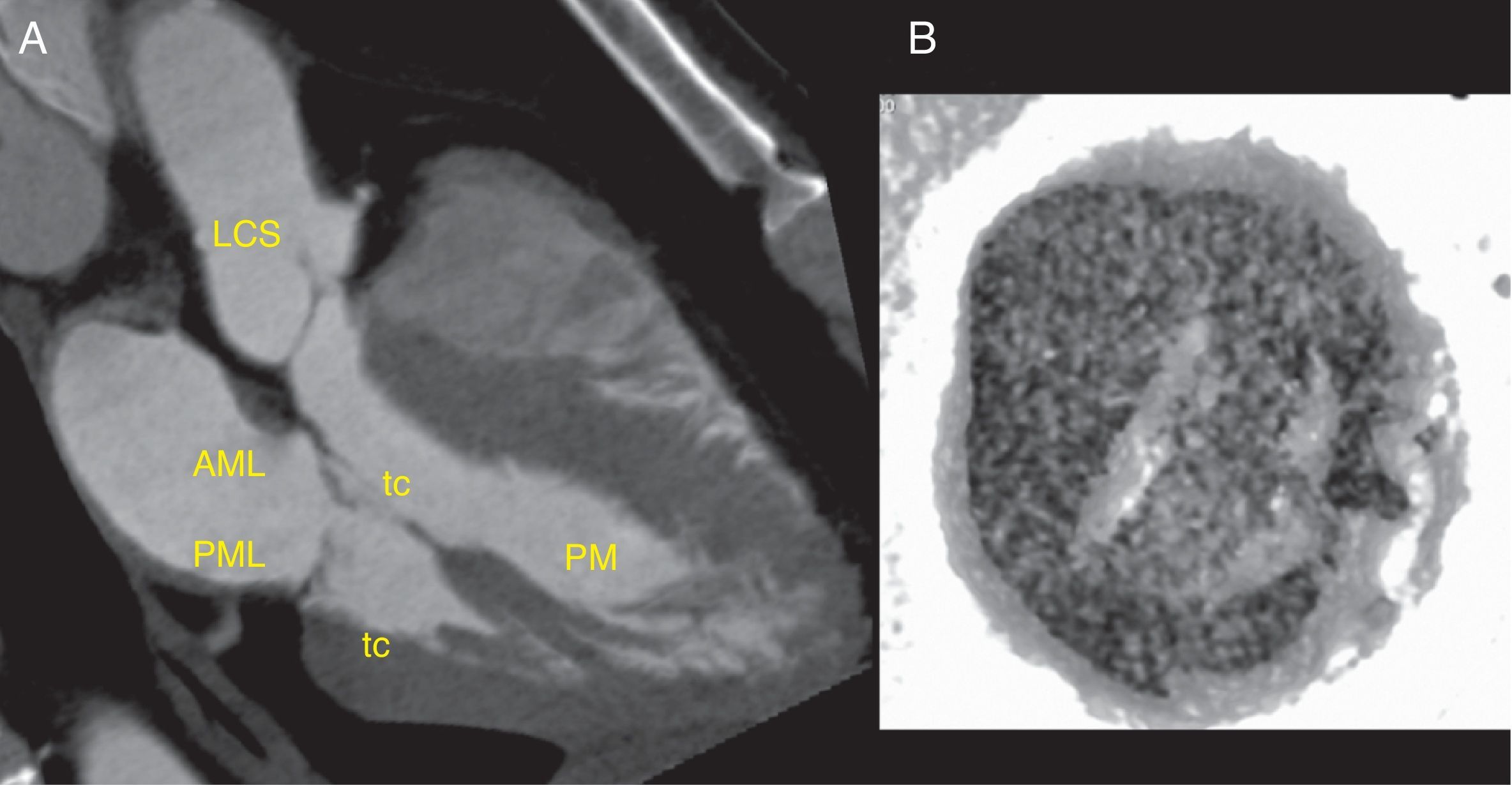
Mitral valveThe mitral valve has an anterior or aortic leaflet, with a semicircular shape, and a posterior or mural leaflet, with a crescent shape (Fig. 2). Each leaflet is divided into 3 scalops termed according to their proximity to the left atrial appendage.5,7 The leaflets are anchored on the periphery of the mitral annulus. Annular calcification is a common finding in the elderly, without implying leaflet dysfunction. During ventricular diastole, the mitral valve area is typically 4–6cm2.5
Mitral valve anatomy. (A) Thick slab minimum-intensity projection in a dedicated three-chamber view shows the mitral valve in mid-diastole. The anterior mitral leaflet (AML) is in continuity with the left coronary sinus (LCS). The posterior mitral leaflet (PML) is independient. The tendinous chords (tc) connect the mitral leaflets to the papillary muscles (PM), which attach to the trabeculated myocardium. (B) Inverted volume reconstruction shows an open mitral valve during mid-diastole viewed from the ventricle, note the typical “smile” appearance.
The chordae tendineae are attached to the ventricular surface of the free margins of both leaflets and are in continuity with the two papillary muscles, anterolateral and posteromedial, which attach to the non-compacted myocardium of the left ventricle (Fig. 2). This subvalvular apparatus supports the leaflets during ventricular contraction, preventing valve eversion and blood reflux into the atrium (parachute appearance).7
Pulmonary valveThe pulmonary valve is similar to the aortic valve, with thinner leaflets due to lower pressures, and it has no arteries arising from its sinuses. During ventricular systole, the area of the normal pulmonary valve is approximately 2cm2/m2 of body surface.5
Tricuspid valveThe subvalvular apparatus is similar to that of the mitral valve, but it has three leaflets and three groups of chordae tendineae and papillary muscles (septal, anterior and posterior). The leaflets are thin and the annulus is more apical than the mitral one.5
Technical considerations of cardiac computed tomography in valvular diseaseCoronary calcium scoreCoronary artery calcifications, more common in elderly patients with degenerative aortic stenosis, may hinder or prevent the analysis of non-invasive coronary angiography.8 In this group of patients, an initial coronary calcium scoring may help decide whether to perform a coronary angiography.
Medication used in coronary angiographyArtifacts caused by heart rate variability in mitral valve disease and those caused by pulsatile movement in oartic and mitral insufficiency can be partially reduced with beta blockers, which reduce and stabilize heart rate.9 These drugs may worsen the hemodynamic status in patients with valve disease with impaired ventricular function or critical valve stenosis and preserved function. Administration should be based on heart rate, blood pressure, ECG and echochardiographic findings. Drugs with short active life (esmolol, atenolol) and patient monitoring during the administration are recommended. These considerations are applicable to the use of vasodilatadors (nitroglycerin).
