Supplement "Advances in Musculoskeletal Radiology"
More infoTo evaluate differences in measurements of the lateral recesses and foramina in degenerative lumbar segments on MR images in symptomatic patients obtained with the patient standing versus lying down and to analyze the relationship between possible differences and patients’ symptoms.
Material and methodsWe studied 207 disc levels in 175 patients aged between 17 and 75 years (median: 47 years) with low back pain. All patients underwent MRI in the decubitus position with their legs extended, followed by MRI in the standing position. We calculated the difference in the measurements of the lateral recesses (in mm) and in the foramina (area in mm2 and smallest diameter in mm) obtained in the two positions. To eliminate the effects of possible errors in measurement, we selected cases in which the difference between the measurements obtained in the two positions was ≥10%; we used Student’s t-tests for paired samples to analyze the entire group and subgroups of patients according to age, sex, grade of disc degeneration, and postural predominance of symptoms.
ResultsOverall, the measurements of the spaces were lower when patients were standing. For the lateral recesses, we observed differences ≥10% in 68 (33%) right recesses and in 65 (31.5%) left recesses; when patients were standing, decreases were much more common than increases (26% vs. 7%, respectively, on the right side and 24% vs. 7.5%, respectively, on the left side; p < 0.005). For the foramina, decreases in both the area and in the smallest diameter were also more common than increases when patients were standing: on the right side, areas decreased in 23% and increased in 4%, and smallest diameters decreased in 20% and increased 6%; on the left side, areas decreased in 24% and increased in 4%, and smallest diameters decreased in 17% and increased in 8% (p < 0.005). Considering the group of patients in whom the postural predominance of symptoms was known, we found significant differences in patients whose symptoms occurred predominantly or exclusively when standing, but not in the small group of patients whose symptoms occurred predominantly while lying. We found no differences between sexes in the changes in measurements of the recesses or foramina with standing. The differences between the measurements obtained in different positions were significant in patients aged >40 years, but not in younger groups of patients. Differences in relation to the grade of disc degeneration were significant only in intermediate grades (groups 3–6 in the Griffith classification system).
ConclusionMRI obtained with patients standing can show decreases in the lateral recesses and foramina related to the predominance of symptoms while standing, especially in patients aged >40 years with Griffith disc degeneration grade 3–6, thus providing additional information in the study of patients who have low back pain when standing in whom the findings on conventional studies are inconclusive or discrepant with their symptoms. Further studies are necessary to help better define the value of upright MRI studies for degenerative lumbar disease.
Evaluar los cambios en las medidas de recesos laterales y forámenes de conjunción en segmentos degenerativos lumbares de pacientes sintomáticos estudiados mediante resonancia magnética posicional en decúbito y bipedestación, así como su posible relación con la clínica.
Material y métodosSe estudiaron 207 niveles discales de 175 pacientes de 17 a 75 años (mediana: 47 años) con clínica de dolor lumbar. A todos los pacientes se les realizó, en el mismo procedimiento, resonancia magnética en decúbito con piernas estiradas seguida de resonancia magnética en bipedestación. Se determinó la diferencia de las medidas de sus recesos laterales (en milímetros) y forámenes de conjunción (áreas en milímetros cuadrados y diámetros inferiores en milímetros) entre ambas posiciones. Para los casos con diferencias significativas (≥10% de aumento o disminución entre posiciones, margen fijado para el factor de error de medición) se empleó el test t de Student pareado, presentándose análisis globales y en subgrupos de edad, sexo, grado de degeneración discal y predominancia postural de la clínica.
ResultadosEn el conjunto de niveles, las medias de las medidas de las distintas variables fueron menores en bipedestación. Para los recesos laterales, se observaron diferencias ≥10% en 68 recesos derechos (33%) y 65 izquierdos (31,5%), con clara predominancia de la disminución en bipedestación (disminución en el 26% de niveles vs. aumento en el 7% en los recesos derechos, 24% vs. 7,5% en los izquierdos), con valores de p < 0,005. Con respecto a los forámenes, también predominancia de la disminución en bipedestación de áreas y diámetros inferiores en ambos lados (derechos: áreas 23% disminuciones vs. 4% aumentos, diámetros inferiores 20% vs. 6%. Izquierdos: áreas 24% disminuciones vs. 4% aumentos, diámetros inferiores 17% vs. 8%), igualmente con valores de p < 0,005. Considerando el grupo de pacientes en los que se conocía la predominancia postural de la clínica, existieron diferencias estadísticamente significativas en el grupo de pacientes con clínica predominante o exclusiva en bipedestación, no así en el pequeño grupo de pacientes con clínica en decúbito. No hay diferencias entre sexos en los cambios en bipedestación en los recesos o áreas foraminales. En relación con la edad, las diferencias entre posiciones no son estadísticamente significativas en los grupos más jóvenes, sí en los grupos de mayores de 40 años. Las diferencias en función del grado de degeneración discal solo fueron estadísticamente significativas en grados intermedios (grupos 3–6 de Griffith).
ConclusiónLa resonancia magnética en posición vertical puede evidenciar disminución en recesos laterales y forámenes de conjunción acorde a la predominancia de la sintomatología en bipedestación, preferentemente para el grupo de pacientes mayores de 40 años y con grados de degeneración discal 3 a 6 de Griffith. Ello puede suponer información adicional en el estudio de pacientes con dolor lumbar en bipedestación en caso de resultados no concluyentes o discrepantes con la clínica en los estudios convencionales. Son necesarios ulteriores estudios para ayudar a precisar mejor el valor de la resonancia magnética en bipedestación para el estudio de la patología degenerativa lumbar.
Degenerative conditions that affect different elements of the lumbar spine are a significant cause of low back pain and radiculopathy and are a major indication for performing imaging tests, including magnetic resonance imaging (MRI).1 Disc protrusion, focal or diffuse, can cause compression of the nerve roots in the lateral recesses, in the intervertebral foramen or where the nerve roots exit the spinal column.2 Facet joint degeneration is also a common cause of radicular pain.3
Most of the symptoms in patients with lumbar disease occur primarily in weight-bearing positions, such as sitting or standing. Discogenic pain, the main cause of nonspecific low back pain, particularly chronic pain, has a clear mechanical influence: it is caused by positions and activities that increase intradiscal pressure and stress on the annulus fibrosus, and is relieved by lying down. Radicular pain is acute and lancing. It is most often caused by disc herniation and, to a lesser extent, spinal and foraminal stenosis. Facet joint pain should be suspected in patients over 60 who have pain on standing or walking, and the pain is relieved by sitting and increases with ipsilateral extension and palpation. Degenerative instability (in its different phases of dysfunction, instability or re-stabilisation) is closely related to chronic pain and involves a series of abnormal movements potentially related to changes in posture.4–6
Images obtained in the supine position do not provide adequate information about the physiological changes that occur in a weight-bearing position, in a sitting position or with changes in position.7–9 Most conventional imaging techniques do not have adequate precision to accurately identify the actual cause of the pain, which is not only frustrating for the patient and physician, but also makes it extremely difficult for clinicians to design a specific treatment plan for the patient.10 The poor correlation between imaging findings and the clinical presentation of patients with painful degenerative disease of the spine is a well-documented problem.11 It has led many working groups to investigate the role of new techniques, including positional MRI (pMRI) and dynamic MRI (kMRI) scans.12
Many studies have shown that changes occur in standing or sitting positions with respect to lying down in the lumbar spine's different spaces and structures, such as the disc space, the spinal canal, the lateral recesses and the foramina. These changes can compromise neural structures related to pain. Based on the assumption that the degenerated segment is unstable with altered biomechanics, and that the instability is associated with mechanical low back pain, we wanted to determine whether or not being in an upright position with weight-bearing was sufficient to cause quantifiable differences in the MRI measurements of lateral recesses and foramina, by comparing standing scans to supine scans with legs extended. Our aim was to find out whether these changes were associated with certain factors or circumstances in certain patients, and whether these patients might benefit from the study using this technique.
