To describe the radiologic findings of extrapulmonary air in the chest and to review atypical and unusual causes of extrapulmonary air, emphasizing the importance of the diagnosis in managing these patients.
ConclusionIn this article, we review a series of cases collected at our center that manifest with extrapulmonary air in the thorax, paying special attention to atypical and uncommon causes. We discuss the causes of extrapulmonary according to its location: mediastinum (spontaneous pneumomediastinum with pneumorrhachis, tracheal rupture, dehiscence of the bronchial anastomosis after lung transplantation, intramucosal esophageal dissection, Boerhaave syndrome, tracheoesophageal fistula in patients with esophageal tumors, bronchial perforation and esophagorespiratory fistula due to lymph-node rupture, and acute mediastinitis), pericardium (pneumopericardium in patients with lung tumors), cardiovascular (venous air embolism), pleura (bronchopleural fistulas, spontaneous pneumothorax in patients with malignant pleural mesotheliomas and primary lung tumors, and bilateral pneumothorax after unilateral lung biopsy), and thoracic wall (infections, transdiaphragmatic intercostal hernia, and subcutaneous emphysema after lung biopsy).
Describir los hallazgos radiológicos y revisar las causas atípicas e inusuales de aire torácico extrapulmonar, incidiendo en la importancia del diagnóstico para el manejo de estos pacientes.
ConclusiónEn este artículo revisaremos una serie de casos recogidos en nuestro centro que se manifiestan como aire torácico extrapulmonar, con especial atención a las causas atípicas e infrecuentes. Según su localización anatómica, las clasificaremos en: localización mediastínica (neumomediastino espontáneo con neumorraquis, rotura traqueal, dehiscencia de sutura bronquial en trasplante pulmonar, disección intramucosa esofágica, síndrome de Boerhaave, fístula traqueoesofágica en neoplasia esofágica, perforación bronquial y fístula esofagorrespiratoria por rotura de ganglio linfático, y mediastinitis aguda), localización pericárdica (neumopericardio en neoplasia pulmonar), localización cardiovascular (embolia venosa aérea), localización pleural (fístulas broncopleurales, neumotórax espontáneo en mesotelioma pleural maligno y neoplasia pulmonar primaria, y neumotórax bilateral posbiopsia pulmonar unilateral) y localización en pared torácica (infecciones, hernia intercostal transdiafragmática y enfisema subcutáneo posbiopsia pulmonar).
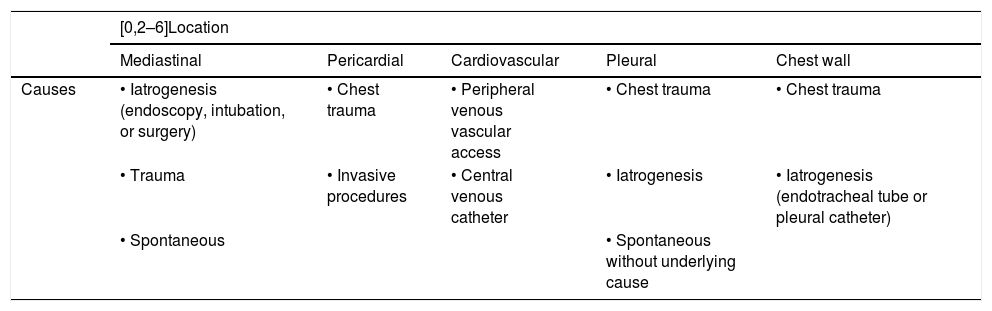
