To describe the detection of cortical areas and subcortical pathways involved in language observed in MRI activation studies and tractography in a 3T MRI scanner and to correlate the findings of these functional studies with direct intraoperative cortical and subcortical stimulation.
Materials and methodsWe present a series of 14 patients with focal brain tumors adjacent to eloquent brain areas. All patients underwent neuropsychological evaluation before and after surgery. All patients underwent MRI examination including structural sequences, perfusion imaging, spectroscopy, functional imaging to determine activation of motor and language areas, and 3D tractography. All patients underwent cortical mapping through cortical and subcortical stimulation during the operation to resect the tumor. Postoperative follow-up studies were done 24h after surgery.
ResultsThe correlation of motor function and of the corticospinal tract determined by functional MRI and tractography with intraoperative mapping of cortical and subcortical motor areas was complete. The eloquent brain areas of language expression and reception were strongly correlated with intraoperative cortical mapping in all but two cases (a high grade infiltrating glioma and a low grade glioma located in the frontal lobe). 3D tractography identified the arcuate fasciculus, the lateral part of the superior longitudinal fasciculus, the subcallosal fasciculus, the inferior fronto-occipital fasciculus, and the optic radiations, which made it possible to mark the limits of the resection. The correlation with the subcortical mapping of the anatomic arrangement of the fasciculi with respect to the lesions was complete.
ConclusionThe best treatment for brain tumors is maximum resection without associated deficits, so high quality functional studies are necessary for preoperative planning.
Describir, con estudios funcionales de activación y tractografía en una RM de 3 Teslas (3T), las áreas corticales y vías subcorticales implicadas en el lenguaje, y mostrar la buena correlación de estos estudios funcionales con la estimulación directa cortical y subcortical intraoperatoria.
Material y métodosPresentamos una serie de 14 pacientes con lesiones focales cerebrales junto a áreas elocuentes. Todos los pacientes se evaluaron neuropsicológicamente antes y después de la cirugía, se estudiaron con RM con secuencias estructurales, de perfusión, espectroscopia, resonancia magnética funcional y del lenguaje y tractografía 3D, y se sometieron a un mapeo cortical de estimulación cortical y subcortical y resección de la lesión. Se hizo un control posquirúrgico a las 24h.
ResultadosLa correlación funcional motora y del haz corticoespinal con el mapeo intraoperatorio cortical y subcortical motor fue completa. Las áreas elocuentes del lenguaje expresivo y del lenguaje receptivo presentaron una alta correlación con el mapeo cortical intraoperatorio en todos los casos menos 2, un glioma infiltrativo de alto grado y un glioma de bajo grado frontal. La tractografía 3D identificó los fascículos arcuato, frontoparietal, subcalloso, frontooccipital inferior y las radiaciones ópticas, lo que permitió marcar los límites de la resección. La correlación con el mapeo subcortical en la disposición anatómica de los fascículos con respecto a las lesiones, fue completa.
ConclusiónLa máxima resección tumoral sin déficits asociados es el mejor tratamiento posible ante un tumor cerebral, lo que resalta la necesidad de estudios funcionales de alta calidad en la planificación prequirúrgica.
The main clinical indication of the functional activation and tractography studies is presurgery planning of focal brain lesions in eloquent areas. Motor paradigms of functional magnetic resonance (fMR) are simple and consistent, and easy to reproduce, therefore they have been widely validated and correlated in medical literature with the cortical and subcortical mapping techniques.1,2 However, language is a complex cognitive task and the experimental paradigms used present greater complexity and variability among individuals; hence, its routine application is not easy. Intraoperative surgical mappings have shown that the classic Broca and Wernicke language areas are not only basic,3–6 but also that there is a much more complex network that implies larger areas of cortical activation7,8 and broad subcortical areas.9–12
Our article's interest is the confirmation that the new functional activation sequences and diffusion tensor image (DTI) in 3T MR provide, in a non-invasive manner, a reliable presurgery cortical and subcortical map,13–16 upon comparing our data with intraoperative mapping. These techniques are indispensable for an adequate surgical planning, especially in the centers where intraoperative mapping is not performed. As far as we know, this is one of the largest series of image test correlation with cortical and subcortical mapping.
Materials and methodsPatientsWe selected the patients in whom, both the functional studies and the intraoperative mapping, were satisfactory (adequate collaboration on the part of the patient in the making of the paradigms, absence of alterations due to excessive movement, absence of consecutive crises in intraoperative stimulation), and that is why our series is limited to 14 patients with expansive intracerebral tumoral lesions in whom a presurgery study was conducted with functional neuroimage and, as reference standard, a cortical and subcortical intraoperative electrical stimulation. All the patients signed their informed consent for the diagnostic tests. Permission from the Hospital Ethics Committee was not requested for it was a retrospective study.
Activation functional studyFunctional paradigmsA motor functional study was carried out with alternating movements of opposition of the fingers to the thumb (finger-tapping), prior to the functional study of language, because routinely intraoperative approach begins by mapping the sensorimotor cortex, identifying the central groove and confirming the patient's response.
Of the various paradigms described as applicable to the study of language,17,18 in our center we use 3 paradigms for the functional study of language: (1) the paradigm of “verbal generation” (relating actions with the names of objects presented visually. The patient must try to verbalize without moving the face in the activation periods. In the rest periods, in order to perform an automatic task, the patients count forward); (2) the paradigm of “passive listening” (listening and understanding a text; the patient is informed that comprehension questions will be asked later. In the rest periods, the patient listens to an inverted, incomprehensible text); and (3) the paradigm of “text reading” (reading the text presented. In the rest periods an unreadable text is presented visually). The first paradigm aims at activating the expressive or motor areas of language. The other two tasks seek to activate the receptive and reading areas of language.
Data acquisitionAcquisition was performed in a 3T scanner (GE Medical Systems, Milwaukee, WI, USA) using an echo-planar gradient sequence (ET: 35ms; RT: 3000ms; 90° angle; matrix: 64×64; 1 acquisition; FOV: 24cm; cut thickness: 4.0mm; spacing: 0.0). The reference anatomical image is a 3D-SPGR-T1 on an axial plane. A block design was used alternating periods of activity and rest, beginning with activity. 6 activity-rest cycles were made, with 10 rest phases and 10 activity phases lasting 30s per period, 120 volumes were obtained.
Post processingFunctional images post processing was performed with the 3D software Brainwave (General Electric Health Care, Milwaukee, WI, USA). The anatomical images 3D-SPGR T1 were segmented automatically eliminating the soft parts. The functional image volumes obtained were aligned by means of the Air method by Woods et al.17 (maximum translation 0.22mm/maximum rotation 0.07°). They were later processed with a Gauss spatial filter (full width at half maximum [FWHM]=8.8mm). The functional data were analyzed according to a linear regression model generating a t-test map, which becomes an activation Z map. The statistical threshold used is that of Z=4.51451 with a value of p=0.05, and only the activation areas above that threshold are represented. The activated areas were represented in a parametric map fused with the anatomical images and represented in a 3D volume.
3D-DTI studiesA diffusion spin-echo echo-planar sequence was used with 25–45 directions in the axial, sagittal and coronal planes (ET: minimum; RT: 6000ms; matrix: 128×128; 1 acquisition; FOV: 24cm; thickness: 4mm; spacing: 1.5mm; maximum value of b: 1000). 3D reconstruction uses a FuncTool 3D Fiber Tracking software (General Electric Health Care, Milwaukee, WI, USA). The white substance bundles, obtained by tractography with the seed method,17 were: lower frontal occipital fasciculus, arcuate fasciculus, subcallous fasciculus, frontal occipital bundle and lower longitudinal fasciculus. The corticospinal bundle and optical radiations were routinely added.
Intraoperative cortical and subcortical electrical stimulationThe tumor had been located by means of intraoperative echography in all the patients, under local anesthesia, delimiting the borders and marking them with the letters A, B, C and D. Mapping with cortical electrical stimulation began in the sensorimotor cortex, around Rolando's groove. Subsequently, language was mapped with different paradigms (verbalizing, naming objects, reading a text). Intensity of electrical stimulation ranged from 1 to 2mA, to a maximum of 8mA, which induces crisis. Stimulation was considered positive if an alteration in verbal discourse occurred after 3 stimulations in a row in an area of 0.5cm×0.5cm. It was marked with numbered white tags. On a second time, the lesion was resected, and the subcortical region was reached. Electrical stimulation of these subcortical bundles caused language alterations similar to the cortical areas. In this moment, the subcortical functional bundles that had already been mapped were followed from their cortical origin and the resection was resumed until the eloquent subcortical surrounded the surgical cavity.
Rolando's operculum (which delimits the motor facial cortex), the anterior and ascending branches of the sylvian fissure (which delimit Broca's area and the ventral premotor cortex) were considered as reference anatomical marks in language mapping, and finally, the horizontal sylvian branch along with the posterior third of the superior temporal groove (which mark Wernicke's area).
Intraoperative image-mapping correlationCortical correlation was considered positive when there was an anatomical concordance with the circumvolutions activated in the 3D fMR study with a margin for error below 0.5cm. The reference anatomical marks described previously were used in mapping language in order to identify the functional activation areas. Subcortical correlation was considered positive when the spatial disposition of the subcortical bundles (cranial, caudal, medial, lateral, anterior or posterior) corresponded to that of the subcortical mapping.
Post surgery controlA control MR study was carried out at 24h, and a radiological and neuropsychological control at 3 months.