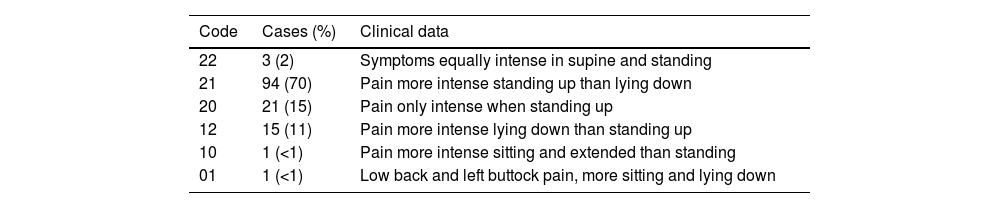
Material and methodsIn total, 175 patients aged 17–75 years (median 47) with symptoms of mainly chronic, mechanical low back pain (lumbago, sciatica or lumbosciatica), lasting months or years, referred to our department for lumbar MRI in standing position, were studied. The patients came mainly from neurosurgery, trauma and pain units, and many of them had previously been examined using imaging techniques, including conventional MRI. All the patients in the study had symptoms at the time of the test, although clinical information about the posture in which the pain predominated was only available in 115 patients. Pain intensity was coded, as reported by the patient, with a value of 2 (maximum), 1 (moderate-slight) and 0 (no pain), in a two-digit code, where the first digit corresponds to symptoms in standing position and the second to symptoms lying down. In the vast majority (85%) of the 115 patients for whom we knew the predominance and intensity of the symptoms, they occurred or worsened when standing (groups 21, 20 and 10) (Table 1).
Clinical data of the levels included, expressed as absolute value and as a percentage of the total number of cases with clinical information (N = 135), according to the position in which the symptoms manifest or intensify.a
| Code | Cases (%) | Clinical data |
|---|---|---|
| 22 | 3 (2) | Symptoms equally intense in supine and standing |
| 21 | 94 (70) | Pain more intense standing up than lying down |
| 20 | 21 (15) | Pain only intense when standing up |
| 12 | 15 (11) | Pain more intense lying down than standing up |
| 10 | 1 (<1) | Pain more intense sitting and extended than standing |
| 01 | 1 (<1) | Low back and left buttock pain, more sitting and lying down |
The studies were performed on a 0.6 T Stand-Up TM MRI® (Fonar Corp, Melville, NY), with a top/front-open design, which incorporates a scanning table with tilt, translation and elevation functions, allowing the patient to be placed in weight-bearing upright and horizontal positions. A flexible surface antenna was used in the lumbar area. The scans were performed first supine with legs extended and then standing. To help them tolerate the position, patients were allowed to rest their forearms on a support in front of them slightly below chest height, although without leaning their weight on the support. The mean duration of the scans was 15 min for supine and 10 min for standing.
The study protocol was the same in all cases:
Scan in supine position
- •
Sagittal T2-weighted TSE: TR 2000, TE 140, 4-mm slice thickness, 0.4-mm gap. FOV 28 cm and matrix 256 × 256.
- •
Axial T2-weighted TSE: five slices obtained for each lumbar segment analysed, with TR 2000-300, TE 120, FOV 25 mm, matrix 256 × 224, 4-mm slice thickness and 0.5-mm gap.
- •
Sagittal T1-weighted: TR 350-400, TE 25, 4-mm slice thickness, FOV 44 and matrix 512 × 256.
Scan in standing position
- •
Sagittal T2-weighted TSE: same protocol as for supine.
- •
Axial T2-weighted TSE: same protocol as for supine.
In each patient, for taking measurements, we considered the level or levels where there were signs of prominent degenerative involvement (loss of signal and height in T2-weighted sequences according to the Griffith classification,13 bulging-protrusion-disc herniation, facet joint degeneration, spinal canal stenosis, degenerative listhesis and/or Modic type I and II changes). None of the cases was post-traumatic or diagnosed as cancer or infectious disease. All cases that had factors making it impossible to compare the chosen levels between positions (accentuated scoliosis and motion artifacts or surgical material in one or more sequences to be compared) were excluded. In the end, 207 disc levels were included, belonging to the 175 patients in the study. Of these, 135 disc levels corresponded to the 115 patients in whom the postural predominance of the symptoms was known.
Image analysis (taking measurements) was performed by a single examiner. The intraclass correlation coefficients for the different parameters ranged from 0.92 to 0.99.
Recess measurements: in axial slices, we determined the distances between the disc margin lined by the posterior vertebral ligament (anterior limit) and the cranial part of the facet joint lined by the ligamentum flavum. This corresponded to zone I as described by Anderson and McNeill.14
Foramen measurements: in sagittal slices, the smallest diameters and areas of both foramina, right and left, were measured in each analysed segment. We considered as first choice Anderson and McNeill zone II, at the level of the pedicles, as upper and lower limits, but when it was estimated that there might be differences at that level due to discrepancies between positions in the level of slice, some foramina lateral to the pedicle in zone III were considered.
Data analysis: to take into account the possible error factor (changes in measurements not attributable to the difference between supine and standing), only increases or decreases greater than or equal to 10% in the standing measurement compared to the supine measurement were considered significant. Therefore, we analysed the statistical significance of the differences of 10% or higher between the values of the variables in the two postures using paired Student’s t-tests. We considered as statistically significant a confidence interval greater than 95% (p < 0.05).
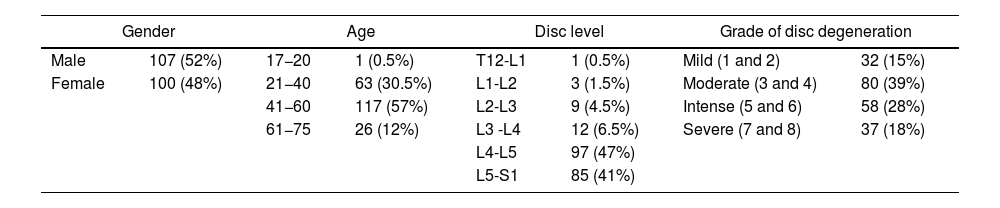
Results and discussionThe number of significant changes with respect to gender, age, disc level and grade of disc degeneration is shown in Table 2. L4-L5 (97; 47%) and L5-S1 (85; 41%) accounted for most of the levels analysed. The eight grades of disc degeneration according to the Griffith classification system13 were condensed into four groups: mild (grades 1 and 2); moderate (grades 3 and 4); intense (grades 5 and 6); and severe (grades 7 and 8). Most of the cases analysed were in the moderate and intense groups (Griffith 3–6).