The presence of thoracic air in an ectopic location can be seen in different pathologies (Table 1). In this article we will present the atypical and unusual causes of extrapulmonary thoracic air. To do so, we will classify the causes by their location: mediastinal, pericardial, cardiovascular, pleural and in the chest wall.
Most common causes of ectopic extrapulmonary thoracic air.
| [0,2–6]Location | |||||
|---|---|---|---|---|---|
| Mediastinal | Pericardial | Cardiovascular | Pleural | Chest wall | |
| Causes | • Iatrogenesis (endoscopy, intubation, or surgery) | • Chest trauma | • Peripheral venous vascular access | • Chest trauma | • Chest trauma |
| • Trauma | • Invasive procedures | • Central venous catheter | • Iatrogenesis | • Iatrogenesis (endotracheal tube or pleural catheter) | |
| • Spontaneous | • Spontaneous without underlying cause | ||||
From an anatomical point of view, there are no barriers in the subcutaneous tissue in any part of the body or in the perivertebral space, so air can circulate freely. The visceral and retropharyngeal cervical spaces communicate with the mediastinum, and the thoracic cavity connects with the abdomen through the diaphragmatic hiatus.1
Mediastinal locationThe origin of the pneumomediastinum (PM) may be intrathoracic (airway stenosis or obstruction, Valsalva manoeuvre, trauma or alveolar rupture) or extrathoracic (paranasal sinus fracture, tooth extraction or intestinal perforation).2
A chest X-ray frequently reveals the presence of air anterior to the pericardium in the lateral view (pneumopericardium). In the posteroanterior projection we are able to identify a continuous air collection between the two hemidiaphragms (continuous diaphragm sign). Air in the mediastinum may also surround the pulmonary arteries (ring-around-the-artery sign), the aorta (tubular artery sign), or a main bronchus (double bronchial wall sign).3
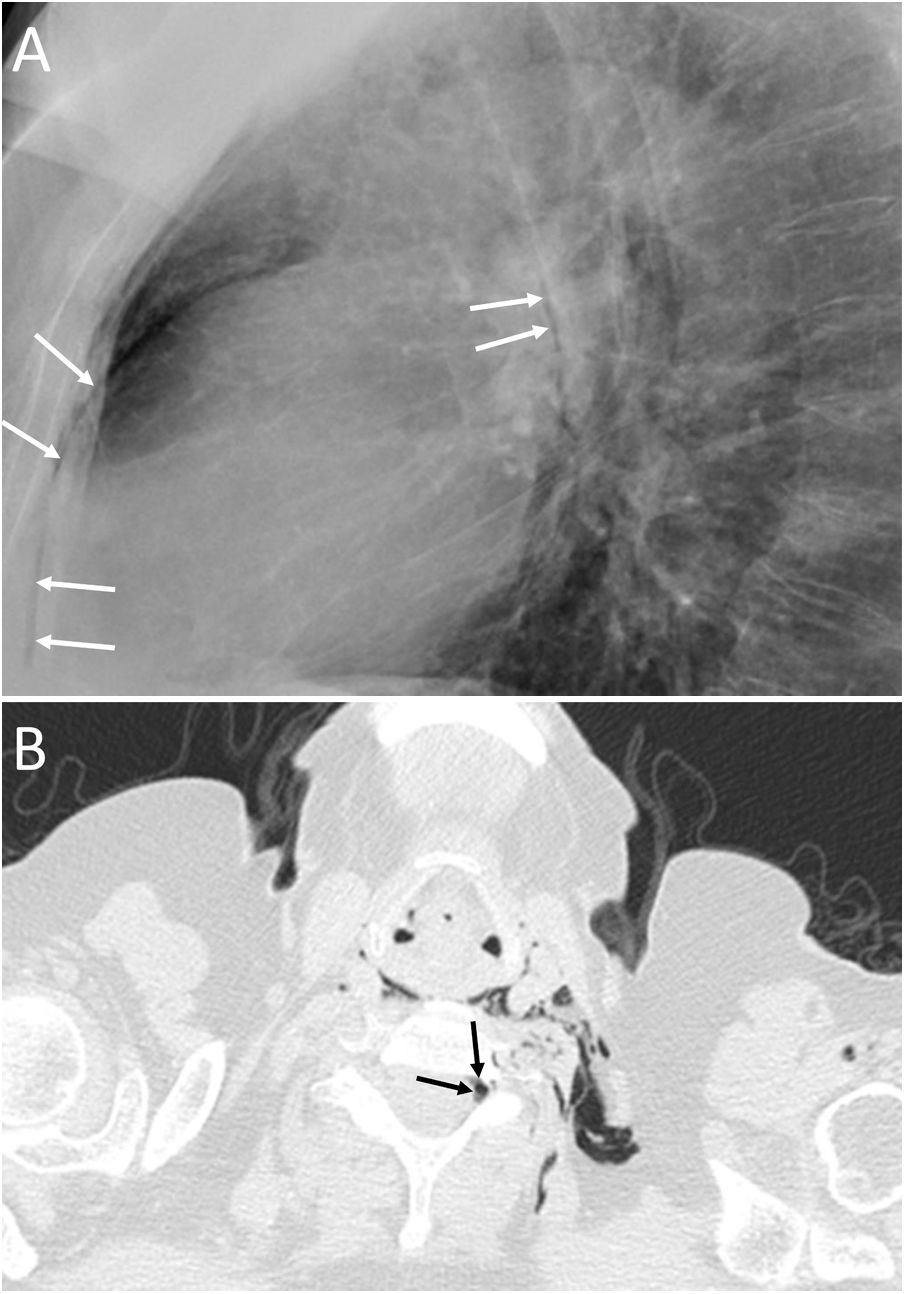
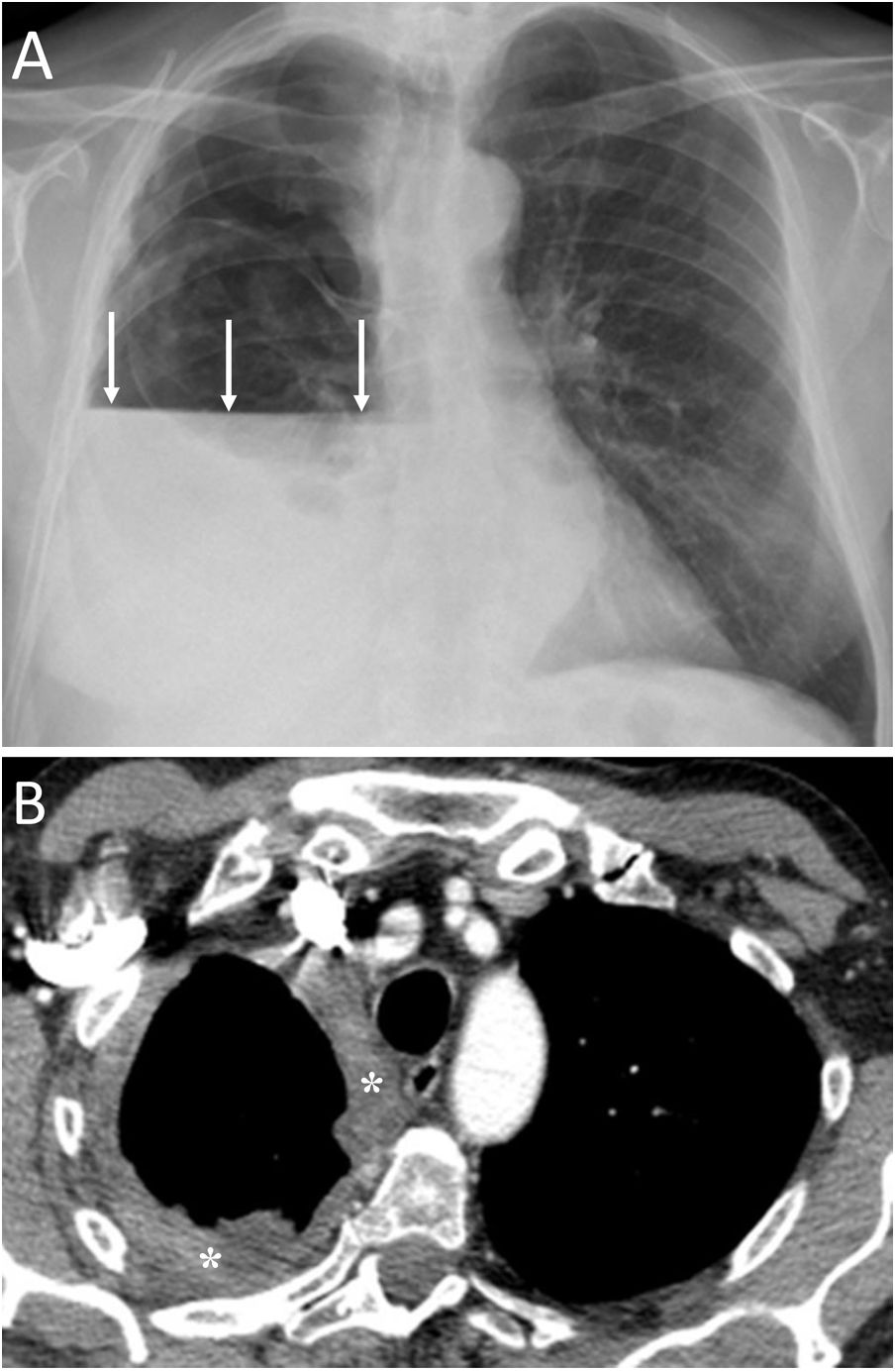
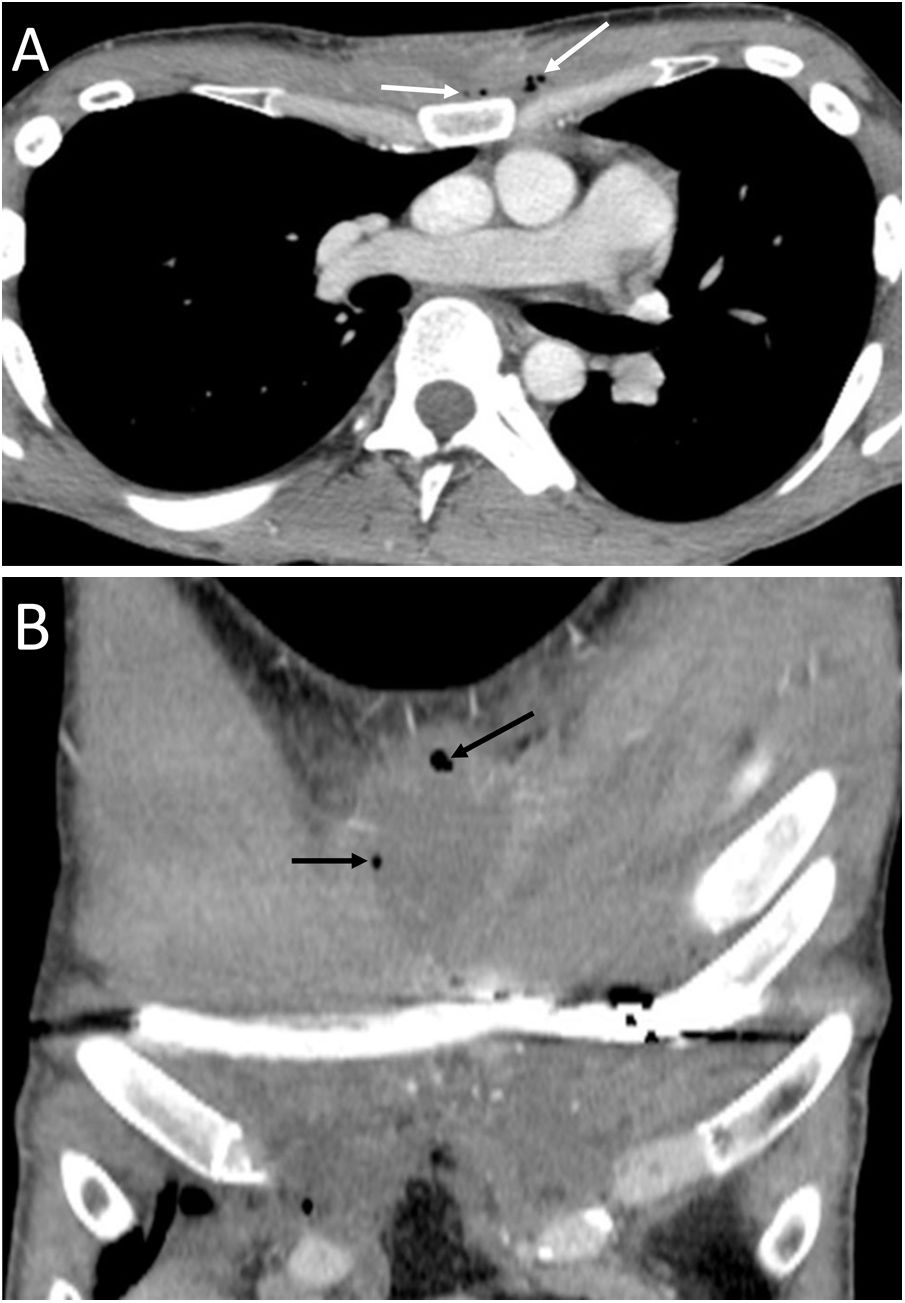
Spontaneous PM is not due to an apparent cause such as chest trauma. Rarely, it can be associated with pneumorrhachis, which is the presence of air in the spinal canal4 (Fig. 1). It is due to the passage of air from the submandibular and retropharyngeal spaces to the epidural space through the neural foramina.1 In general, this condition is benign and self-limited.1
A 63-year-old woman with dyspnoea. A) The chest X-ray (lateral projection) showed pneumomediastinum with air delimiting the anterior wall of the intrathoracic trachea and the cardiac silhouette (white arrows). B) The computed tomography also revealed the presence of air in the medullary canal (black arrows). Subsequent radiological studies showed resolution of the extrapulmonary thoracic air (not shown).
