Gastric cancer is the fifth most common cancer in the world. The most common histologic subtype is adenocarcinoma. Gastric adenocarcinomas are staged using the American Joint Committee on Cancer’s 8th TNM classification. The perigastric ligaments, mesentery, omentum, and potential spaces between the parietal and visceral peritoneal linings play are important structures for staging. The spread of disease is influenced by the location of the tumor within the stomach, as well as by the anatomy related to the ligaments and lymph vessels. CT is the imaging modality of choice for the preoperative clinical staging of gastric cancer, and it is essential for planning treatment. To be able to do an adequate imaging workup, radiologists need to know the different pathways through which gastric cancer can spread: lymphatic, subperitoneal, direct invasion, transperitoneal, hematogenous, and extramural venous invasion.
El cáncer gástrico es el quinto cáncer más frecuente en el mundo. El subtipo histológico más frecuente es el adenocarcinoma. Para su estatificación se utiliza la 8.ª edición de la clasificación TNM de la American Joint Comittee on Cancer. Los ligamentos perigástricos, mesenterio, omento y espacios potenciales entre los revestimientos peritoneales parietal y visceral, son estructuras con gran implicación en la estadificación. La diseminación de la enfermedad se ve afectada por la localización del tumor en el estómago, así como por la anatomía ligamentaria y linfática. La tomografía computarizada es la modalidad de imagen de elección para la estadificación clínica preoperatoria del cáncer gástrico, y es esencial para la planificación del tratamiento. Existen múltiples vías de diseminación en el cáncer gástrico que se deben conocer para poder realizar una correcta valoración radiológica: linfática, subperitoneal, invasión directa, transperitoneal, hematógena e invasión venosa extramural.
Gastric adenocarcinomas (GA) are malignant epithelial tumours that originate in the gastric glands of the mucosa and account for 95% of malignant stomach tumours1. It is the fifth most common cancer worldwide and the fourth most common cause of cancer deaths2. The average age at diagnosis is 68 years and it is more common in men than in women (2:1 ratio)1,3. The highest incidence rates are observed in East Asia (especially Japan) and Eastern Europe2. The main risk factor is Helicobacter pylori (HP) infection, especially in non-cardia gastric cancers2,3. According to several studies, it is a persistent risk factor even after the infection has been treated, due to possible histological changes in the mucosa4–6. Other risk factors worth noting are a low intake of fruit and vegetables, a high consumption of salt, processed meat, alcohol or tobacco, metabolic syndrome or a previous partial gastrectomy. The principal risk factors for gastric cardia adenocarcinomas are obesity and gastro-oesophageal reflux2,3. Approximately 30% of GAs are located in the fundus and cardia, 30% in the corpus, 30% in the antrum and 10% present as a diffuse infiltrative disease1. The most commonly used histological grading systems are the Lauren classification and the World Health Organization's (WHO) Classification of Tumours. The former is the most widely used and divides GAs into subtypes: intestinal (54%), diffuse (32%) and indeterminate. Intestinal GAs are more frequently found in the elderly and men, affect the antrum, and are generally associated with intestinal metaplasia and HP2,7–9. The diffuse type is more common in young people and slightly more prevalent in women. It affects the body to a greater extent and has a worse prognosis2,7–9. The WHO classification draws a distinction between tubular, papillary and mucinous GAs (equivalent to the intestinal type) and signet ring carcinomas and poorly cohesive carcinomas (equivalent to the diffuse type)7,9.
The aims of this article are to review GAs using the latest edition of the American Joint Committee on Cancer’s (AJCC) tumour-node-metastasis (TNM) staging system and discuss the dissemination pathways of GA disease spread and their importance in tumour staging.
Anatomy reviewFrom an anatomical perspective, the stomach is divided into five parts: cardia, fundus, corpus, antrum and pylorus. The gastric wall is made up of 5layers: mucosa (which is further divided into epithelium, lamina propria and muscularis mucosae), submucosa, muscularis propria, subserosa and serosa. The normal wall thickness is 5mm when the stomach is distended and 10mm when it is only mildly distended10,11. Although an endoscopic ultrasound can differentiate between the five layers, only three layers can be visualised on computed tomography (CT). On CT, the mucosa is seen as a thin hyperdense layer (1−3mm), the submucosa as a hypodense middle layer of variable thickness, and the muscularis, subserosa and serosa are seen as a single tenuously hyperdense layer10,12,13.
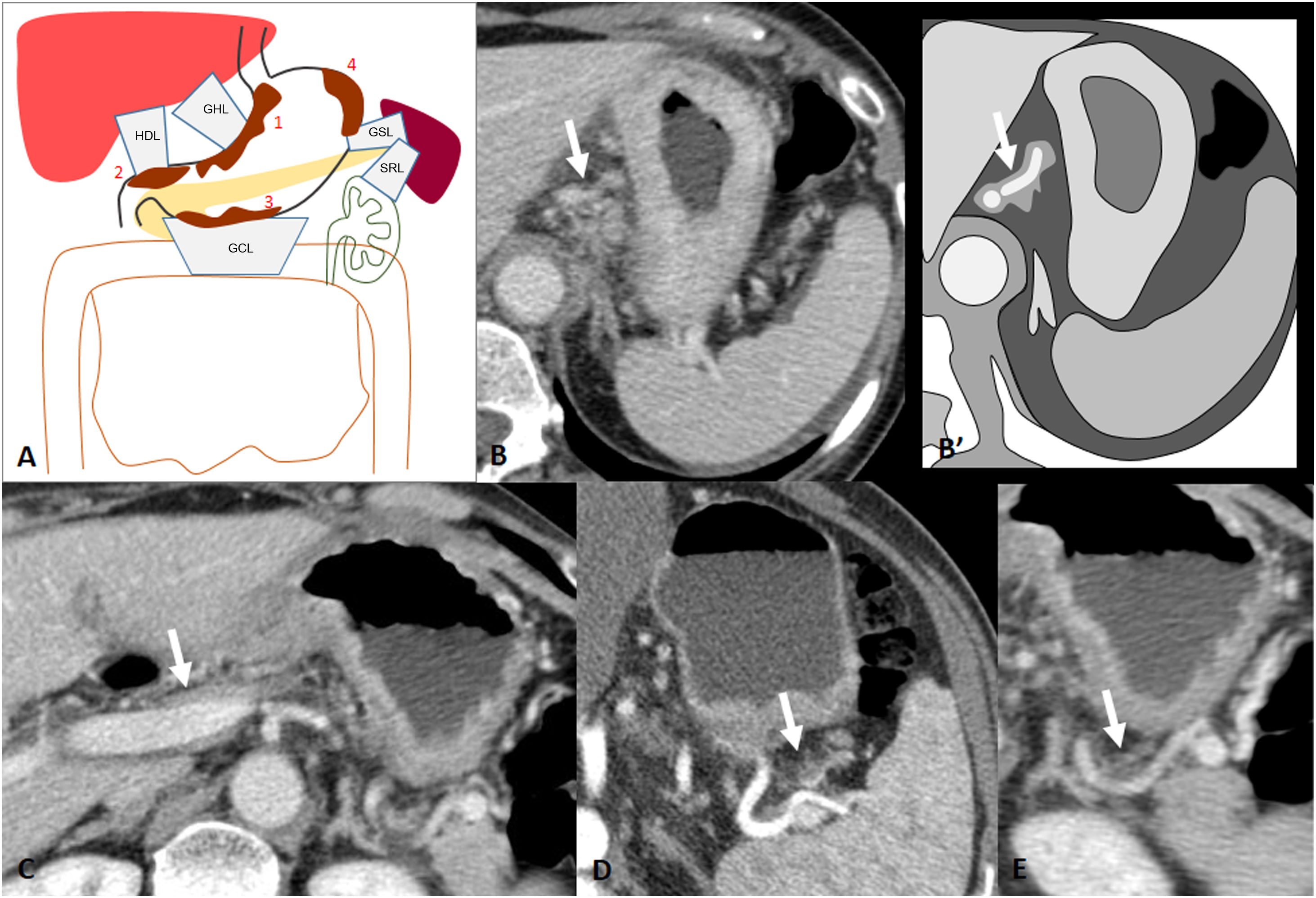
Outside the stomach, but in intimate contact with it, is the peritoneum, which reflects and folds to cover the organs and includes ligaments, the mesentery, the omentum, and the potential spaces between the parietal and visceral peritoneal linings. The subperitoneal space is the continuous interconnecting space beneath the peritoneum which contains the ligaments, mesentery and suspended abdominopelvic organs. In imaging, and more specifically with CT, we use the vascular structures in each ligament to recognise their locations (Table 1) (Fig. 1)1.