Number of levels included, expressed as absolute value and as a percentage of the total number of cases (N = 207) according to gender, age group, disc level and grade of disc degeneration.
| Gender | Age | Disc level | Grade of disc degeneration | ||||
|---|---|---|---|---|---|---|---|
| Male | 107 (52%) | 17−20 | 1 (0.5%) | T12-L1 | 1 (0.5%) | Mild (1 and 2) | 32 (15%) |
| Female | 100 (48%) | 21−40 | 63 (30.5%) | L1-L2 | 3 (1.5%) | Moderate (3 and 4) | 80 (39%) |
| 41−60 | 117 (57%) | L2-L3 | 9 (4.5%) | Intense (5 and 6) | 58 (28%) | ||
| 61−75 | 26 (12%) | L3 -L4 | 12 (6.5%) | Severe (7 and 8) | 37 (18%) | ||
| L4-L5 | 97 (47%) | ||||||
| L5-S1 | 85 (41%) | ||||||
There were no significant differences (above a threshold of 10%) following the change from lying down to standing at most of the levels assessed. When variations were detected, the measurements of the different parameters either increased or decreased with the change from lying to standing, but predominantly decreased (Table 3), which suggests a compromise of space. The mean changes in the foramina and recesses between positions and their confidence intervals were analysed for all levels (Table 4) and according to the different variables (gender, age, grade of disc degeneration and postural predominance of the symptoms, Tables 5–8). When analysing the changes according to the different variables, different behaviour was found with the different age groups, grade of disc degeneration and postural predominance of the symptoms, but not with gender. We believe that the results support the significance of instability as abnormal, unpredictable movement patterns of the vertebral segment due, in this case, to gravity-dependent loading.
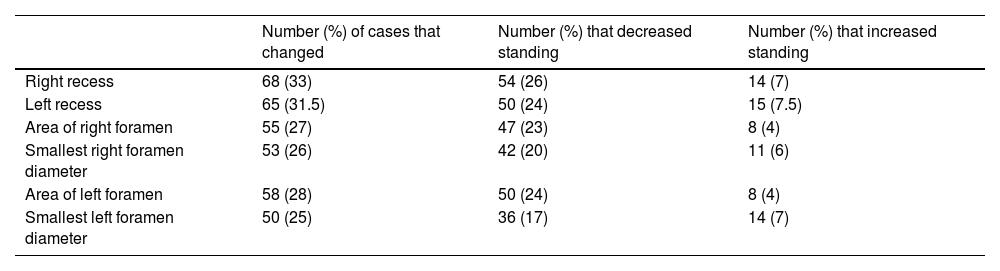
Number of cases, expressed as absolute value and as a percentage of the total number of cases (N = 207), that decreased or increased with the positional change from supine to standing.
| Number (%) of cases that changed | Number (%) that decreased standing | Number (%) that increased standing | |
|---|---|---|---|
| Right recess | 68 (33) | 54 (26) | 14 (7) |
| Left recess | 65 (31.5) | 50 (24) | 15 (7.5) |
| Area of right foramen | 55 (27) | 47 (23) | 8 (4) |
| Smallest right foramen diameter | 53 (26) | 42 (20) | 11 (6) |
| Area of left foramen | 58 (28) | 50 (24) | 8 (4) |
| Smallest left foramen diameter | 50 (25) | 36 (17) | 14 (7) |
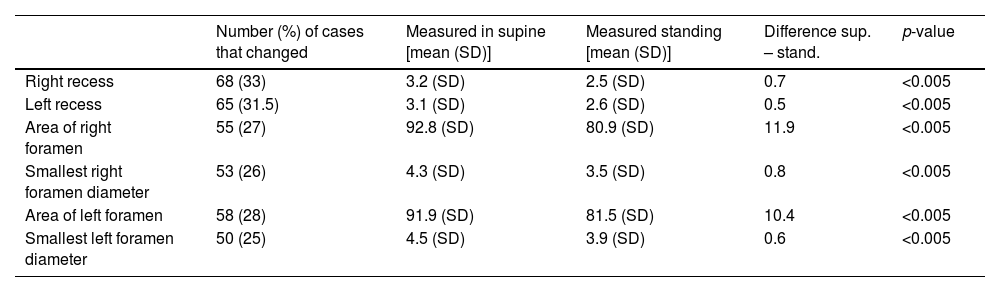
Mean measurements (in millimetres for recesses and foramen diameters, and in millimetres squared for the areas of the foramina) of the parameters that changed ≥10% from supine to standing.
| Number (%) of cases that changed | Measured in supine [mean (SD)] | Measured standing [mean (SD)] | Difference sup. – stand. | p-value | |
|---|---|---|---|---|---|
| Right recess | 68 (33) | 3.2 (SD) | 2.5 (SD) | 0.7 | <0.005 |
| Left recess | 65 (31.5) | 3.1 (SD) | 2.6 (SD) | 0.5 | <0.005 |
| Area of right foramen | 55 (27) | 92.8 (SD) | 80.9 (SD) | 11.9 | <0.005 |
| Smallest right foramen diameter | 53 (26) | 4.3 (SD) | 3.5 (SD) | 0.8 | <0.005 |
| Area of left foramen | 58 (28) | 91.9 (SD) | 81.5 (SD) | 10.4 | <0.005 |
| Smallest left foramen diameter | 50 (25) | 4.5 (SD) | 3.9 (SD) | 0.6 | <0.005 |
p-values using paired Student’s t-test.
SD: standard deviation.
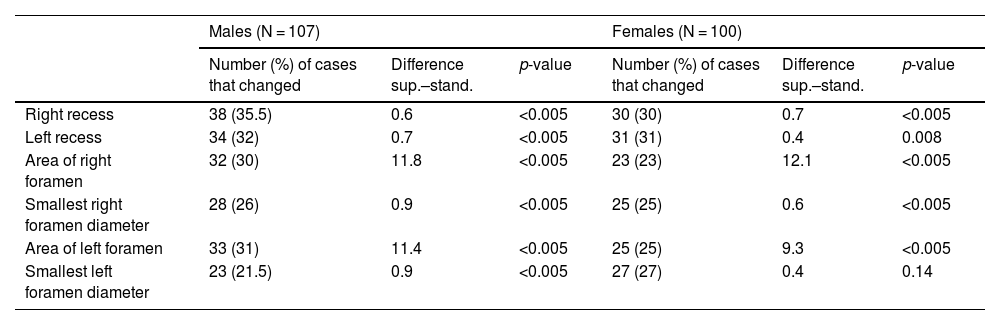
Differences in measurements (in millimetres for recesses and foramen diameters, and in millimetres squared for the areas of the foramina) for cases with ≥10% variation (absolute value and percentage of N in each case) according to gender.
| Males (N = 107) | Females (N = 100) | |||||
|---|---|---|---|---|---|---|
| Number (%) of cases that changed | Difference sup.–stand. | p-value | Number (%) of cases that changed | Difference sup.–stand. | p-value | |
| Right recess | 38 (35.5) | 0.6 | <0.005 | 30 (30) | 0.7 | <0.005 |
| Left recess | 34 (32) | 0.7 | <0.005 | 31 (31) | 0.4 | 0.008 |
| Area of right foramen | 32 (30) | 11.8 | <0.005 | 23 (23) | 12.1 | <0.005 |
| Smallest right foramen diameter | 28 (26) | 0.9 | <0.005 | 25 (25) | 0.6 | <0.005 |
| Area of left foramen | 33 (31) | 11.4 | <0.005 | 25 (25) | 9.3 | <0.005 |
| Smallest left foramen diameter | 23 (21.5) | 0.9 | <0.005 | 27 (27) | 0.4 | 0.14 |
p-values using paired Student's t-test.