Spontaneous PM may be related to an interstitial lung disease such as idiopathic pulmonary fibrosis. In idiopathic pulmonary fibrosis, the presence of extra-alveolar air (both PM and pneumothorax) has been seen in 11.2% of cases, with clinical manifestations ranging from asymptomatic patients to severe respiratory failure.5 PM can also be associated with pneumoretroperitoneum with or without perforation of a hollow abdominal viscus.6
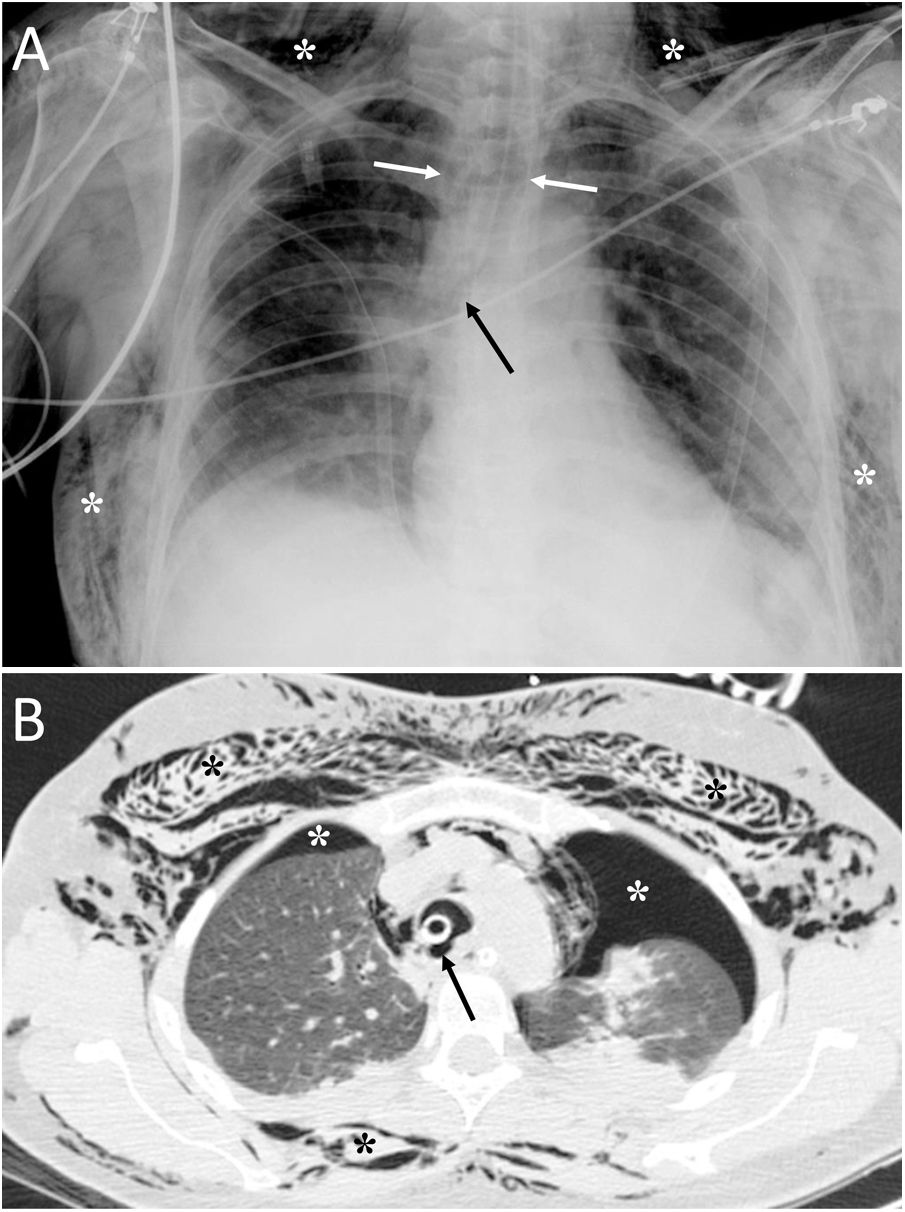
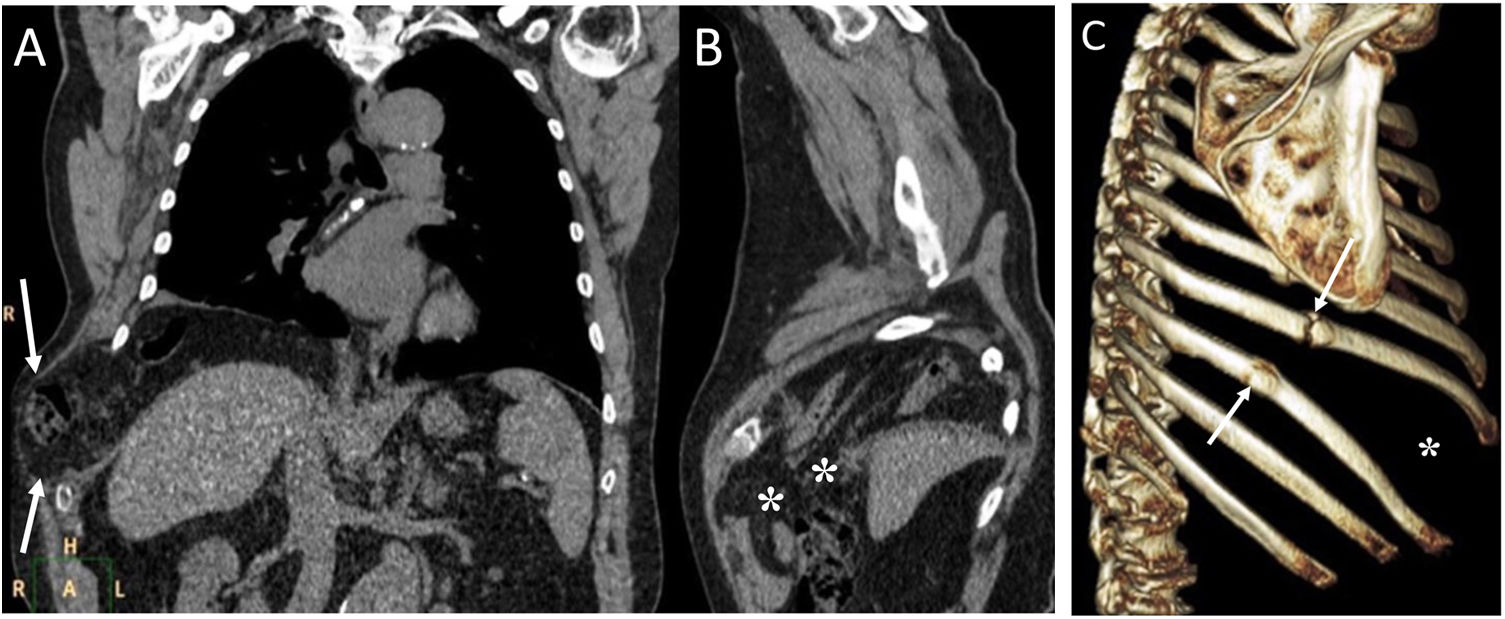
Tracheal rupture is rare, represents 15%–27% of all tracheobronchial lesions and is associated with high morbidity and mortality.7 The most common causes are chest trauma and orotracheal intubation (Fig. 2). Diagnosis can be delayed because the clinical and radiological findings are not specific, leading to complications such as failure of mechanical ventilation, mediastinitis, airway stenosis, bronchiectasis, recurrent infections and impaired lung function.7,8 Tracheal tears are frequently vertical and longitudinal and are located at the junction of the cartilaginous and membranous portions.8 The common and non-specific radiological findings are cervical subcutaneous emphysema and PM. If the cause is orotracheal intubation, a large dilatation and herniation of the endotracheal tube balloon outside the tracheal wall can be seen on computed tomography (CT) as a specific sign.7,8 Other signs would be the visualisation of the endotracheal tube outside the tracheal lumen, a focal defect in the tracheal wall and a deformity of the trachea.7 The CT enables the tracheal tear to be located in 70%–100% of cases, although the definitive diagnosis is made by bronchoscopy.8
A 36-year-old woman with attempted autolysis due to ingestion of benzodiazepines and acute respiratory failure, who presented with subcutaneous cervicofacial and thoracic emphysema after intubation. A) The chest X-ray revealed massive subcutaneous emphysema (white asterisks), hyperdistention of the endotracheal tube balloon (white arrows) and the distal end of the endotracheal tube in the proximal segment of the right main bronchus (black arrow). B) Computed tomography revealed pneumomediastinum with significant subcutaneous emphysema (black asterisks), bilateral pneumothorax (white asterisks) and hyperdistention of the endotracheal tube balloon with deformity of the posterior wall of the intrathoracic trachea (black arrow) due to post-intubation tracheal rupture. Treatment consisted of inserting two pleural drainage tubes for bilateral pneumothorax and selective intubation of the right main bronchus, with extubation at 36 h.