Perigastric ligaments: connected structures, vascular anatomical landmarks and organs at risk of direct invasion.
| Ligament | Course | Associated Vasculature | Potential organ involvement |
|---|---|---|---|
| Gastrohepatic (GHL) | Cardia and the lesser curvature of the stomach to visceral surface of the liver | Gastric vessels | Liver |
| Hepatoduodenal (HDL) | Hepatic hilum to lesser curvature of stomach and proximal duodenum | Hepatic artery and portal vein | LiverPancreatic Head |
| Gastrocolica (GCL) | Greater curvature of stomach to transverse colon | Gastroepiploic vessels | Transverse colon |
| Gastrosplenic (GSL) | Fundus and greater curvature of gastric body to splenic hilum | Short gastric and left gastroepiploic vessels | Spleen |
| Splenorenal (SRL) | Splenic hilum to left anterior pararenal space | Splenic vessels | Pancreatic tail, Descending colonLeft kidney |
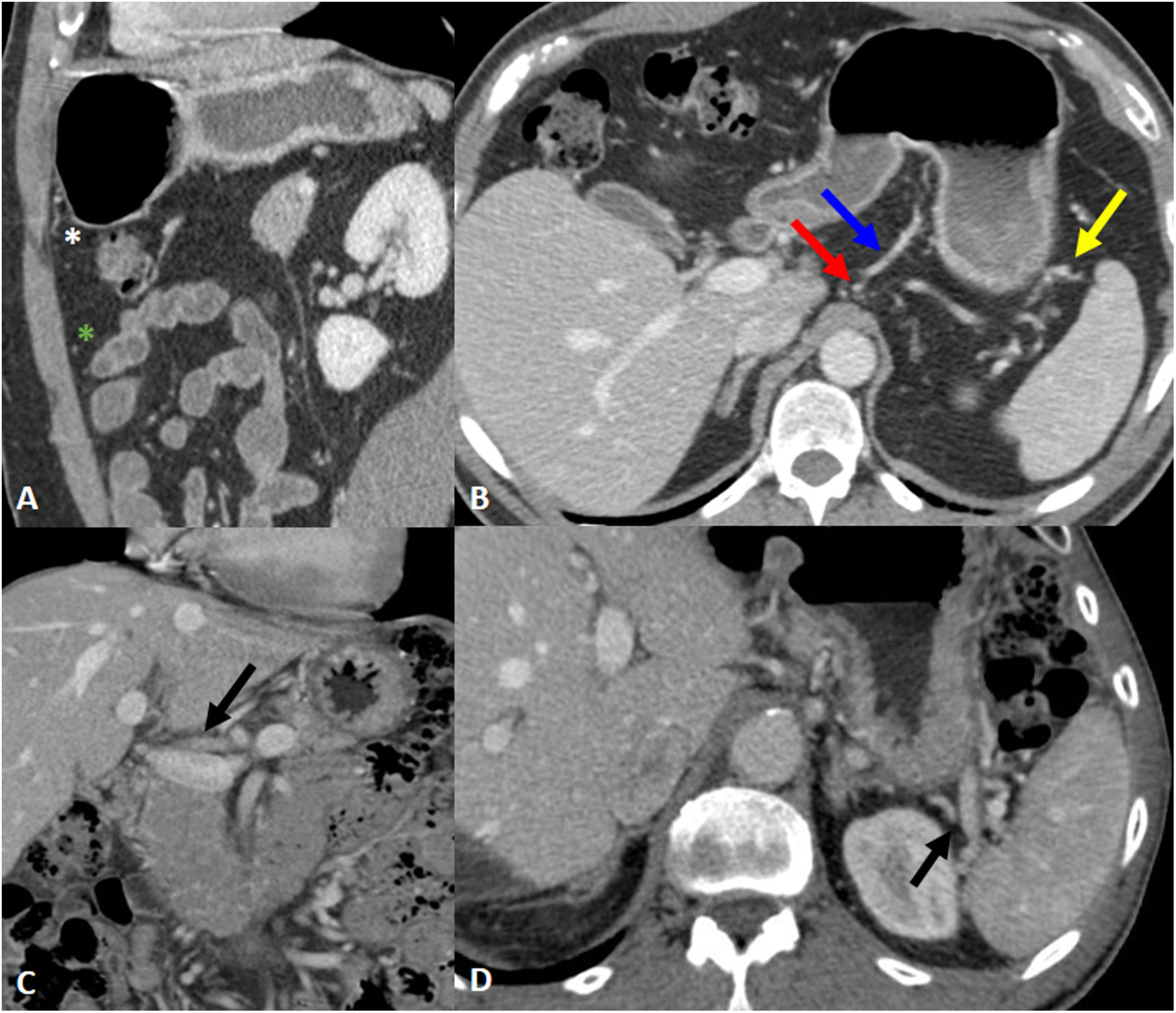
Perigastric ligaments and associated vasculature. A) Gastrocolic ligament; associated vasculature: gastroepiploic vessels (white asterisk). Greater omentum; associated vasculature: epiploic branches (green asterisk). B) Gastrohepatic ligament; associated vasculature: left gastric artery and vein (red and blue arrow). Gastrosplenic ligament; associated vasculature: left gastroepiploic vessels (yellow arrow). C) Hepatoduodenal ligament; associated vasculature: portal vein and hepatic artery (arrow). D) Splenorenal ligament; associated vasculature: splenic vessels (arrow).
The prognosis of GA correlates with the stage of the cancer determined at diagnosis according to the depth of tumour invasion (T), lymph node involvement (N) and metastasis (M)3,14. Gastric cancer (GC) is usually classified into two groups: early GC (EGC) and advanced GC (AGC)1. ECG affects the mucosa and submucosa (T1), irrespective of tumour size and lymph node involvement. Its 5-year survival rate is >90%. AGC affects deeper layers (T2-T4) and has a 5-year survival rate of 7–27%1.
For GC staging, we use the eighth edition of the AJCC and Union for International Cancer Control TNM staging system, published in 2017, applicable to adenocarcinomas, poorly differentiated neuroendocrine carcinomas and mixed adenoneuroendocrine carcinomas14. Before discussing staging, we will specify the three main changes in the eighth TNM staging manual compared to the seventh edition.
- -
It features three separate staging systems for GC14,15: pre-treatment clinical staging (cTNM), pathological staging assigned after surgery (pTNM) and pathological staging assigned after resection following neoadjuvant therapy (ypTNM). The preoperative diagnosis of GC has been found to be inaccurate, especially regarding metastasis to the lymph nodes, and therefore the highly complex pathological stage groups cannot be legitimately assigned preoperatively. Moreover, neoadjuvant chemotherapy is increasingly employed and this is having an impact on the pathological findings of patients who are resected following therapy, and subsequently also the p-staging classifications. Therefore, these p-classifications may not reflect accurate prognoses for patients who are resected without prior chemotherapy.
- -
Some of the pathological (pTNM) staging groups have been modified. Differences in survival rates among stage III patients is more accurately reflected by subdividing the pN3 category into pN3a (7–15 nodes affected) and pN3b (≥16 nodes affected). Therefore, the recommendation is that at least 16 nodes (although more than 30 is desirable) should be removed and pathologically assessed14,15.