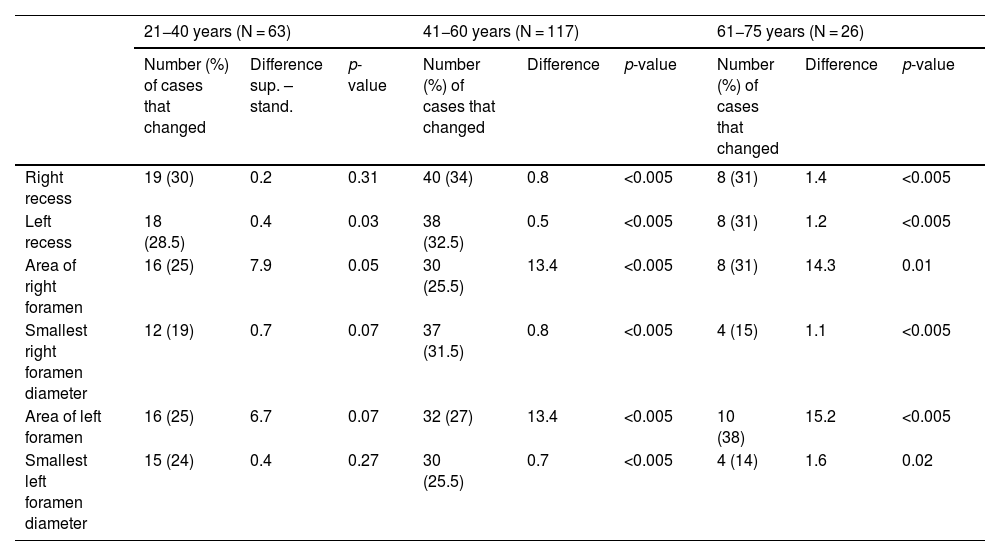
Differences in measurements (in millimetres for recesses and foramen diameters, and in millimetres squared for the areas of the foramina) for cases of ≥10% variation (absolute value and percentage of N in each case) according to age group.
| 21−40 years (N = 63) | 41−60 years (N = 117) | 61−75 years (N = 26) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Number (%) of cases that changed | Difference sup. – stand. | p-value | Number (%) of cases that changed | Difference | p-value | Number (%) of cases that changed | Difference | p-value | |
| Right recess | 19 (30) | 0.2 | 0.31 | 40 (34) | 0.8 | <0.005 | 8 (31) | 1.4 | <0.005 |
| Left recess | 18 (28.5) | 0.4 | 0.03 | 38 (32.5) | 0.5 | <0.005 | 8 (31) | 1.2 | <0.005 |
| Area of right foramen | 16 (25) | 7.9 | 0.05 | 30 (25.5) | 13.4 | <0.005 | 8 (31) | 14.3 | 0.01 |
| Smallest right foramen diameter | 12 (19) | 0.7 | 0.07 | 37 (31.5) | 0.8 | <0.005 | 4 (15) | 1.1 | <0.005 |
| Area of left foramen | 16 (25) | 6.7 | 0.07 | 32 (27) | 13.4 | <0.005 | 10 (38) | 15.2 | <0.005 |
| Smallest left foramen diameter | 15 (24) | 0.4 | 0.27 | 30 (25.5) | 0.7 | <0.005 | 4 (14) | 1.6 | 0.02 |
p-values using paired Student’s t-test.
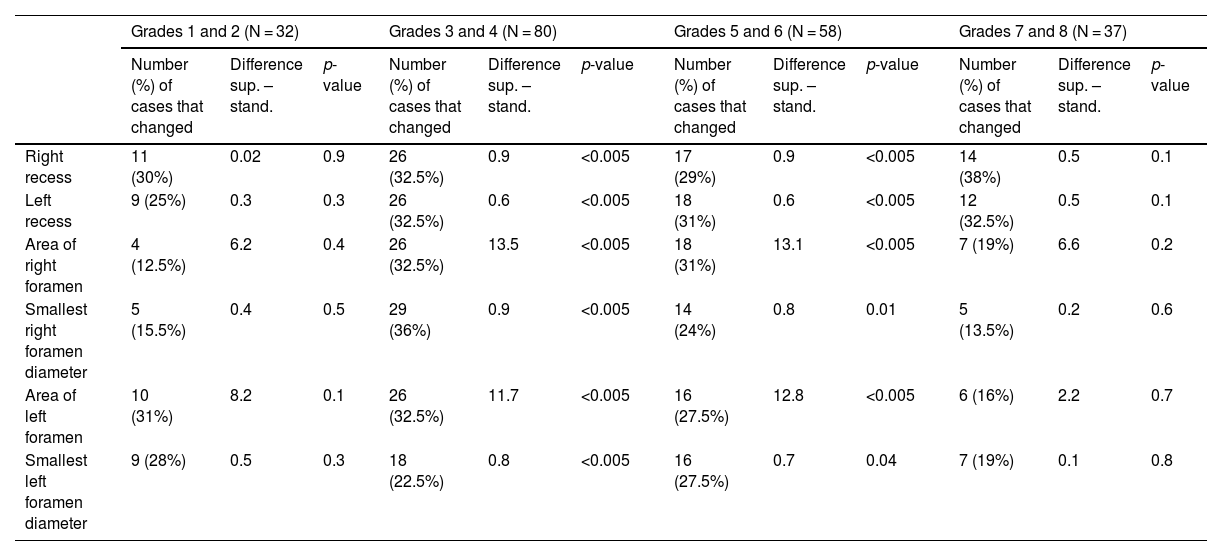
Differences in measurements (in millimetres for recesses and foramen diameters, and in millimetres squared for the areas of the foramina) for cases of ≥10% variation (absolute value and percentage of N in each case) according to grade of disc degeneration.
| Grades 1 and 2 (N = 32) | Grades 3 and 4 (N = 80) | Grades 5 and 6 (N = 58) | Grades 7 and 8 (N = 37) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number (%) of cases that changed | Difference sup. – stand. | p-value | Number (%) of cases that changed | Difference sup. – stand. | p-value | Number (%) of cases that changed | Difference sup. – stand. | p-value | Number (%) of cases that changed | Difference sup. – stand. | p-value | |
| Right recess | 11 (30%) | 0.02 | 0.9 | 26 (32.5%) | 0.9 | <0.005 | 17 (29%) | 0.9 | <0.005 | 14 (38%) | 0.5 | 0.1 |
| Left recess | 9 (25%) | 0.3 | 0.3 | 26 (32.5%) | 0.6 | <0.005 | 18 (31%) | 0.6 | <0.005 | 12 (32.5%) | 0.5 | 0.1 |
| Area of right foramen | 4 (12.5%) | 6.2 | 0.4 | 26 (32.5%) | 13.5 | <0.005 | 18 (31%) | 13.1 | <0.005 | 7 (19%) | 6.6 | 0.2 |
| Smallest right foramen diameter | 5 (15.5%) | 0.4 | 0.5 | 29 (36%) | 0.9 | <0.005 | 14 (24%) | 0.8 | 0.01 | 5 (13.5%) | 0.2 | 0.6 |
| Area of left foramen | 10 (31%) | 8.2 | 0.1 | 26 (32.5%) | 11.7 | <0.005 | 16 (27.5%) | 12.8 | <0.005 | 6 (16%) | 2.2 | 0.7 |
| Smallest left foramen diameter | 9 (28%) | 0.5 | 0.3 | 18 (22.5%) | 0.8 | <0.005 | 16 (27.5%) | 0.7 | 0.04 | 7 (19%) | 0.1 | 0.8 |
p-values using paired Student’s t-test.