Dehiscence of the bronchial suture in lung transplantation is the most frequent complication of the airway in the early postoperative period, typically in the first 2–4 weeks,8,9 and it affects 2%–3% of cases. The main aetiological factor in the appearance of complications of the bronchial anastomosis is ischaemia due to the disruption of the native bronchial circulation, although other causes are recurrent infections and rejection.10 CT can identify direct findings, such as a defect in the bronchial wall or the presence of extraluminal air, and indirect findings such as pneumothorax, PM and loss of volume of the ipsilateral lung.8,10 Dehiscences smaller than 4 mm can be treated conservatively. However, they can progress to stenosis and require stenting, or be fatal. Fibre-optic bronchoscopy identifies mucosal necrosis and is the gold standard diagnostic test.9,10
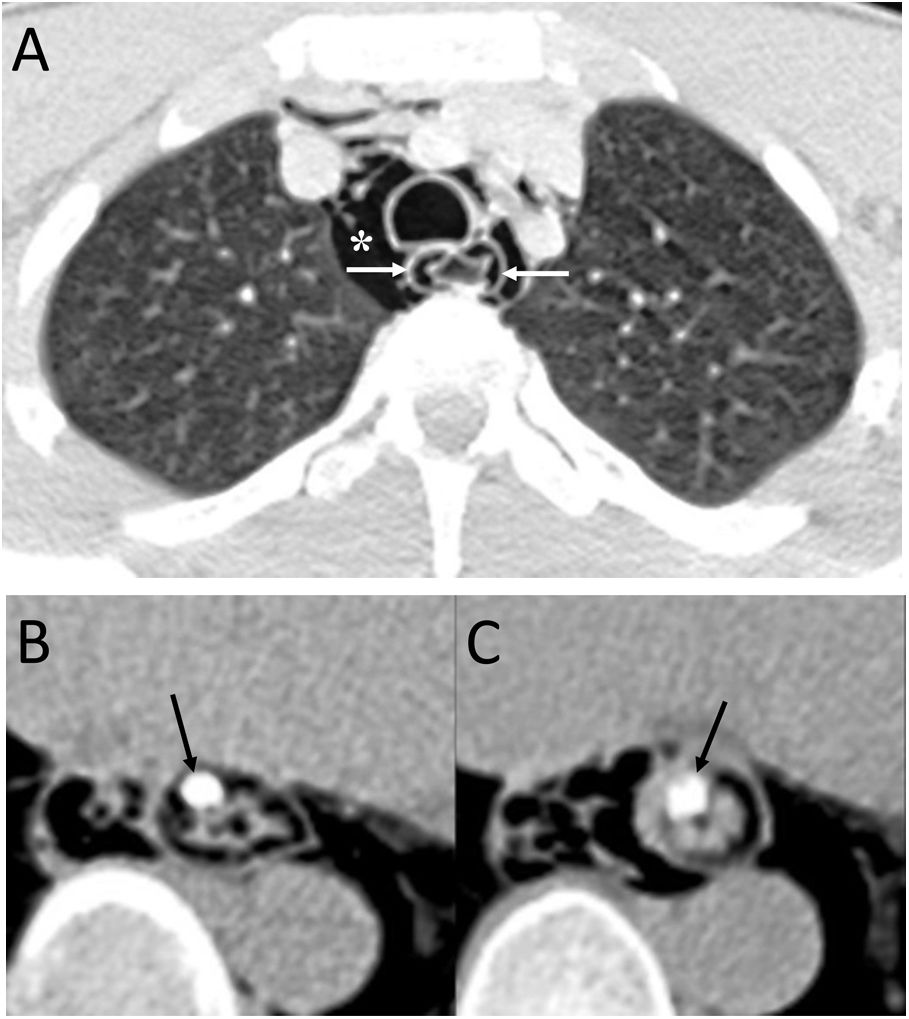
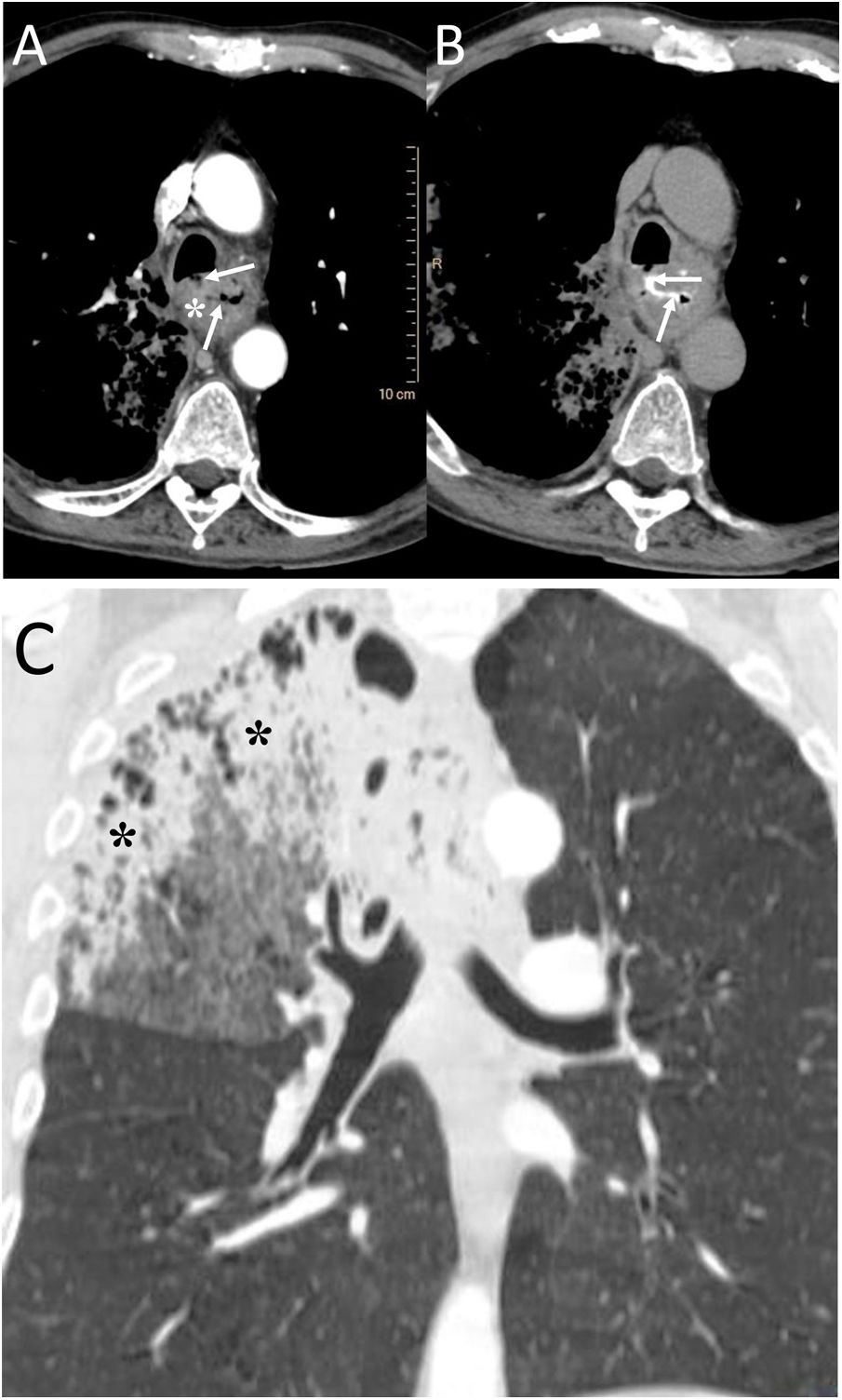
Intramucosal oesophageal dissection consists of the complete separation of the mucosa and/or submucosa layers from the deeper layers of the oesophageal wall, due to an initial trauma to the mucosa and subsequent increase in intraoesophageal pressure.11 The causes can be iatrogenic (endoscopy, transoesophageal echocardiography or haemostatic procedures) or by ingestion of a foreign body.11,12 It has also been seen in older women on anticoagulant treatment or with coagulopathy13 (Fig. 3).
A 20-year-old man with a history of endoscopy to assess food impaction. A) Computed tomography (CT) showed the presence of pneumomediastinum (white asterisk), with air inside the submucosal layer of the oesophageal wall (white arrows) and pneumoperitoneum with retropneumoperitoneum (not shown in the image). B) After the administration of oral contrast, no leakage was identified and it was located within the submucosa layer of the oesophageal wall (black arrows). The diagnosis was an intramucosal oesophageal dissection, and the patient's management was conservative, with complete resolution of the radiological findings on the control CT.
It must be remembered that: intramucosal oesophageal dissection is generally considered a contained form of oesophageal perforation, although the presence of pneumomediastinum may indicate frank perforation.14
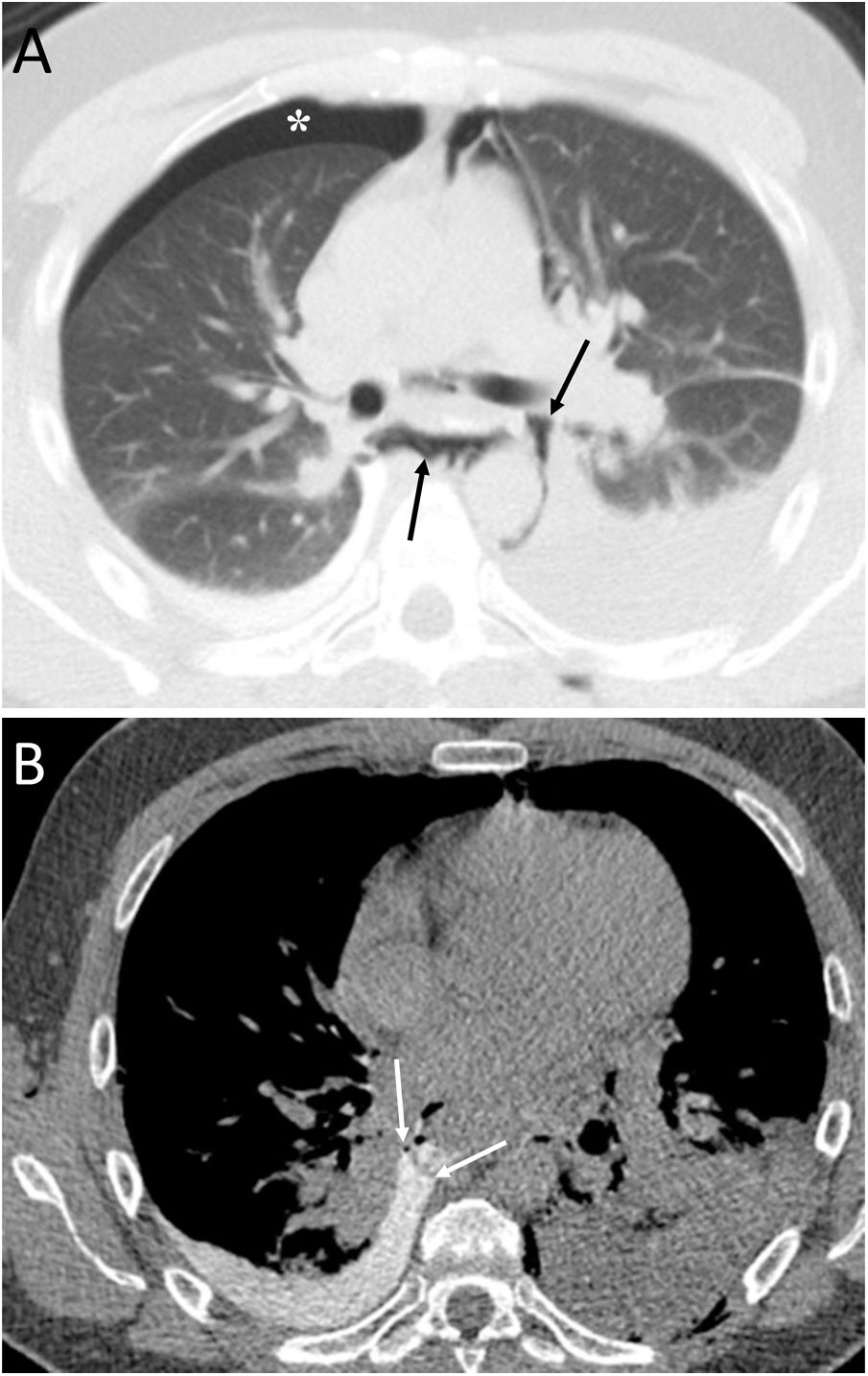
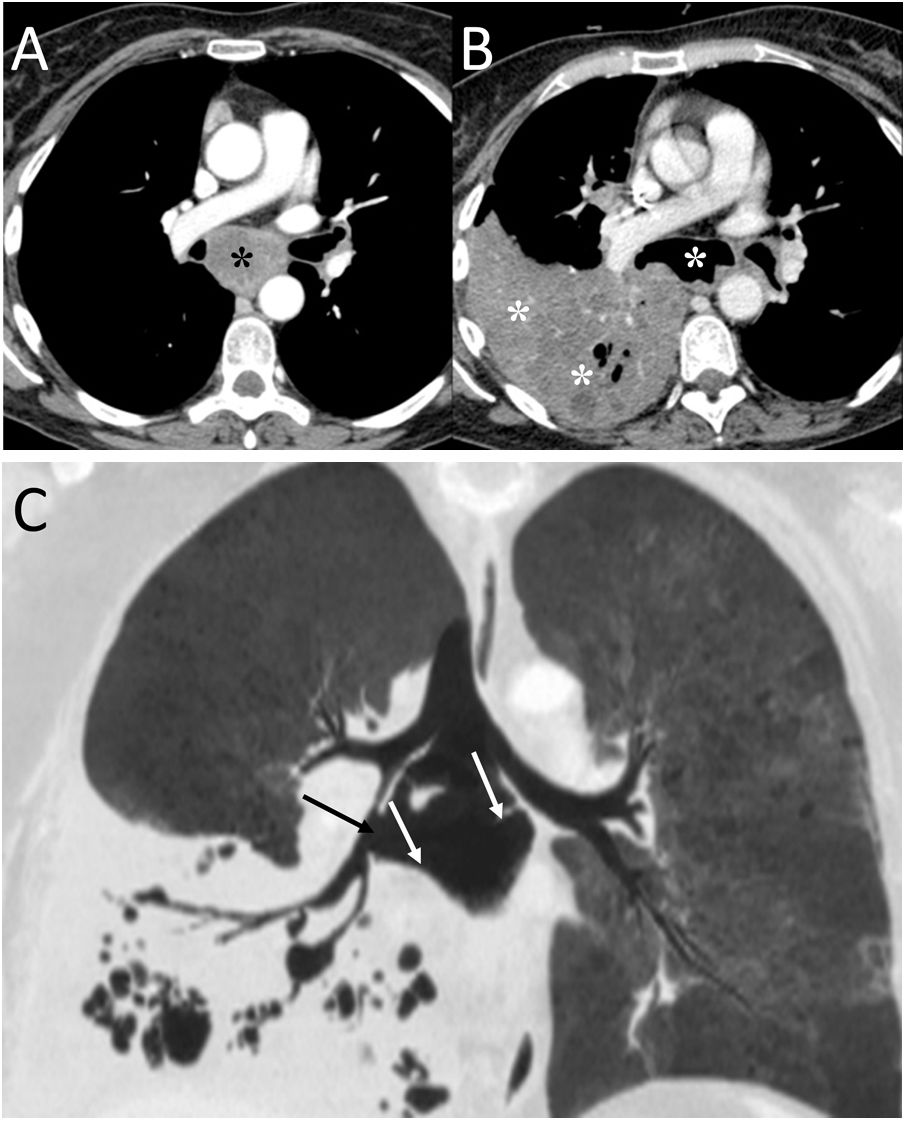
Boerhaave's syndrome (Fig. 4) is a type of oesophageal perforation caused by significant effort or by vomiting that conditions an increase in intraoesophageal pressure. It has a high morbidity and mortality and is more common in middle-aged men with a history of alcoholism (50% of cases).15 The most common clinical picture is epigastric pain after severe vomiting, although Mackler's triad (vomiting, chest pain and subcutaneous emphysema) can be identified.15 The oesophageal wall defect is typically located on the left posterior wall of the lower oesophageal third, about 3−6 cm above the diaphragm, and is usually about 2 cm in length.15,16 Chest X-ray may reveal mediastinal widening, PM, subcutaneous emphysema, hydropneumothorax and pulmonary opacities.15 CT enables the visualisation of typical findings such as the presence of distal peri-oesophageal air, oral contrast extravasation and an oesophagopleural fistula (more frequent on the left).15 Treatment may be conservative, endoscopic or surgical.16