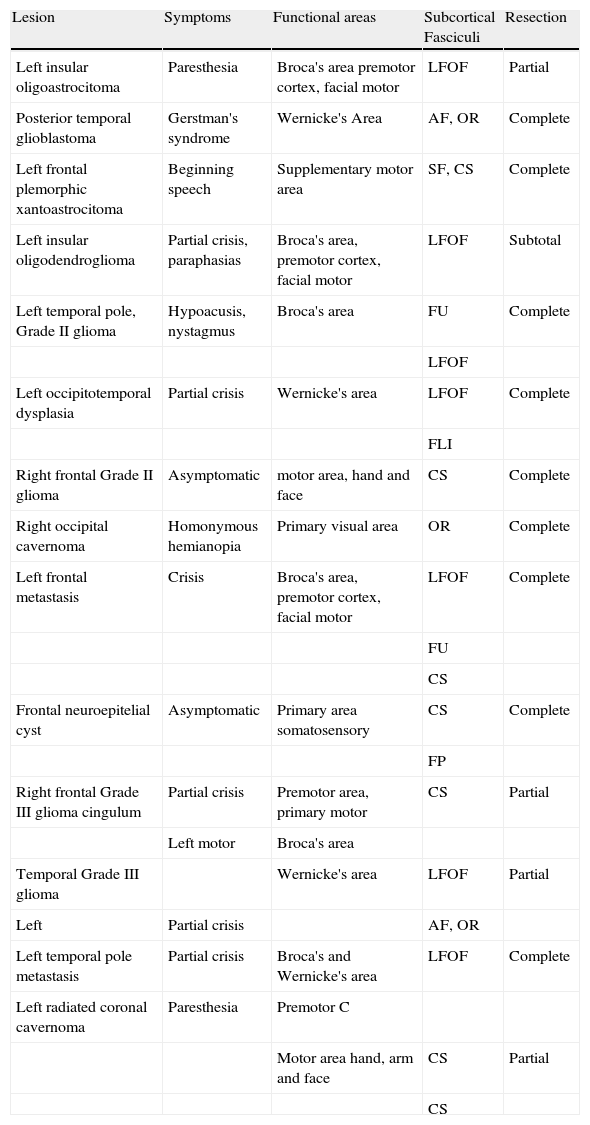
ResultsThe clinical characteristics of functional studies, of the subcortical fasciculi, and the surgical results of the correlated cases appear in Table 1. A positive cortical correlation was found in 12 of the 14 patients studied, and one without complete coincidence in 2 patients (frontal Grade II glioma and cingulum Grade III glioma). In the first, due to a false positive in the functional study caused by the precentral vein, and the second, due to the extensive tumoral infiltration that inhibited functional activation. In the latter case, additionally, intraoperative mapping was partial, since the patient presented a crisis during procedure.
Correlated patients: clinical-radiologic findings.
| Lesion | Symptoms | Functional areas | Subcortical Fasciculi | Resection |
| Left insular oligoastrocitoma | Paresthesia | Broca's area premotor cortex, facial motor | LFOF | Partial |
| Posterior temporal glioblastoma | Gerstman's syndrome | Wernicke's Area | AF, OR | Complete |
| Left frontal plemorphic xantoastrocitoma | Beginning speech | Supplementary motor area | SF, CS | Complete |
| Left insular oligodendroglioma | Partial crisis, paraphasias | Broca's area, premotor cortex, facial motor | LFOF | Subtotal |
| Left temporal pole, Grade II glioma | Hypoacusis, nystagmus | Broca's area | FU | Complete |
| LFOF | ||||
| Left occipitotemporal dysplasia | Partial crisis | Wernicke's area | LFOF | Complete |
| FLI | ||||
| Right frontal Grade II glioma | Asymptomatic | motor area, hand and face | CS | Complete |
| Right occipital cavernoma | Homonymous hemianopia | Primary visual area | OR | Complete |
| Left frontal metastasis | Crisis | Broca's area, premotor cortex, facial motor | LFOF | Complete |
| FU | ||||
| CS | ||||
| Frontal neuroepitelial cyst | Asymptomatic | Primary area somatosensory | CS | Complete |
| FP | ||||
| Right frontal Grade III glioma cingulum | Partial crisis | Premotor area, primary motor | CS | Partial |
| Left motor | Broca's area | |||
| Temporal Grade III glioma | Wernicke's area | LFOF | Partial | |
| Left | Partial crisis | AF, OR | ||
| Left temporal pole metastasis | Partial crisis | Broca's and Wernicke's area | LFOF | Complete |
| Left radiated coronal cavernoma | Paresthesia | Premotor C | ||
| Motor area hand, arm and face | CS | Partial | ||
| CS |
CS, corticospinal bundle; AF, arcuate fasciculus; LFOF, lower frontooccipital fasciculus; LIF, lower longitudinal fasciculus; FP, frontoparietal bundle; SF, subcallous fasciculus; UF, uncinate fasciculus; OR, optic radiations.
Postoperative neuropsychological evaluation of the patients was optimal and no language deficits were appreciated added to the preoperative ones in any of the 14 patients.
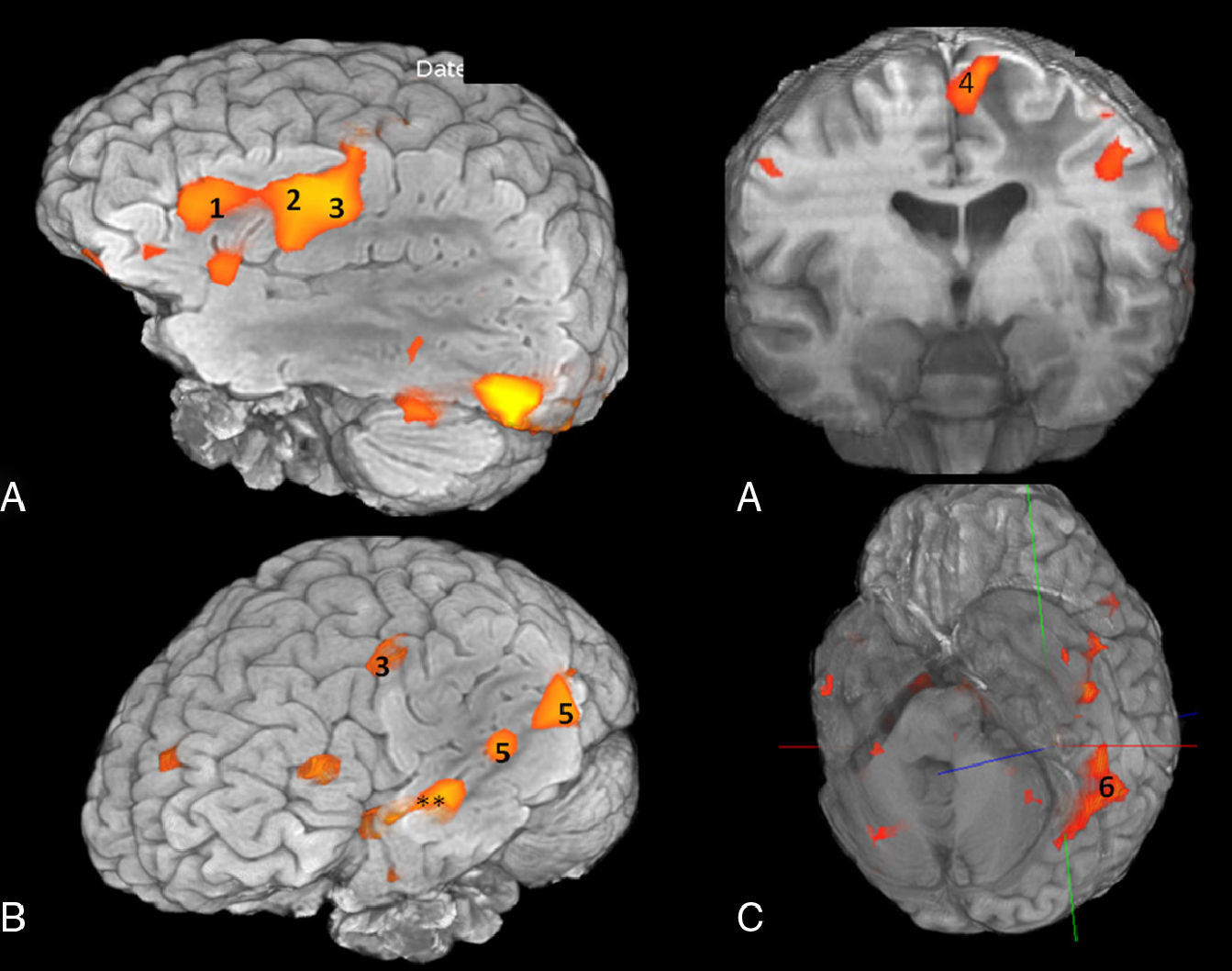
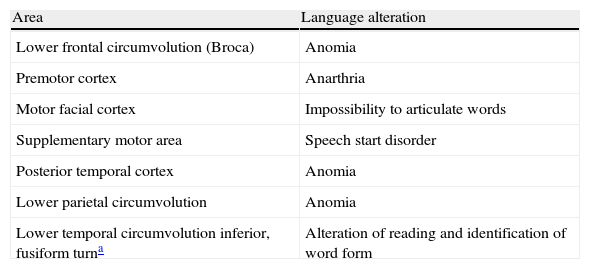
The cortical areas implicated in language (Fig. 1) correlated with cortical stimulation (Table 2) were:
- 1.
Frontal areas: lower frontal circumvolution (Broca's area), triangular and opercular pars; motor facial cortex (lowest margin of ascending frontal circumvolution); premotor cortex (anterior to the motor facial cortex); supplementary motor area (medial to the most cranial part of the precentral groove).
