The fluid-attenuated inversion recovery (FLAIR) sequence forms part of the vast majority of current diagnostic protocols for brain MRI. This sequence enables the suppression of the signal from cerebrospinal fluid, facilitating the detection of disease involving the subarachnoid space. The causes of hyperintensity in the arachnoid space in this sequence can be divided into two main categories: hyperintensity due to disease and hyperintensity due to artifacts. Hyperintensity due to tumors, inflammation, vascular disease, or hypercellularity of the cerebrospinal fluid or hematic contents is well known. However, numerous other non-pathological conditions, mainly due to artifacts, that are also associated with this finding are a potential source of diagnostic errors.
La secuencia fluid attenuated inversion recovery (FLAIR) forma parte hoy en día de la gran mayoría de protocolos diagnósticos de RM cerebral. Esta secuencia de inversión-recuperación permite una supresión de la señal del líquido cefalorraquídeo, lo que facilita la detección de enfermedad que afecta al espacio subaracnoideo. Las causas de hiperintensidad del líquido cefalorraquídeo en esta secuencia pueden subdividirse en 2 grandes grupos, las patológicas y las debidas a artefactos. Son bien conocidas la etiología tumoral, la inflamatoria, la vascular o las debidas a hipercelularidad del líquido cefalorraquídeo o a ocupación por contenido hemático. Sin embargo, existen numerosas condiciones no patológicas, principalmente debidas a artefactos, que se relacionan con este hallazgo constituyendo una potencial fuente de errores diagnósticos.
- 1.
The importance of FLAIR imaging today requires radiologists to be familiar with the various pathological causes of hyperintensity in the subarachnoid space. Analysis of the distribution and other associated findings may help to elucidate its aetiology.
- 2.
Increased signal in the subarachnoid space on FLAIR imaging may be due to the presence of hyperproteic content, haematic content or increased cellularity, either due to lymphocytosis, or inflammatory or tumoural in nature.
- 3.
In some cases, the increased signal displayed is not a pathological finding, but rather is due to artefacts, which must be taken into account to avoid potential diagnostic errors.
- 4.
The long inversion time gives the FLAIR sequence a certain degree of T1 weighting, which facilitates assessment of intravenous contrast enhancement of certain lesions, despite being highly T2-weighted.
- 5.
Contrast-enhanced-FLAIR imaging differentiates between vascular structures and true leptomeningeal lesions, as this sequence does not show uptake of cortical veins, unlike contrast-enhanced T1 sequences, which is particularly relevant in patients with suspected meningitis or leptomeningeal carcinomatosis.
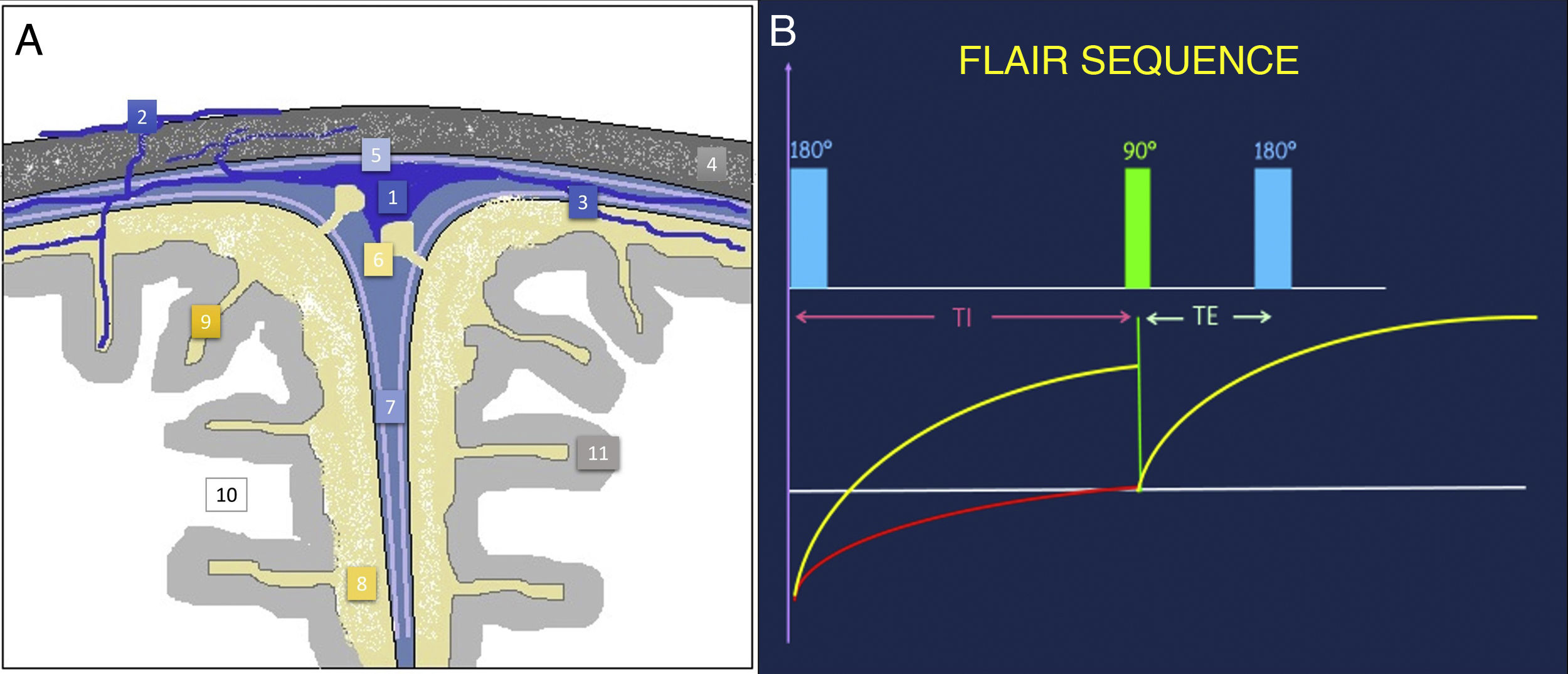
The subarachnoid space (SAS) is the extra-axial space between the deep pia mater and the superficial arachnoid, both known as the leptomeninges (Fig. 1). It contains cerebrospinal fluid (CSF), vascular channels and cranial nerves, as well as a thin web of connective tissue that it supports.
Anatomical illustration of the subarachnoid space and meningeal layers (A). FLAIR sequence acquisition diagram (B). By applying an initial inversion pulse of 180° and using a long TI prior to the initial excitation pulse of 90°, the signal of structures that have a long relaxation time, such as the CSF, can be suppressed. FLAIR: fluid-attenuated inversion recovery; TE: echo time; TI: inversion time; 1: superior sagittal sinus; 2: emissary and diploic veins; 3: pial cerebral veins; 4: skull; 5: dura mater; 6: arachnoid granulations; 7: interhemispheric fissure; 8: subarachnoid space; 9: sulci of the cerebral convexity; 10: cerebral white matter; 11: cerebral grey matter.
Courtesy of Dr Javier Lafuente Martínez.
In the convolutions of the cerebral convexity, where the two membranes are in close proximity, this space is very narrow, almost non-existent. However, in the sulci and in the basal cisterns, the arachnoid is distant from the brain parenchyma, accompanying the dura mater. As such, the SAS is enlarged and becomes visible in the various imaging tests.
FLAIR magnetic resonance imagingThe fluid-attenuated inversion recovery (FLAIR) sequence is a magnetic resonance imaging (MRI) sequence with long repetition times (TR) and echo times (TE) using an inversion-recovery pulse (Fig. 1). With an initial inversion pulse of 180° and a sufficiently long inversion time (TI) (around 2000 ms) prior to the initial excitation pulse of 90°, the CSF signal is suppressed in the resulting images.1 The brain parenchyma shows intensity values superimposable on those of the T2 sequence, with the grey matter being more hyperintense than the white matter.