A 36-year-old woman with abdominal pain after vomiting. A) Computed tomography enabled visualisation of pneumomediastinum (black arrows) and right pneumothorax (white asterisk). B) After the administration of oral contrast, perforation in the distal oesophagus was identified with the passage of oral contrast into the right pleural cavity, due to an oesophagopleural fistula (white arrows) associated with Boerhaave's syndrome. Treatment consisted of surgical repair of the oesophageal perforation.
The most common acquired oesophago-respiratory fistula are tracheo-oesophageal and can be seen in 5%–10% of advanced oesophageal neoplasms, especially after radiotherapy15 (Fig. 5). Clinically, they can manifest as coughing attacks with fluid intake, dry mouth, neck and chest pain, spontaneous cough and sputum with food remains.15 Chest X-ray is non-specific. Endoscopy can identify the fistula and CT can determine the location and extent of the fistula, its cause and the status of the pulmonary parenchyma.15
A 58-year-old man with fever and dysphagia. A and B) The computed tomography showed an oesophageal wall thickening (white asterisk) and a fistulous path between the oesophagus and the intrathoracic trachea, clearly visualised when oral contrast was administered (white arrows). C) In the pulmonary parenchyma window, pulmonary consolidation was visualised in the right upper lobe (black asterisks). The radiological findings indicated an oesophageal neoplasm with tracheo-oesophageal fistula and aspiration pneumonia in the right lung. The patient was treated by placing an oesophageal endoprosthesis, with significant clinical improvement.
Bronchial perforations and tracheo-oesophageal or broncho-oesophageal fistulas can be caused by a ruptured lymph node. If the cause is infectious, it may be due to tuberculosis, histoplasmosis or actinomycosis.17 Bronchial perforation due to tumourous subcarinal adenopathies has also been described18 (Fig. 6). CT is helpful in revealing PM and the underlying cause.
A 48-year-old man with lung neoplasia treated with surgery and chemotherapy. A and B) The computed tomography (CT) scan showed the presence of a subcarinal adenopathy (black asterisk) that in the next control CT scan necrotised and filled with air (white asterisk). In addition, a right lower lobe lung consolidation was seen due to aspiration pneumonia (white asterisks). C) MinIP reconstruction (coronal plane) revealed a fistula between the intermediate bronchus (black arrow) and the necrotic adenopathy (white arrows).
Acute mediastinitis has a high mortality and morbidity. The imaging test of choice for its evaluation is CT, which enables visualisation of these findings: infiltration of the mediastinal fat, diffuse or focal air bubbles in the mediastinum, collections, lymphadenopathy, pleural effusion and empyema.15
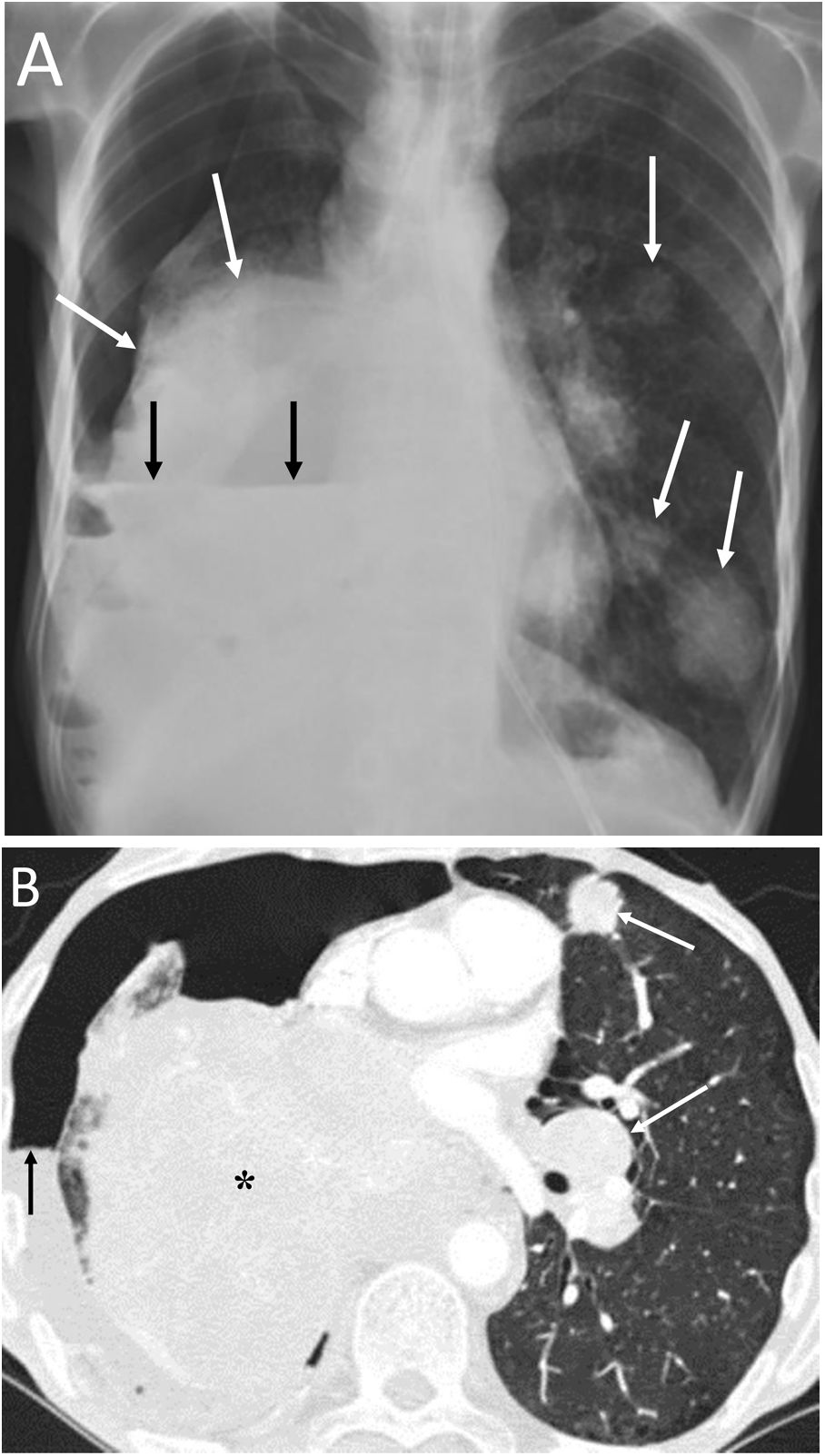
Pericardial locationPneumopericardium (PPC) is caused by invasive procedures (thoracentesis, endotracheal intubation, thoracotomy, etc.), pericarditis, chest trauma, barotrauma and fistulas with air-borne structures (bronchial tree, gastrointestinal tract, and pleural or peritoneal spaces).19,20 In pneumothorax and PM due to barotrauma or blunt trauma, it has been suggested that the aetiopathogenesis could be the rupture of the alveolar walls with air passing towards the hila and/or the pleura, dissecting the peribronchovascular space. If this air spreads