- 2.
Temporoparietal areas: left posterior temporal cortex: posterior third of the superior and meddle temporal circumvolutions; lower left parietal lobe: angular and supramarginal circumvolutions.
- 3.
Occipitotemporal basal areas: lower temporal and fusiform circumvolutions. They are areas related with visual recognition, specialized in identification of the form of words or objects, such as the left turn (“visual word form area” and “visual object-form area”).
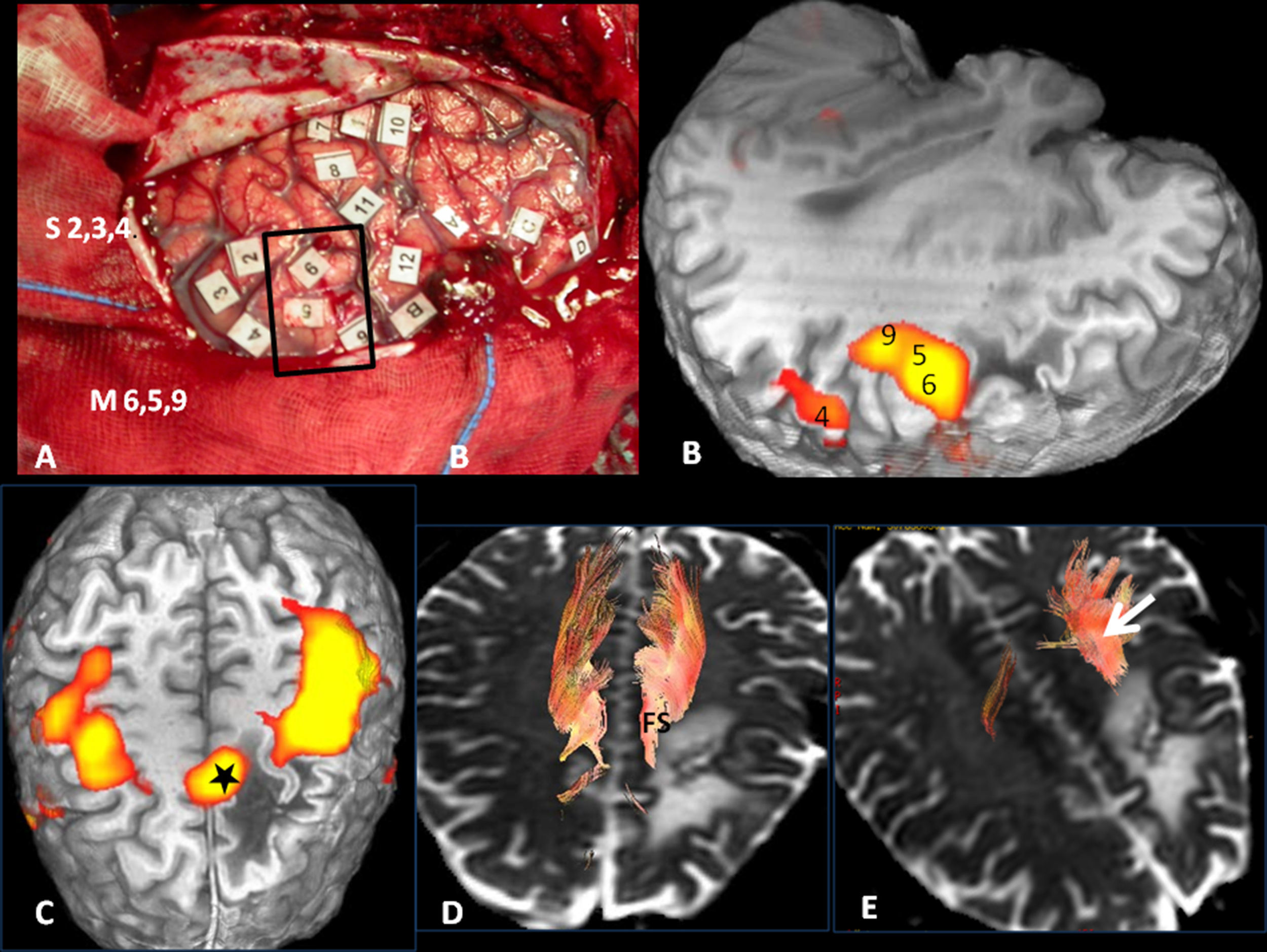
Volumetric functional MR. (A) Frontal cortical areas, (B) temporoparietal areas and (C) basal occipitotemporal areas implicated in language. 1: Broca's area; 2: Premotor cortex; 3: Motor facial cortex; 4: Supplementary motor area; 5: Wernicke's area; 6: Visual word form area. **Auditory association areas.
Cortical electrical stimulation.
| Area | Language alteration |
| Lower frontal circumvolution (Broca) | Anomia |
| Premotor cortex | Anarthria |
| Motor facial cortex | Impossibility to articulate words |
| Supplementary motor area | Speech start disorder |
| Posterior temporal cortex | Anomia |
| Lower parietal circumvolution | Anomia |
| Lower temporal circumvolution inferior, fusiform turna | Alteration of reading and identification of word form |
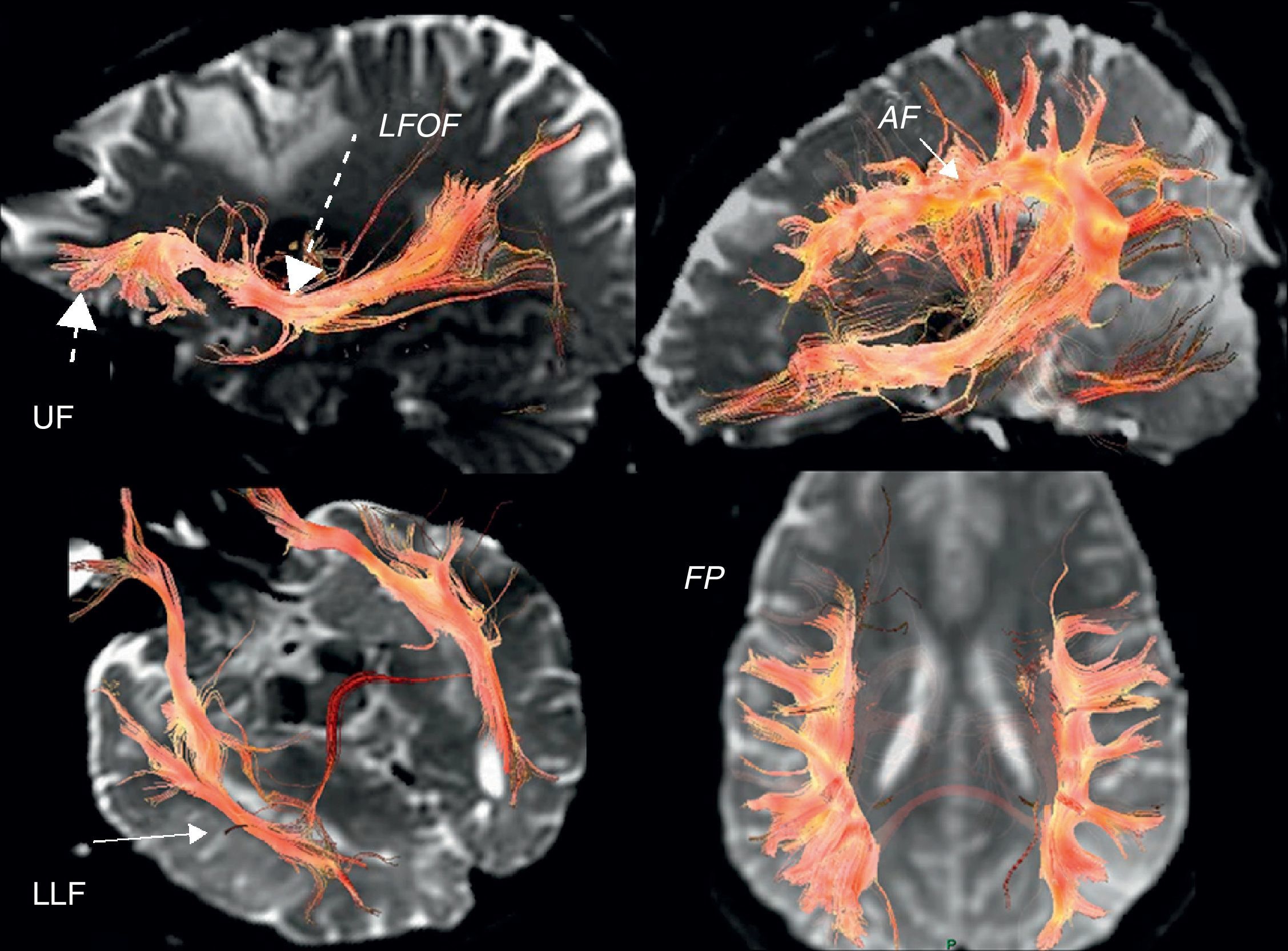
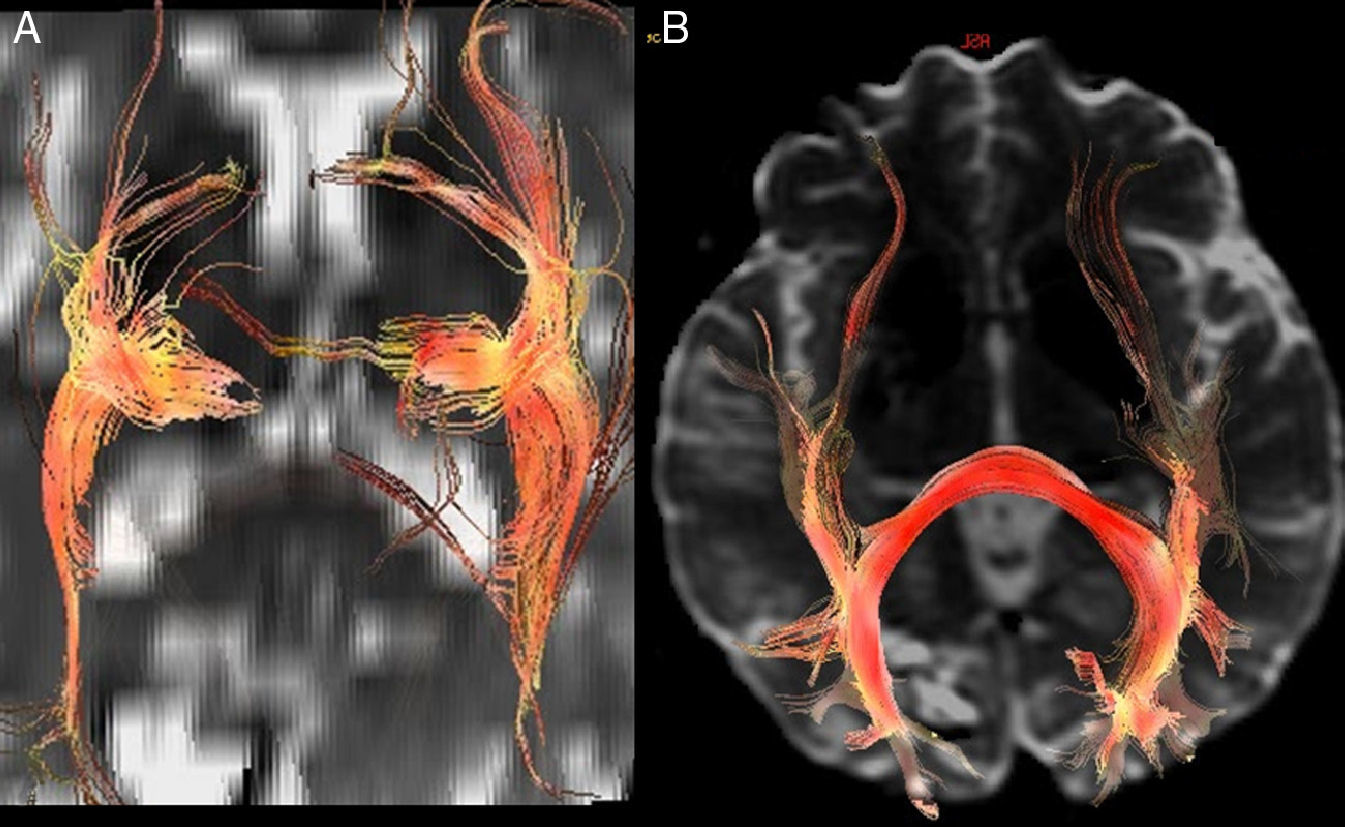
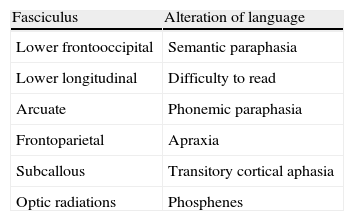