Contrast injectionThe standard biphasic protocol (contrast injection with saline flush) is sufficient for the study of the coronary arteries and heart valves.9 For the study of the right valves, however, a triphasic protocol is recommended where the second phase is a contrast agent-saline mixture that pushes the initial contrast bolus.5,9 This avoids artifacts caused by the mixture of blood with and without contrast coming from the cava veins, making the right ventricle to fill homogeneously with contrast agent. If the dual-syringe pump for simultaneos injection is not available, a similar effect can be achieved by decreasing the saline flow rate.9
Acquisition and reconstruction of raw dataTechniques aimed at reducing the radiation dose, such as prospective and retrospective acquisition with systolic reduction, are not indicated for patients with fast or irregular heart rate (atrial fibrillation), in whom ventricular systole may be needed for the study of the coronary arteries. Neither of these techniques is indicated for the assessment of valve morphology during ventricular systole nor complex valvular pathology (fistulas, endocarditis). The retrospectively acquired raw data are recontructed at 5–10% of the R–R interval (10–20 images per cardiac cycle) in order to detect the moments of best quality of the coronary artery tree and valvular structures.10,11 A cine loop of these phases allows qualitative assessment of the valvular function.
In general terms, the optimal phase of the cardiac cycle for simultaneous assessment of coronary arteries and left valves in most patients (Table 2) depends on the valve and heart rate:
- 1)
The area of the aortic opening is widest during mid-ventricular diastole, 50–100ms after the R wave peak,12 which corresponds to 20–30% of the R–R interval depending on the heart rate and patient. In general, this point coincides with the decrease in the T wave in the electrocardiogram. Earlier reconstructions (0–50ms) keep this maximum area but with severe motion artifacts.12
- 2)
The optimal phase for evaluation of the closed mitral valve during systole is at 5% of the R–R interval.13
- 3)
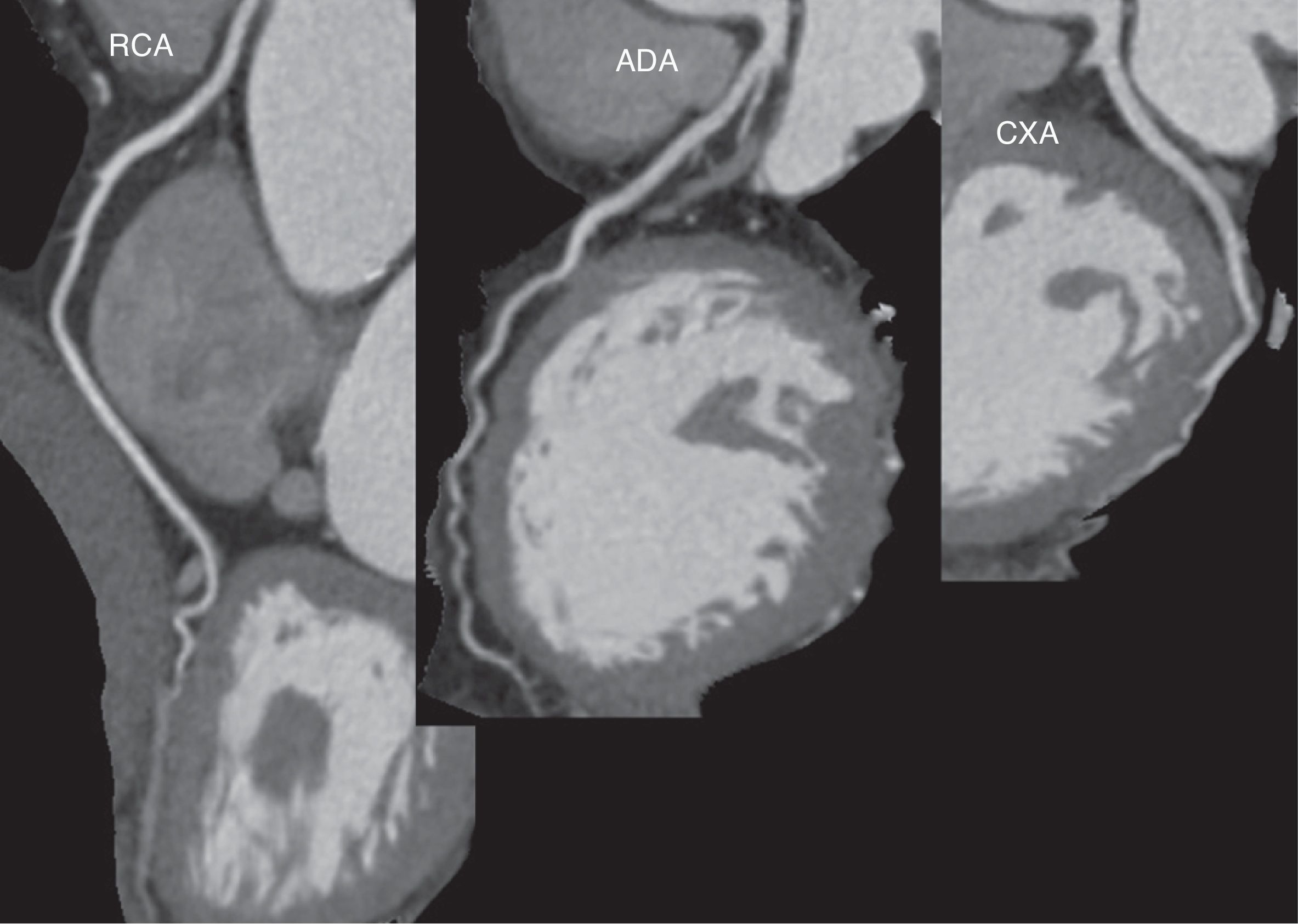
For low heart rate (<65bpm), the optimal phase to visualize the coronary arteries is ventricular mid-diastole, at 60–75% of the R–R interval depending on the heart rate and patient (Fig. 3).9 This point can be used to assess the closed aortic valve and the mitral area.
- 4)
For fast heart rate, the optimal phase to visualize the coronary arteries is variable, from ventricular diastole to ventricular mid-systole, which usually also is the best phase to visualize the aortic valve.
Indicative table of the optimal phases of the cardiac cycle to visualize the coronary arteries and left valves, expressed as relative percentages of the R–R interval.
| Coronary artery | 20–30% if HR>65bpm | 60–75% if HR<65bpm |
| Aortic valve | 20–30% | 60–75% if HR<65bpm |
| Mitral valve | 5% | 60–75% if HR<65bpm |
The optimal points vary depending on the patient, heart rate and equipment. For heart rate greater than 65bpm with poor quality of the diastolic series, acquisition of a series at end-systole is recommended for assessment of the coronary arteries.
HR: heart rate; bpm: beats per minute.