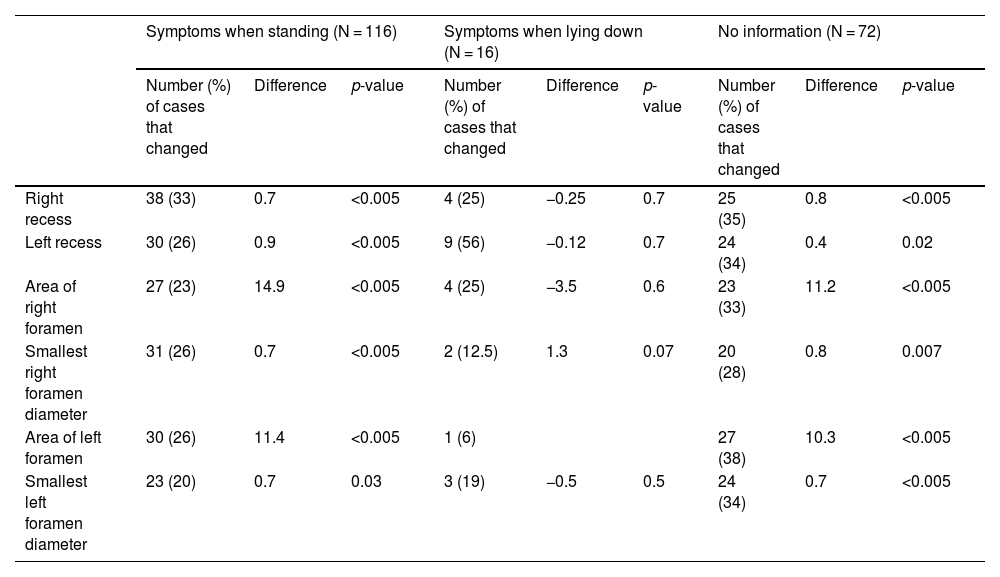
Differences in measurements (in millimetres for recesses and foramen diameters, and in millimetres squared for the areas of the foramina) for cases of ≥10% variation (absolute value and percentage of N in each case) according to postural predominance of the symptoms.
| Symptoms when standing (N = 116) | Symptoms when lying down (N = 16) | No information (N = 72) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Number (%) of cases that changed | Difference | p-value | Number (%) of cases that changed | Difference | p-value | Number (%) of cases that changed | Difference | p-value | |
| Right recess | 38 (33) | 0.7 | <0.005 | 4 (25) | −0.25 | 0.7 | 25 (35) | 0.8 | <0.005 |
| Left recess | 30 (26) | 0.9 | <0.005 | 9 (56) | −0.12 | 0.7 | 24 (34) | 0.4 | 0.02 |
| Area of right foramen | 27 (23) | 14.9 | <0.005 | 4 (25) | −3.5 | 0.6 | 23 (33) | 11.2 | <0.005 |
| Smallest right foramen diameter | 31 (26) | 0.7 | <0.005 | 2 (12.5) | 1.3 | 0.07 | 20 (28) | 0.8 | 0.007 |
| Area of left foramen | 30 (26) | 11.4 | <0.005 | 1 (6) | 27 (38) | 10.3 | <0.005 | ||
| Smallest left foramen diameter | 23 (20) | 0.7 | 0.03 | 3 (19) | −0.5 | 0.5 | 24 (34) | 0.7 | <0.005 |
p-values using paired Student’s t-test.
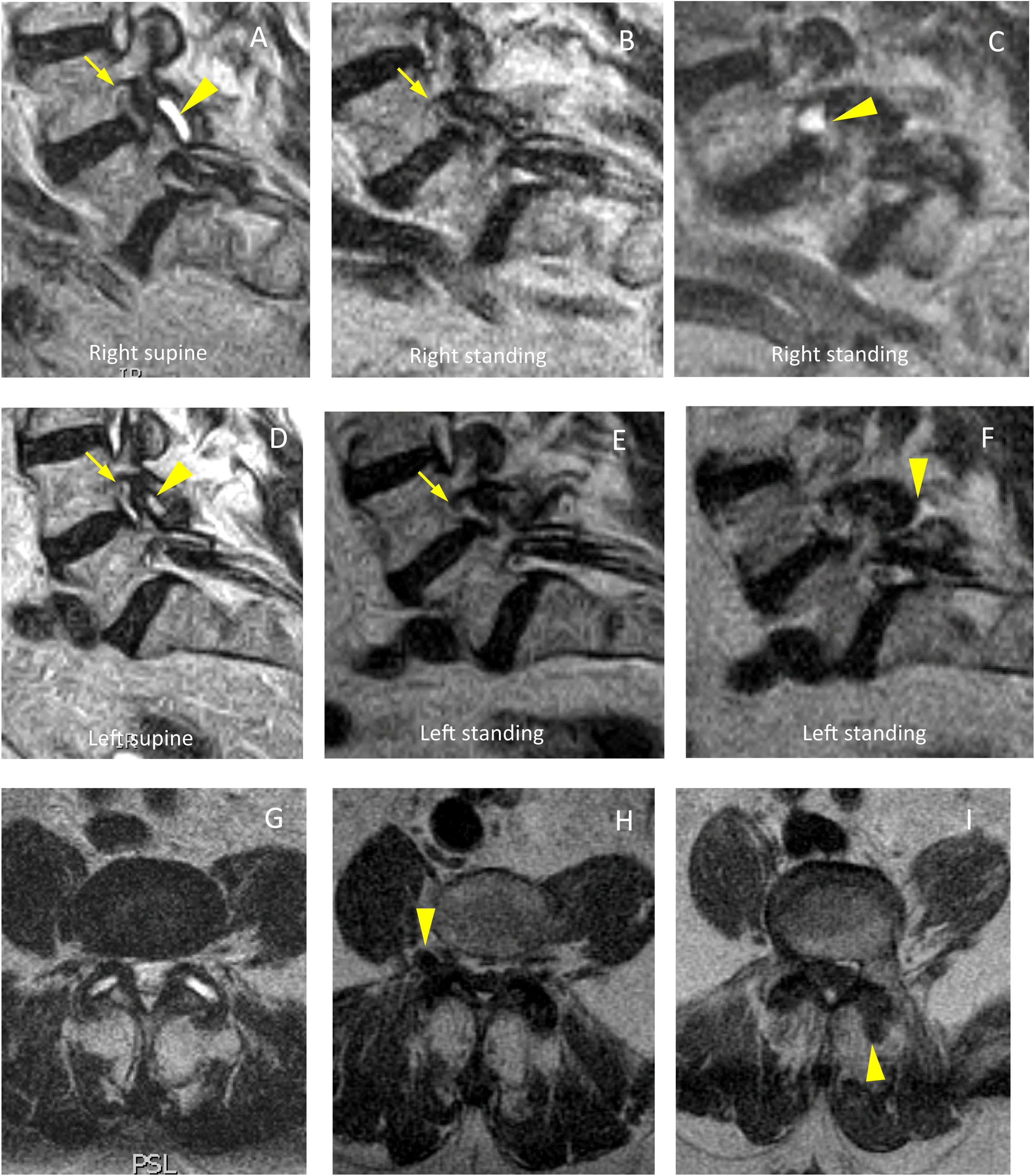
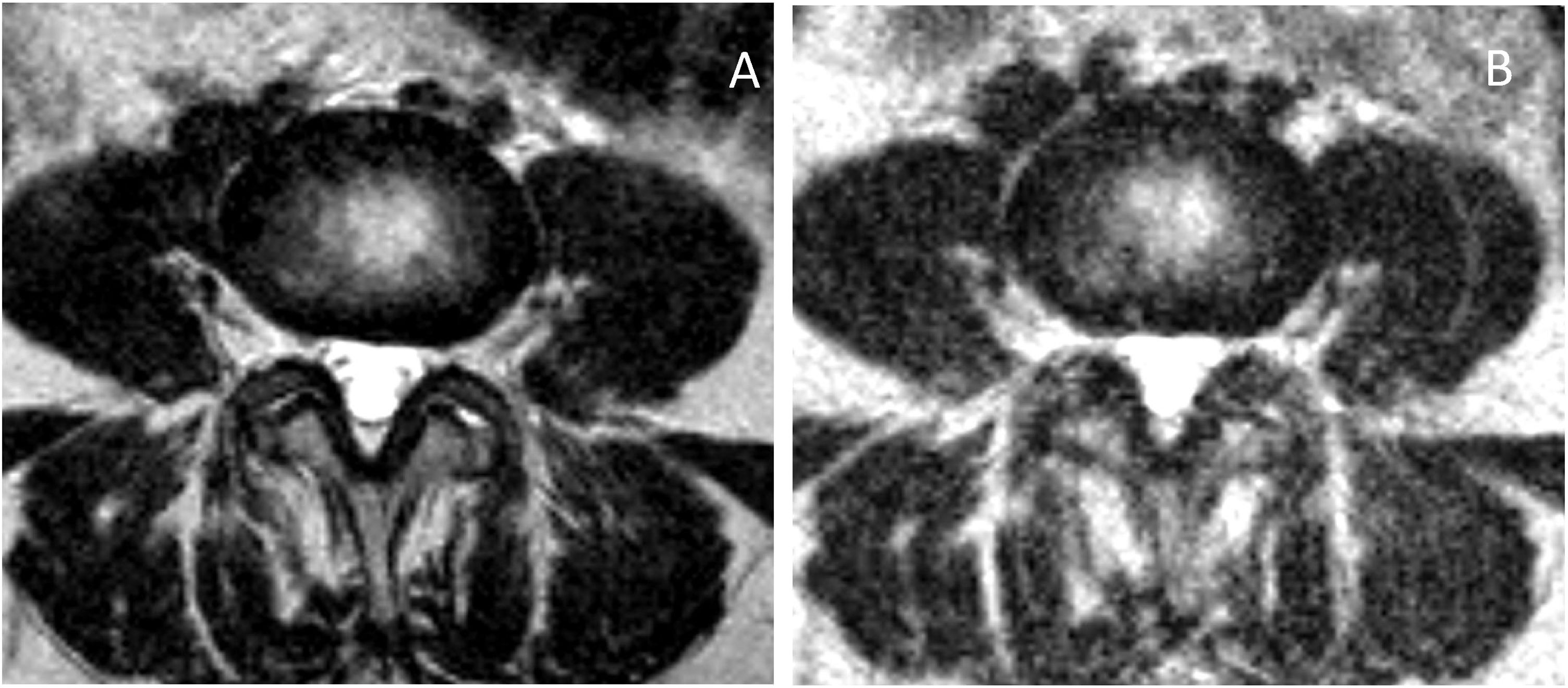
The foramina are structures in which we expect a postural difference that may be related to the symptoms (Fig. 1). In changes from lying to standing, the foraminal area will potentially be sensitive to changes originating mainly in the ligamenta flava (posterior limit of the foraminal area, where the capsule of the facet joint can also undergo change) or the disc (anterior limit at the level of the smallest foramen diameters). Reduction of the foramina in extension compared to flexion has been well described in cadaveric models and in multiple subjective and objective studies.8,15–22 Statistically significant differences have been described with regard to changes from lying to standing in the decreases in foraminal areas in both asymptomatic21 and symptomatic patients, and even more so in an upright position with hyperlordosis.23 Other studies describe a decrease in width and foraminal area in symptomatic patients compared to healthy volunteers.12 The term “occult foraminal stenosis” describes stenosis visualised only under weight-bearing conditions, and it is always associated with degenerative disc and facet joint disease.24 In our study, statistically significant differences were found for the decrease in areas and diameters bilaterally in standing scans in all patients (see Table 4). In terms of variables, we found clearly differentiated results when taking into account age and the grade of disc degeneration. By age, the results were different in the 41-to-60 