In the case of subcortical “functional” fasciculi, there was 100% concordance. The correlated bundles were (Table 3) (Fig. 2):
- 1.
Arcuate fasciculus: medial part of the superior longitudinal fasciculus, connecting the frontal lateral and parietotemporal.
- 2.
Frontoparietal fasciculus or bundle: lateral part of the superior longitudinal fasciculus, connecting Broca's area with the lower parietal lobe.
- 3.
Lower frontooccipital fasciculus: it connects medial occipito-temporoparietal and prefrontal areas. In its anterior third it is located cranially to the ceiling of the temporal horn and the optic radiations. In its posterior temporooccipital segment it is lateral in relation to the ventricular occipital horn, becoming part of the sagittal stratum. It ends in the medial temporoccipital cortex.
- 4.
Lower longitudinal fasciculus: in its left temporooccipital tract it is involved in identifying the form of objects or “visual object-form area”.
- 5.
Subcallous fasciculus: it surrounds the ventricular frontal horn and connects the supplementary motor area with the cingulum and the caudate nucleus.
3D tractography. Functional subcortical fasciculi implicated in language. AF: arcuate fasciculus (short continuous arrow); LFOF: lower frontooccipital fasciculus (long discontinuous arrow); LLF: lower longitudinal fasciculus (long continuous arrow); FP: frontoparietal bundle; UF: uncinate fasciculus (short discontinuous arrow).