- -
A change is made to the classification of tumours of the gastroesophageal junction (GEJ): in the current staging manual, tumours that involve the GEJ with epicentres located >2cm away from the GEJ (former Siewert type III), or, those which having epicentres within 2cm of it, do not involve the GEJ are classified and staged as GC14,15.
Clinical staging is reached using a combination of the following techniques:
- -
Endoscopy±endoscopic ultrasound (EUS): useful for assessing cT and cN, but not for cM. The overall accuracy of EUS for cT ranges from 65% to 92% (lower when differentiating between T1a and T1b: 63.6%). The overall accuracy for cN ranges from 66% to 90%. This technique is less useful for staging tumours in the antrum1,16
- -
Multidetector computed tomography (MDCT) of the chest, abdomen and pelvis: the test of choice for preoperative clinical staging1,3.
- -
Magnetic resonance imaging: this technique is to be considered given the absence of ionising radiation and the superior tissue contrast resolution which results in an equally or more accurate cT staging17. Moreover, several studies have demonstrated its usefulness in detecting liver metastases and peritoneal carcinomatosis18–21. However, it is not routinely used due to technical limitations and lower availability1.
- -
18F-FDG PET/CT: useful for staging distant metastases. However, it has several limitations. Its spatial resolution is low, so it is unsuitable for assessing locoregional disease and metastases that are <1cm. Moreover, mucinous, signet ring cell and poorly differentiated adenocarcinomas tend to show a lower uptake of 18F-FDG. Other factors, such as inflammation or infection, may give false-positive results. Clinical guidelines do not recommend their routine use1,3.
- -
Diagnostic laparoscopy±peritoneal lavage: exploration recommended in patients with potentially resectable GAs (stages IB-III) to exclude radiologically occult metastatic disease3.
An assessment must be carried out by a multidisciplinary team prior to any treatment3. Complete surgical resection of the gastric tumour and adjacent adenopathies with negative margins is the only proven curative treatment for GAs3. The radiologist has an extremely important role in the pre-surgical clinical staging of GAs, as the visual findings determine how the patient is treated. Less invasive surgical endoscopic techniques are excluded if nodal disease is present. Laparoscopic surgery is excluded if there is perigastric ligament involvement, and the invasion of the tumour into adjacent structures or metastases limit the resectability of Gas1,3.
Clinical staging by MDCTTable 2 shows the clinical staging categories for gastric cancer from the latest AJCC cancer staging manual and the corresponding CT appearances. Table 3 shows the clinical prognostic stage groups from the same staging system14. In the eighth edition of the AJCC's cancer staging system, tumour invasion is divided into three groups: superficial invasion (cT1/cT2), deep invasion (cT3/cT4a) and invasion of adjacent structures (cT4b). Stage cT4b is classified into the cIVA prognostic stage group even in the absence of metastases. This is unusual in the current staging system as ‘stage IV’ almost always means distant metastasis. In addition, the N category is divided into cN0 (no lymph node involvement) and cN+ (with lymph node involvement), regardless of the number of lymph nodes involved14,15.
Clinical TNM classifications from the AJCC Cancer Staging Manual (8th ed.) for gastric cancer and associated CT appearances.
| cTNM from 8th edition of the AJCC staging manual for gastric cancer | ||
|---|---|---|
| Staging Category | Definition | CT Appearance |
| T1a | Tumour invades lamina propria or muscularis mucosae | Not visible on CT images |
| T1b | Tumour invades submucosa | Mucosal thickening and enhancementHypoattenuating submucosal stripe remains visible |
| T2 | Tumour invades muscularis propia | Loss of submucosal hypoattenuating stripe but smooth outer gastric wall |
| T3 | Tumour penetrates subserosal connective tissue | Inability to discriminate between the gastric mass and the outer enhancing layer of the stomachMildly blurred but generally smooth outer gastric wallA few small linear areas of fat strandingNodular or sheetlike soft-tissue thickening within perigastric ligaments |
| T4a | Tumour invades the serosa (visceral peritoneum) | Nodular or irregular serosal surfaceinfiltration of surrounding peritoneal fatHyperattenuating serosa sign. |
| T4b | Tumour invades adjacent structures (spleen, transverse colon, liver, diaphragm, pancreas, abdominal wall, adrenal gland, kidney, small intestine and retroperitoneum) | Direct invasion into adjacent organs and structures, deformity or irregular contour or loss of fat planes between tumour and adjacent organa |
| N0 | No regional lymph node metastasis | |
| N+ | Metastasis in regional lymph node(s) | >6mm in perigastric lymph nodesb>8mm in extra-perigastric lymph nodesbRounded shapeHeterogeneous or intense enhancementA cluster of three or more nodes |
| M0 | No distant metastasis | |
| M1 | Distant metastasis involving distant nodes, non-direct extension into other organs, or peritoneal carcinomatosis | Distant (non-regional) lymph nodes, non-direct extension into other organs, or peritoneal carcinomatosis (ascites, peritoneal nodules, plaques, thickening, fat stranding and/or peritoneal enhancement) |
While each radiology centre tailors its CT scan protocol to its own resources, it is useful to be familiar with the protocol proposed by the Spanish Society for Abdominal Diagnostic Imaging (SEDIA) (Table 4)22. Debate surrounds the optimal protocol for assessing GAs by MDCT. The literature supports a dynamic multiphase study with images obtained in two or three phases1,22–26. Triple-phase CT scan protocols capture images not only of the abdomen in the late arterial phase (at 40s after contrast administration) and chest, abdomen and pelvis in the portal venous phase (at 70s), but also of the abdomen in a late or equilibrium phase at 3−4min after contrast administration23–26. Intravenous contrast is generally considered to provide maximum enhancement of the tumour in the arterial phase, while it is used in the venous phase to assess lymph node involvement and metastases1,27–29. However, CT enhancement patterns appear to be affected by tumour histology, especially by fibrosis23,28. Intramural fibrosis of undifferentiated GAs is reportedly associated with increased and peak delayed-phase enhancement23. On the other hand, while it is less widely used, there are publications that advocate for the use of CT-gastroscopy or virtual gastroscopy (VG) of the upper abdomen during an initial non-IV contrast phase following the administration of 3−7g of effervescent granules with 5−10ml of water to distend the stomach with gas23–26. Several studies also affirm that adding VG to the dynamic MDCT study improves the accuracy of preoperative staging of GAs, especially in EGC24,25. Although the spectrum of protocols employed in the literature is wide, arterial and portal venous phase acquisitions are included in them all.
CT protocol proposed by The Spanish Society for Abdominal Imaging Diagnosis (SEDIA) for stomach cancer.
| Fast for 8h |
| MDCT with at least 16 rows |
| Slice thickness: 0.6−1mm and multiplanar reconstructions of 3mm |
| Distention of the gastric lumen: |
| Neutral oral contrast: water (most frequently used. 1−1.5l 15−30 mins prior to test) or methylcellulose |
| Negative oral contrast: gas (CT-gastroscopy). Less commonly used |
| Spasmolytics: hyoscine butylbromide or glucagon |
| Position: supine, or prone in cardia and antrum tumours. |
| IV contrast is injected: 350mg/mL iodine (amount: 1.8−2 cc/kg) at 4mL/s + 30ml saline. 2 phases: |
| The late arterial phase at 40s in the upper abdomen from the start of contrast administration. If the chest is included in this phase, it is excluded in the venous phase. |
| Venous portal phase of chest+abdomen+pelvis at 70s from the start of contrast administration |
Imaging findings that should lead us to suspect GA include polypoid lesions, with or without associated ulceration; focal gastric wall thickening > 15mm; diffuse thickening; abnormal gastric wall enhancement; the obliteration of gastric wall layers; and extragastric involvement1. GAs that present as polypoid lesions can be confused with gastric polyps: hyperplastic or inflammatory (the most common), fundic gland polyps or adenomatous polyps29,30. Submucosal lesions that may be mistaken for GAs include carcinoid tumours, glomus tumours and metastases. These can all present on CT scans as small hyperenhancing lesions in both the late arterial and portal venous phases, although they are less common in the stomach than GAs29,31–33. Gastrointestinal stromal tumours originate in the muscle layer and small tumours are seen on CT as hypervascular lesions similar to carcinoid tumours. Most are benign and, even if malignant, do not usually show lymph node involvement29. Diffuse gastric thickening is typical in signet ring cell carcinomas, a subset of GAs characterised by an infiltration of all mural layers that may not be detected. It frequently progresses from the distal half of the stomach into the corpus/fundus, producing scirrhous infiltration of the gastric wall (linitis plastica)1,29. In some cases it can be difficult to differentiate between diffuse thickening and gastritis or lymphoma with imaging and therefore a biopsy is required1,29.
