Cavernous malformations (cavernomas) are hamartomatous lesions formed by sinusoidal vascular spaces, with no cerebral parenchyma between them. Seizures are the most usual clinical presentation. They are dynamic lesions, producing changes throughout their evolution. The majority are located in the supratentorial region, but up to 20% of cases they are found in the posterior fossa. In computed tomography (CT) and in magnetic resonance (MR) their typical presentation is as a well defined round or oval lesion, with or without a minimal mass effect or oedema, with little or no contrast enhancement. Their appearance in magnetic resonance imaging (MRI) will depend on the stage of the haemorrhage, a T2 echo gradient being the most sensitive sequence. Angiography do not usually detect cavernomas. However, it may demonstrate a venous developmental anomaly. Cavernomas may present with atypical characteristics, as regards their size, appearance, location and number.
Las malformaciones cavernosas (cavernomas) son lesiones hamartomatosas formadas por espacios vasculares sinusoidales sin parénquima cerebral entre ellos. Las crisis son su presentación clínica más habitual. Son lesiones dinámicas en las cuales se producen cambios a lo largo del tiempo. La mayoría son de localización supratentorial, pero hasta un 20% de los casos se presentan en la fosa posterior. Tanto en la tomografía computarizada como en la resonancia magnética (RM) su presentación típica es como una lesión redondeada u ovoidea, bien definida, sin o con un mínimo efecto masa o edema, y con poco o ningún realce. Su apariencia en la RM dependerá del estadio de la hemorragia, siendo la secuencia más sensible el eco de gradiente T2. El cavernoma no es visible en la arteriografía. No obstante, ésta puede demostrar una anomalía del desarrollo venoso asociada. Los cavernomas pueden presentar características atípicas en cuanto a su tamaño, apariencia, localización y número.