One of its main applications is the assessment of subtle changes occurring in the brain parenchyma close to or in contact with CSF, such as the grey matter on the periphery of the hemispheres or the periventricular white matter.
Although the resulting FLAIR images are highly enhanced on T2, the long TI conditions a certain degree of enhancement on T1. This makes it possible to assess the enhancement of lesions or anatomical structures with this sequence when paramagnetic intravenous contrast is administered beforehand.2
One of the most widely accepted uses of this contrast-enhanced FLAIR sequence is to differentiate between vascular structures and true leptomeningeal lesions when contrast-enhanced T1 images are inconclusive, since the FLAIR sequence does not identify cortical vein uptake that can be confused with pathological leptomeningeal enhancement, which is especially relevant in patients with suspected meningitis or leptomeningeal carcinomatosis.2
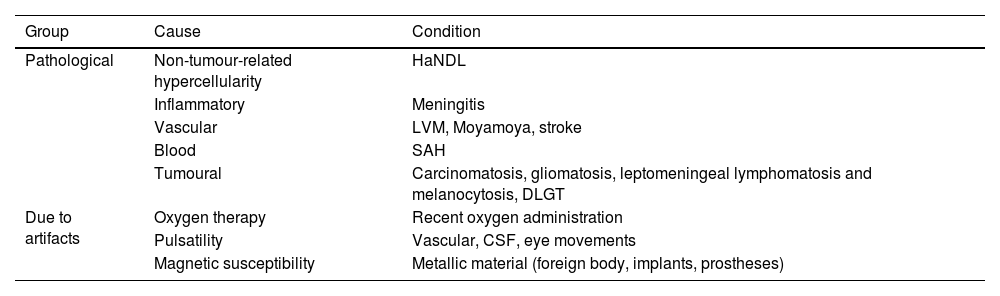
Causes of FLAIR hyperintensity in the SASCSF hyperintensity in the SAS on the FLAIR sequence is a non-specific finding in itself that has been described in numerous entities that can be divided into two large groups (Table 1):
- –
Pathological: which include aetiology due to CSF hypercellularity (HaNDL [Headache and Neurological Deficits with cerebrospinal fluid Lymphocytosis]), inflammatory aetiology (meningitis), vascular aetiology (leptomeningeal venous malformation, Moyamoya disease or in hypoperfusion in stroke), aetiology due to occupation by haematic content (subarachnoid haemorrhage [SAH]) or tumoural aetiology (carcinomatosis, leptomeningeal melanosis and diffuse leptomeningeal glioneuronal tumour).
- –
From artefacts: in patients receiving supplemental oxygen, from eye, CSF or vascular pulsatility movements, or from magnetic susceptibility artefacts induced by metallic material (e.g. dental implants) or by adjacent bony structures of the skull base. It is important to be aware of this group so as not to confuse it with leptomeningeal disease, which will lead to an error in the definitive diagnosis.
Summary table of the main causes of signal hyperintensity in the subarachnoid space on FLAIR imaging.
| Group | Cause | Condition |
|---|---|---|
| Pathological | Non-tumour-related hypercellularity | HaNDL |
| Inflammatory | Meningitis | |
| Vascular | LVM, Moyamoya, stroke | |
| Blood | SAH | |
| Tumoural | Carcinomatosis, gliomatosis, leptomeningeal lymphomatosis and melanocytosis, DLGT | |
| Due to artifacts | Oxygen therapy | Recent oxygen administration |
| Pulsatility | Vascular, CSF, eye movements | |
| Magnetic susceptibility | Metallic material (foreign body, implants, prostheses) |
CSF: cerebrospinal fluid; DLGT: diffuse leptomeningeal glioneuronal tumour; HaNDL: Headache and Neurological Deficits with cerebrospinal fluid Lymphocytosis; LVM: leptomeningeal venous malformations; SAS: subarachnoid haemorrhage.
Given the importance and routine use of the FLAIR sequence today, radiologists should be familiar with the various pathological causes of hyperintensity in the SAS. Analysis of the distribution and other associated findings may help to elucidate its aetiology.
However, on many occasions there will be no other findings to help us establish a definitive diagnosis, in which case correlation with the clinical history and CSF analysis will be necessary.
Generally speaking, the CSF signal in this sequence will not be suppressed when other substances or structures are present in the CSF (e.g. proteinaceous material, red blood cells, inflammatory or tumour cells, slowed vascular flow in ectatic vessels, etc.). This results in a prolongation of the T2 relaxation time or a shortening of the T1 relaxation time, with consequent hyperintensity in this sequence.1
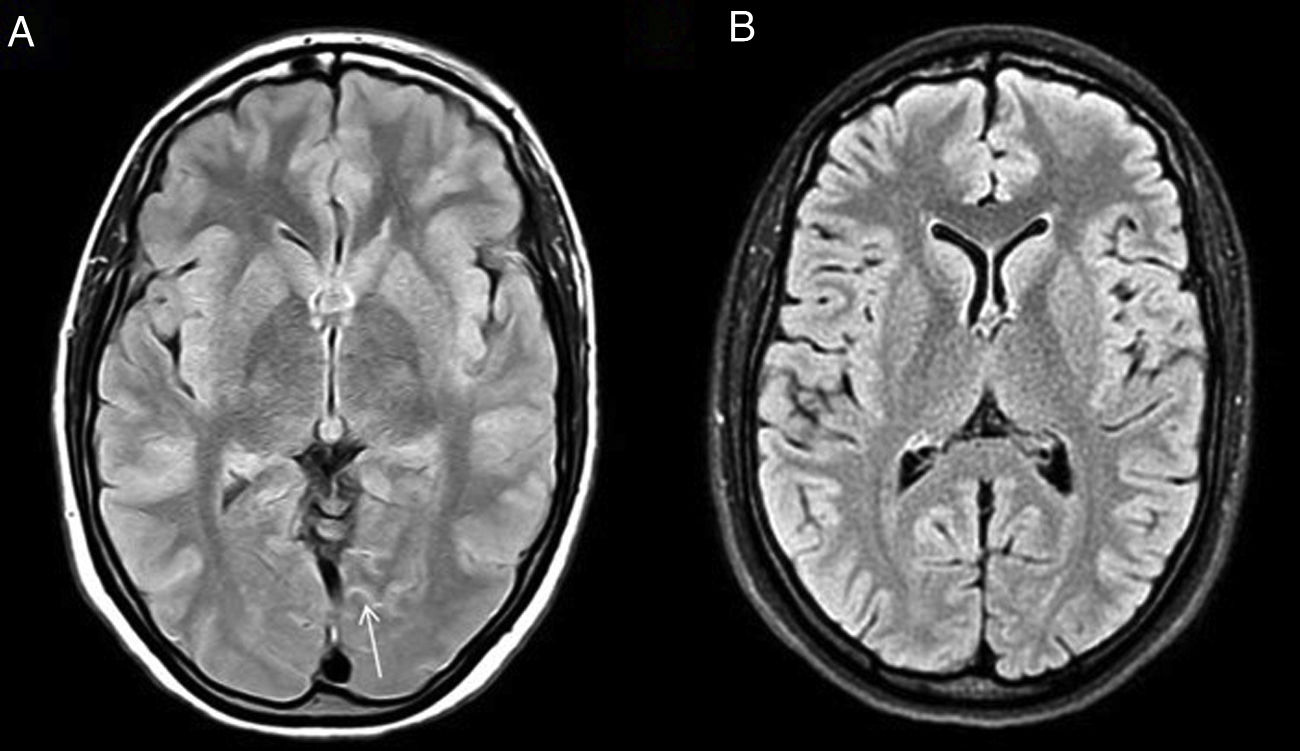
HaNDLHeadache with Neurological Deficits and CSF Lymphocytosis (HaNDL) is characterised by bouts of pain, associated with focal motor, sensory or language neurological symptoms and CSF lymphocytic pleocytosis. It is considered to be a benign disorder due to its reversible and self-limiting nature.3 Its aetiology is unknown, although the neurovascular alterations that occur are very similar to those of migraine, which is sometimes one of the clinical differential diagnoses.