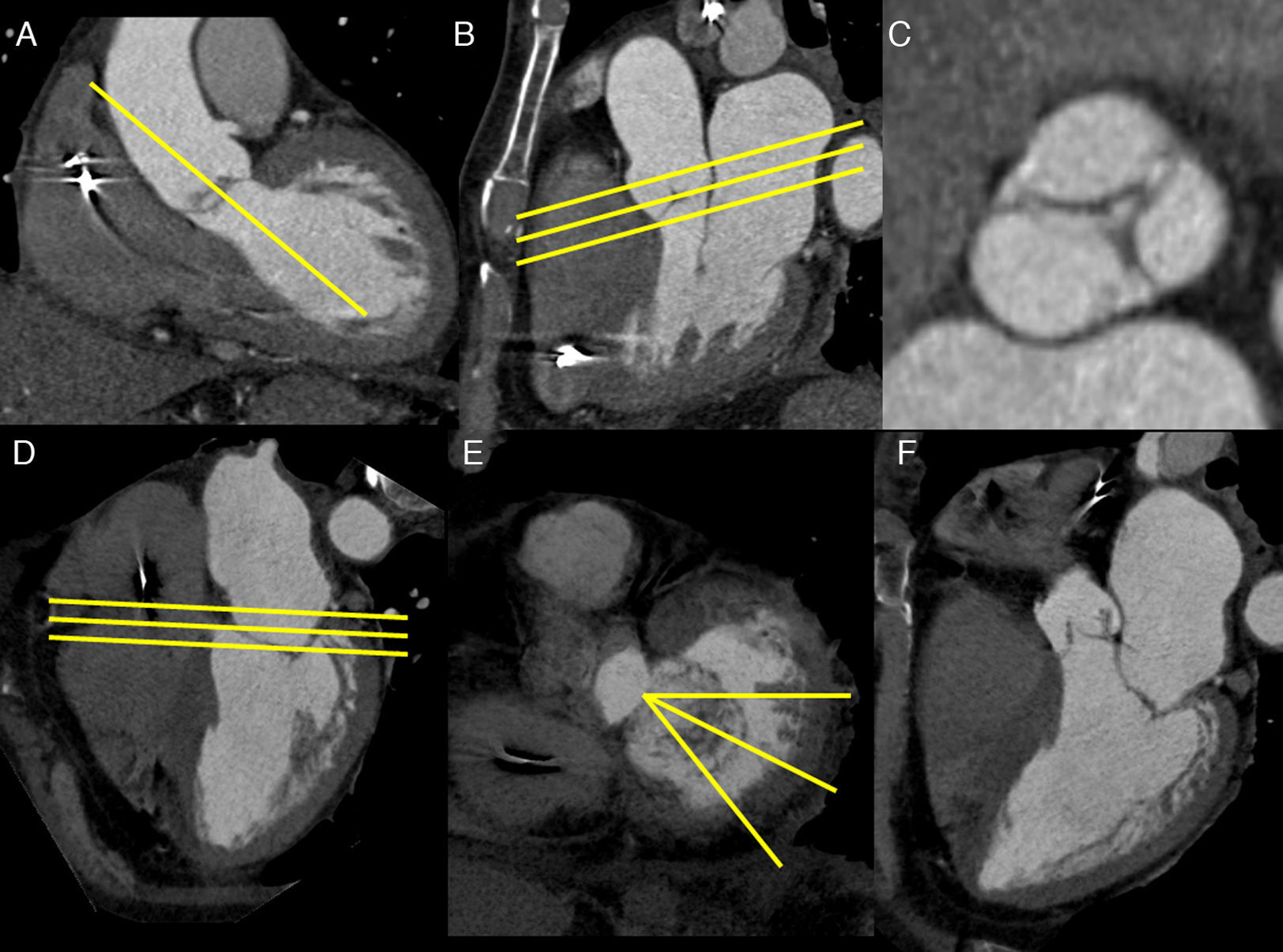
Parallel images, orthogonal to the left ventricular outflow tract are recommended for the assessment of the aortic valve and its area (aortic valve planimetry) (Fig. 4).12
Image acquisition using aortic planimetry (A–C), mitral planimetry (D–E), and mitral radial images (D–F). An oblique parasagittal plane is created on a coronal image (A) parallel to the left ventricular outflow tract (yellow line). The resultant plane (B), similar to the three-chamber view, allows the creation of a series of parallel planes, orthogonal to the left ventricular outflow tract (yellow lines), which corresponds to the aortic planimetry (C). On the four-chamber view (D), planes are drawn parallel to the mitral annulus and orifice (yellow line), and mitral planimetry is obtained (E). On the planimetry, a series of planes are drawn from the center of the anterior mitral leaflet that slice perpendicularly the line of apposition of both leaflets (yellow lines), which allow accurate evaluation of the mitral valve closure (F).
For assessment of the mitral valve and its area, images parallel to the mitral valve opening, including part of the left atrium and ventricle, are recommended. They can be obtained from a long axis image of the left ventricle (mitral planimetry) (Fig. 4).10,13 For evaluation of mitral insufficiency, the three-chamber view is indicated with addition of a series of radial images obtained from the mitral planimetry, starting from the center of the anterior mitral leaflet and slicing perpendicularly through the line of apposition of both leaflets (Fig. 4).13 For assessment of mitral valve prolapse, two- and three-chamber views are recommended.14
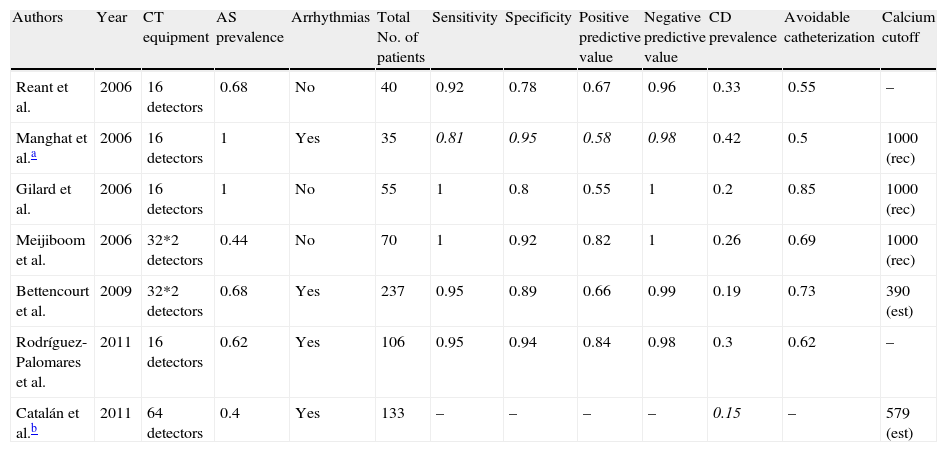
Preoperative coronary angiography with cardiac computed tomographyA number of studies published up to 2006 suggested that non-invasive coronary angiography was able to rule out coronary disease before valve surgery with a sensitivity of 92–100%, a specificity of 78–92%, positive predictive value of 55–82%, and negative predictive value of 96–100% (Table 3).15–18 Nonetheless, these trials were based on small series of patients whose only or predominant condition was aortic stenosis. Patients with arrhythmias, fast heart rate and high calcium levels (usually values >1000Agatston units [AU]) were generally excluded. More recently, one Portuguese trial19 and one Spanish trial20 have confirmed the excellent yield of the technique in large series of patients (237 and 106, respectively) with different types of valve diseases. In the Spanish trial, 38% of patients had a valve disease different from degenerative oartic stenosis (Table 3).20 In order to obtain homogeneous results that could be used as a reference for the technique, a CT scanner with only 16-detectors was used throughout the study. Despite this, catheterization could have been avoided in 64% of patients (Fig. 3).20 At the same time, another Spanish group assessed 133 patients before non-coronary cardiac surgery, including patients with non-valvular conditions.21 The absence of significant coronary artery disease at CCT was confirmed by clinical follow-up of patients, and not by invasive coronary angiography, and none of the patients showed adverse events that could be attributed to underdiagnosed coronary artery disease after an average of 1000 days.21 Both Spanish trials included patients with arrhythmias controlled with medication20,21 while maintaining a positive predictive value of 99%20 without reducing the quality of the test.21
Comparison of results reported by the main studies on non-invasive coronary angiography with CCT in patients with valvular disease.