Cavernomas are non-encapsulated, well-defined, vascular hamartomatous lesions formed by sinusoidal vascular spaces with no cerebral parenchyma between them. They are one of the four major types of vascular malformations of the central nervous system, together with developmental venous anomalies, arteriovenous malformations, and capillary telangiectasias.1 The literature offers a wide range of synonyms of cavernoma, such as cavernous angioma, cavernous malformations, and angiographically occult vascular malformation. Although cavernomas were first classified as a rare condition, they are now becoming a more and more common finding in neuroimaging studies, particularly after the advent of magnetic resonance imaging (MRI).
Since they are normally asymptomatic, their actual incidence is not well known. According to series of autopsies, carvernomas occur in about 0.4% of subjects, accounting for 5–13% of all cerebral vascular malformations, and are only second to venous developmental anomalies in incidence.2 Cavernomas occur equally in males and females and usually appear between the second and fifth decade of life.3
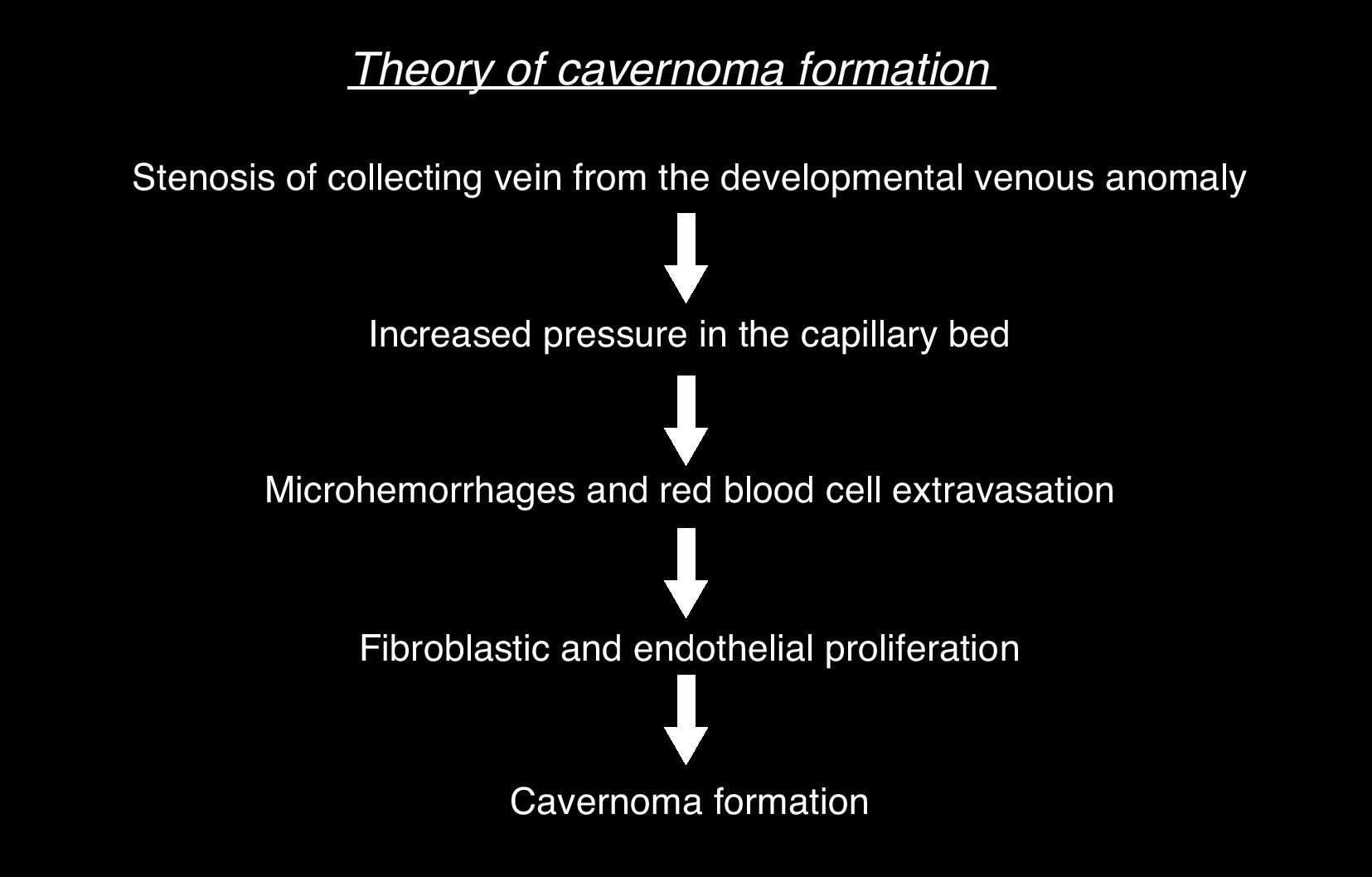
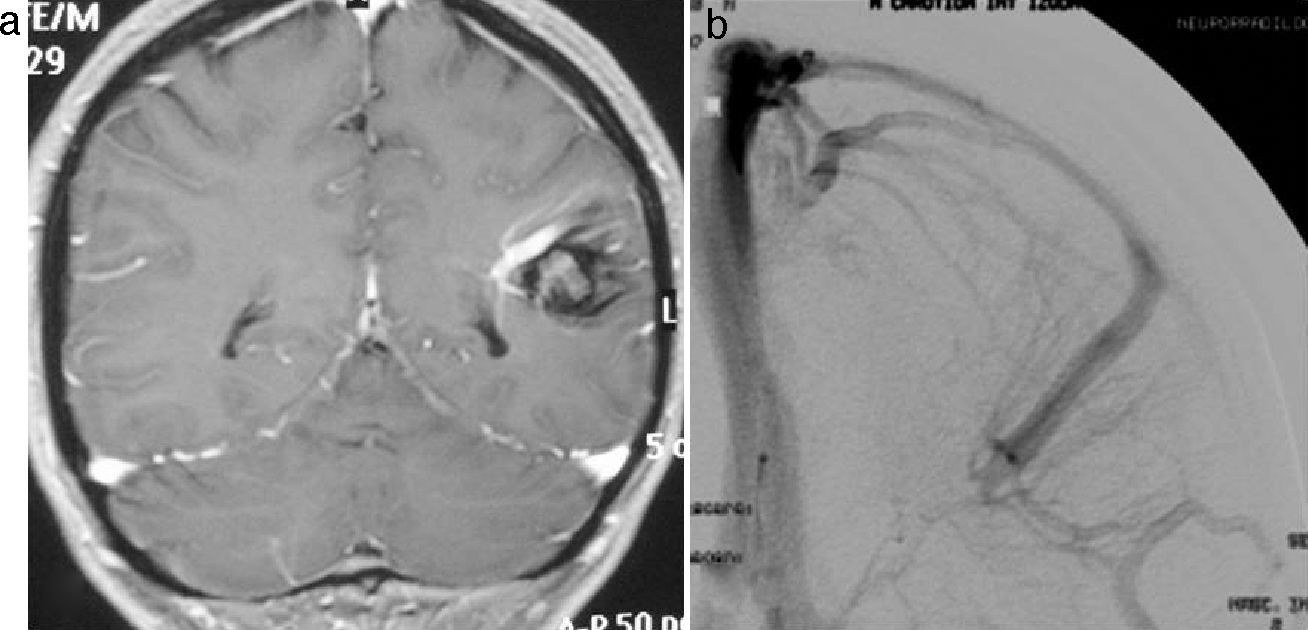
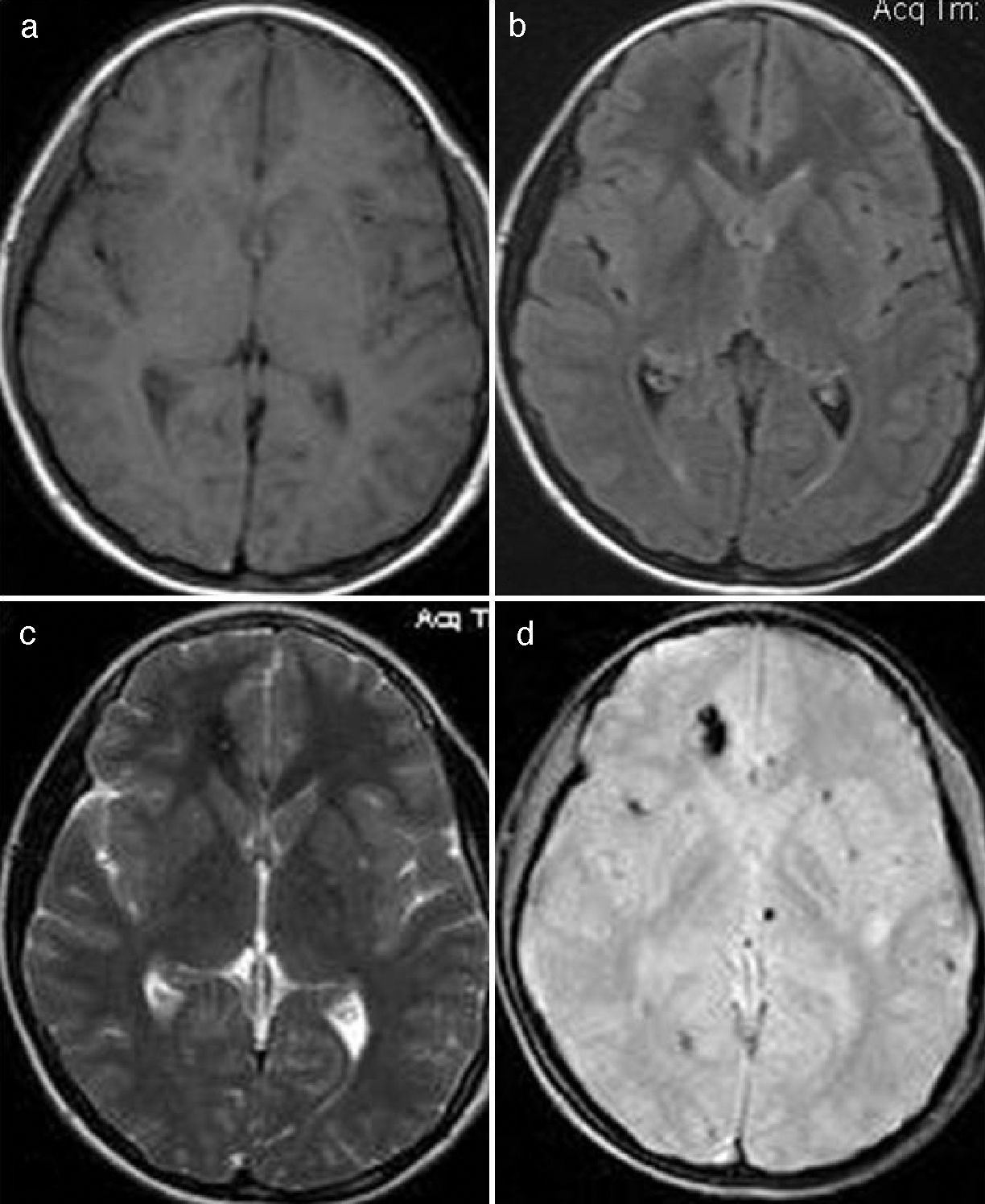
All cavernomas were initially thought to have a congenital cause. However, it has been shown that they are dynamic lesions that undergo changes overtime (de novo appearance, growth, size reduction).4 On some occasions, carcinomas disappear after a hemorrhage.5 Several factors associated with de novo formation of carcinoma have been reported6: previous cranial irradiation, infection of specific viruses, influence of hormones, genetic causes, seeding along the needle track during biopsy, and venous developmental anomalies.7 The association of cavernoma and developmental venous anomaly should be considered since both disorders co-occur in about 30% of cases according to the medical literature.8 In this respect, it would be very useful to complete the radiological studies with the injection of intravenous contrast material because, unlike cavernoma, developmental venous anomaly shows intense enhancement (Fig. 1). There are also studies reporting the rare association between cavernoma, developmental venous anomaly, and capillary telangiectasia as spectrum of one same disorder. Capillary telangiectasia is best detected following intravenous contrast administration.9 Based on a number of histological and immunohistochemical studies, a theory has been suggested for cavernoma formation in case of previous developmental venous anomaly10,11 (Fig. 2).