Neuroradiology plays a key role in cases where other more serious diseases such as ischaemic stroke, meningitis or vasculitis cannot be ruled out due to the clinical presentation. However, its utility in confirming the disease is minimal.
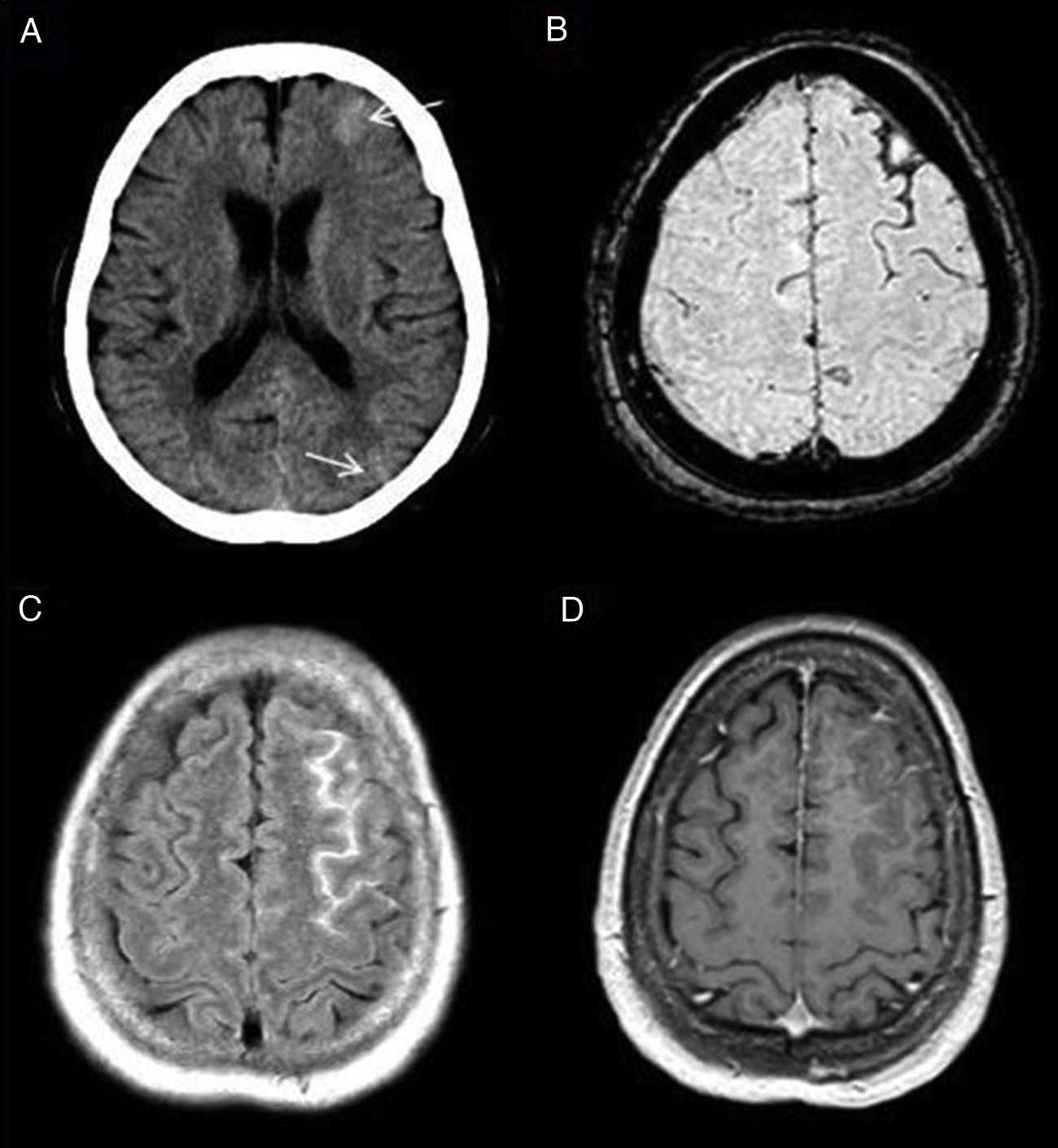
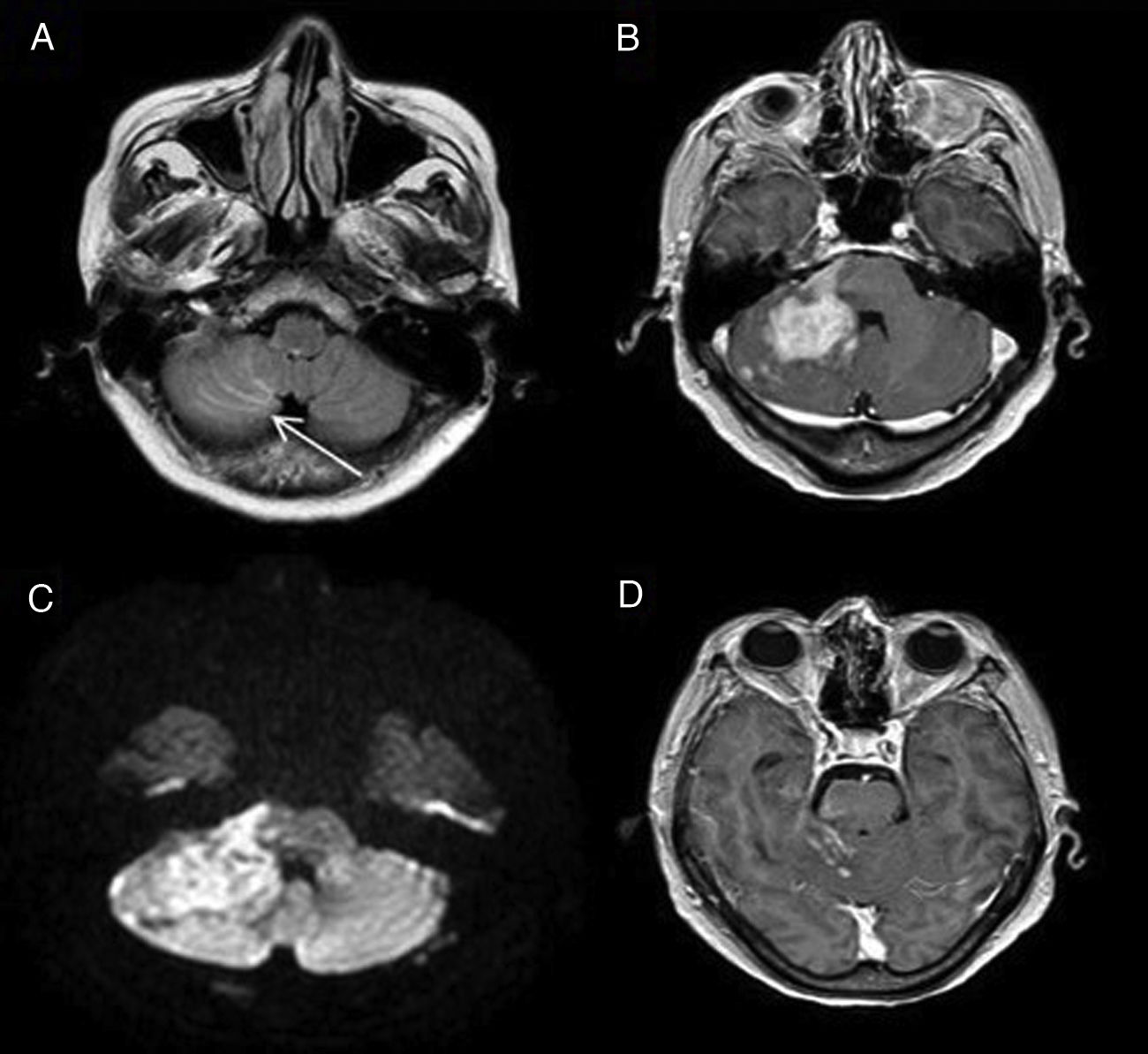
Some of the signs described in this disease, all of them non-specific, are: focal cerebral oedema, the finding of hypoperfusion in temporo-occipital regions and the identification of areas of FLAIR hyperintensity in the SAS (Fig. 2).4 Another more recently described sign, the decreased venous signal in the symptomatic hemisphere shown on magnetic susceptibility sequences, may be useful in the differential diagnosis with migraine with aura, which by contrast shows a prominence of venous structures on this sequence.5
MeningitisThe diagnosis of suspicion is primarily clinical, while confirmation is obtained by biochemical and cellular analysis of CSF.