| Authors | Year | CT equipment | AS prevalence | Arrhythmias | Total No. of patients | Sensitivity | Specificity | Positive predictive value | Negative predictive value | CD prevalence | Avoidable catheterization | Calcium cutoff |
| Reant et al. | 2006 | 16 detectors | 0.68 | No | 40 | 0.92 | 0.78 | 0.67 | 0.96 | 0.33 | 0.55 | – |
| Manghat et al.a | 2006 | 16 detectors | 1 | Yes | 35 | 0.81 | 0.95 | 0.58 | 0.98 | 0.42 | 0.5 | 1000 (rec) |
| Gilard et al. | 2006 | 16 detectors | 1 | No | 55 | 1 | 0.8 | 0.55 | 1 | 0.2 | 0.85 | 1000 (rec) |
| Meijiboom et al. | 2006 | 32*2 detectors | 0.44 | No | 70 | 1 | 0.92 | 0.82 | 1 | 0.26 | 0.69 | 1000 (rec) |
| Bettencourt et al. | 2009 | 32*2 detectors | 0.68 | Yes | 237 | 0.95 | 0.89 | 0.66 | 0.99 | 0.19 | 0.73 | 390 (est) |
| Rodríguez-Palomares et al. | 2011 | 16 detectors | 0.62 | Yes | 106 | 0.95 | 0.94 | 0.84 | 0.98 | 0.3 | 0.62 | – |
| Catalán et al.b | 2011 | 64 detectors | 0.4 | Yes | 133 | – | – | – | – | 0.15 | – | 579 (est) |
Quantitative values are expressed as percentages, with the exception of the number of patients, in nominal values, and the calcium cutoff recommended or used, expressed in Agatston units.
AS: aortic stenosis; CD: coronary disease; (rec): cutoff value recommended and used in the inclusion criteria of the group; (est): recommended value estimated from the results of the study.
All the studies cited considered the presence of severe coronary artery calcification to be the major obstacle to interpreting a non-invasive coronary angiography. A number of studies15–18 have suggested that a calcium scoring >1000AU is more likely to result in an inconclusive or non-diagnostic coronary angiography, thus forcing catheterization. In view of this situation, these studies recommended discontinuing the use of non-invasive coronary angiography. More recent studies suggest lower score cutoffs of 579AU21 and 390AU.19 This latter value is partially explained by the high prevalence of aortic estenosis and coronary artery disease of the series studied.
Current scientific evidence indicates that non-invasive coronary angiography is an appropriate test to assess the coronary tree before non-coronary surgery in patients with intermediate risk of coronary artery disease, but its utility remains uncertain in low-risk patients.4 Similarly, there is currently no consensus regarding the value of calcium scores >400AU to avoid non-invasive coronary angiography.4 We recommend assessment of calcium score images because peripheral calcifications in proximal segments do not prevent evaluation of the coronary angiography in most cases.18