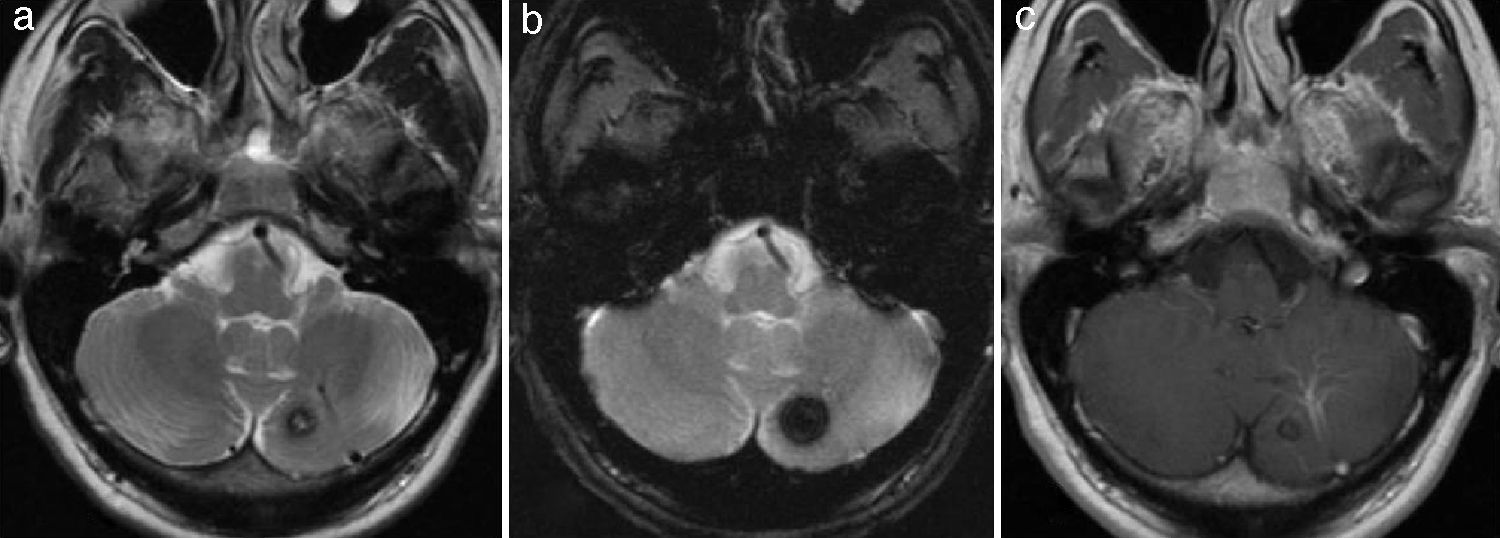
Cavernoma in the left cerebellar hemisphere with associated developmental venous anomaly whose collecting vein drains into the left transverse sinus. (a) and b) T2-weighted cranial MR image and T2 gradient-echo cranial MR image in the axial plane. Cavernoma in the left cerebellar hemisphere. An associated developmental venous anomaly can be guessed in (a). (c) Axial T1-weighted sequence clearly shows enhancement of the developmental venous anomaly and absence of enhancement of the cavernoma after administration of intravenous gadolinium.
Recent genetic studies have provided evidence of dysfunction of specific genes involved in angiogenesis in patients with inherited forms of cerebral cavernomas. These genes encode proteins that interact at the junction of endothelial cells. These patients would present with increased vascular permeability caused by the absence or dysfunction of the junctions between the endothelial cells. Three genes associated with familial forms of cerebral cavernoma have been identified to date. These genes have been named after the abbreviation CCM (cerebral cavernous malformations): CCM1 (KRIT1), CCM2 (MGC4607), and CCM3 (PDCD10). Of all three, gen CCM3 is associated with a higher risk of hemorrhage, and thus, with appearance of the disease at an earlier age.12
Histological featuresOn histological examination, cavernomas are composed of dilated vascular channels variable in size lined by a thin and weak epithelium that lacks elastic and muscular layers predisposing to hemorrhage. The channels are surrounded by a reactive collagenous matrix. There might be internal calcifications. The main histologic feature of cavernomas is the absence of cerebral parenchyma between the interstices of the lesions, which distinguishes cavernomas from capillary telangiectasias.10
Macroscopically, cavernomas are bluish nodules containing areas of bleeding at different stages. Due to recurrent bleeding, carvernomas are surrounded by a pseudocapsule of gliotic brain that is stained with hemosiderin (Fig. 3). As will be discussed below, all these histologic features condition the radiological appearance of cavernomas.
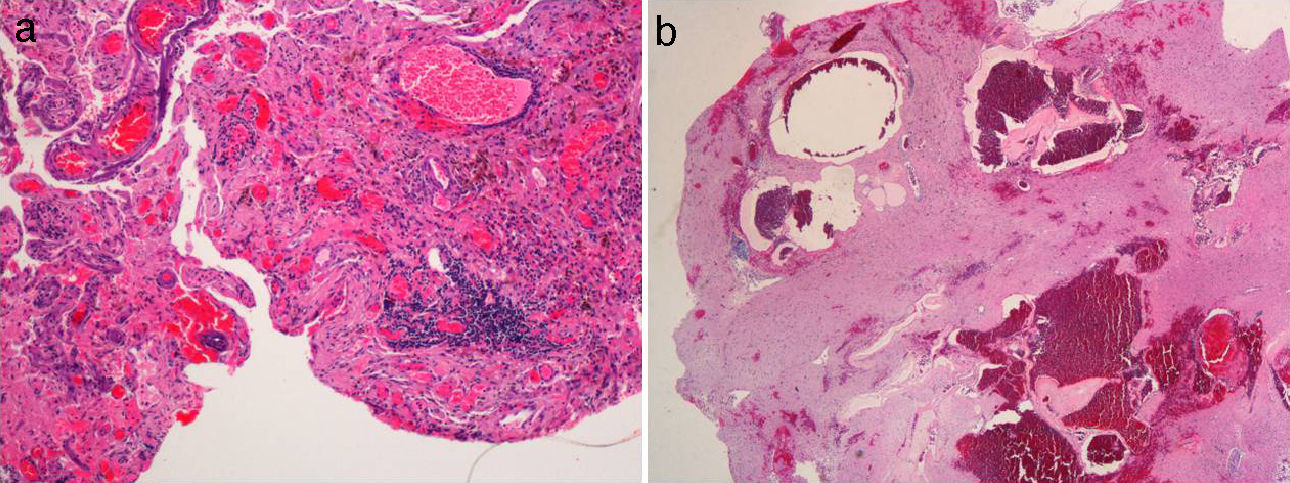
Histologic features of the cavernoma. (a) Microscopic histologic section dyed with hematoxylin–eosin showing congestive dilated vascular lumina with frequent signs of thrombosis, adjacent cerebral parenchyma with reactive gliosis, and hemosiderin depositions. (b) Macro photography of the same lesion shows a bluish nodule with hemorrhages at different stages.