Imaging tests are relegated to the background, as in many cases they show non-specific findings. However, brain MRI is increasingly used to support this diagnosis. Some features on imaging are6,7:
- –
Leptomeningeal inflammation (Fig. 3) in the form of nodular or linear and focal or diffuse contrast uptake in the SAS, due to a rupture or altered permeability of the blood-brain barrier.
- –
Areas of hyperintensity on FLAIR or diffusion-weighted imaging (DWI) in the SAS, resulting from pathological deposition of protein content and increased cellularity.
- –
Cerebritis resulting from contiguous inflammatory changes in the adjacent parenchyma and development of intra-axial (brain abscess) or extra-axial (empyema) collections.
In recent years, an intravenous contrast-enhanced 3 D-FLAIR sequence has been included in meningitis protocols because it has shown greater sensitivity in detecting involvement of the SAS than gadolinium-enhanced T1-weighted imaging and a greater ability to differentiate between true pathological contrast uptake and enhancement of a pial vascular structure.2,8
Moyamoya diseaseThis is a non-atherosclerotic idiopathic cerebral vasculopathy characterised by progressive stenosis of the intracranial segment of the internal carotid arteries and their terminal branches, usually bilateral, with a consequent reduction in oxygen supply to the brain. In an attempt to compensate for this deficit, a proliferation of a network of anomalous collateral vessels occurs. It is called Moyamoya syndrome when there are certain associated pre-existing conditions, such as sickle cell anaemia, neurofibromatosis type 1, Down's syndrome, etc.
Imaging tests reveal vascular stenoses and in many cases generalised brain atrophy and infarcts in border territories by haemodynamic mechanism secondary to the stenoses. Cerebral neovascularisation will be responsible for other findings:9,12
- –
Ivy sign: identified in 80% of symptomatic Moyamoya patients and 30% of asymptomatic patients.10 It is a meandering hyperintensity in the sulci on FLAIR imaging (Fig. 4) and on contrast-enhanced T1 sequences, where it is easier to detect. This sign is an indicator of the dynamic state of the leptomeningeal collaterals and a predictor of Moyamoya disease, especially in those diseases that predispose to its development. It is caused by slowed flow in the engorged pial collaterals, which, from less affected regions, attempt to irrigate the hypoperfused territory.10,11
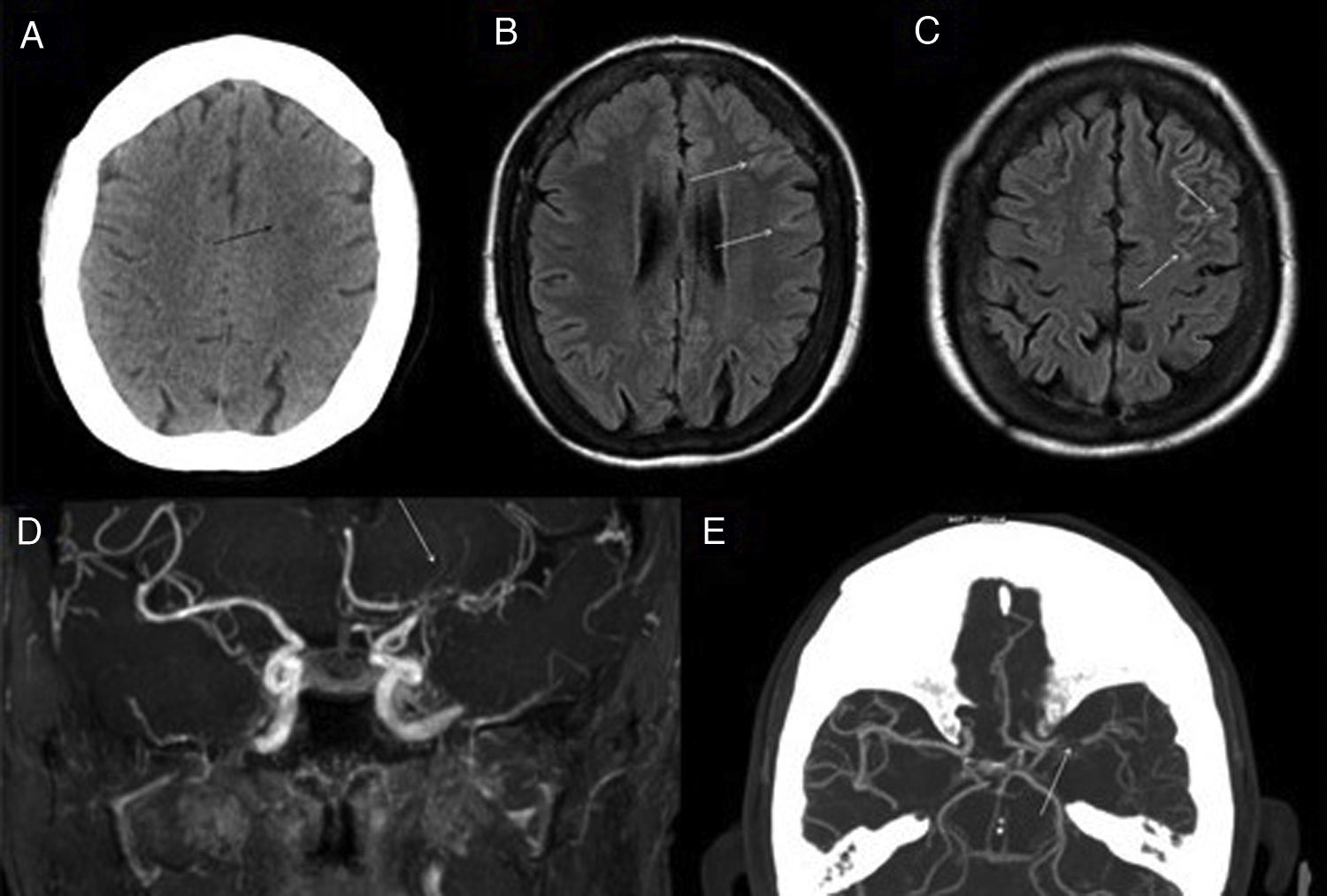
Figure 4.Moyamoya disease. 60-year-old woman with stenosis of major intracranial vessels, with Moyamoya pattern. Cranial CT scan without IVC (A) shows small ischaemic lesions (black arrow) of undetermined chronology in the subcortical border zone. FLAIR images (B and C) show discrete linear signal hyperintensity in the adjacent convexity sulci (white arrows), reflecting the increased pial collaterality. MRI angiography (D) confirms stenosis of the supraclinoid segment of the left internal carotid artery (ICA) and M1 segment of the left middle cerebral artery (MCA) (white arrow). CT angiography (E) also shows the proliferation of a deep vascular network surrounding the stenosed segments (white arrow).
(0.13MB). - –
Prominent medullary veins and small foci of microbleeding in deep regions.