Another bundle identified was the uncinate fasciculus (Fig. 2) which connects the anterior frontotemporal cortex, which apparently is not involved in language. It serves as point of reference in locating the lower frontooccipital fasciculus and it is the point of departure for Meyer's ansa and the optic radiations (Fig. 3) (posterior temporooccipital tract forming part of the sagittal stratum, it lies laterally to the occipital horn, with a medial curvature toward the calcarine fissure).
Figs. 4–6 show a case representative of correlation. More cases may be found in Figs. 7–14 only available in the on-line version of Appendix B.
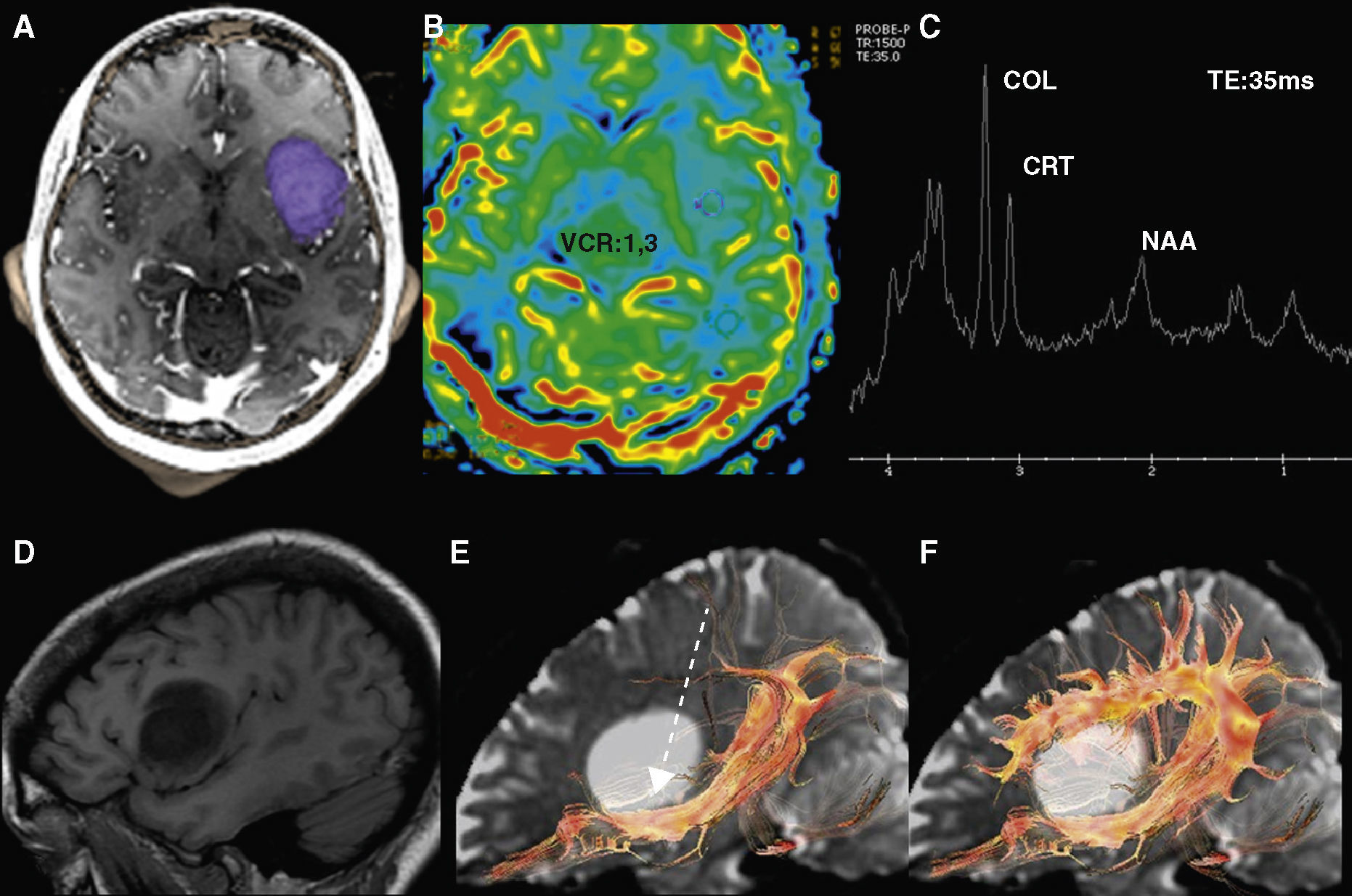
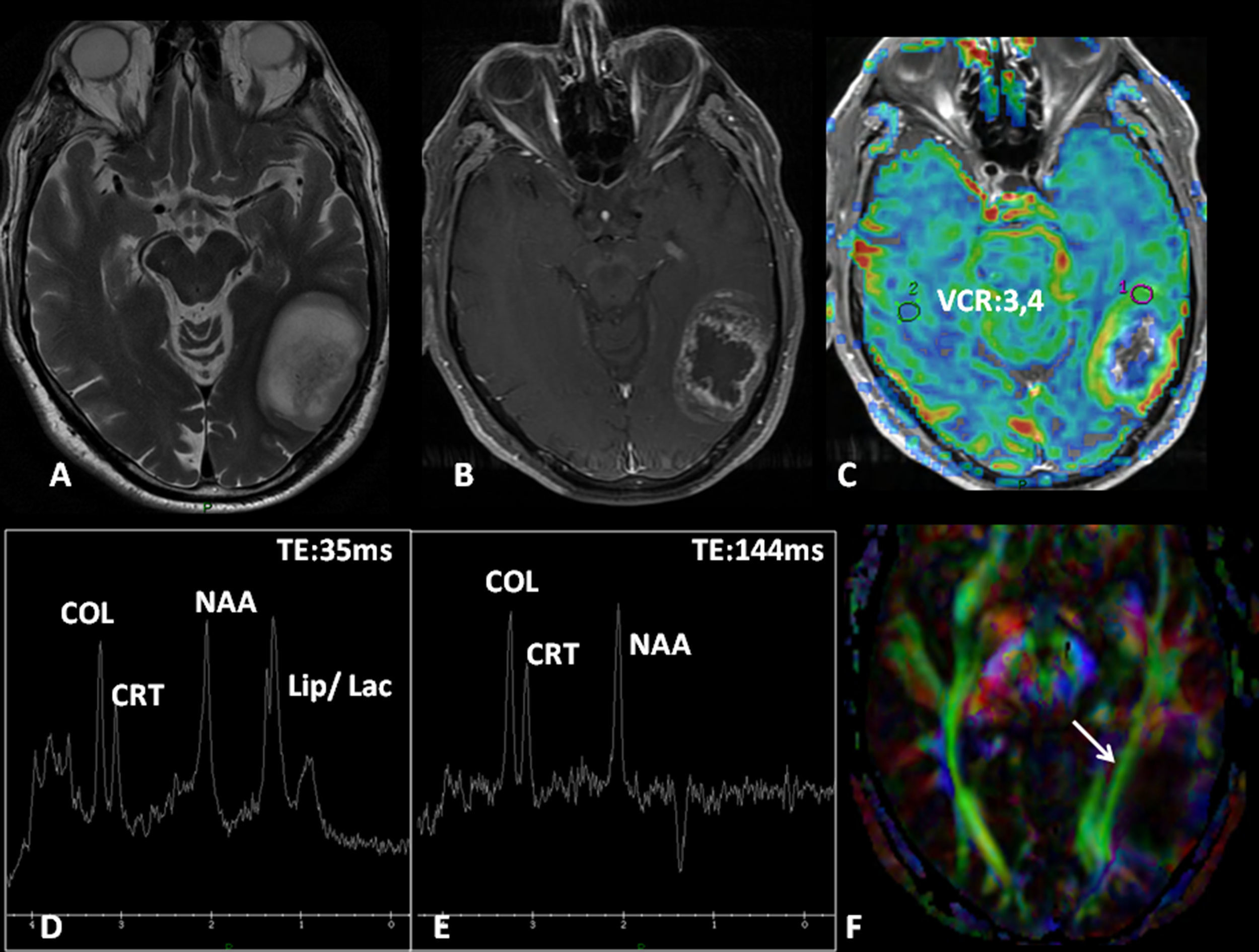
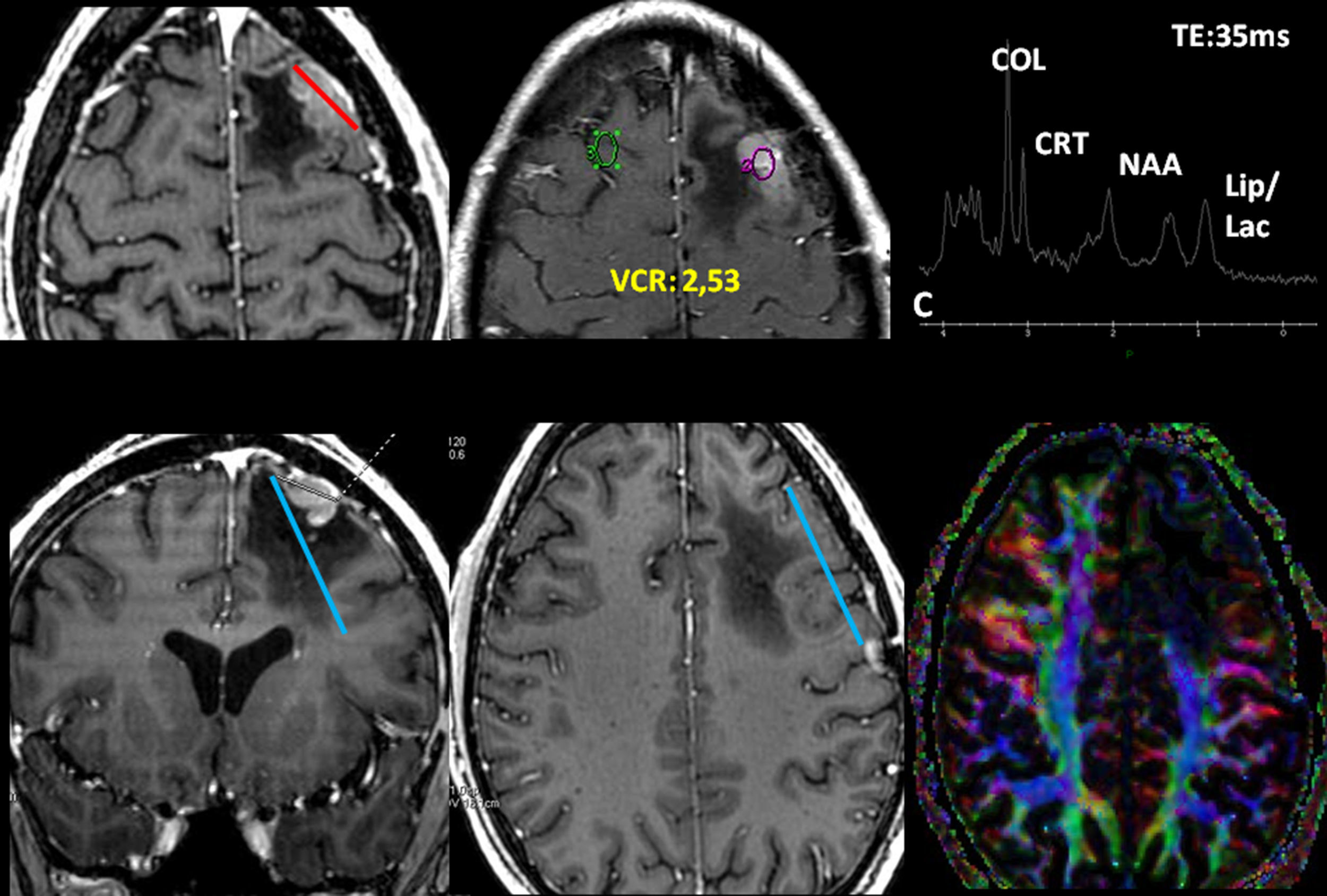
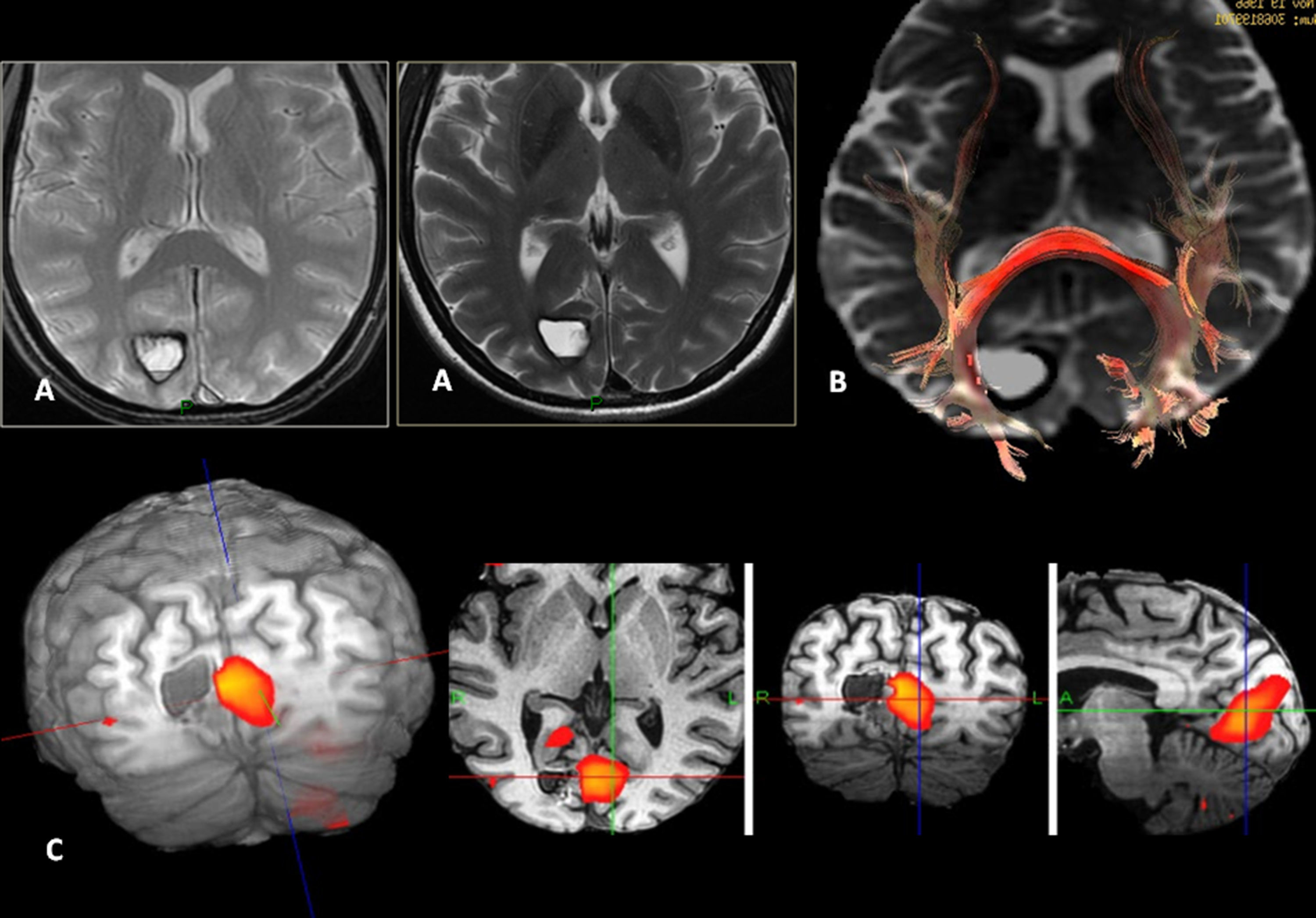
Low-grade oligodendroglioma in the left insular region. 25-Year-old patient with a history of a traffic accident and paresthesia in the upper right limb. (A) Left insular lesion with a tumoral volume of 25cm3. (B) In the perfusion map, he has a relative brain blood volume (RBV) of 1.3. (C) In the spectroscopy-MR study, he presents an increase of cholina and myoinositol, and a discrete reduction of N-acetyl-aspartate. These are data indicative of a low-grade glioma. (D) The T1 sagittal plane shows the relation of the lesion with frontal operculum. (E) The 3D tractography shows an infiltration of the anterior third of the lower frontooccipital fasciculus (discontinuous arrow). (F) The arcuate fasciculus is complete.