Cavernomas may affect any part of the brain and their clinical manifestations mainly depend on their localization.
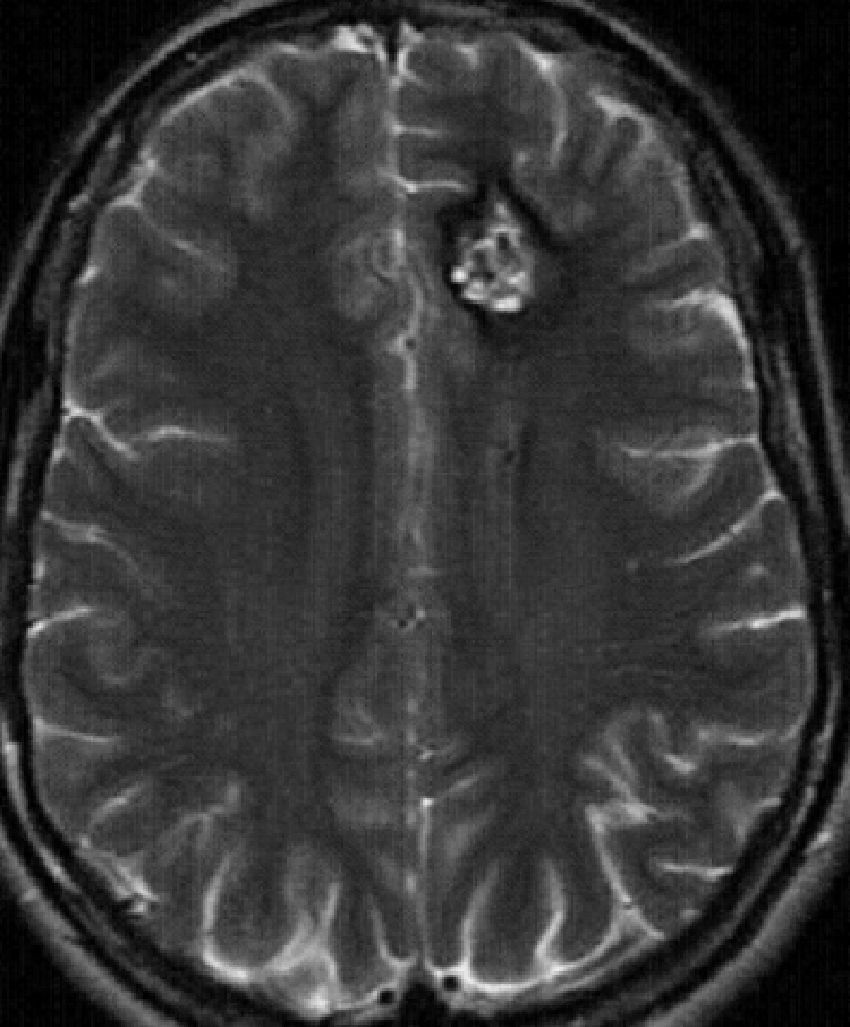
Supratentorial cavernomas are the most common, accounting for approximately 80% of cases. They mainly affect the subcortical region and the frontal and temporal lobes are the most commonly affected (Fig. 4). Seizures are the most frequent clinical manifestation of cavernomas, generally associated with hemorrhages. However, they can also be associated with headaches, other focal neurological deficits, and hemorrhages.3,13
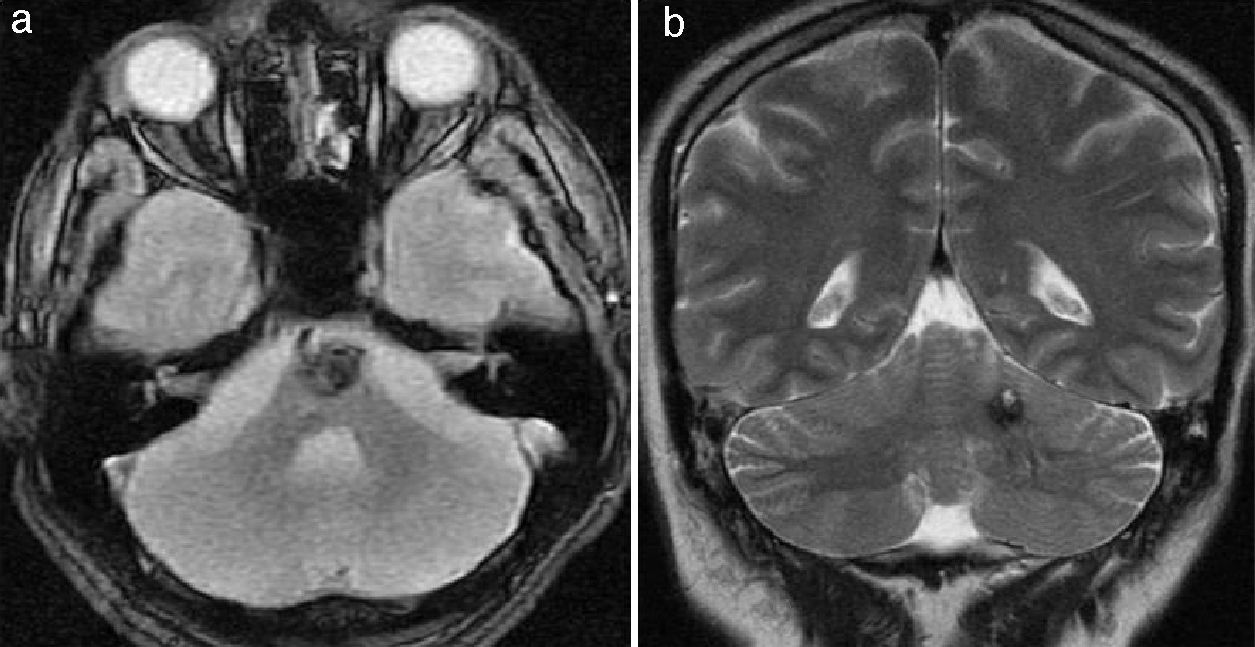
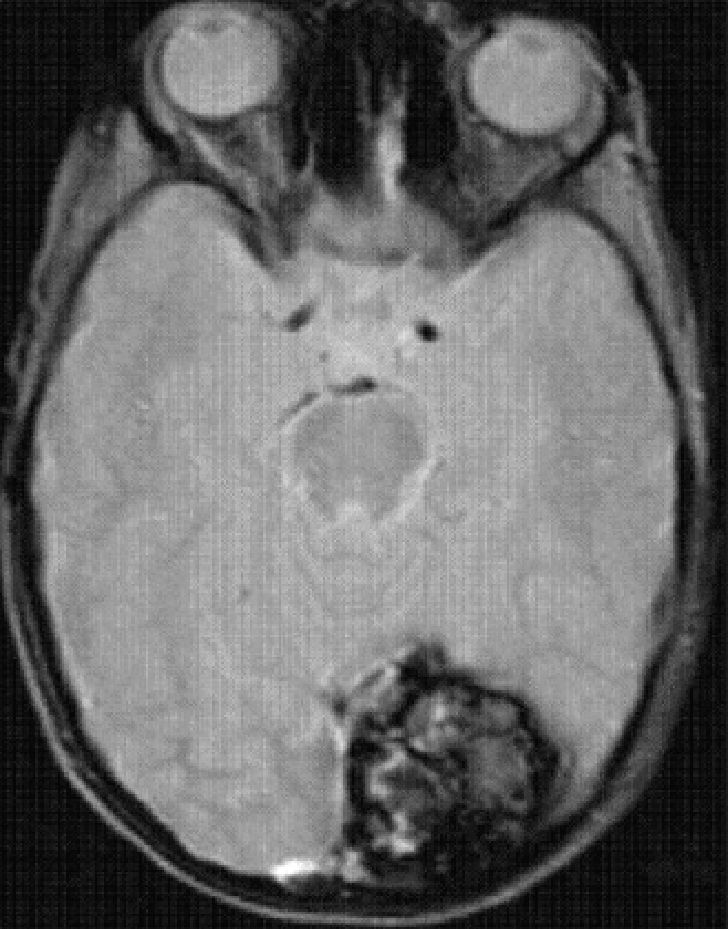
In the posterior fossa, cavernomas mostly affect the protuberance and cerebellar hemispheres (Fig. 5) and their common manifestations are focal neurological deficits, such as anomalies in the pairs of cranial nerves and changes in sensitivity and ataxia. The first clinical manifestations of intraventricular cavernomas (very rare) usually involve intracranial hypertension caused by obstruction of the cerebrospinal fluid due to recurrent hemorrhages.14