- –
Transdural collateral arteries from branches of the middle meningeal artery.
The term Moyamoya pattern or phenomenon is an angiographic concept in which multiple entities, whether congenital or acquired, can cause progressive stenosis of the distal end of the internal carotid artery with subsequent development of leptomeningeal collateral vessels, including Moyamoya disease.11,12
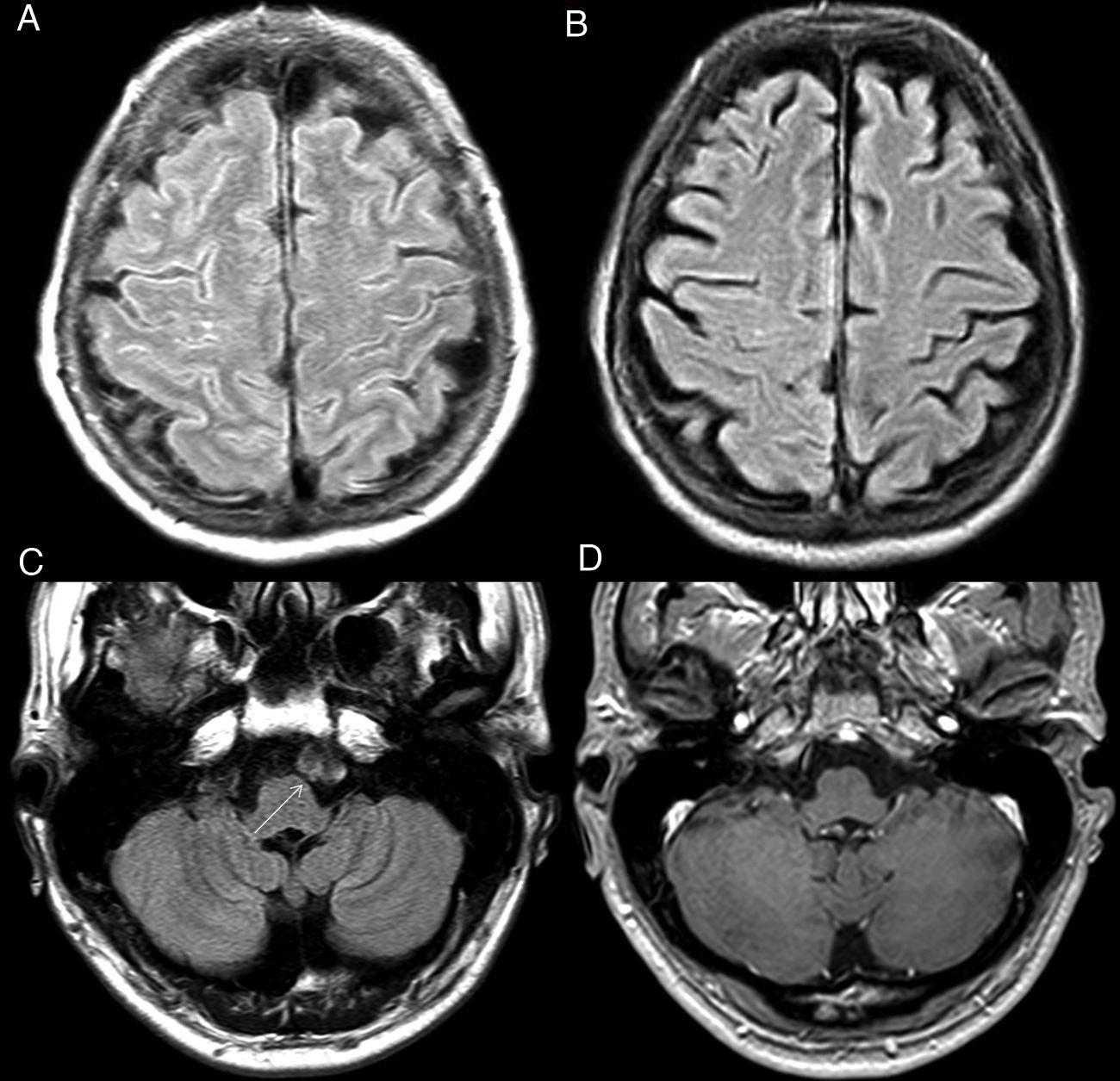
Leptomeningeal venous malformation (LVM)This is a manifestation that is categorised as a cerebrofacial venous metameric syndrome,13,14 in which patients develop a constellation of venous vascular malformations in the soft tissue, bone, dura mater and nerve structures, including the brain and eyeball. Within this group, Sturge-Weber syndrome is the best known and is characterised by the triad of leptomeningeal venous malformations, "port-wine stain" facial capillary malformation and sometimes ipsolateral glaucoma, which are the defining criteria of this disease.15
For treatment planning and follow-up, the diagnosis of glaucoma and intracranial involvement, even if asymptomatic, is essential in at-risk patients.15,16
LVM is a vascular malformation dysplasia of superficial veins showing slowed flow and reminiscent of Moyamoya disease on imaging (Fig. 5), although with a different pattern of involvement and pathophysiology. The most specific direct sign is fine gyral enhancement on gadolinium-enhanced T1 sequences. However, this enhancement is not always present and in early stages of the disease other more subtle, indirect findings should be sought, which may be suggestive of LVM:13–16
- –
Hyperintensity on FLAIR imaging in the convexity sulci.
- –
Inversion of intensity values in the underlying white matter on both T1- and T2-weighted sequences.
- –
Hypertrophic choroid plexus, with debate as to whether this is due to a venous vascular malformation or secondary to an attempt to compensate for diminished venous return.
- –
Retinal venous vascular malformation that can lead to glaucoma.
Sturge-Weber syndrome. Extensive gyral and leptomeningeal calcification (black arrow) in the left parietal lobe, visualised on cranial CT scan without IVC (A). Control MRI 5 years later (B-D): T2* FFE sequence (B) shows susceptibility artefact (*) caused by calcifications; FLAIR sequence (C) and T1 after IVC administration show focal cortical atrophy, SAS hypersignal and leptomeningeal enhancement (white arrow in D) in the sulci of the left parietal convexity, reflecting the leptomeningeal venous malformation typical of this disease. Frontal hyperostosis and subtle cutaneous hyperintensity corresponding to the port-wine stain on the left side (white arrow in C) are also seen.
Courtesy of Dr Lancharro Zapata and Dr Aguado del Hoyo.