A CT report of a GA should include the following aspects:
- -
Tumour site: anatomical (cardia, fundus, body, antrum and pylorus) and circumferential (anterior, posterior, greater or lesser curvature, or circumferential involvement)22. Distance to the GEJ should be provided. For middle-third tumours of the stomach, a subtotal gastrectomy is recommended when there is a macroscopic proximal margin of at least 5cm between the proximal tumour margin and the GEJ, and of 8cm for diffuse GAs.
- -
Tumour size: the tumour’s greatest dimension should be provided. While this data does not feature in the staging system, it is an important prognostic factor. If the tumour is >5cm, it will generally require adjuvant therapy22.
- -
Depth of tumour invasion (cT staging): - Depth of wall invasion (cT staging): although the use of CT scans has historically been limited to detecting EGC (T1), the accuracy rates for differentiating between T1 and T2 have improved to 65-82% in recent years due to multiplanar reconstructions, thin slices and successful gastric distention26,34. The rate for detecting AGC by CT (≥T2) ranges from 85% to 95%26. Differentiating between cT3/T4a can be an imaging challenge. Direct perigastric fat infiltration is suggestive of T4a, but the differential diagnosis should take into account inflammation, vascular or lymphatic engorgement and desmoplastic reaction1. The ‘hyperattenuating serosa sign’, visualised on CT as a focal or diffuse hyperattenuating thickening of the serosal layer, suggests its infiltration and therefore T4a (Fig. 2)35,36. While it is difficult to distinguish between T3/T4a, it should be noted that the role of the radiologist in clinical staging is to categorise the T in cT1/T2 or cT3/T4a. The clinical stage group is the same for cT3 and cT4a14,15.
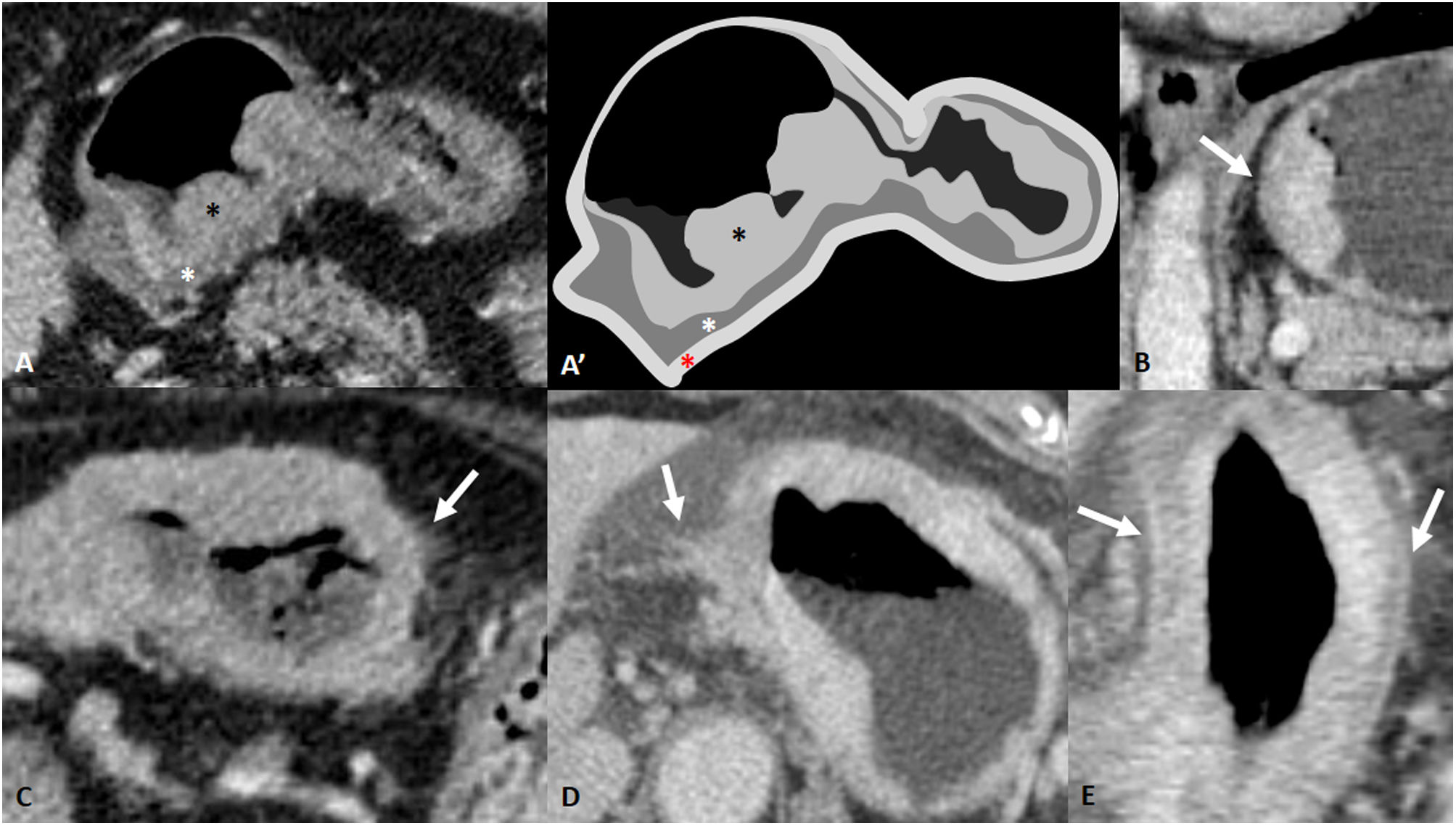
Figure 2.Depth of wall invasion (cT). A and A') GA cT1b in CT and drawing of the same slice. Polypoid thickening of gastric mucosa (black asterisk) with preservation of the hypodense submucosal layer (white asterisk). The red asterisk indicates the outermost tenuously hyperdense layer corresponding to the muscularis propria, subserosa and serosa. Post-surgery confirmation: pT1b. B) cT2. Gastric mural thickening with loss of the submucosal layer but a smooth border of the outer layer is visible. C) Mild blurring of the outer wall and minimal fat stranding. Possible cT3/T4a. Neoadjuvant chemotherapy was administered and surgery was performed. It proved to be a ypT3N0. D) cT4a: irregular wall surface with clear direct invasion of the fat. E) cT4a: diffuse gastric mural thickening with hyperattenuating serosa sign.
(0.21MB). - -
Regional lymph node involvement (cN): preoperative clinical staging should identify whether or not there is regional lymph node involvement: cN0 or cN+. The number of lymph nodes is not important, but rather whether there is lymph node involvement and their location14. Regional lymph nodes are classified into two groups: perigastric and extra-perigastric. Distant lymph node involvement is considered metastasis (M1) (Fig. 3)14,37. There is no clear consensus on the threshold for pathological size. An upper limit of 6mm for perigastric lymph nodes and 8mm for extraperigastric lymph nodes is generally accepted. A round shape, intense or heterogeneous enhancement, and a cluster of more than 3 nodes also raise suspicions3,14. It is generally accepted that any lymph node >10mm and >85–100 HU in the portal phase is considered positive, regardless of its location7. The sensitivity of CT for nodal involvement is variable, ranging from 63 to 92%3. Further information on Iymph node involvement will be provided in the section on lymphatic spread.