A number of factors have been reported that modify the natural history and behaviour of these lesions, increasing the risk of hemorrhage, and thus, conditioning more aggressive clinical presentations (multiple lesions, female sex, CCM3 genotype, diagnosis before 35 years of age, infratentorial location, association with a developmental venous anomaly, and lesions >1cm in diameter15).
According to the series, up to 40% of cavernomas are asymptomatic and incidentally discovered during radiological examinations.2,3,15
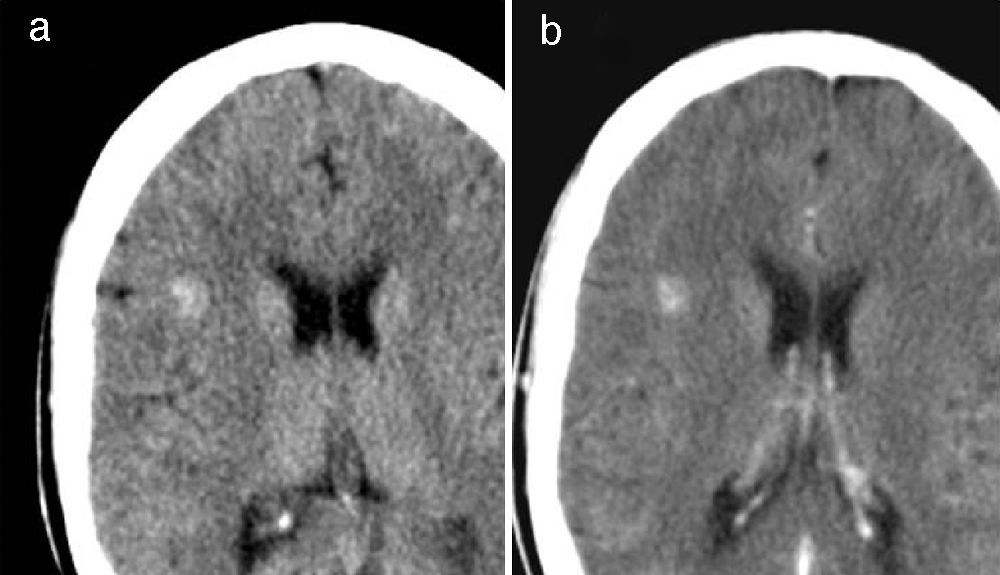
Typical radiological featuresComputerized tomography (CT), with or without contrast administration, detects only 30–50% of lesions, leading to underdiagnosis of the disease if CT is the only imaging modality used.16 Cavernomas usually manifest as high-density and well-defined lesions rounded or ovoid in shape, sometimes with internal calcifications. The surrounding brain parenchyma usually remains normal. There is usually minimal or no mass effect on the adjacent structures and no vasogenic edema associated. Mild or no enhancement is observed following intravenous contrast administration17 (Fig. 6).
Cavernoma located in the right suprasylvian area, a typical CT image. (a) Unenhanced axial CT. (b) Similar axial image after contrast injection shows a well-defined, high-density, rounded lesion with no associated edema or mass effect and no enhancement after contrast administration.