As the disease progresses, leptomeningeal uptake decreases and atrophy of the affected hemisphere and gyral calcifications exhibiting "tram track" appearance underlying the location of the LVMs, best assessed by CT, appear. In addition, the prevalence of venous occlusion, thrombosis and vascular steal phenomena that favour the development of gliosis and parenchymal malignancy increases.16,17
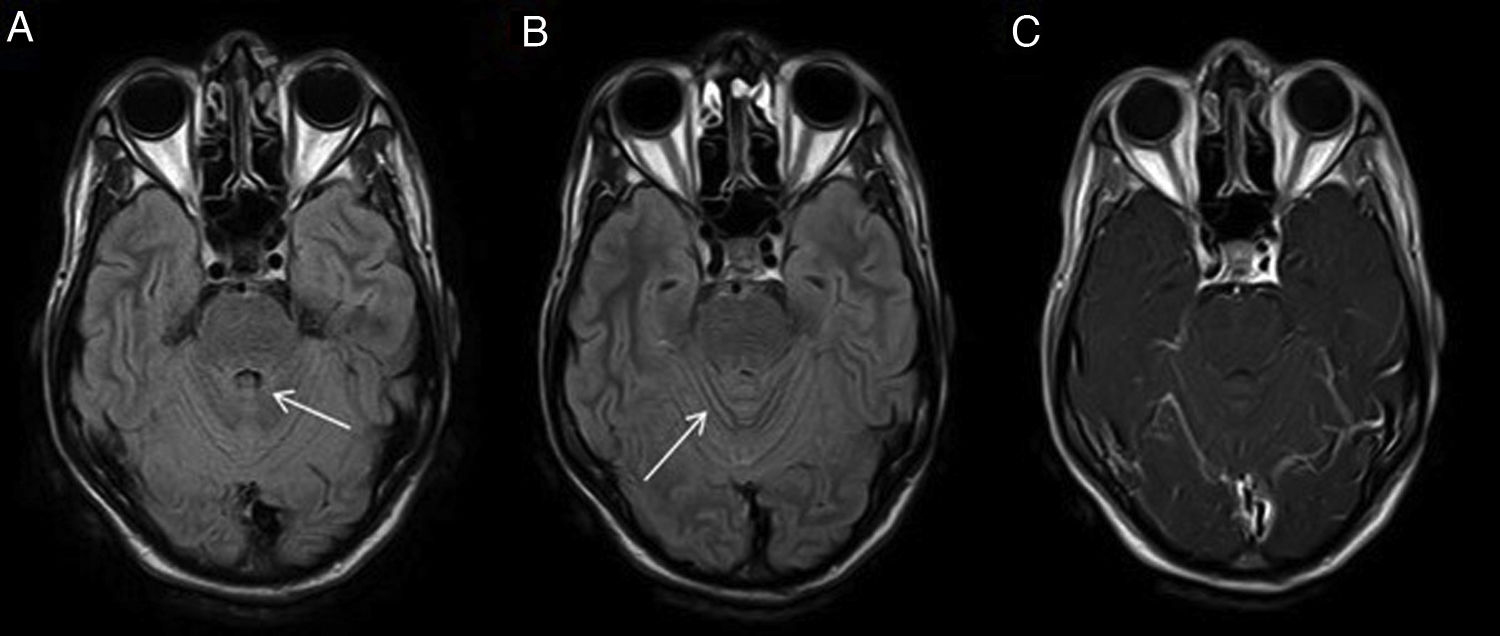
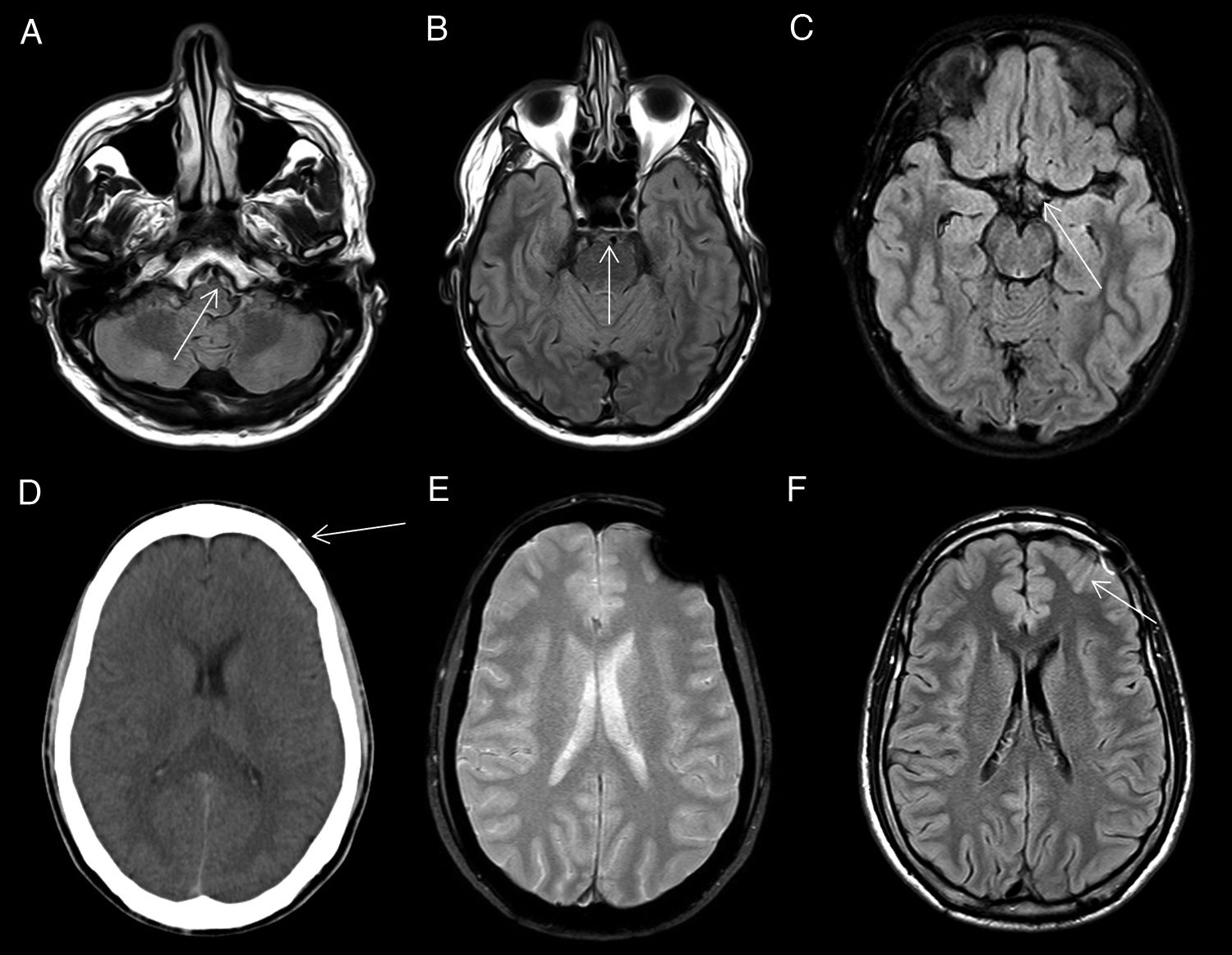
Subarachnoid haemorrhage (SAH)In imaging tests (Fig. 6), acute SAH is difficult to detect in T1- or T2-weighted sequences, with the FLAIR sequence demonstrating a higher sensitivity, as high or higher than CT, especially in the subacute phase and in anatomical sites difficult to assess by CT, due to beam hardening artefacts such as the posterior fossa.18,19 Moreover, haemoglobin and haemosiderin degradation products condition susceptibility artefacts in T2* gradient-echo sequences and magnetic susceptibility sequences such as susceptibility-weighted imaging (SWI) that help characterise foci of chronic SAH (superficial haemosiderosis).