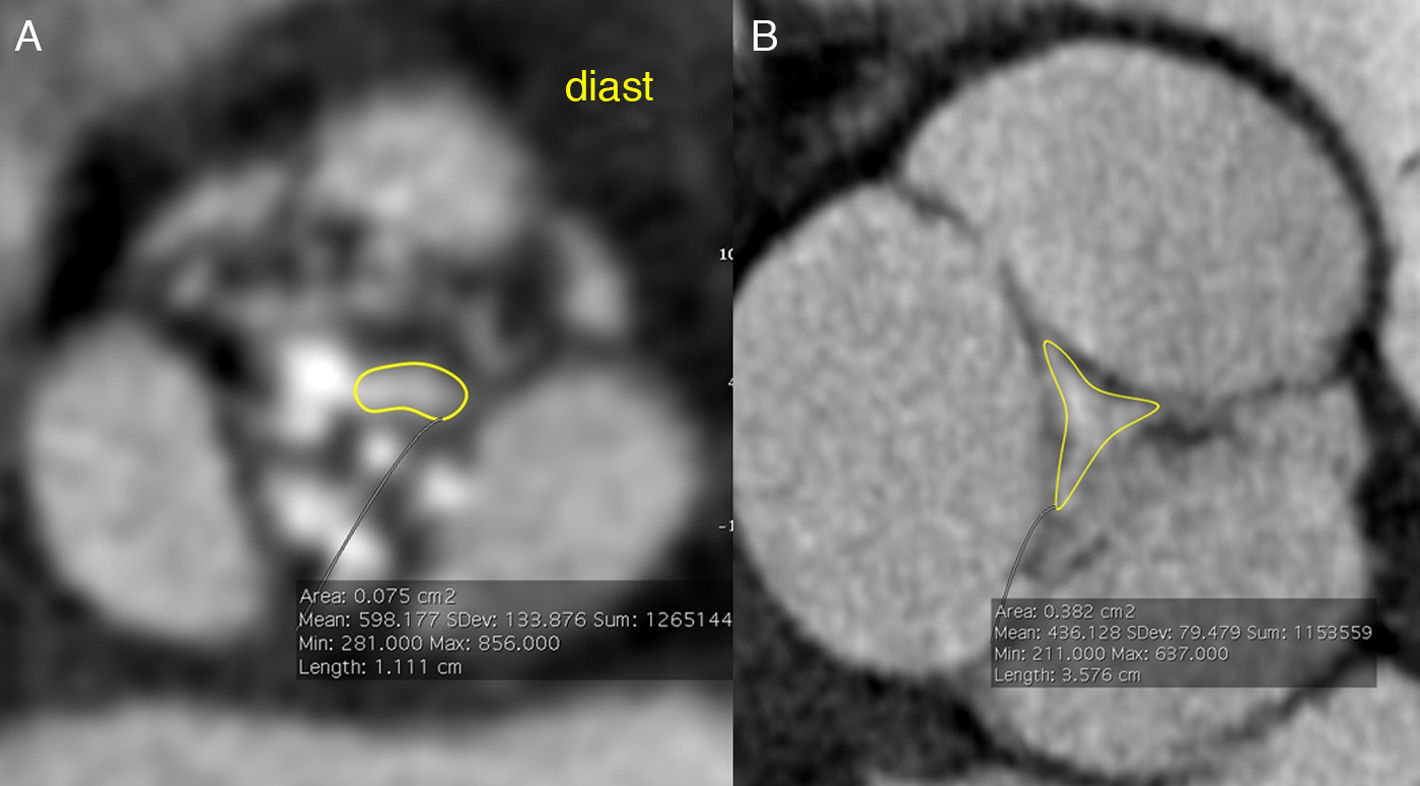
Assessment of aortic valve diseaseAn aortic valve area <1cm2 is considered diagnostic for severe aortic stenosis.1,5 The area can be measured by transesophageal echocardiography as the geometric valve area or by transthoracic echocardiography as effective valve area, using the continuity equation. The geometric area is greater than the effective area due to convergence of the flow stream in the center of the orifice. However, the continuity equation has limitations because it integrates the velocity–time curves of the blood flow at the left ventricular outflow tract and at the aortic valve during ventricular systole, which vary in relation to the hemodynamic status of the patient.1 In addition, the measurements in the outflow tract are calculated assuming a circular shape, rather than elliptical.22,23Leaflet calcification in aortic stenosis may hinder the proper assessment of the orifice with transesophageal echocardiography, but it does not affect the quantitative analysis of aortic planimetry with CCT (Fig. 5)24 or cardiac magnetic resonance (CMR). In fact, the aortic valve calcium score is correlated with aortic valve area and severity of aortic stenosis, having a proven prognostic value for this condition.24–27
Image of aortic planimetry during systole at the level of the coronary sinuses shows critical degenarative aortic stenosis, with a maximum valve area <1cm2. The minimum orifice delimited by the yellow dotted line is <1cm2. Note thickening and calcification of leaflets (white arrows), more marked in the left leaftlet (red arrow).
CCT allows 3D evaluation of the aortic valve providing better spatial resolution than CMR (voxels of 0.4mm3 and 1.2mm3, respectively).5 However, 64-detector CT scanners do not provide optimal temporal resolution, although dual-source scanners provide values close to those of CMR (150–200ms, 75ms and 25–50ms, respectively).5 Unlike CMR, CCT can be used in patients with implanted pacemakers and defibrillators, but it does not provide information about valve flow.