around the pulmonary veins and arteries, it can reach the pericardium. Another possible cause of air in the pericardium in chest trauma would be direct communication with the pleura or tracheobronchial tree through a pericardial rupture or tear.19 Most cases of PPC are due to iatrogenesis or trauma, spontaneous PPC being very rare.20 In the case of pulmonary neoplasia (Fig. 7), the possible routes are: direct invasion of the necrotic tumour that causes a bronchopericardial fistula, trauma due to bronchoscopy or thoracentesis, and rupture to the pericadium of a focus of necrosis.20
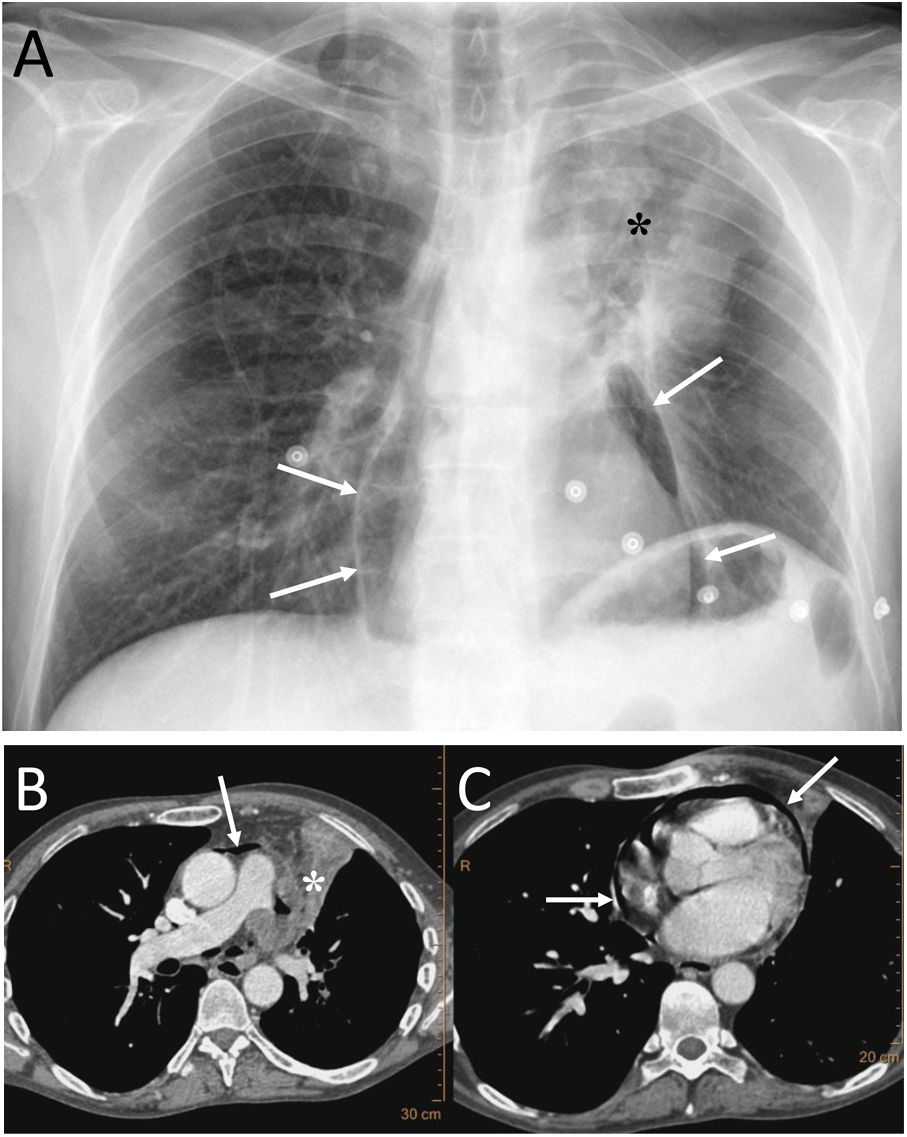
A 53-year-old man with squamous cell carcinoma of the lung presented with chest pain, fever and productive cough. A) The chest X-ray showed a lung mass in the left upper lobe (black asterisk) and pneumopericardium (white arrows). B and C) Computed tomography showed progression of the upper left lung mass (asterisk) and pneumopericardium secondary to the direct extension of the tumour to the pericardium (white arrows).
It must be remembered that: chest X-ray shows air around the cardiac silhouette, limited superiorly by the lower border of the aortic arch, without extension around the trachea, bronchi or cervical region. CT enables confirmation of the diagnosis and identification of the possible cause. Treatment will be conservative if the pneumopericardium is small and the patient is stable and asymptomatic.21
Cardiovascular locationVenous air embolism is a potentially serious complication of intravenous fluid injection or vascular trauma, with an incidence of 11.7%–23% after intravenous contrast administration in CT scans.22,23 Usually, there are no symptoms because the amount of air administered is small, but a rapid injection of air of about 100−200 ml can be fatal.22,23 Air can be introduced at various times during patient preparation: when inserting the intravenous needle (images without intravenous contrast from CT suggest this origin), at the connection between the needle and the contrast injector, and finally, if there are small air bubbles mixed in with the contrast.22 The most common locations on CT (with the patient in the supine position) are the most anterior vascular structures that, in descending order, would be: the trunk of the main pulmonary artery, the superior vena cava, the right ventricle, the subclavian or brachiocephalic veins and the right atrium.22 Air embolism can also be caused by a transthoracic lung biopsy due to the passage of air into the pulmonary veins (Fig. 8).22,23
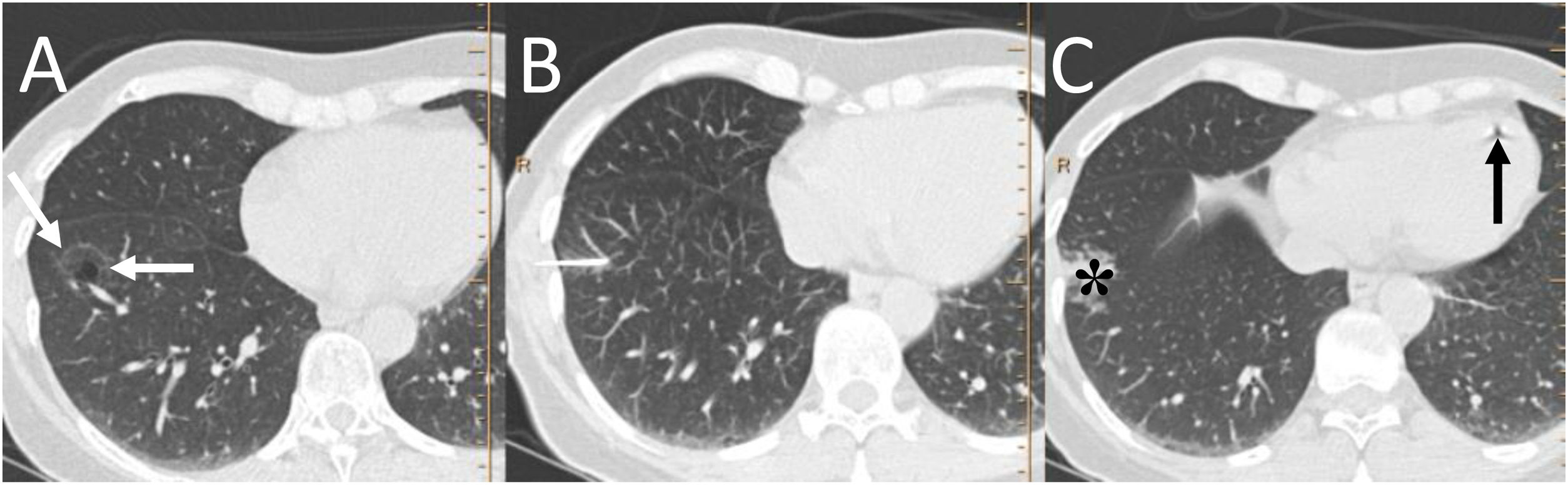
A 57-year-old woman with a pulmonary nodule awaiting surgery. A) Computed tomography (CT) showed a subsolid pulmonary nodule in the right lower lobe (white arrows). B and C) Preoperative marking was performed with CT-guided radiotracer injection, identifying pulmonary haemorrhage (black asterisk) and air bubbles in the right ventricle post-procedure (black arrow). When performing the subsequent scintigraphy, no focal deposit of the intrapulmonary radiotracer was identified and its distribution was observed in the liver and kidneys. The injection of the radiotracer was considered to be intravascular in a branch of the left inferior pulmonary vein. The procedure was repeated without incident and the intraoperative biopsy of the lesion showed adenocarcinoma in situ, for which a lower left lobectomy was performed.