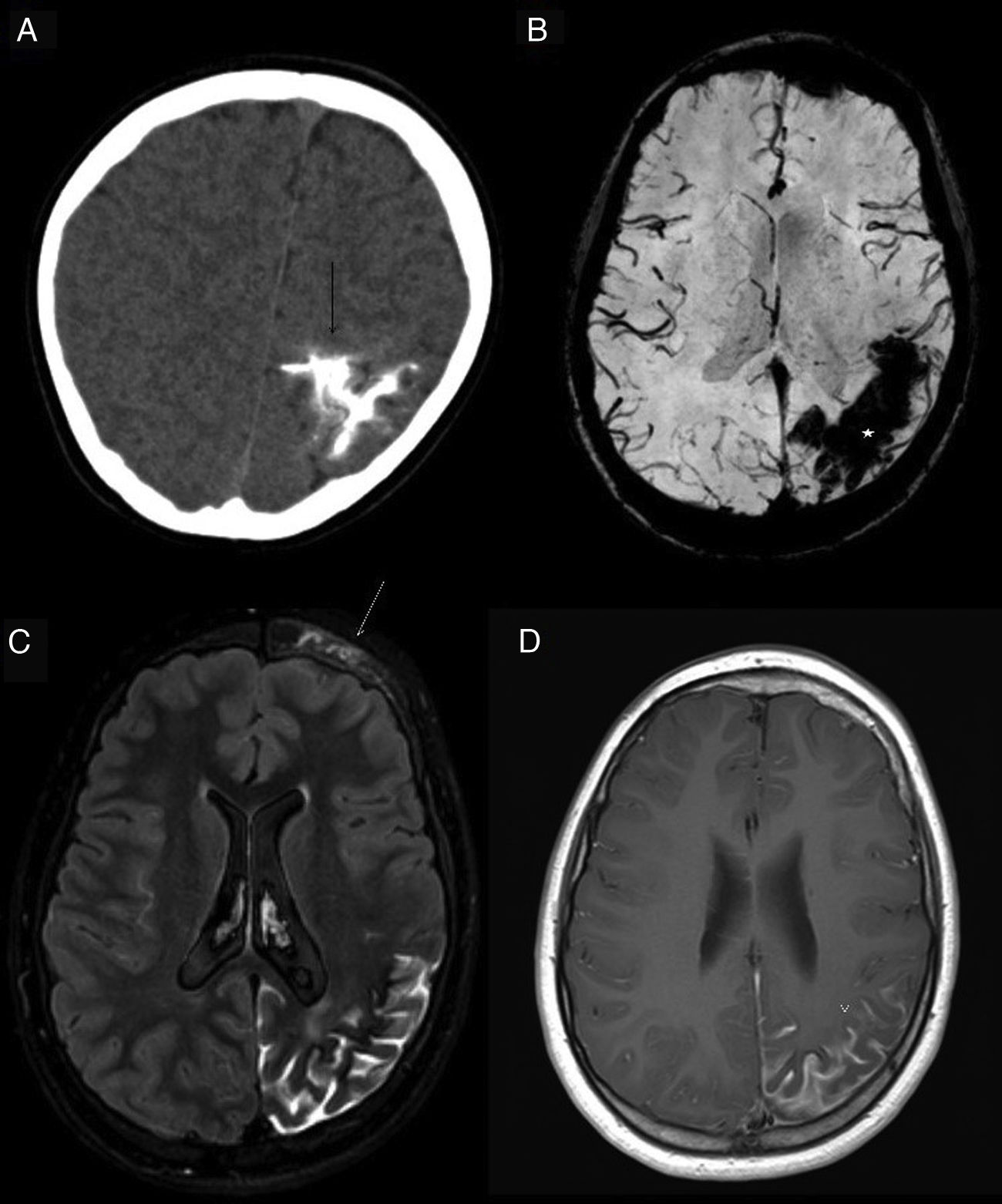
Subarachnoid haemorrhage (SAH). The CT image shows a faint hyperdensity in the left superior frontal and left parieto-occipital sulci (arrows) in relation to non-traumatic SAH (A). The haematic content of the superior frontal and prerolandic sulci produces a susceptibility hypointensity in the T2*FFE sequence (B) and a hyperintensity in the FLAIR sequence (C). No enhancement on T1 sequence with IVC (D). No aneurysms or vascular anomalies were found on digital subtraction angiography.
Nevertheless, CT and CT angiography, together with clinical suspicion and lumbar puncture for CSF analysis, remain the modalities of choice for the diagnosis of SAH and its possible causes, with MRI being relegated to a secondary role if the initial scan is inconclusive or for differential diagnosis.
Leptomeningeal carcinomatosisLeptomeningeal metastases occur from the spread of a tumour to the CSF, arachnoid or pia mater in approximately 5–8% of cancer patients. They are most common in lung cancer, breast cancer and melanoma, but also occur in tumours of the central nervous system (CNS) with dissemination through the CSF, characteristic of such conditions as medulloblastoma and ependymoma.
Imaging tests are unequivocally useful for their detection, especially as they are often asymptomatic patients and CSF cytology has a significant percentage of false negatives. The main findings are:20,21
- –
Hyperintensity in FLAIR (Fig. 7) and in DWI with low apparent diffusion coefficient (ADC) values in the SAS, similar to that explained in the case of meningitis, mainly due to increased tumour cellularity and protein content.
Figure 7.Leptomeningeal carcinomatosis. Patient with a history of lung cancer with signs of leptomeningeal dissemination with signal hyperintensity in bilateral frontoparietal convexity sulci on FLAIR imaging (A), which show enhancement with intravenous contrast in both the FLAIR sequence with contrast (B) and in gadolinium-enhanced T1 with magnetisation transfer (arrow in C). Secondary leptomeningeal melanosis. Patient with metastatic melanoma with signs of bilateral leptomeningeal infiltration on SPIR FLAIR images (arrows in D) and with areas of leptomeningeal uptake on FLAIR (E) and gadolinium-enhanced T1-weighted sequences with magnetisation transfer (F).
(0.17MB). - –
Nodular or linear, focal or diffuse thickening of the leptomeninges, which will show up on T1 sequences after intravenous contrast administration. As previously mentioned, the shortening of the T1 relaxation time exerted by gadolinium can be exploited in contrast-enhanced FLAIR sequences to better delineate carcinomatous lesions. However, the added value of this technique is open to much debate and its use is not yet standardised in protocols for patients with this diagnostic suspicion. However, an intravenous contrast-enhanced 3 D-FLAIR sequence is now being included in carcinomatosis protocols to help differentiate between true pathological contrast uptake or enhancement of a pial vascular structure.8
- –
Non-resorptive hydrocephalus is not uncommon as a form of disease onset and results from infiltration of the arachnoid villi.
A rare condition, leptomeningeal melanocytosis (Fig. 7) may show overlapping findings on MRI studies. It consists of primary (originating in neuroectodermal tissue) or secondary (in patients with metastatic melanoma) neoplastic proliferation of melanocytes in the pia mater or arachnoid at any location in the CNS. Due to the paramagnetic properties of melanin, the hyperintensity of lesions on T1-weighted sequences can provide diagnostic guidance.22,23
Diffuse leptomeningeal glioneuronal tumour (DLGT)This is a very rare entity with a very poor prognosis defined by focal or diffuse neoplastic proliferation of oligodendroglial-like cells with evidence of neuronal differentiation in a subset of cases located in the meninges, with no evidence of primary tumour in the CNS. Most are low-grade lesions histologically, but in some cases anaplasia may manifest.24
Pre-mortem diagnosis is difficult. Some of its manifestations on imaging tests should be known since CSF cytology for malignant cells is often negative:24,25
- –
Leptomeningeal enhancement on contrast-enhanced sequences, particularly in the basal cisterns with extension to the surface of the brain and spinal cord.
- –
Focal or diffuse FLAIR signal hyperintensity in the SAS.
- –
Numerous, millimetric subpial cysts on the inferior surface of the temporal and frontal lobes, posterior fossa and spinal cord. It is thought that they may correspond to dilated perivascular spaces.
- –
Non-resorptive hydrocephalus and signs of intracranial hypertension.
Much more frequent than its primary counterpart, it consists of contiguous infiltration of the meninges by glial tumour cells from a primary CNS tumour and poses differential diagnosis problems in few patients.