MRI is the primary imaging technique for cavernoma diagnosis and evaluation, showing a clearly higher sensitivity than CT. The appearance of cavernomas varies depending on the stage of the hemorrhage.17 The most common finding of uncomplicated cavernomas is known as popcorn lesion involving a nucleus with heterogeneous signal in T1- and T2-weighted images (due to thrombosis, hemorrhage, fibrosis, and calcification) surrounded by a complete hemosiderin ring with lower signal intensity in T2-weighted sequences (Fig. 7). The most sensitive sequence to detect cavernomas is T2-weighted gradient-echo sequences.18 The lesion is said to bloom (it is more clearly visualized) due to magnetic susceptibility effects caused by hemoglobin degradation products. It has recently been demonstrated that MRI performed with high-field (3T) systems provide better morphological characterization of these lesions, while new susceptibility-weighted imaging (SWI) is more sensitive to detect small lesions than conventional sequences.19 As with CT, the surrounding parenchyma is usually normal and the mass effect, edema, and enhancement following administration of intravenous gadolinium rarely occur.
Cavernoma located in the right suprasylvian area, a typical MR image (the same case as in Fig. 6). (a) and (b) Axial T1- and T2-weighted RM images, respectively, show the typical popcorn appearance of a cavernoma with hypointense peripheral rim in T2-weighted sequence. (c) Note the blooming in the axial T2-weighted gradient-echo sequence.
Arteriography is indicated when there are doubts about the causes of cerebral haemorrhage, when the diagnosis of cavernoma cannot be reliably made using CT or MRI, with a view to ruling out an arteriovenous malformation as the cause of hemorrhage. Arteriography is not indicated when the MRI shows the typical image of cavernoma. Cavernomas, thrombosed arteriovenous malformations, and capillary telangiectasia make up the group of angiographically occult vascular malformations, whose main common feature is the absence of vascular anomalies due to slow blood flow. This is by far the most common feature.20,21 Arteriography is very sensitive to demonstrate developmental venous anomalies associated with cavernomas (Fig. 8), but MRI scanning following intravenous contrast administration is reliable enough to rule it out.
Left parietal cavernoma with associated developmental venous anomaly. (a) Coronal T1-weighted MR image after administration of intravenous gadolinium showing both the cavernoma and the enhancing developmental venous anomaly. (b) The arteriography clearly shows the anatomy of the developmental venous anomaly, whereas the cavernoma remains occult.
Cavernomas may present with atypical radiological features in terms of appearance, size, location, and number.17
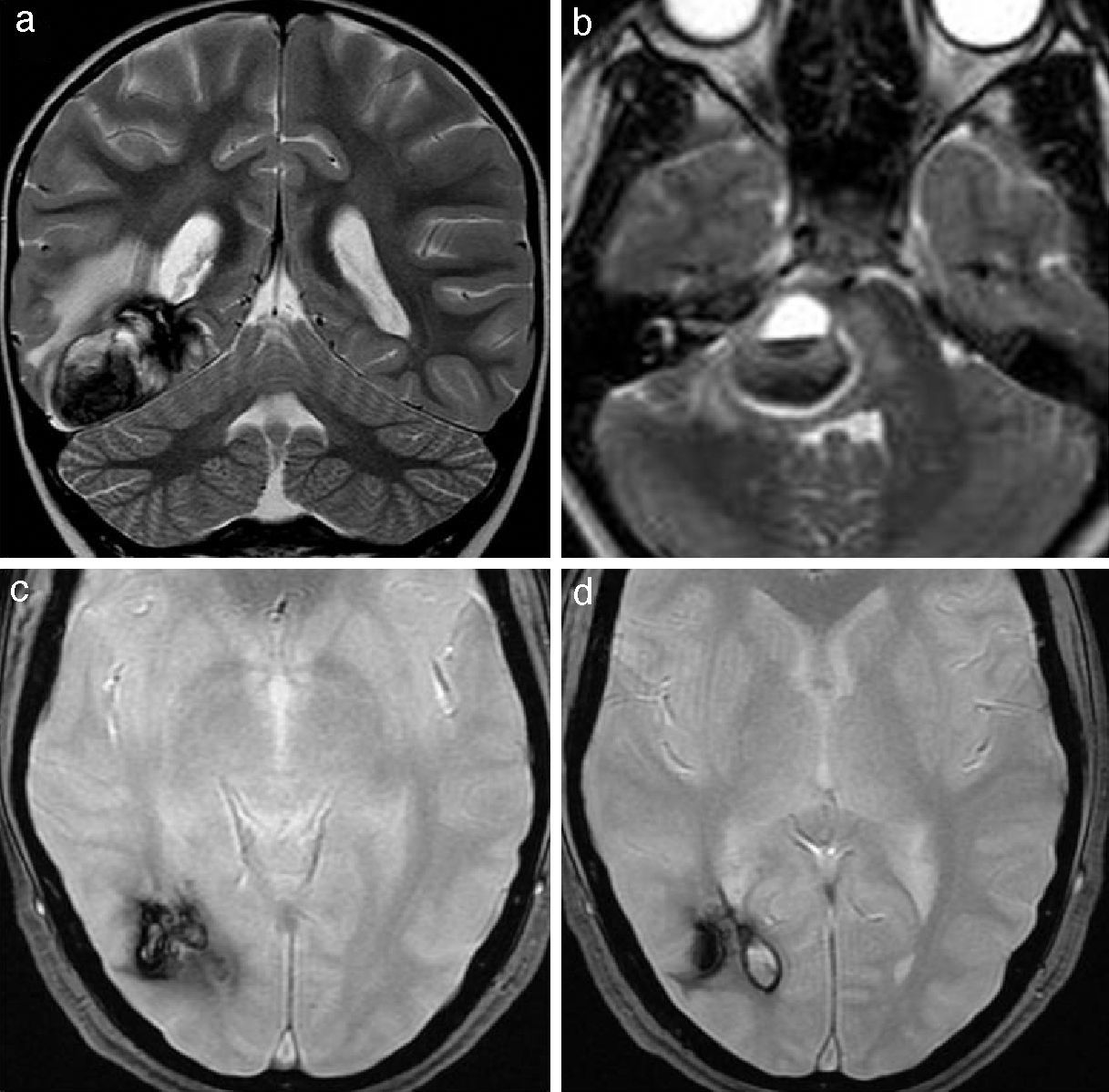
In case of recent hemorrhage, which usually co-occurs with clinical manifestations, cavernomas may lack its typical appearance in the different imaging modalities showing atypical characteristics such as associated vasogenic edema, mass effect on adjacent structures, loss of the hypointense peripheral hemosiderin ring, fluid-fluid level, or presence of perilesional hemorrhage17 (Fig. 9). In these cases, serial images are useful if the hemorrhage does not undergo surgery.22,23
Cavernomas with an atypical radiological appearance. (A) Axial T2-weighted cranial RM image shows a large right occipital cavernoma with vasogenic edema and a mild mass effect on the occipital horn of the right lateral ventricle. (b) Coronal T2-weighted RM image shows a cavernoma in the right hemi-protuberance with signs of recent haemorrhage. The image also shows a fluid-fluid level inside the cavernoma, loss of the peripheral low-intensity rim, vasogenic edema, and mass effect on the IV ventricle. (c) and (d) Axial T2-weighted gradient-echo sequence. Intraventricular haemorrhage caused by previous bleeding of a cavernoma located adjacent to the occipital horn of the right lateral ventricle.