It must be remembered that: treatment for symptomatic air embolism consists of placing the patient in the left lateral decubitus position and administering 100% oxygen.
In case of left-right shunt, there is an increased risk of arterial embolism with neurological deficit or myocardial ischaemia.22
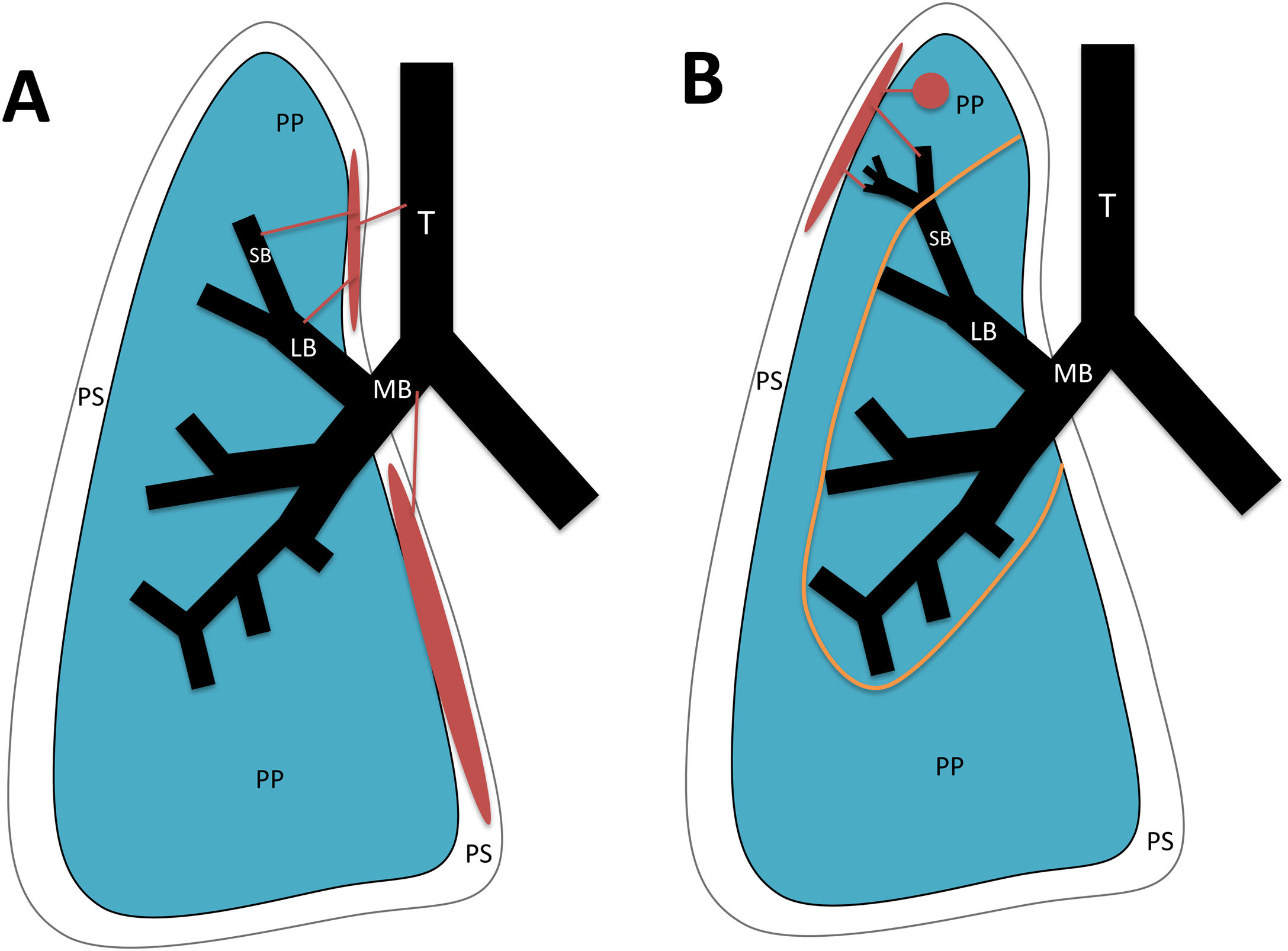
Pleural locationBronchopleural fistulas consist of a direct communication between the pleural space and the bronchial tree or pulmonary parenchyma.24 They can be of two types (Fig. 9):
- 1
Central: connection between the pleura and the trachea or bronchi. The main causes are lung resection, lung transplant and trauma to the tracheobronchial tree. Dehiscence of the bronchial stump suture due to anatomic resection is the most common cause of bronchopleural fistulas.
- 2
Peripheral: connection between the pleura and the airway distal to the segmental bronchi or pulmonary parenchyma. Possible causes are necrotising pneumonia, empyema, previous radiation therapy, ruptured bulla or pulmonary cyst, interventional procedures, tuberculosis, aspergillosis, granulomatosis with polyangiitis and sarcoidosis.25
Types of bronchopleural fistula (in red). A) Central: between the pleural space and the trachea, main bronchi, lobar bronchi or segmental bronchi. B) Peripheral: between the pleural space and the airway distal to the segmental bronchi or pulmonary parenchyma. T: Trachea. MB: main bronchus. LB: lobar bronchus. SB: segmental bronchus. PP: pulmonary parenchyma. PS: pleural space. Orange line: boundary between central and peripheral bronchopleural fistulas.
It must be remembered that: a hydropneumothorax usually appears on chest X-ray. The appearance or increase of air in the pneumonectomy cavity suggests bronchopleural fistulas due to suture dehiscence.24,25 CT enables direct visualisation of the fistula in large fistula tracts, although the presence of air bubbles adjacent to the bronchial suture or changes in the pleural air-fluid level suggest small bronchopleural fistulas.25
Spontaneous pneumothorax (PTX) is a very rare presentation of malignant pleural mesothelioma (Fig. 10) and is more common in patients over 40 years of age.26 Possible mechanisms are: rupture of necrotic tumour nodules, obstruction of peripheral pulmonary bronchi by tumour nodules with formation of subpleural bullae, and invasion of the pulmonary parenchyma by mesothelioma.27 Pleural drainage is usually ineffective and the lung cannot be re-expanded.27
A 69-year-old man with progressive dyspnoea and chest pain. A) The chest X-ray showed an air-fluid level that occupied the entire right hemithorax, indicative of a hydropneumothorax (white arrows). B) Computed tomography after pleural drainage showed the presence of nodular pleural thickening with involvement of the mediastinal pleura (white asterisks). Videothoracoscopy revealed the presence of multiple right pleural nodules, a biopsy of which was positive for pleural mesothelioma.
Spontaneous PTX has been described in 0.03%−0.05% of patients with primary lung neoplasia (Fig. 11), with a clear predominance of males (90%).28 Presentation of pulmonary neoplasia as spontaneous PTX appears in patients with advanced disease.28 An underlying lung neoplasm should be ruled out in patients under 40 years of age with recurrent pneumothorax, or in patients over 40 years with a history of smoking, chronic bronchitis or emphysema, with incomplete lung expansion or a lung mass on chest radiograph after pleural drainage.28
A 57-year-old man with a disseminated lung neoplasm undergoing chemotherapy. A) The chest X-ray revealed a right lung mass with left lung nodules (white arrows) and an air-fluid level in the right hemithorax due to hydropneumothorax (black arrows). B) The computed tomography scan revealed a large right lung mass (black asterisk), lung nodules and masses and contralateral hilar adenopathies (white arrows), and the right hydropneumothorax (black arrow). The patient died within a few days.