The geometric area measured by CCT has demonstrated excellent correlation with the effective area measured by transthoracic echocardiography, with a slight overestimation partially attributed to the methodological differences between the two techniques.24 In one study, valve planimetry and measurement of geometric valve area by CCT were feasible in all patients, but when using transesophageal echocardiography they were feasible in only 78% and 58% of patients, respectively.28 In the cases that could be compared, for the detection of moderate to severe aortic estenosis with CCT, the sensitivity, specificity, positive predictive value and negative predictive value were 98%, 92%, 98% and 92%, repectively, with excellent interobserver agreement and between techniques.28
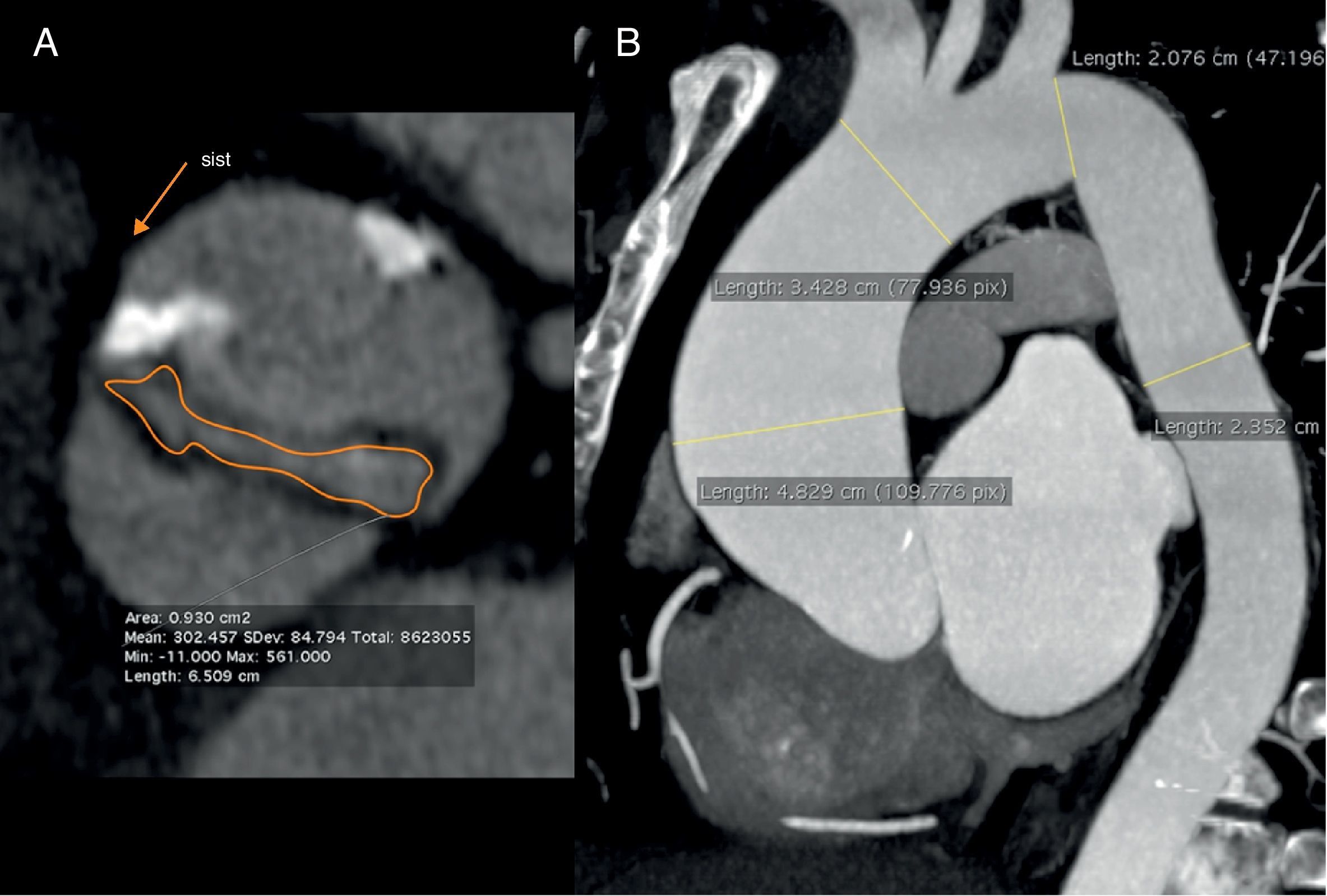
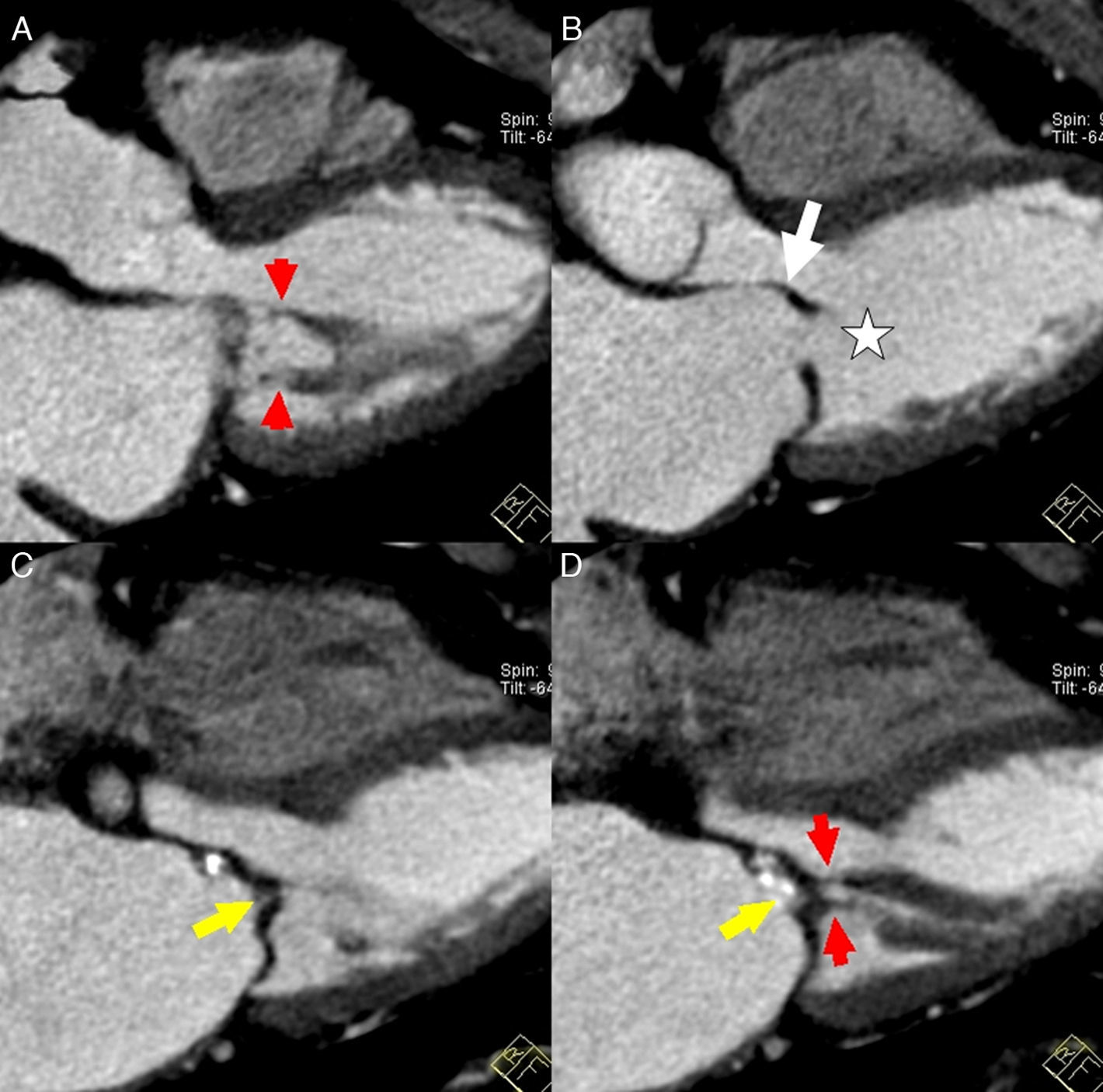
In classic aortic insufficiency, the aortic leaflets fail to close completely during ventricular diastole and blood flows back through the orifice. This orifice can be visualized by transesophageal or transthoracic echocardiography, CCT and CMR (Fig. 6). Aortic insufficiency may occur alone or associated with aortic stenosis (Table 1).2 In a series of patients referred for non-invasive echocardiography who also underwent transthoracic echocardiography, the sensitivity, specificity, positive and negative predictive value of CCT for the detection and quantification of moderate and severe aortic insufficiency was 95%, 100%, 100% and 98%, respectively, with good correlation between area and retrograde flow at CCT and transthoracic echocardiography.29,30 However, a recent study using dual-source CT reported that classification of aortic insufficiency based on the orifice area was not accurate to differentiate mild and moderate aortic insufficiency31. This difficult evaluation of the mild forms of aortic insufficiency could be explainced by the presence of partial volume effect of calcium around a small regurgitation orifice and by the cases of bicuspid valves.30,31 Retrospective acquisition allows estimation of the aortic regurgitation volume in the absence of other serious valvular diseases by means of quantitative analysis of the volumes and function of both ventricles,32 in a similar fashion to MR imaging.
Images of aortic planimetry during diastole show two different types of aortic insufficiency. (A) A patient with a double degenerative valve lesion. Note thickening and calcification of the leaflets that result in retraction and the presence of a regurgitation orifice. (B) The dilation of the aortic root prevents proper aortic leaflet closure. Note the large size of the coronary sinuses with thin leaflets. Manual delimitation for the estimation of the valve area is shown in both cases.