Leptomeningeal lymphomatosisLymphoproliferative syndromes can also involve the meningeal layers either primarily (primary leptomeningeal lymphoma) or secondarily either by contiguity (Fig. 8) or by haematogenous spread. Its imaging manifestations are superimposable to other neoplastic processes affecting the leptomeninges (carcinomatosis or gliomatosis).26
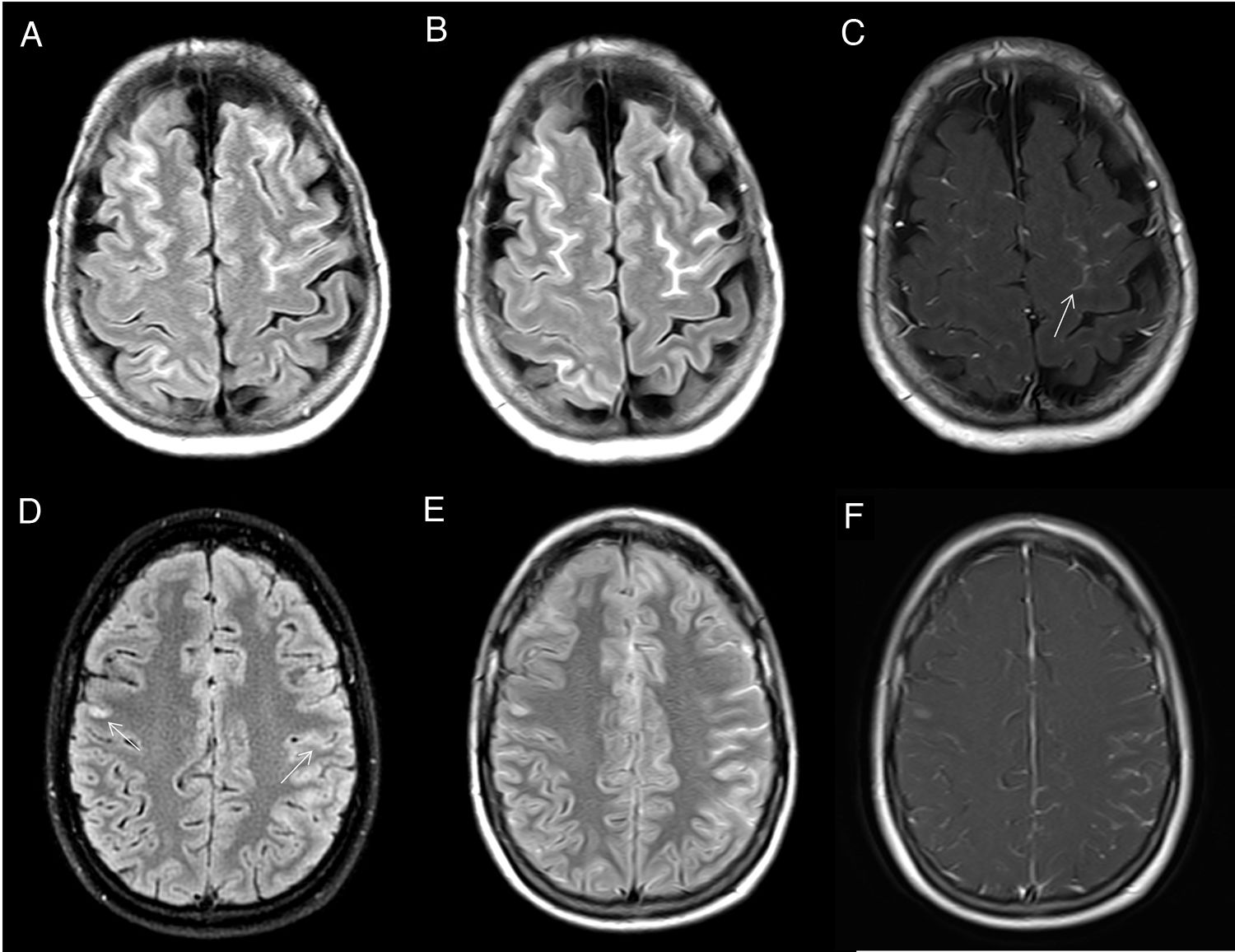
Secondary leptomeningeal lymphomatosis by contiguity. The FLAIR image (A) shows hyperintensity in the sulci between the cerebellar folia (arrow) due to leptomeningeal infiltration contiguous to a cerebellar lymphomatous mass showing intense enhancement on the gadolinium-enhanced T1-weighted sequence (B) and diffusion restriction (C). Leptomeningeal involvement shows uptake in the T1-weighted sequence after Gd administration (D).
FLAIR images have several artefacts that can degrade image quality and simulate disease.24 Some of the most frequent are as follows.
CSF pulsatilityCSF pulsation artefacts in inversion-recovery sequences are a very common source of diagnostic error in brain MRI. The pulsatile CSF flow gives rise to the influx of protons that have potentially not received that inversion pulse, with consequent failure of CSF signal suppression. This artefact is especially common in the basal cisterns (Fig. 9) (prepontine or pontocerebellar cistern) and in the third and fourth ventricles, and less common and intense in the sulci of the convexity where CSF flow is diminished.27
CSF pulsatility artifact. Axial FLAIR images show non-pathological CSF signal hyperintensity in the peribulbar (A), prepontine (B) and suprasellar (C) cisterns. Magnetic susceptibility artifact. The CT image (D) shows a millimetre-sized foreign body of metallic density in the subcutaneous cellular tissue (arrow) causing a magnetic susceptibility artefact on T2* gradient-echo sequences (E) and a linear signal hyperintensity artefact on FLAIR (F) in the underlying subarachnoid space (arrow).
Metallic foreign bodies (shrapnel, brackets, etc.) and bony structures of the base of the skull (floor of the anterior and middle cranial fossa) cause magnetic susceptibility artefacts in the form of hyperintensity in conventional FLAIR sequences (Fig. 9), as well as a marked and more extensive hypointensity that hinder the assessment of adjacent structures.27
The origin of these artefacts can be confused with pathological findings, so it is advisable to consult previous CT studies, which will often facilitate the detection of the metallic material causing the artefact.
Oxygen therapyOxygen is a paramagnetic substance and its increase in CSF will cause a reduction in T1 relaxation time. For this reason, it is not uncommon to observe an increased CSF signal on FLAIR imaging in patients receiving supplemental oxygen (Fig. 10).28
Oxygen therapy artifact. Patient who was receiving supplemental oxygen. The FLAIR sequence (A) shows a very faint and diffuse increase in CSF intensity in the frontoparietal convexity sulci that disappeared in the control MRI after withdrawal of oxygen therapy (B). Artefact due to vascular pulsatility. Pulsatility artefact in the cistern of the bulbocerebellar angle on the left side (C), produced by the throbbing of the basilar artery, simulating a lesion. This image is not identified in the T1-weighted sequence with IVC (D).
Many studies have shown that the diffusion of oxygen from the blood to the CSF and the resulting signal hyperintensity are dependent on the delivery methods and the concentration of inhaled O2. Higher fractions of inspired oxygen (FiO2) will increase blood oxygen tension and subsequently diffusion into the CSF.29
Vascular pulsatilityIntracranial vessel pulsatility can generate motion artefacts in FLAIR images. These are well-defined spectral images that are projected onto the CSF and neighbouring anatomical structures in the form of a hyperintensity that mimics the size, shape and alignment of the responsible vessel, and could be mistaken for CSF disease (Fig. 10). It is important to mention that this artefact is not unique to the FLAIR sequence or to intracranial vessels.27
It has also been linked to spontaneous eye movements, respiratory movements or heartbeat in cardio-MRI studies. The distance between the false images is related to the TR and the periodicity with which the movement is reproduced.
ConclusionsCSF hyperintensity in the SAS on FLAIR imaging can be found in numerous entities with different clinical repercussions, some pathological and some due to artefacts, of which the radiologist should be aware. The reported clinical context, CSF analysis and other coexisting findings on MRI scan will be helpful in arriving at a definitive diagnosis.
FundingThe authors declare that they have not received funding of any kind for drafting this manuscript.
Authors’ contribution- 1
Responsible for study integrity: JMB.
- 2
Study conception: JMB, IGM, PFG and IHH.
- 3
Study design: JMB and IGM.
- 4
Data collection: JMB, IGM and PFG.
- 5
Data analysis and interpretation: N/A.
- 6
Statistical processing: N/A.
- 7
Literature search: JMB, IGM, PFG and IHH.
- 8
Drafting of the article: JMB.
- 9
Critical review of the manuscript with intellectually relevant contributions: IGM, PFG and IHH.
- 10
Approval of the final version: JMB, IGM, PFG and IHH.
The authors declare that they have no conflicts of interest.