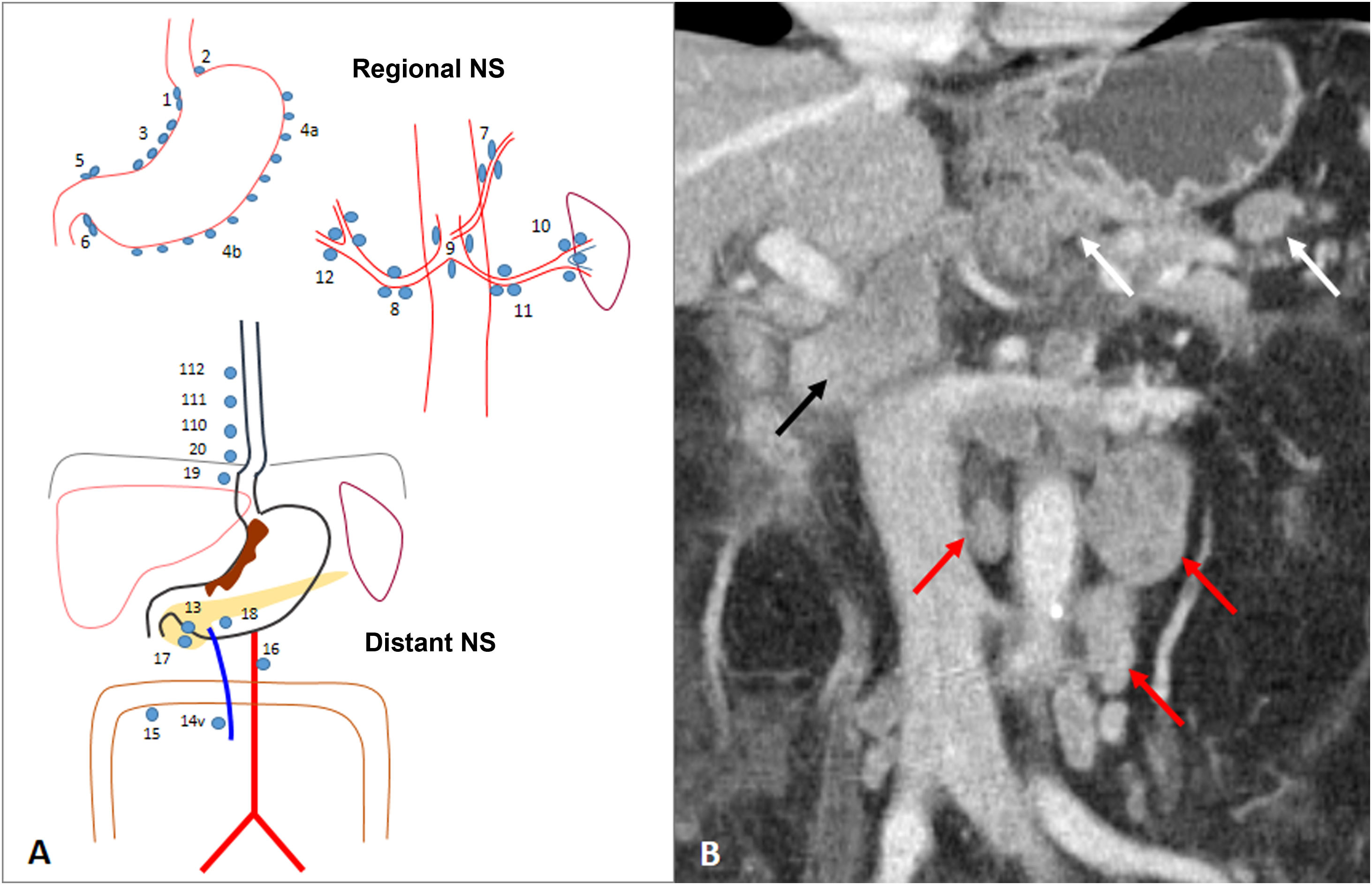
Figure 3.Lymph node involvement (cN). A) Diagram of nodal stations (NS) for gastric cancer, initially proposed by the Japanese Gastric Cancer Association (JGCA) and widely used today. Perigastric regional NS: right paracardial (1), left paracardial (2), lesser curvature (3), greater curvature (4), suprapyloric (5), infrapyloric (6). Extraperigastric regional NS: left gastric artery (7), common hepatic artery (8), coeliac artery (9), splenic hilum (10), splenic artery (11), hepatoduodenal ligament (12). Distant NS: posterior to pancreatic head (13), superior mesenteric vein (14v), transverse colon (15), para-aortic (16), anterior to the pancreatic head (17), inferior border of the pancreatic body (18), infradiaphragmatic along the subphrenic artery (19), paraesophageal in the diaphragmatic hiatus (20), paraesophageal in lower thorax (110), supradiaphragmatic (111), posterior mediastinum (112). If the gastric tumour invades the oesophagus, stations 19, 20, 110, and 111 are considered regional. Station 14v is considered regional in the JGCA classification and distant in the 8th AJCC classification. B) Subcardial GA with regional perigastric adenopathy (white arrows), extraperigastric adenopathy in the hepatic hilum (black arrow) and distant inter aorto-cava and para-aortic adenopathy (red arrows). The latter are compatible with cM1.
(0.37MB). - -
Distant metastatis (cM): the absence of metastatic involvement is described as M0 and the presence of it as M1. The latter may present as distant adenopathy, nondirect extension into other organs, or peritoneal carcinomatosis14. The sensitivity and specificity of CT in detecting liver metastases is 74% and 99%, respectively1.
- -
Extramural venous invasion (further information will be provided in the section on pathways of disease spread).
While the site of the tumour in the stomach can help predict the pattern of spread of the disease, familiarity with the different routes of spread of GA is necessary for accurate pre-surgical staging.
Lymphatic spreadLymphatic spread is the most common way for AGs to disseminate. It occurs in 74–88% of patients with GA and is observed in up to 14% of patients with EGC and invasion limited to the mucosa (T1a)38. Lymphatic drainage of the stomach depends on the primary tumour. It is complex and multidirectional39. Regardless of the site of the tumour, the most frequently affected lymph node stations are 3 (lesser curvature), 4 (greater curvature) and 7 (left gastric artery)9. When there is only one regional lymph node involved, the affected lymph node station is located on the same side as the tumour in 83–92% of cases39. The greater the lymph node involvement, the less predictable the spread. Metastatic involvement in extraperigastric nodes without perigastric node involvement is called skip metastasis. This is observed in 5–14% of cases and there is a higher incidence in upper-middle third AGCs (Fig. 4)39.