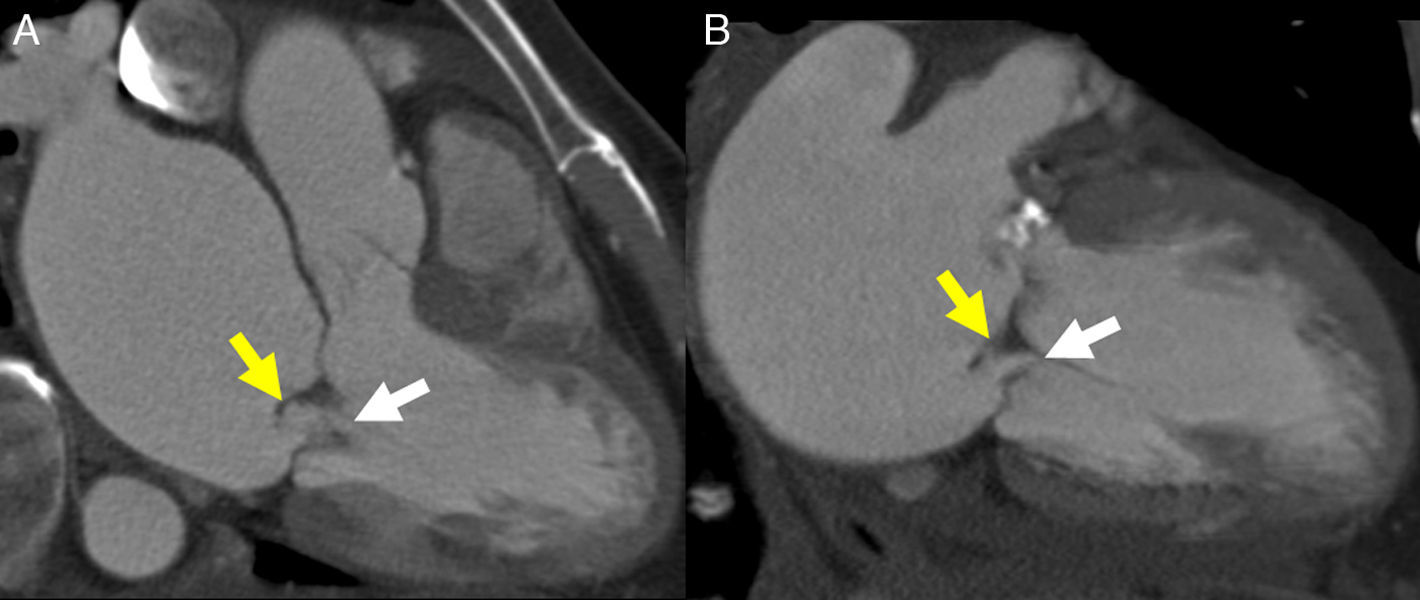
CCT is highly accurate for the identification of naturally perfect bicuspid aortic valves during ventricular diastole,33 but for imperfect valves, the “fish-mouth” appearance has to be visualized during ventricular systole (Fig. 7). In these cases, the thoracic aorta should be included in the study to rule out dilation or coarctation of the ascending aorta, usually associated to bicuspid valve (Fig. 7).
Image of aortic planimetry during systole at the level of the coronary sinuses (A) shows critical degenerative stenosis of a naturally perfect (with two sinuses) aortic valve, with a maximum valve area <1cm2. The maximum intensity projection (MIP) in the oblique parasagittal plane (B) during diastole allows accurate evaluation of the aneurysm in the ascending aorta associated with the bicuspid valve.
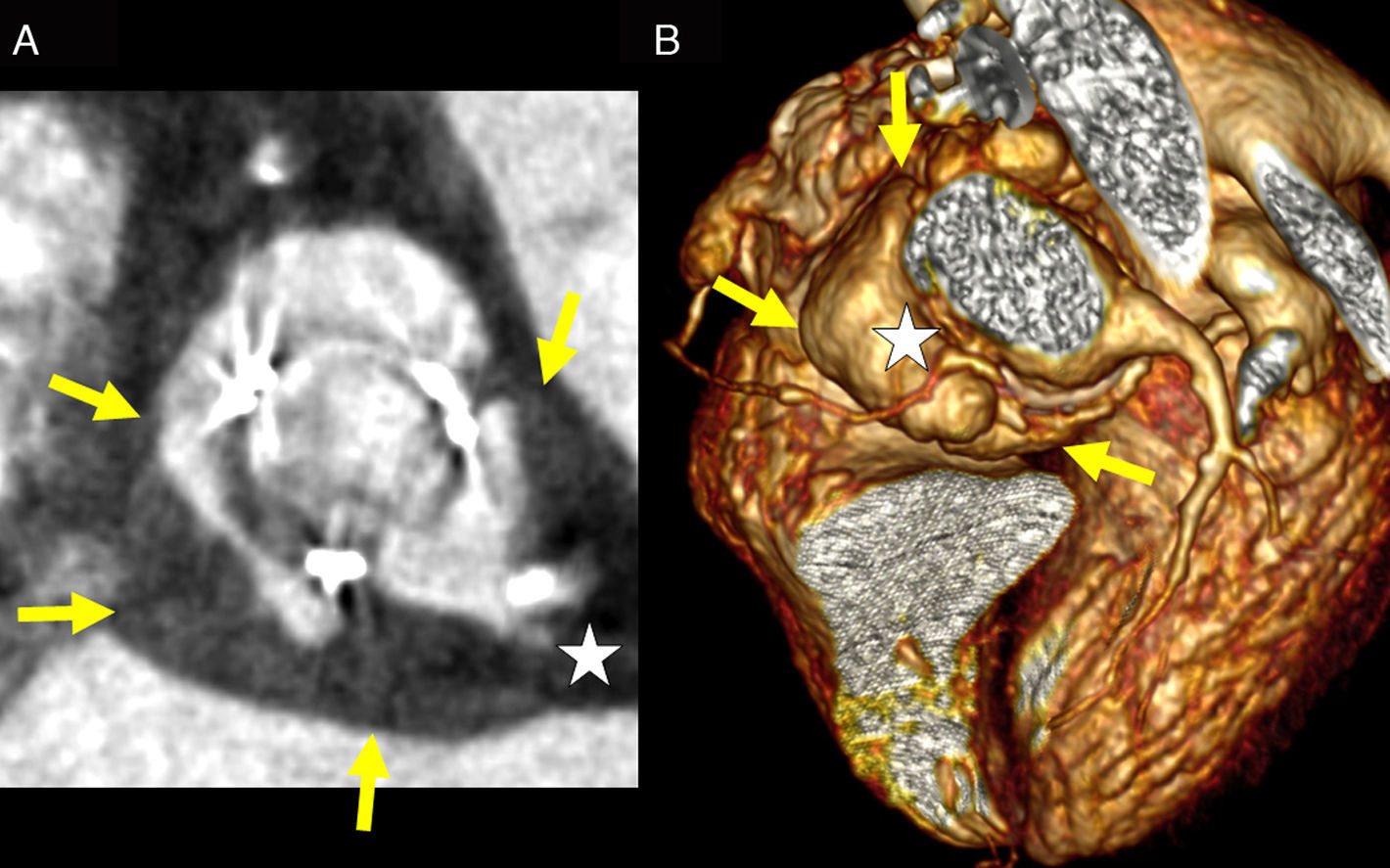
CCT complements the findings of transesophageal echocardiography in endocarditis with paravalvular involvement, as it allows an accurate 3D evaluation of abscessses, pseudoaneurysms and fistulas (Fig. 8).34 In addition, non-invasive coronary angiography is particularly important in patients with endocarditis because catheterization poses a high risk of embolization of vegetations or perforation of abscesses.34
Two cases of aortic valve endocarditis. Image of aortic planimetry at the level of the coronary sinuses showing an abscess of the aortic root secondary to endocarditis on a biological valve prosthesis (A). The yellow arrows delimit the outer margin of the abscess that corresponds to the hypondense wall thickening. Anterior projection of a volume reconstruction showing a pseudoaneurysm of the aortic root secondary to endocarditis on a biological prosthesis (B). The yellow arrows delimit the outer margin of the pseudoaneurysm. Note the involvement of the common trunk in (A) and the cranial displacement of the proximal right coronary artery in (B) (white stars).