When treating a hemorrhagic lesion in the central nervous system with associated edema, Yung et al. demonstrate that the presence of a hyperintense peripheral rim in T1-weighted sequences may be highly suggestive of cavernoma. This rim was observed in 62% of hemorrhages secondary to cavernomas, only in 6% of hemorrhages secondary to metastasis and it was not identified in hemorrhages caused by primary tumours or in primary hemorrhages. For this reason, the presence of this rim in a haemorrhage in the central nervous system suggests that a cavernoma is highly likely to be the cause of the hemorrhage.24
Most cavernomas present with a size <3cm and many of them are millimetric. Nevertheless, large lesions or the so-called giant cavernomas may occur25 (Fig. 10).
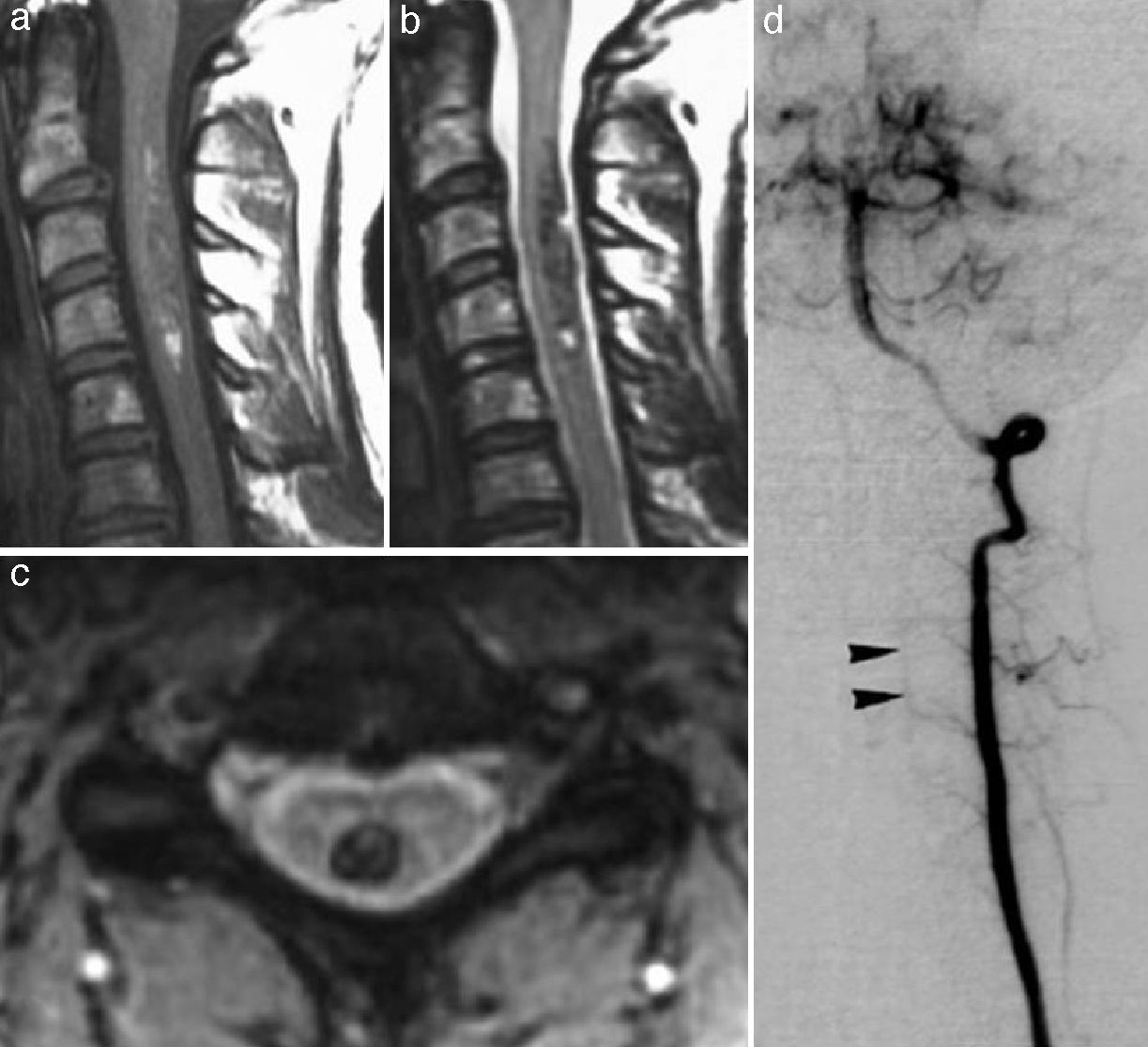
Cavernomas have been reported in many different locations. They may be found in the spinal cord, most commonly in the cervical region, usually co-occurring with multiple brain lesions26 (Fig. 11). Subarachnoid, intraventricular, subdural and even extradural lesions as well as lesions in the sinuses of the dura mater have been reported.27
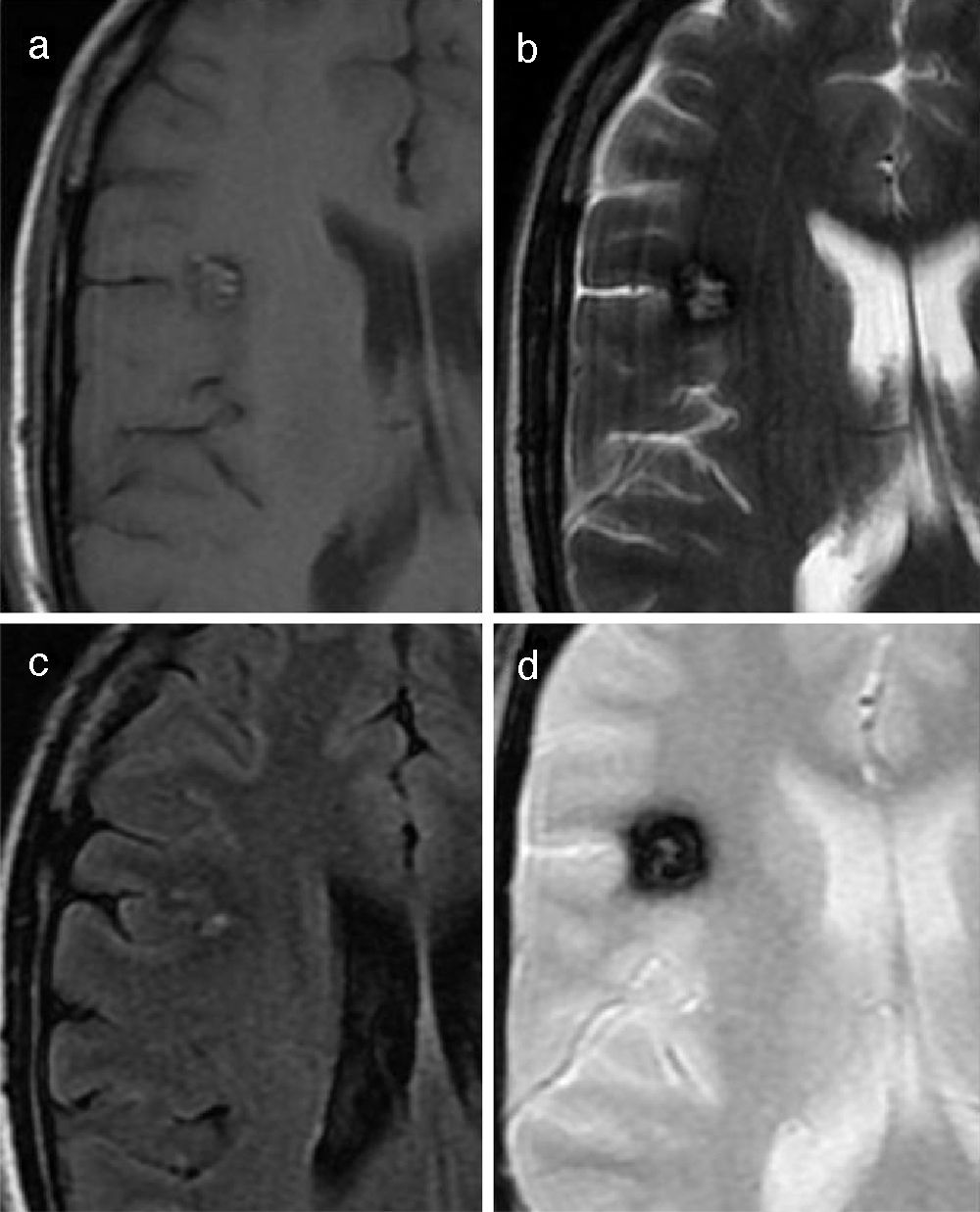
In approximately 15–20% of cases, more than one lesion is diagnosed, a phenomenon which is known as multiple cavernomatosis. These cases are associated with a family history of cavernoma in over 80% of patients, whereas only 15% of multiple lesions have been reported in cases with no family history. Multiple lesions involve a higher risk of bleeding, and thus, they clinically manifest in patients at an earlier age.28,29 In cases of multiple cavernomas, T2 gradient-echo sequences (and more recently, susceptibility-weighted sequences) usually detect a higher number of lesions than the rest of sequences due to the blooming of small lesions by the magnetic susceptibility effect due to millimetric hemosiderin depositions18 (Fig. 12). Therefore, when punctuate hemosiderin depositions are seen in T2 gradient-echo sequences, multiple cavernomatosis should be included in the differential diagnosis, together with hypertensive angiopathy, amyloid angiopathy, hemorrhagic metastasis, vasculitis of the central nervous system, hemorrhagic diffuse axonal injury, and radiation-induced telangiectasia.
Typical multiple cavernomatosis. (a) T1-weighted sequence. (b) T2-weighted FLAIR sequence. (c) T2-weighted sequence. (d) T2-weighted gradient-echo sequence. All sequences are visualized in the axial plane. Note the superior performance of T2-weighted gradient-echo sequence to visualize these lesions (including those that had been previously identified and many others) due to the magnetic susceptibility artifact secondary to hemoglobin degradation products.