age group and the over-61 s (where the decrease when standing was statistically significant) compared to the 21-to-40 age group (see Table 6). For the grade of disc degeneration, there was a clear difference in the moderate and intense degeneration groups (Griffith 3–6, with statistically significant decreases in standing in all the parameters on both sides), compared to the mild-grade groups (1 and 2) and more severely affected (7 and 8) (see Table 7). This suggests that in the group with a lesser grade of degenerative disease, there is less postural effect and, at the other extreme, in the more severe grades of disc degeneration, to a certain extent segments are perhaps less unstable compared to less degenerated discs because they are in the re-stabilisation phase.4–6 For the postural predominance of the symptoms, statistical significance was found for patients with symptoms predominantly in standing position (see Table 8), but not for foraminal changes in patients with symptoms lying down. We believe there is a correlation in the decreases in foraminal smallest diameters and area with respect to the positional predominance of the symptoms. However, the results suggest a multifactorial component that goes beyond the mere morphological changes visualised in anatomical structures by MRI (Fig. 2). The well-documented anatomy of the intervertebral foramina, the neurovascular relationships and the exquisite complexity of the foraminal ligaments25–27 give us an idea of the challenges still to be overcome in imaging of the lumbar spine.
Bilateral foraminal stenosis associated with instability with L4-L5 facet joint degeneration. (A) Sagittal slice in supine position at the level of the right foramen, with foraminal stenosis (arrow), thickening of the ligamentum flavum and facet joint fluid (arrowhead). (B) Sagittal slice in standing position at the same level, with the foramen of similar size (arrow), not visualising the image of facet joint fluid at that level. At a more lateral parasagittal level (C), an extraforaminal cyst can be seen, an expression of the displacement of the facet joint fluid. (D) Sagittal slice in supine position at the level of the left foramen, with foraminal stenosis (arrow) as well as facet joint fluid (arrowhead). (E) Sagittal slice in standing position at the same level, with the foramen of similar size (arrow), with the synovial joint fluid at that level not clearly visible. At an adjacent level (F), posterior fluid displacement is visible (arrowhead). (G) Axial slice in supine. (H) Axial slice with T1-weighted sequence in standing position at a lower level, where the right-sided displaced cyst is evident (arrowhead). (I) Lower consecutive slice in standing position, with visualisation of the left-sided fluid displaced posteriorly (arrowhead).