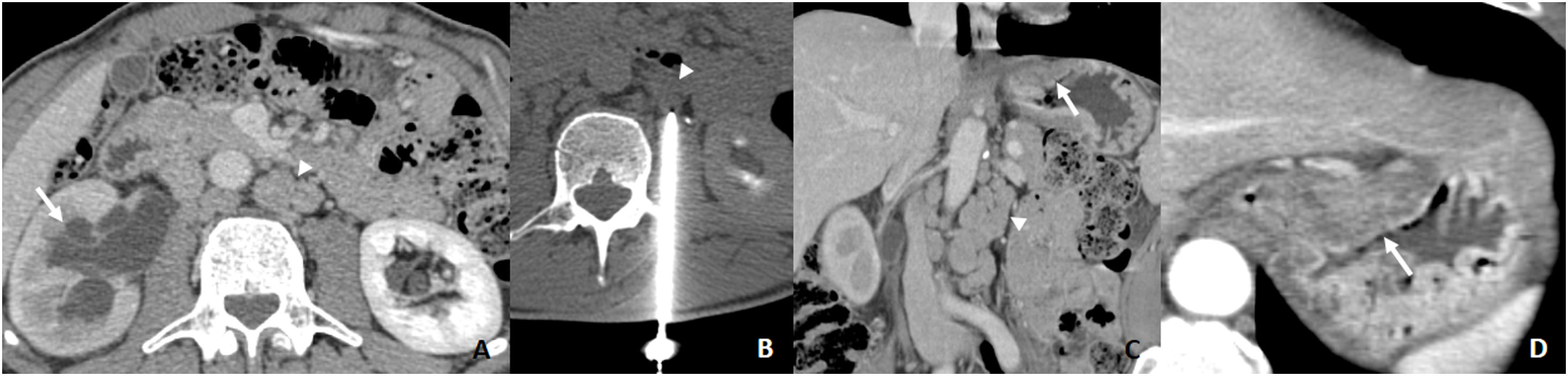
A) CT requested due to abdominal pain. Right hydronephrosis with ureteral obstruction (arrow) and retroperitoneal adenopathy (arrowhead). Suspected urological neoplasm. B) 18G CNB of left para-aortic adenopathy (arrowhead). The result suggests gastrointestinal carcinoma. C and D) CT scan showing suspicious subcardial thickening which proves to be an intestinal type of GA (arrow) with retroperitoneal metastases due to skip metastases (arrowhead).
GA can spread in the subperitoneal space between the layers of the peritoneum, along arteries, veins or nerves within the ligaments and mesentery1. Cancer with local dissemination that penetrates from the subserosal connective tissue to the perigastric ligaments and omentum, without invasion of the visceral peritoneum or serosal layer, is classified as T314. Disease along the perigastric ligaments can be seen on CT as nodular or sheetlike soft-tissue thickening within the ligaments1. This finding excludes patients with operable GAs from laparoscopic surgery.
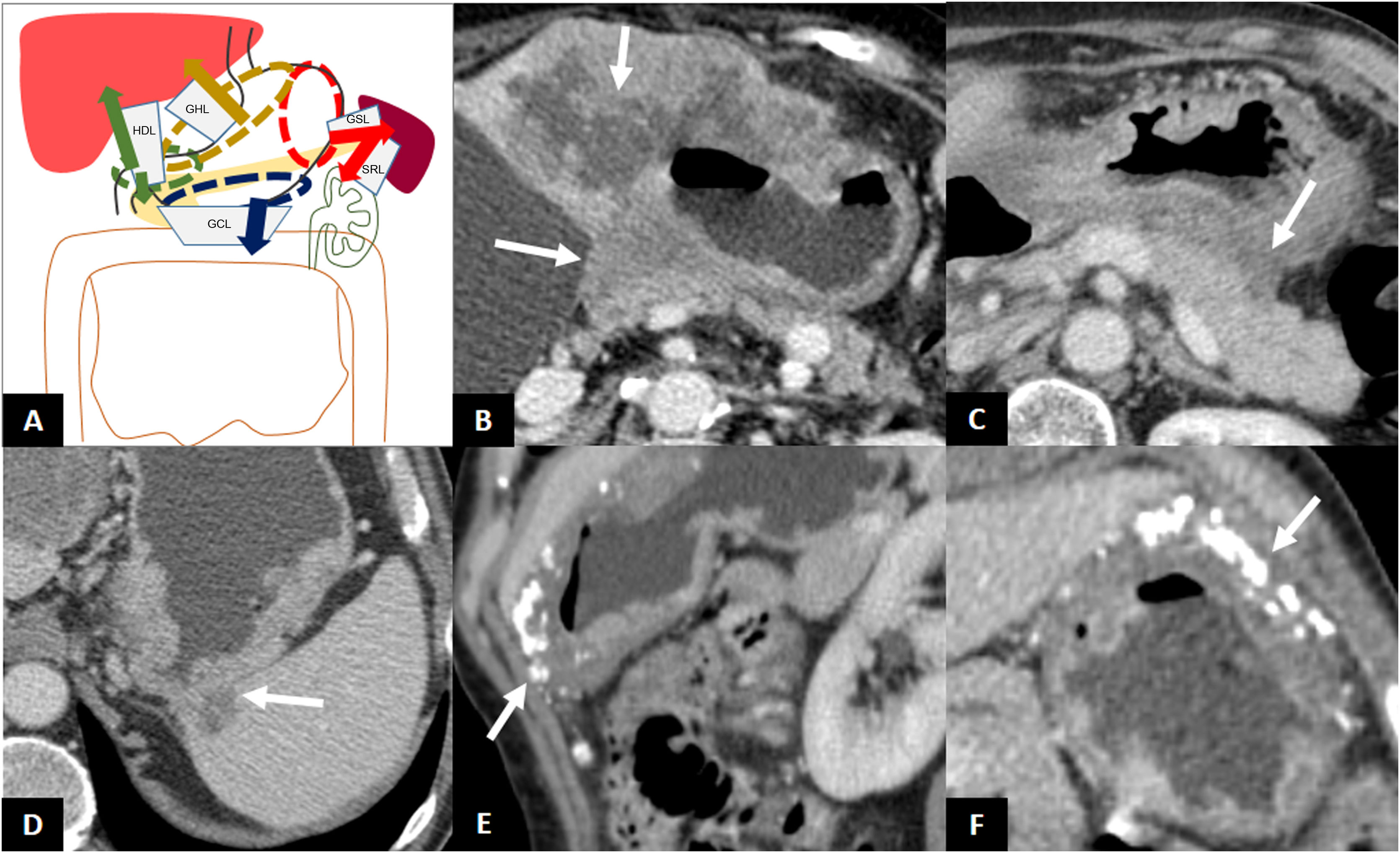
GAs located in the cardia and the lesser curvature can disseminate along the gastrohepatic ligament (GHL), those located in the antrum or pylorus along the hepatoduodenal ligament (HDL), those located in the greater curvature along the gastrocolic ligament (GCL) (and spread to the greater omentum), and those located in the fundus and the upper region of the greater curvature along the gastrosplenic ligament (GSL), and from there to the splenorenal ligament (SRL) (Fig. 5)1.
Subperitoneal dissemination. A) Illustration of the perigastric ligaments as possible routes of subperitoneal dissemination of gastric adenocarcinoma. GSL: gastrosplenic ligament; GCL: gastrocolic ligament; SRL: splenorenal ligament; GHL: gastrohepatic ligament; HDL: hepatoduodenal ligament; 1: neoplasm in cardia and lesser curvature; 2: antro-pyloric neoplasm; 3: neoplasm in greater curvature; 4: neoplasm in fundus and upper region of the greater curvature. B) Infiltration of the GHL: increased nodular density surrounding the left gastric vessels. B') Illustration of B showing soft tissue surrounding the left gastric vessels suggesting infiltration of the ligament (arrow). C) Infiltration of the HDL: soft tissue surrounding the portal vein and hepatic artery in the hepatic hilum. D) GSL infiltration: increased density surrounding the short gastric and left gastroepiploic vessels. E) SRL infiltration: soft tissue surrounding the splenic vessels.
The tumour may extend contiguously beyond the serosa and invade the perigastric fat, adjacent ligaments and even reach another organ. The anatomical site of the primary tumour can help in predicting which perigastric ligaments and organs may be invaded1. Tumours of the cardia, lesser curvature and antrum may invade the left hepatic lobe via the GHL. Tumours of the pylorus and gastric antrum may invade the liver or pancreatic head via the HDL. Tumours of the greater curvature may invade the transverse colon via the GCL. Tumours of the fundus and upper greater curvature may invade the spleen through the GSL and pancreatic tail, the descending colon and the left kidney via the splenorenal ligament (SRL)1. The clinical stage T4b is implicit when direct invasion of an adjacent organ is detected (Fig. 6) (Table 1)14.