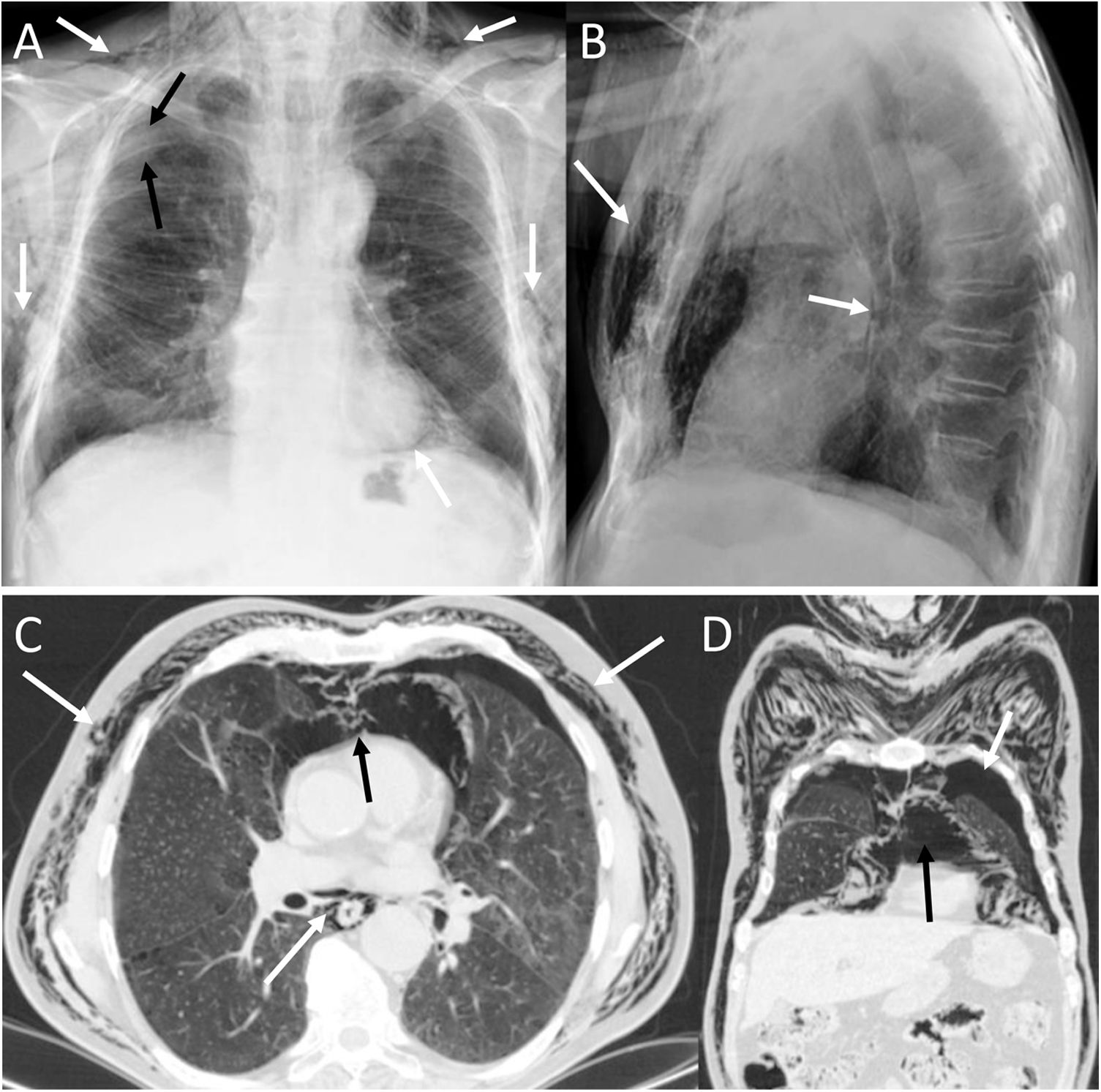
Bilateral spontaneous PTX, which represents 1.3% of spontaneous PTX, has also been described as a complication of a unilateral transthoracic puncture29 (Fig. 12).
A 72-year-old man with a pulmonary nodule in the right upper lobe. Percutaneous fine needle (22 G) lung puncture cytology was of a non-small cell lung neoplasm. A and B) The chest X-ray 72 h after the procedure revealed signs of pneumomediastinum and significant subcutaneous emphysema (white arrows). The biopsied pulmonary nodule can be seen in the periphery of the right upper lobe in the posteroanterior view of the chest X-ray (black arrows). B and C) Computed tomography confirmed pneumomediastinum and subcutaneous emphysema and showed predominantly left bilateral pneumothorax (white arrows) with communication between both pleural spaces in the anterior junctional line (black arrows), probably of congenital origin, since there was no previous thoracic surgery. The subcutaneous emphysema was drained through an incision in the chest wall and multiple pleural adhesions were identified during the surgical procedure of right upper lobectomy.
It must be remembered that: exceptionally, there is communication between the two pleural spaces in the anterior junctional line, which results in a single pleural space. This may be secondary to previous surgery with median sternotomy ("iatrogenic buffalo chest'') or a communication of congenital origin.29,30
Communication between the two pleural spaces after heart and lung transplantation has been observed in 33%–40% of these patients, which can lead to bilateral PTX.30 CT can identify communication between the two pleural spaces. Treatment will usually consist of unilateral pleural drainage.30
Chest wall locationChest wall infections are rare and the most common causative organisms are Staphylococcus aureus and Pseudomonas aeruginosa.31 The prognosis will depend on an early diagnosis, the degree of immunosuppression of the patient, the microorganism causing the infection and its extension.31 Chest X-ray may show an extrapulmonary mass and CT enables visualisation of collections with or without air and rib destruction (Fig. 13).
A 29-year-old man with previous surgery to correct pectus excavatum, who presented with oedema in the left chest wall and spontaneous discharge of purulent material from the surgical wound. A) Computed tomography with intravenous contrast showed a presternal hypodense collection in the subcutaneous cellular tissue with air bubbles (white arrows). B) The multiplanar reconstruction in the coronal plane clearly showed this collection with air bubbles (black arrows) corresponding to a chest wall abscess.
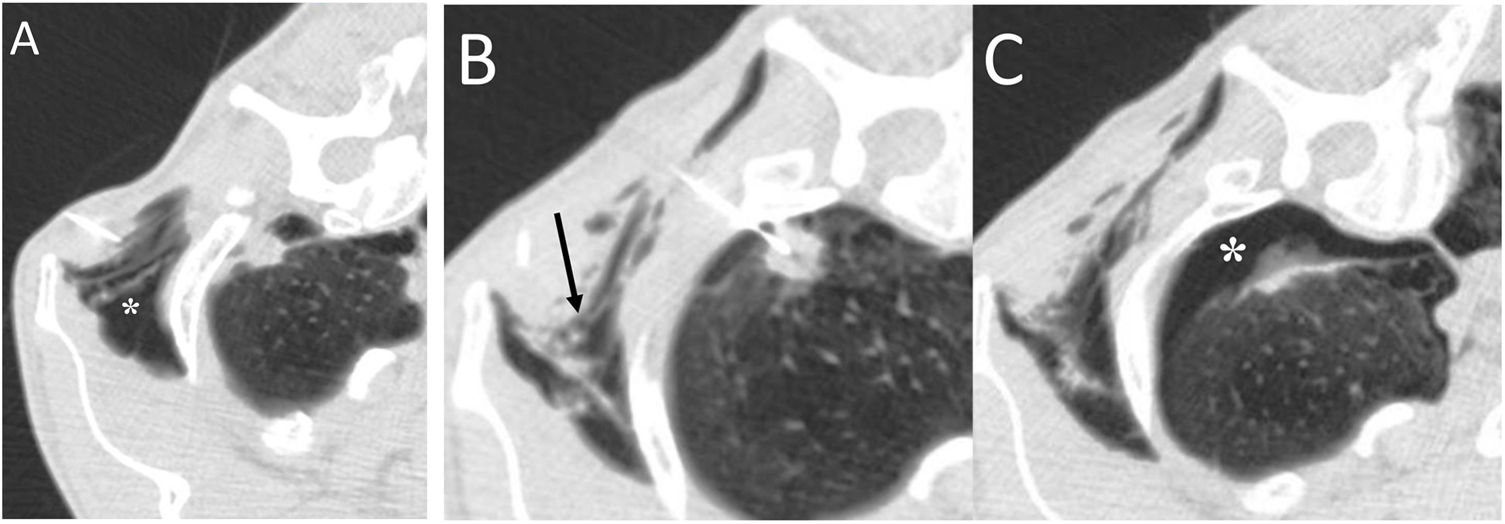
Transdiaphragmatic intercostal hernia consists of herniation of abdominal contents through the thoracic wall through a diaphragmatic defect. It is most common after trauma, but it can also appear after coughing attacks or massage.32 Chest X-ray and CT can identify rib fractures, when present, with increased intercostal space secondary to them and the presence of intestinal loops in the chest wall (Fig. 14). The treatment of choice will be surgery through simultaneous repair of the diaphragmatic and intercostal defects.32
A 55-year-old man with a mass on the right chest wall of more than two years of evolution. A) Computed tomography (multiplanar reconstruction in coronal and sagittal planes) revealed a right transdiaphragmatic intercostal hernia with colon and abdominal fat (white arrows). C) Multiplanar reconstruction in the sagittal plane enabled identification of the diaphragmatic defect (white asterisks). B) The 3D (sagittal plane) showed fracture calluses in the lateral arches of the eighth and ninth right ribs (white arrows) with widening of the intercostal space (white asterisk).
Subcutaneous emphysema (SE) most often originates from a PM,33 although it is also associated with PTX. Possible causes would be: trauma, surgery, thoracentesis, image-guided lung biopsies (Figs. 12 and 15), lung infections or neoplasms, and radiofrequency lung ablations.33 The incidence of SE after CT-guided lung biopsy is 2.1%.30 The risk of PTX and SE increases the greater distance from the lung injury to the pleura and the smaller the size of the injury.33
A 60-year-old man with a right apical pulmonary nodule biopsied by fine needle puncture (22 G). A) After the injection of the local anaesthetic, a subcutaneous emphysema (white asterisk) appeared, although the needle and the plunger were previously purged of air. B) The subcutaneous emphysema was partially drained to prevent its progression and facilitate puncture (black arrow). C) Finally, the procedure was successfully performed with the appearance of a pneumothorax (white asterisk). The patient remained asymptomatic at all times.
The identification and localisation of ectopic extrapulmonary intrathoracic air is important in the differential diagnosis of the disease causing it. Chest X-ray and CT are the most useful tests for the evaluation of these patients. There are multiple atypical and infrequent causes of extrapulmonary thoracic air and its correct diagnosis has a potential impact on therapeutic management.
Authorship- 1
Responsible for the integrity of the study: DVP.
- 2
Study concept: DVP.
- 3
Study design: DVP.
- 4
Data collection: DVP, OP, ALS, LC, EP, JA.
- 5
Data analysis and interpretation: DVP, OP, ALS, LC, EP, JA.
- 6
Statistical processing: not applicable.
- 7
Literature search: DVP.
- 8
Drafting of the article: DVP.
- 9
Critical review of the manuscript with intellectually relevant contributions: DVP, OP, ALS, LC, EP, JA.
- 10
Approval of the final version: DVP, OP, ALS, LC, EP, JA.
The authors declare that they have no conflicts of interest.
Please cite this article as: Varona Porres D, Persiva O, Sánchez AL, Cabanzo L, Pallisa E, Andreu J. Buscando la burbuja: aire torácico extrapulmonar atípico e inusual. Radiología. 2021;63:358–369.