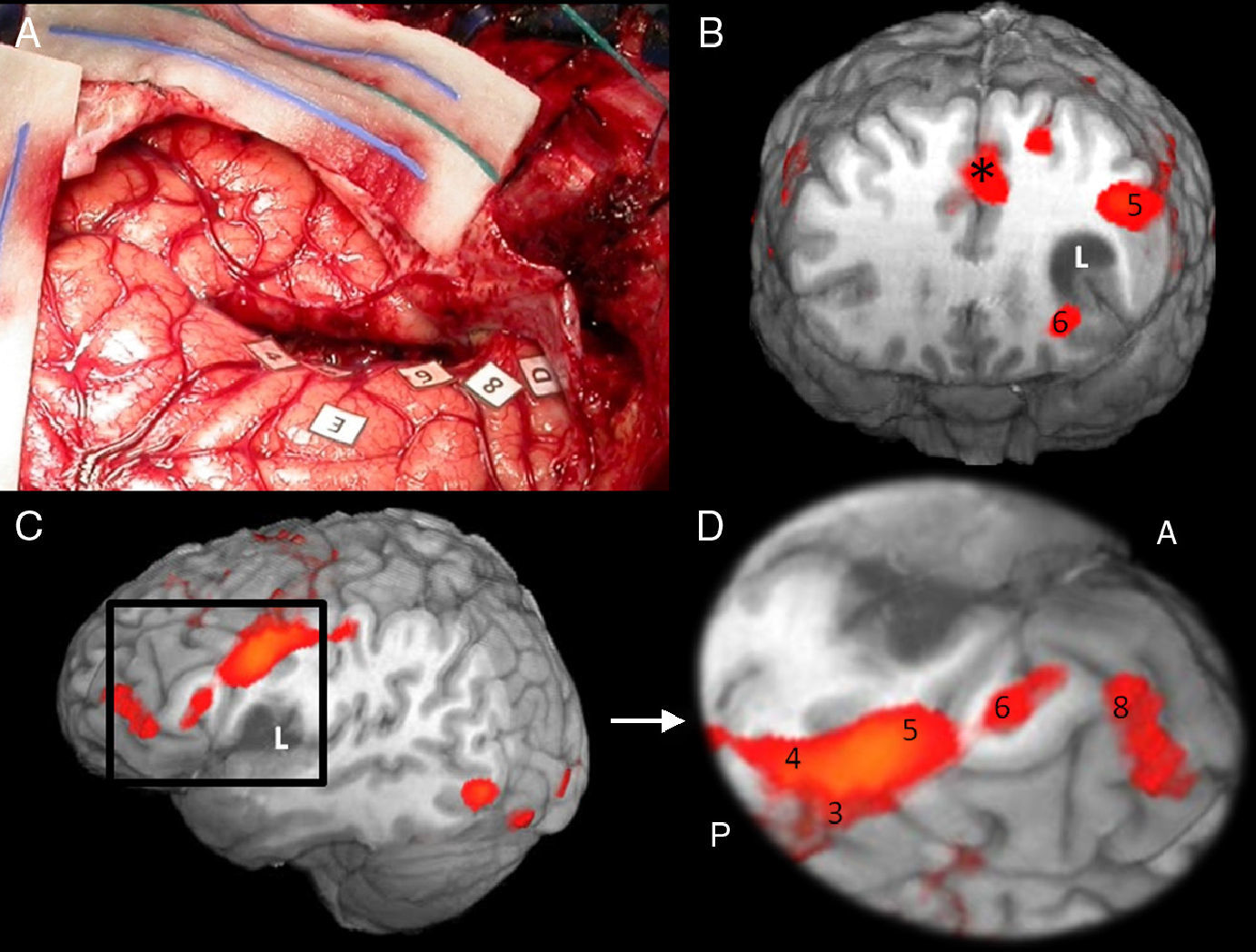
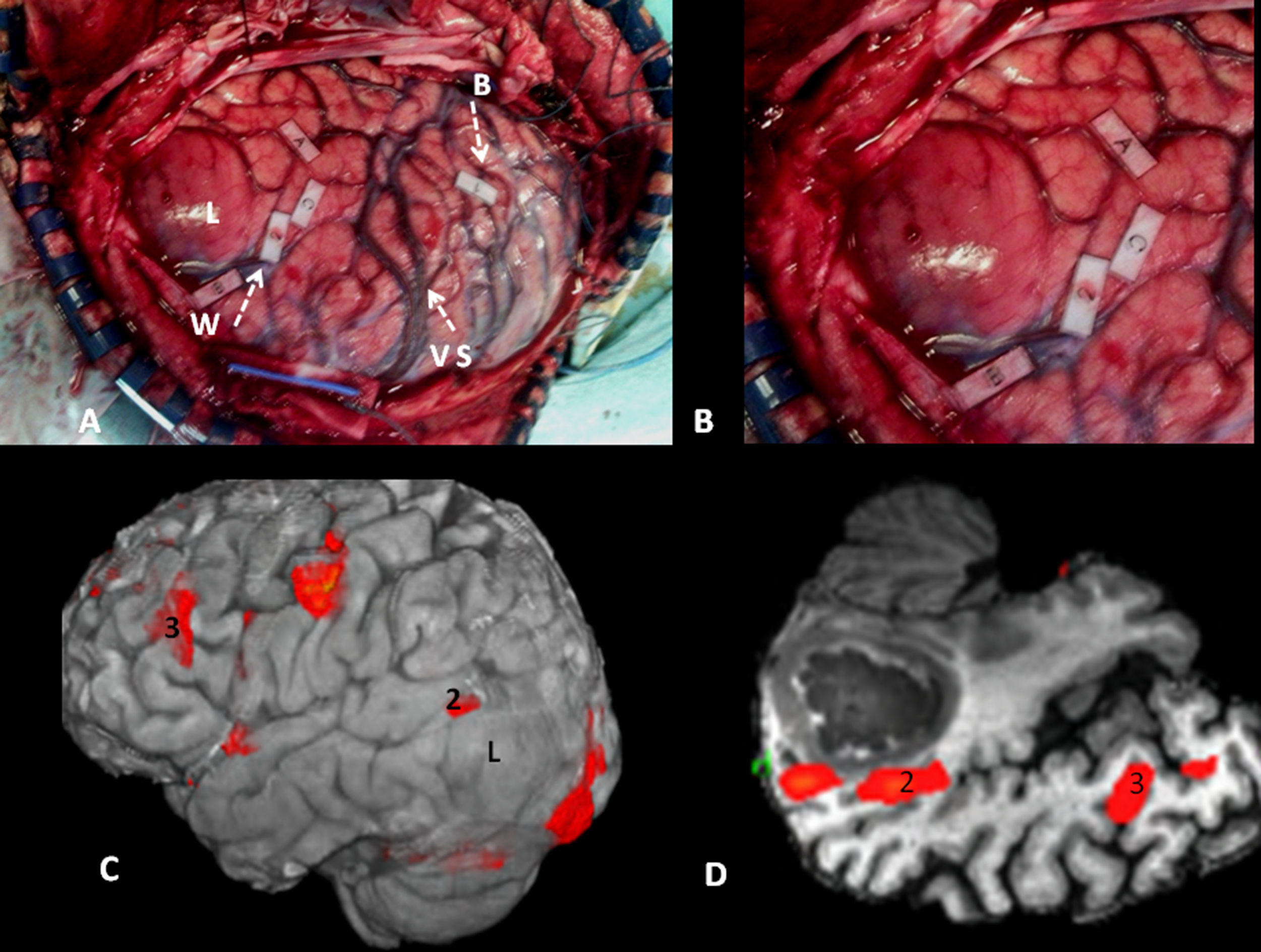
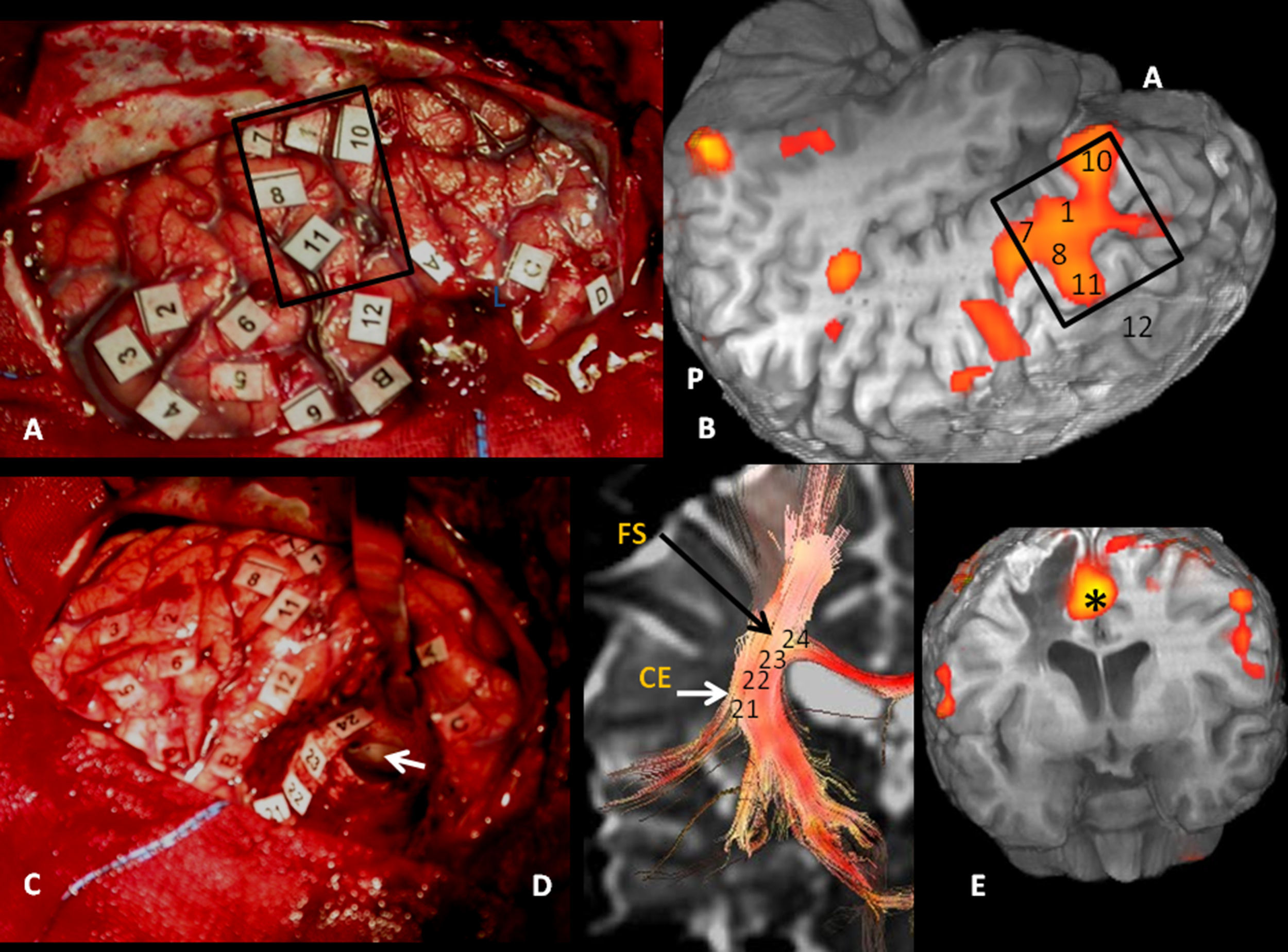
Cortical correlation between functional MR and the surgical electrical mapping in the same patient from Fig. 4. (A) Intraoperative mapping. Patient in right lateral recumbent position. 4: Motor facial cortex; 3: Premotor cortex; 5, 6: Broca's area; 8: Frontal eye field. Correlation with functional MR (B, C and D): activation areas coincidental with cortical mapping. *Supplementary motor area. A: anterior; P: posterior.
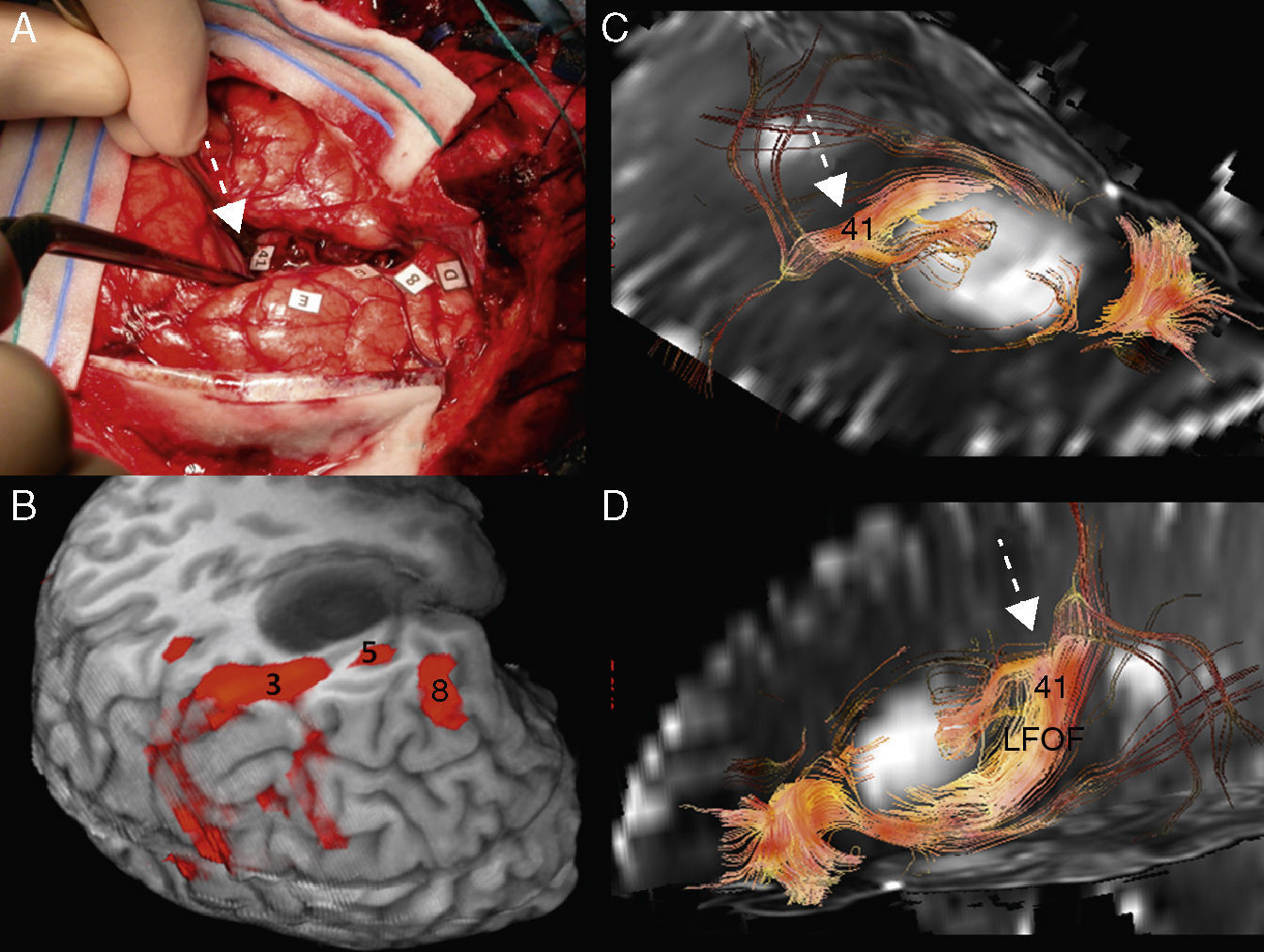
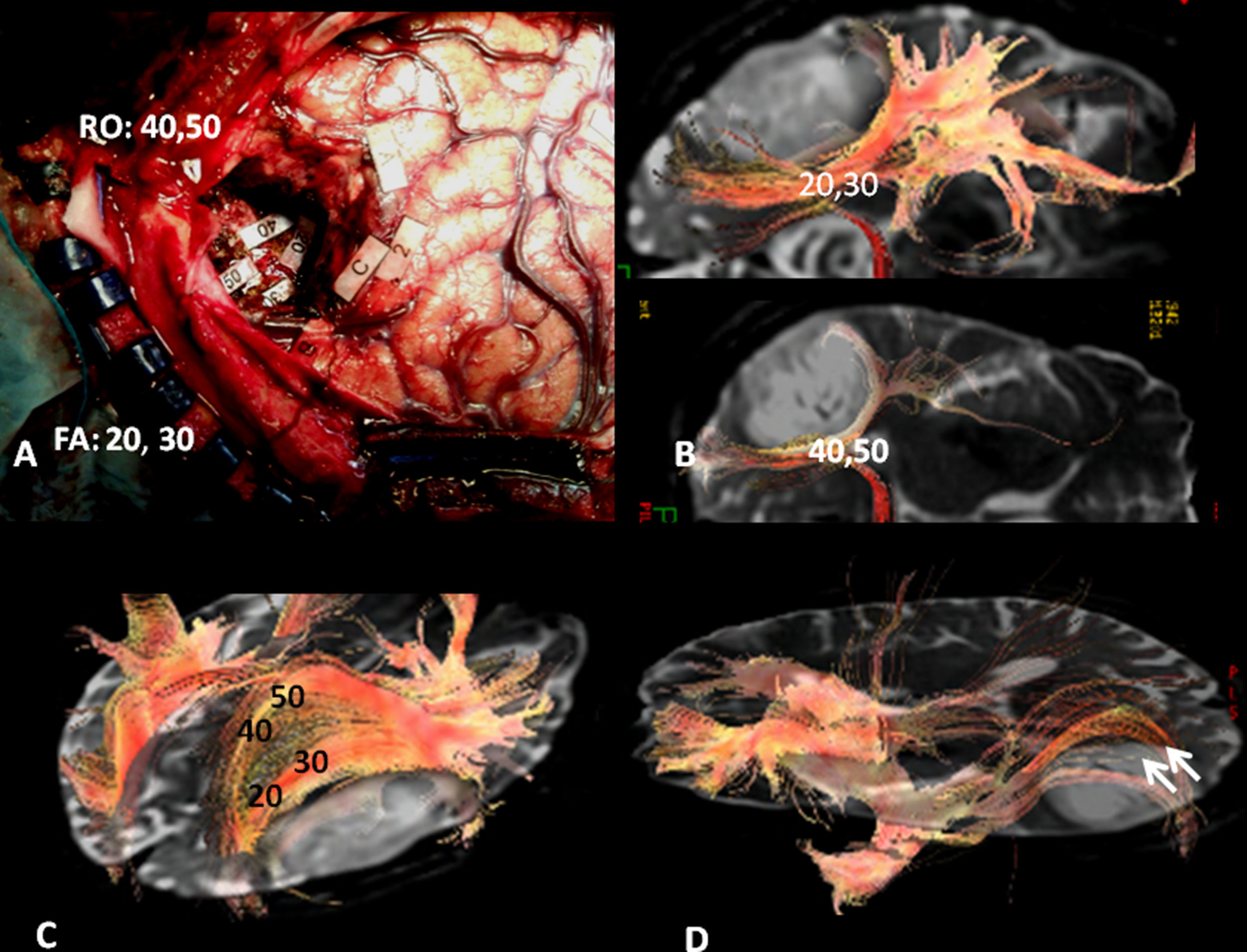
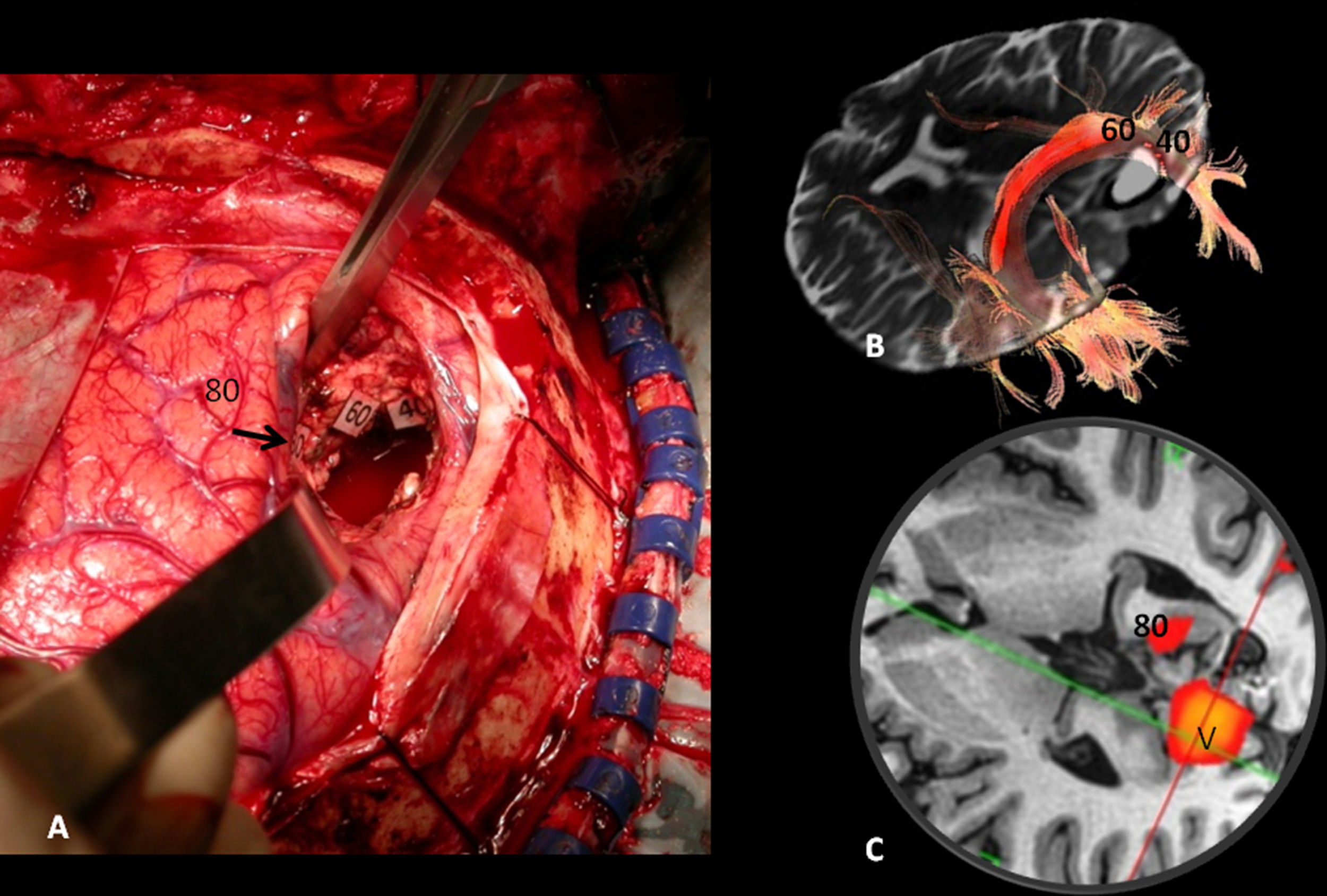
Subcortical correlation between diffusion tensor image (DTI) and the surgical electrical mapping in the same patient from Figs. 4 and 5. (A) Intraoperative subcortical mapping. Patient in right lateral recumbent position. 41: Lower frontooccipital fasciculus (LFOF). (B) Cortical correlation with functional MR: 3: premotor cortex; 5: Broca's area; 8: frontal eye field. 3D-DTI sagittal reconstructions of LFOF. (C and D) The posterior margin of the LFOF (41) (arrow) limits posterior resection of tumor.