Direct invasion. A) Illustration of the perigastric ligaments as possible routes of direct invasion of gastric adenocarcinoma. B) Direct invasion of the left hepatic lobe and pancreatic body. C) Absence of fat plane between the stomach and the pancreatic body and tail indicating possible direct infiltration of the pancreas. Exploratory laparoscopy demonstrated absence of infiltration. D) Direct invasion of the spleen through the GSL. E and F) Thickening of the greater curvature of the stomach with calcifications and direct invasion of the GCL and greater omentum, without encroaching on the colon.
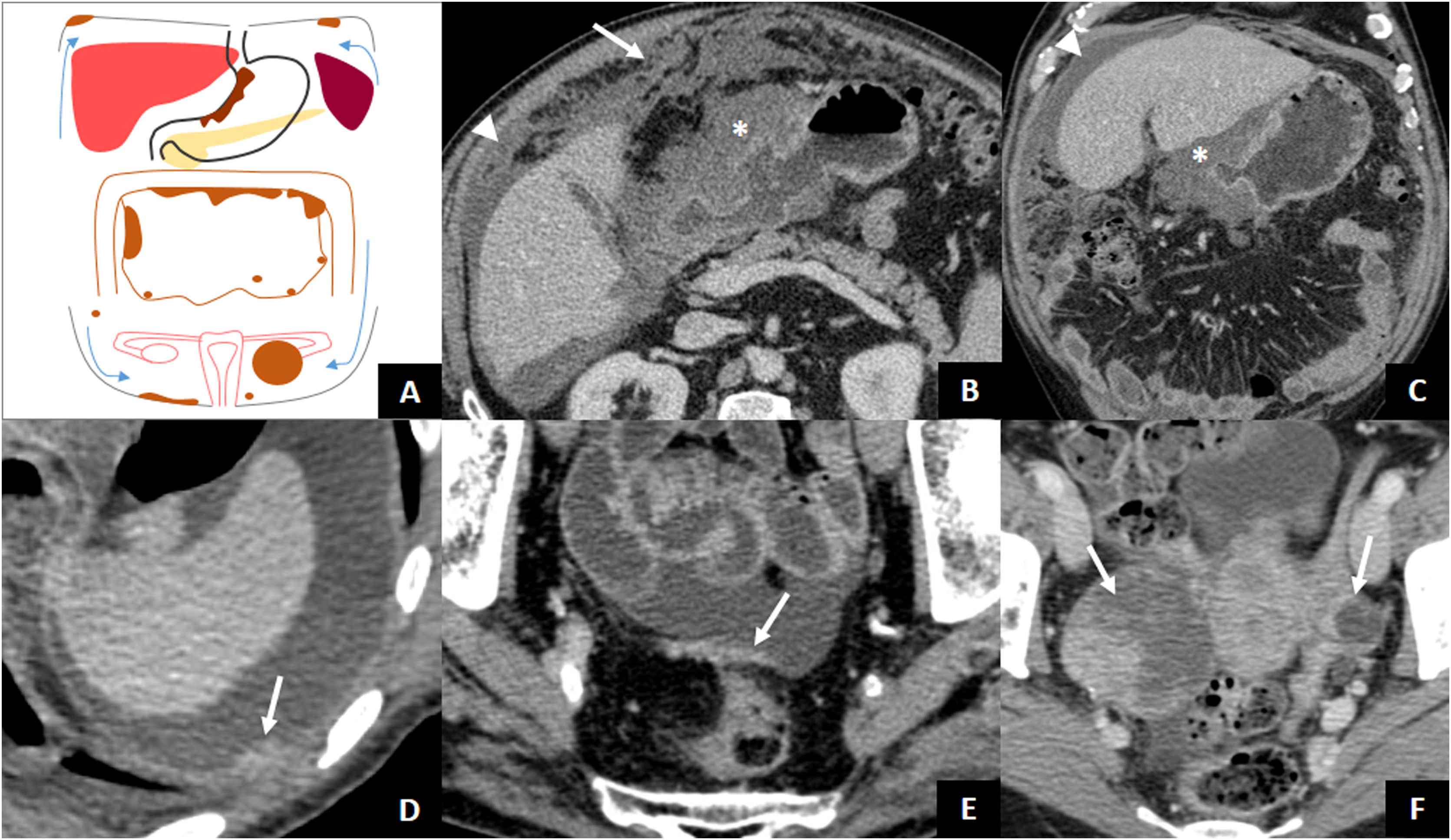
By penetrating the peritoneal layers, GAs can spread within the peritoneal cavity. Fifty percent of patients with tumours infiltrating the muscle layer or beyond (≥ T2) present with peritoneal spread at diagnosis1. Evidence of peritoneal metastatic involvement means that the disease is incurable, so a thorough assessment of the peritoneum is essential. Imaging findings that are indicative of peritoneal carcinomatosis include ascites (the most common finding), nodules or plaques on the peritoneal surface, intra-abdominal fat stranding, and irregular thickening and enhancement of the peritoneum40. Special attention should be paid to the dependent portions of the peritoneal cavity, such as the pouch of Douglas or the rectovesical space, or areas where a large amount of peritoneal fluid is absorbed (subdiaphragmatic surface and omentum), as ascites favours subperitoneal dissemination to these regions (Fig. 7)41,42. Ovarian metastases from AG (Krukenberg tumours) are usually of the signet ring cell histological type and may be due to intraperitoneal spread of AGC or lymphatic spread when there is no serosal invasion43. In patients with tumour recurrence after surgery with curative intent, the most common type of recurrence is peritoneal spread44.
Transperitoneal dissemination. A) Illustration of a GA with transperitoneal spread presented with peritoneal carcinomatosis (subdiaphragmatic and pelvic implants, plaque of pelvic peritoneal thickening, omental cake) and Krukenberg tumour on the left. The arrows point to the direction of the ascites. B and C) Locally advanced antro-pyloric GA (asterisks), diffuse type with signet ring cells, with peritoneal carcinomatosis: ascites (arrowheads) and striking omental thickening or omental cake (arrow). D) Left subdiaphragmatic peritoneal implant (arrow) and ascites indicating tumour recurrence of the operated GA. E) Peritoneal thickening in hyperintense plaque (arrow) due to peritoneal carcinomatosis. F) Diffuse type GA with signet ring cells with bilateral Krukenberg tumour (arrows).
The main limitation of using CT scans is the difficulty in detecting subtle peritoneal dissemination. Therefore, diagnostic laparoscopy is recommended for patients with potentially resectable GAs to avoid invasive surgery in cases of occult peritoneal disease3. From an imaging perspective, we can assess carcinomatosis with the peritoneal cancer index (PCI), a scoring system for the evaluation of the distribution and size of tumour implants, ranging from 0 to 39 points. The abdomen is divided into 9 regions and there are also 4 enteric regions. The largest implant in each region is selected and assigned a score from 0 to 3 (0: no implants, 1: <0.5cm, 2: 0.5−5cm, and 3: >5cm)45. Several studies have recently reported that MRI offers greater sensitivity and specificity in the detection of peritoneal metastases than CT18–20,45.
Haematogenous disseminationHaematogenous dissemination depends on factors intrinsic to the tumour cells and a way of communicating with their surrounding microenvironment that favours the formation of a pre-metastatic niche. From this niche, tumour cells invade the extracellular matrix and enter the vessel lumen, through which they disseminate through the circulatory system to reach another organ and develop distant metastases46,47.