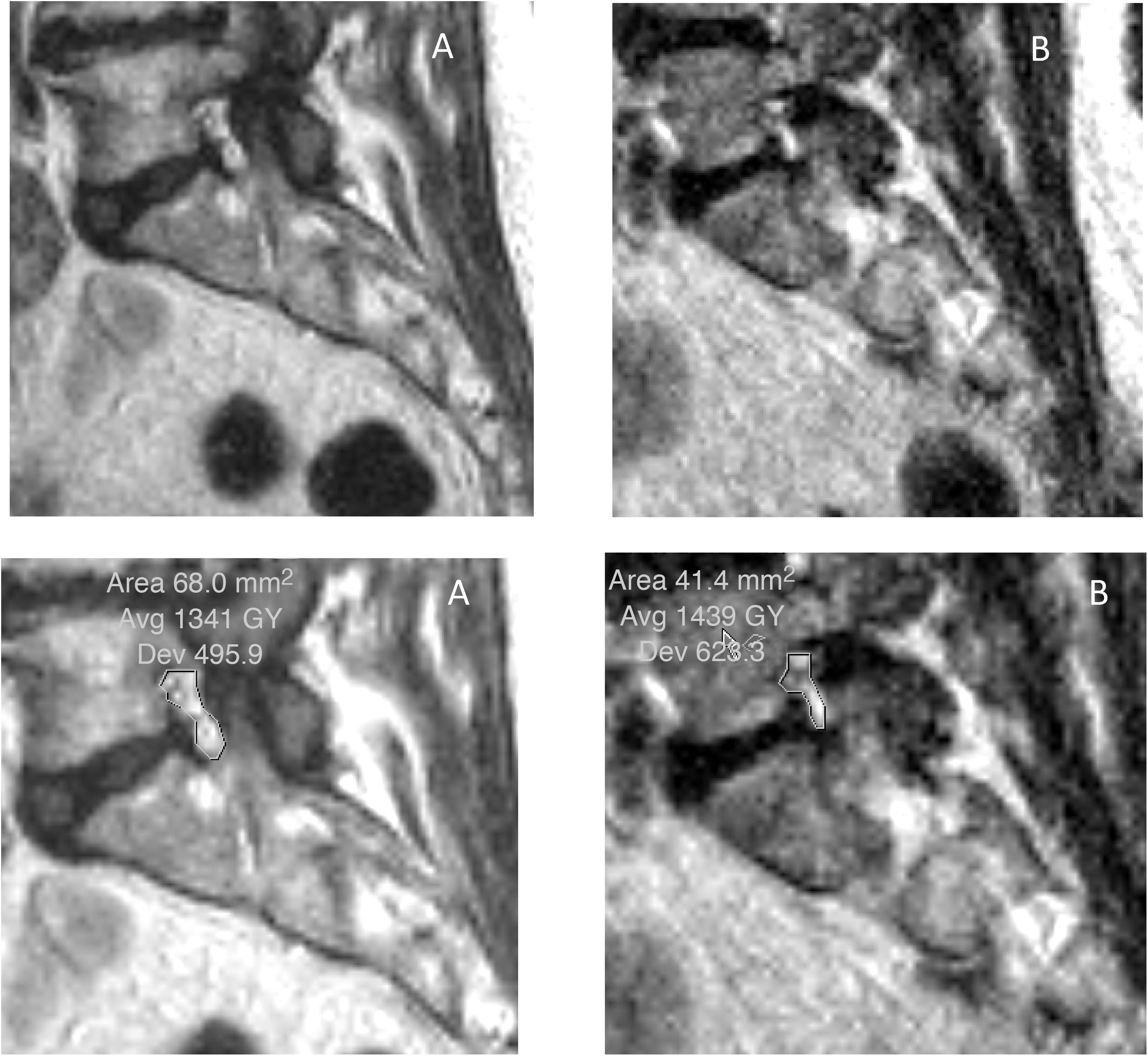
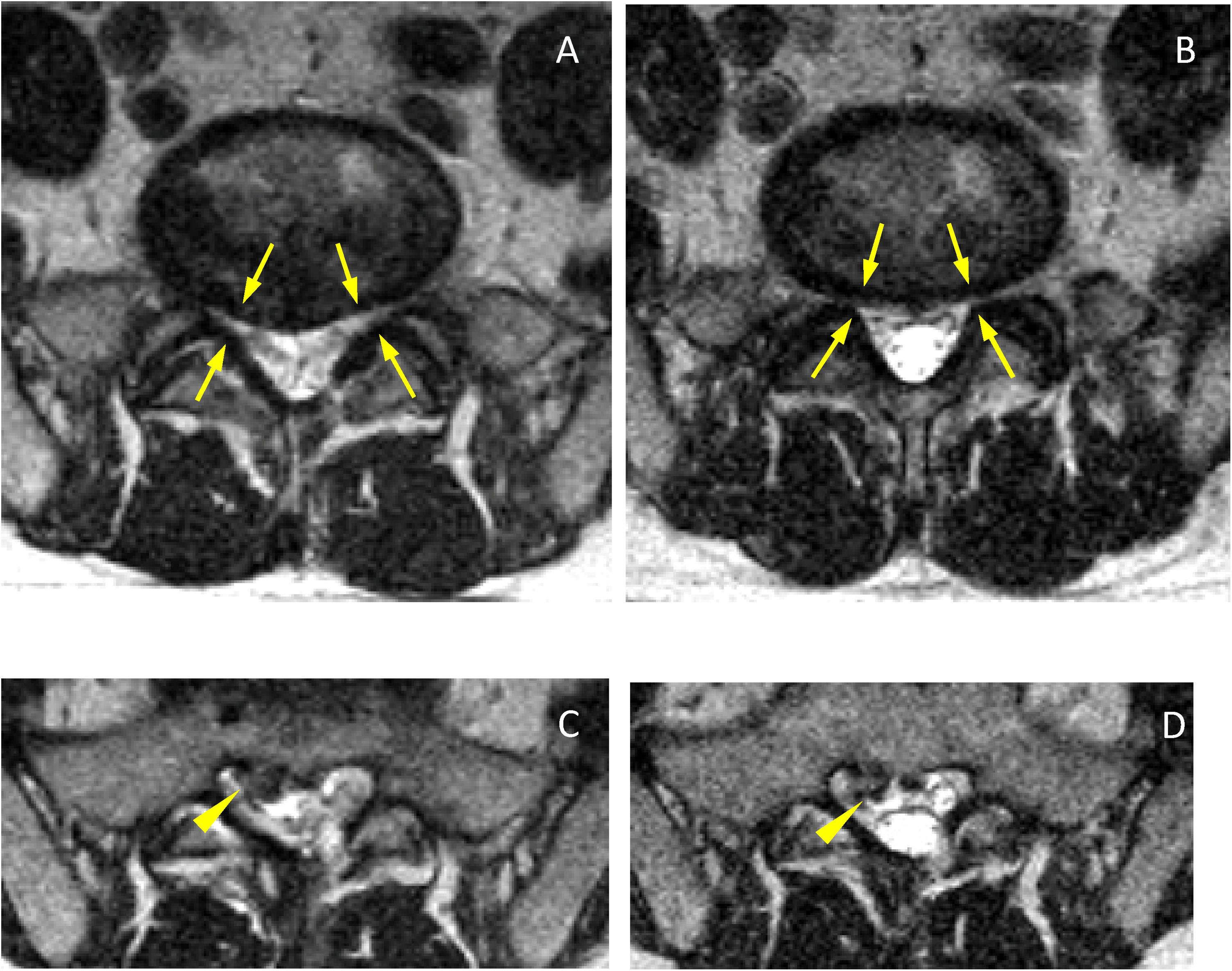
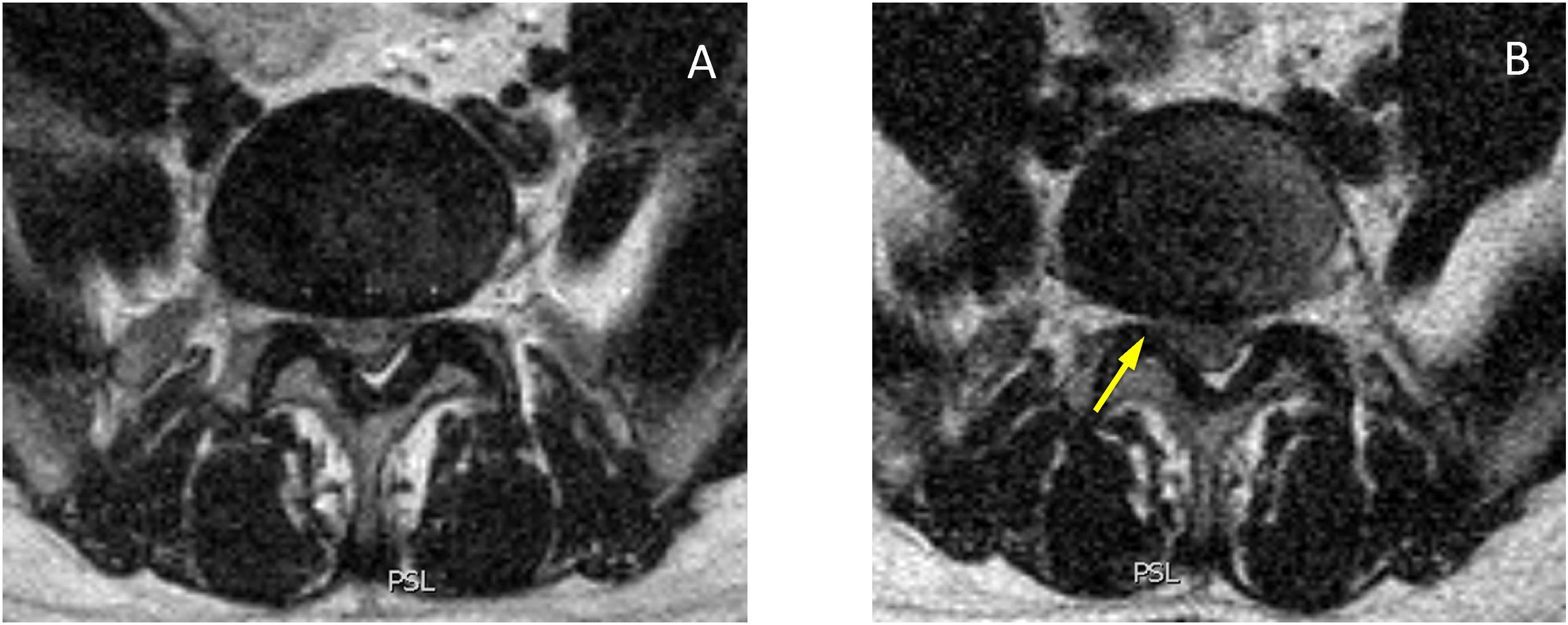
In the lateral recesses, the findings were comparable to those obtained in the foraminal variations. We found statistical significance for the decrease when standing for the entire group of patients (see Table 4), in the age groups over 40 (see Table 6), in the groups with moderate and intense degeneration (Griffith 3–6) (see Table 7) and for patients with symptoms predominantly in standing position (see Table 8). The lateral recesses are potentially susceptible to change due to changes in the discs, facet joints and lateral regions of the ligamenta flava (Fig. 3). In addition to potential facet joint changes, we believe that both disc bulging (considered non-pathological in the Gilbert study28) and disc protrusion (focal or broad-based) are important positional findings for reducing the size of the lateral recesses, foramina and spinal canal. In transferring from the supine position to the standing position, an increase in the compressive force on the discs is expected, as a result of gravity and muscle activation29 (Figs. 4 and 5). Moreover, the increased extension in the standing position creates an added effect to the bulging-protrusion of the disc, an effect that we consider comparable in the supine position with legs extended. We consider the impairment of the annulus fibrosus to be of great importance in the loading function suffered by the degenerated disc, with or without annular tears. We believe that disc bulging may play an important role, with statistically significant differences for all cases overall, in patients aged over 41 and discs with Griffith degeneration grades 3–6. The change from supine to standing position would be of little consequence in younger patients, and so slightly degenerated discs (with minimal or slight changes in the nucleus pulposus) would be relatively undamaged. Little positional change was also seen in the more degenerated discs. Zou et al. describe how more degenerated discs are less predictable than healthier discs. The response of the degenerated disc to postural changes is influenced by structural and degenerative changes that make it very difficult to anticipate a typical or unique response to such positional changes.30 Further investigations are pending to assess the effect of flexion-extension on this and other findings.
Axial slices at the level of the posterior disc margin L5-S1 in supine (A) and standing (B). Reduction of both lateral recesses (arrows), even at a lower level a right posterior-medial hernia is visible (C, arrowhead) which does not increase significantly in standing position (D, arrowhead). In the standing sections, the increase in CSF and the greater diameter of the dural sac (B and D) can be seen.