Surgical treatment is the choice in brain tumors. Although low-grade glial tumors with volumes below 10cm3 rarely suffer transformation to high grade, its treatment with adjuvant therapies such as chemotherapy or radiotherapy is more effective. Optimization of functional studies and DTI allows for an adequate resolution of the cortical areas and those of the fine subcortical bundles,15,18 as well as for a “reliable” valuation of neuronal activity.
Our article's interest, which shows one of the most extensive series of image test correlation with cortical and subcortical mapping, is confirmation that fMR and DTI offer the neurosurgeon a reliable and realistic preoperative cortical and subcortical map.
The paradigms used in our center are similar to those used in the literature, mainly those of verbal fluency, reading and passive listening,19,20 which may be reproduced in all the patients.
The limitation of functional studies continues to be the inability to quantify accurately the activation area, although this situation is probably just a matter of time. It must be remembered that this quantification is not possible either in cortical or subcortical mapping. Perhaps the best validation of these studies is pre- and post surgery neuropsychological evaluation of the patients without changes being perceived after surgery, as it happened in our patients.
As a conclusion, fMR and DTI sequences allow for adequate presurgery planning of the brain lesions near the eloquent areas. The great correlation between these techniques and surgical cortico-subcortical mapping is indicative of the great progress made by these image techniques.
Authors- 1
Person responsible for the study's integrity: MJP, SGR and MRR.
- 2
Conception of the study: MJP and SGR.
- 3
Design of the study: MJP, SGR, MRR, VMV and CRO.
- 4
Data acquisition: MJP, SGR and MRR.
- 5
Data analysis and interpretation: MJP and SGR.
- 6
Statistical treatment: Not applicable.
- 7
Bibliographic search: MRR.
- 8
Writing of the paper: MJP.
- 9
Critical revision of the manuscript with intellectually relevant contributions: MJP, SGR, MRR, VMV and CRO.
- 10
Approval of the final version: MJP, SGR, MRR, VMV and CRO.
The authors declare that they do not have any conflict of interests.
The authors thank the team of Resonance Technicians from the Image Diagnosis Department.
Roberto García (GE Spain).
Please cite this article as: Jiménez de la Peña M, Gil Robles S, Recio Rodríguez M, Ruiz Ocaña C, Martínez de Vega V. Mapa cortical y subcortical del lenguaje. Correlación de la resonancia magnética funcional y tractografía en 3T con la estimulación intraoperatoria cortical y subcortical en tumores cerebrales localizados en áreas elocuentes. Radiología. 2013;55:505–513.