CCT provides additional information regarding the origin and functional repercussion of aortic valve disease. Diffuse thickening of the left ventricular myocardium and dilation of the ascending aorta at CCT are indirect findings suggestive of aortic stenosis, while left ventricular dilation associated with these findings is more characteristic of aortic insufficiency.5 Proper closure of the aortic valve should be routinely assessed on CCT images regardless of the initial suspected diagnosis.
Calcifications of aortic valve are incidentally detected in 13–30% of patients referred for non-invasive coronary angiography.27 These calcifications must be reported and in case of retrospective acquisition, the aortic valve area must be measured. An echocardiography is recommended in patients with areas <2–3cm2.5
Assessment of mitral valve diseaseRheumatic disease is the most common cause of mitral stenosis in our setting. Mitral stenosis may occur alone or in association with mitral insufficiency or other valve diseases.2 CCT can detect the morphological changes typical of mitral stenosis such as thickening and calcification of leaflets with margin fusion and simultaneous subvalvular involvement with retraction of chordae tendineae and papillary muscles (Fig. 9). This results in a “fish-mouth” appearance on short-axis images, while the open anterior leaflet shows a “hockey-stick” appearance on three-chamber images (Fig. 9).5,35
Images parallel to the three-chamber plane during diastole show one case of severe mitral stenosis of rheumatic origin (A–D). Note shortening and thickening of papillary chords (red arrows) and retraction of leaflets that appear diffusely thickened and calcified. Visualization of a small valve orifice in this plane (white star) and fusion of commissures (yellow arrows) suggest stenosis. Note the associated atrial dilation and the “hockey-stick” opening (white arrow) of the anterior mitral leaflet, typical of rheumatic disease.
The mitral valve area determined by mitral planimetry with CCT is systematically larger than that determined by transesophageal echocardiography and catheterization, but it does not prevent discrimination between grades of MS.11 In a series of 28 patients with MS, values of mitral valve area <1.7cm2 allowed detection of moderate to severe MS with a sensitivity, specificity, positive predictive value, and negative predictive value of 73%, 88%, 80% and 83%, respectively.11
Chronic mitral insufficiency is more common and has a multiple etiology. The acute form is rare and is caused by ischemic rupture of the chords. In a series of 44 patients with and without mitral insufficiency, CCT correctly detected all the cases of insufficiency diagnosed with transthoracic echocardiography.10 Similarly, there was good correlation between the regurgitant orifice area measured by CCT, transesophageal echocardiography and ventriculography.10 Mitral valve prolapse, defined as a displacement of mitral valve leaflets toward the left atrium ≥2mm below the mitral annulus, is one of the causes of mitral insufficiency that can be identified with CCT (Fig. 10).5,14 The combined use of two- and three-chamber views at CCT yields an accuracy of 95% for the diagnosis of mitral valve prolapse with respect to transesophageal echocardiography.14 In the absence of other valve diseases, CCT allows quantitative assessment of the severity of mitral insufficiency by calculating the regurgitant volume and regurgitant fraction.35
Mitral insufficiency in three-chamber (A) and two-chamber (B) views in diastole show a closure defect of the mitral valve (white arrow), with partial prolapse of the anterior leaflet toward the right atrium (yellow arrow). The linear hypondense image corresponds to a fragment of ruptured tendinous chord dragged by retrograde flow. Note dilation of left atrium and right chambers secondary to increased retrograde pressure.
Echocardiography and CMR imaging are usually sufficient for the evaluation of mitral pathology, consigning CCT to a secondary role except for the evaluation of calcified lesions, such as the caseous calcification of the mitral annulus.36 Left atrial dilation with wall calcification or thrombus in the atrial appendage, left ventricular dilation, signs of congestion in the lung parenchyma and right ventricular hypertrophy are indirect sign that, when found on a CT study, will force the evaluation of the mitral valve.5
Assessment of right valve diseasesCCT is not appropriate for assessment of right valves because of their characteristically thin leaflets and because homogeneous opacification of the right cavities is difficult to achieve. Nonetheless, a large number of findings of right valve disease can be identified using CCT.5
Postoperative evaluation of heart valvesArtifacts induced by prosthetic valves and surgical material do not prevent the acquisition of a CT with diagnostic quality, which is a good complement to echocardiography.4,37 In case of suspected bileaflet mechanical valve dysfunction, CCT allows the detection and quantification of leaflet restriction,38–40 as well as thrombus and pannus formation.41,42 In valve bioprosthesis, CCT allows the detection of leaftlet degeneration with calcification.41 In the absence of these morphological abnormalities, the dysfunction may be attributed to patient-prosthesis mismatch.41
Finally, CCT provides the surgeon with relevant information before reoperative cardiac surgery, including the state of previous coronary shunts and the degree of mediastinal fibrosis, and it can help plan the clamping of ascending aorta.43–45
ConclusionCCT allows evaluation of the coronary tree before heart valve surgery, and qualitative and quantitative assessment of aortic and mitral valve pathology, which is useful in patients with poor image quality at echocardiography. In addition, CCT is an excellent technique for the evaluation of patients after valve surgery or before reoperative cardiac procedures.
The heart valves should be routinely assessed on CCT images, regardless of the initial suspected diagnosis, and any pathologic findings should be included in the radiological report.
Ethical responsibilitiesProtection of humans and animal subjects. The authors declare that no experiments involving animals or humans have been carried out for this study.
Confidentiality of dataThe authors declare that no data from patients are shown in this article.
Right to privacy and informed consentThe authors declare that no data from patients are shown in this article.
Authorship- 1.
Responsible for the integrity of the study: HC.
- 2.
Conception of the study: HC and VP.
- 3.
Design of the study: HC and VP.
- 4.
Acquisition of data: HC and AR.
- 5.
Analysis and interpretation of data: HC, AR and JR.
- 6.
Statistical analysis: N/A.
- 7.
Bibliographic search: HC.
- 8.
Writing of the paper: HC.
- 9.
Critical review with intellectually relevant contributions: HC, AR, VP and JR.
- 10.
Approval of the final version: HC, AR, VP and JR.
The authors declare not having any conflicts of interest.
Please cite this article: Cuéllar H, et al. La tomografía computarizada cardiaca en la afección valvular. Radiología. 2012. http://dx.doi.org/10.1016/j.rx.2012.05.006.