Axial slices with sp T2 in supine (A) and standing (B). Low-signal material corresponding to nucleus pulposus forming a large central disc herniation that occupies a large part of the spinal canal slice. Greater involvement of the right recess in standing position (arrow), with less impact on the left recess and without significant changes in hernia dimensions.
Our study has some limitations. It would have been preferable to have clinical data on all the cases in the study. We were not able to rely with sufficient precision on the clinical information pertaining to pain to establish the specific type of pain or to use any of the pain recording systems published in the scientific literature. The number of cases in some subgroups when considering the changes according to variables was very small, thereby limiting the value of the results in terms of significance. Although the image quality was optimal, we found limitations in the degree of precision of the high magnetic field devices, an unavoidable factor with open-design devices. The artifacts obtained by involuntary movements when in the standing position were much greater than those obtained in routine studies lying down (Fig. 4B). We had to halt some scans due to poor tolerance to the test, but there were no falls, blackouts or other complications of note.
ConclusionIn most of the patients in our series, there were no significant changes in the measurements of their recesses or foramina when standing compared to lying down with legs extended. However, there can be a decrease in the upright MRI measurements in the lateral recesses and foramina when the symptoms are predominantly in standing position, and this decrease is found particularly for the group of patients aged over 40 and with Griffith grades 3–6 disc degeneration. This may provide additional information in the study of patients with low back pain when standing if results are inconclusive or inconsistent with the symptoms in conventional studies. Further studies are necessary to help better define the value of standing MRI for the study of degenerative lumbar disease.
Authorship- 1
Person responsible for the integrity of the study: MGI.
- 2
Study conception: MGI and AFP.
- 3
Study design: AFP and MGI.
- 4
Data collection: MGI, AFP, MSFLP, MM and PFG.
- 5
Data analysis and interpretation: MGI, AFP, MSFLP, MM and PFG.
- 6
Statistical processing: MGI and AFP.
- 7
Literature search: MGI, AFP, MSFLP, MM and PFG.
- 8
Drafting of the article: MGI and AFP.
- 9
Critical review of the manuscript with intellectually relevant contributions: AFP, PFG, MSFLP and MM.
- 10
Approval of the final version: AFP, MSFLP, MM and PFG.
The authors declare that they have no conflicts of interest.