Cavernomas associated with epilepsy seizures are normally managed with conservative medical treatment of antiepileptic drugs. Resection of a cavernoma is indicated when seizures cannot be controlled with pharmacological treatment, the adverse effects of the drugs are too serious, or the patient fails to follow the treatment. A number of studies have reported seizure-free rates of 60% using pharmacological treatment,30 whereas surgical treatment provides a seizure-free rate of approximately 80%.31 Those lesions that initially manifest with hemorrhage should be managed with conservative treatment. However, surgical resection should also be considered if the hemorrhage causes serious neurological signs or symptoms, or if rebleeding occurs.
Surgical treatment is particularly indicated in superficial and supratentorial lesions that can be easily approached. Biopsy can be performed when diagnosis is not entirely reliable. It should be noted that resection of the entire lesion is mandatory because partial resection entails a higher risk of haemorrhage than that associated with conservative treatment.32
In spite of their difficult surgical approach and risk of sequelae, brainstem lesions and basal ganglia lesions should be more aggressively managed since they have a greater tendency toward bleeding and higher sequela and mortality rates. There is controversy as to what is the most appropriate therapeutic treatment for these lesions. Surgical resection should be performed. However, the high morbidity and mortality rates associated have recently given way to stereotactic radiosurgery as an alternative for treatment of lesions with difficult surgical approach, with lower morbidity mortality rates.33
ConclusionsCavernomas are vascular malformations that the radiologist usually faces in daily practice. They present with typical radiological features, especially on MRI, associated with subacute or chronic blood products. For this reason, T2 gradient-echo sequences are of great help for carvernoma identification. Cavernomas are quite commonly associated with developmental venous anomaly, and thus, MRI studies are necessary for better visualization. However, cavernomas may manifest with atypical features, usually associated with recent hemorrhage. In these cases, follow-up is very useful to confirm diagnosis. Knowledge of these features is crucial to make a correct diagnosis, which enables optimization of the different treatment strategies.
Authorship- 1.
Responsible for the integrity of the study: JJCV.
- 2.
Conception of the study: JJCV, LCA, FBM and JIGL.
- 3.
Design of the study: JJCV, LCA, FBM and JIGL.
- 4.
Acquisition of data: JJCV.
- 5.
Analysis and interpretation of data: JJCV, LCA, FBM and JIGL.
- 6.
Statistical analysis: N/A.
- 7.
Bibliographic search: JJCV, LCA, FBM, JIGL and JGSSG.
- 8.
Drafting of the paper: JJCV, LCA, FBM, JIGL and JGSSG.
- 9.
Critical review with intellectually relevant contributions: JJCV, LCA, FBM, JIGL and JGSSG
- 10.
Approval of the final version: JJCV, LCA, FBM, JIGL and JGSSG.
The authors declare not having any conflict of interest.
Please cite this article as: Cortés Vela JJ, et al. Malformaciones cavernosas intracraneales: espectro de manifestaciones neurorradiológicas. Radiología. 2012. doi:10.1016/j.rx.2011.09.016.