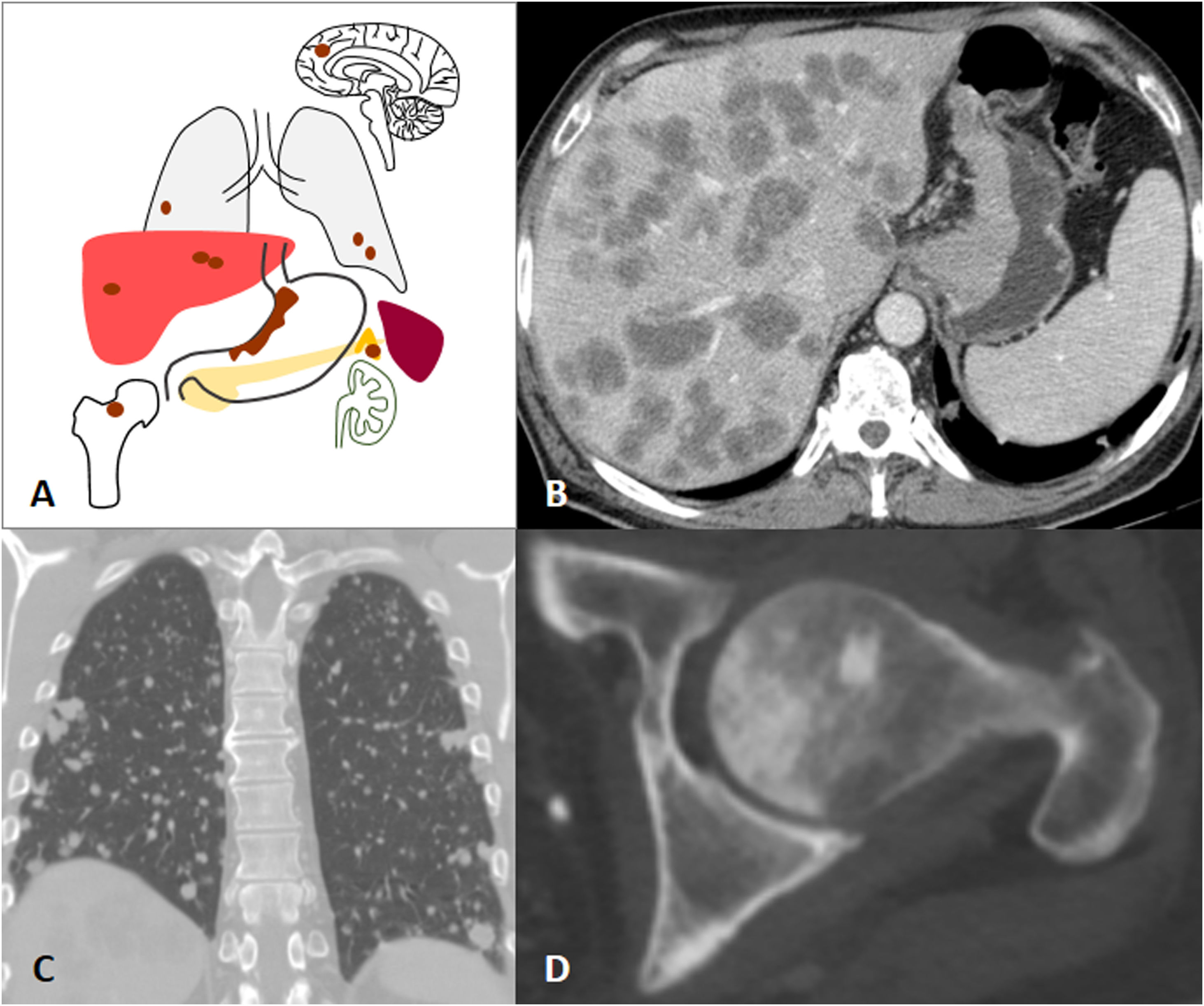
Since the stomach drains through the portal venous system, haematogenous metastases most commonly occur in the liver. Clinical guidelines recommend CT and sometimes PET-CT as the imaging tests of choice for the detection of metastases3,48. However, the detection of liver metastases by MRI has increased in recent years. A recent review and meta-analysis demonstrated that the sensitivity of contrast-enhanced MRI with liver-specific agents was higher than that of intravenous contrast-enhanced CT for the detection of liver metastases in primary gastrointestinal and colorectal neoplasms, especially in lesions smaller than 10mm21. Liver metastases of GAs are usually hypovascular and present as nodular hypodense lesions on CT. On MRI, restricted diffusion in Diffusion-Weighted Imaging and low signal in the hepatobiliary phase following the administration of a liver-specific contrast agent are the main diagnostic features for the detection of liver metastases18,21,49. Other possible sites of GA metastasis are the lungs, bone, the adrenal glands or CNS (Fig. 8)1.
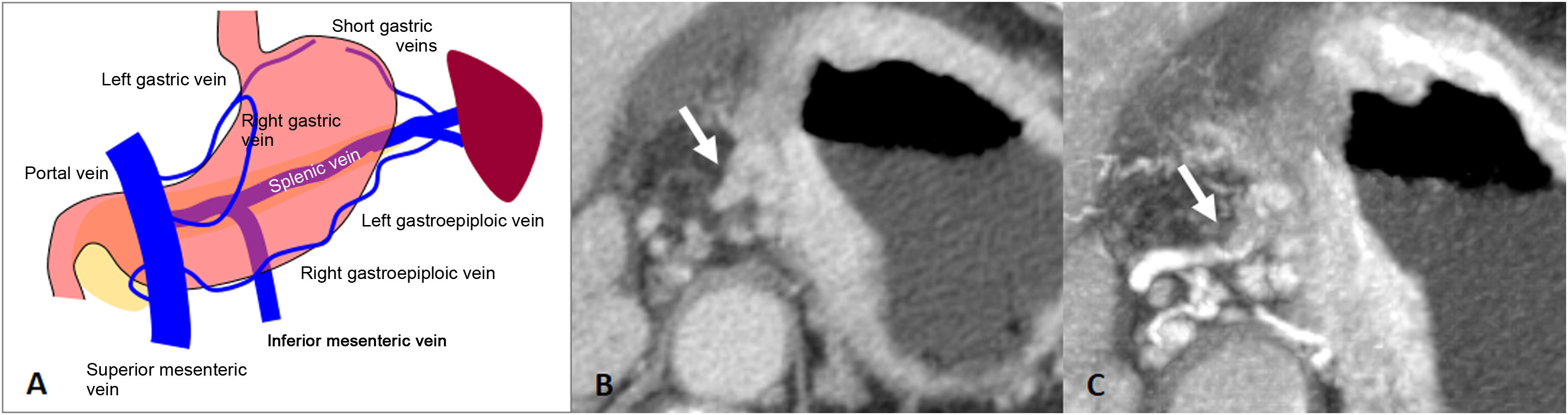
The intramural gastric veins arise from a capillary bed of the mucosa that drains into the submucosal layer forming a venous plexus. They penetrate the gastric serosa to form the gastric veins, eventually forming the left and right gastroepiploic veins, and the right and left gastric veins. These end up draining into the superior mesenteric vein, splenic vein or directly into the portal vein50.
A direct malignant invasion of the perigastric veins or invasion through the perigastric veins extending beyond the wall of the gastrointestinal tract is known as extramural venous invasion (EVI). Histologically, it is defined as the presence of tumour cells in veins beyond the muscularis propria, and although its diagnosis is primarily histological, advances in imaging techniques such as MDCT have made it possible to assess the existence of EVI prior to surgery50. It is frequently associated with extramural periarterial, perineural and lymphatic invasion. It is not a criterion of the AJCC system, but is considered a poor prognostic factor, associated with increased recurrence and shorter survival time51–53
There are 3 types of EVI. a) intraluminal (the most frequent): tumoural spread within the vessel (tubular configuration of the vessel), sometimes distending it; b) floating: tumour within the lumen but with free-floating edges; and c) infiltrative: the tumour infiltrates the vessel wall by contiguity50. EVI is typically seen on CT as a tubular or nodular soft-tissue thickening extending from the tumour along the perigastric vessels50. Multiplanar reconstructions or Maximum Intensity Projection (MIP) can be helpful in assessing EVI (Fig. 9). The detection of EVI by CT has been shown to be an independent predictor of poor survival in patients with AGC52,53.
Extramural venous invasion. A) Illustration of the gastric venous system. B) Diffuse gastric adenocarcinoma with EVI of the left gastric vessels in the gastrohepatic ligament (arrow). C) Same case as in B with MIP reconstruction of the arterial phase, where the tubular thickening of the left gastric vein is more evident.
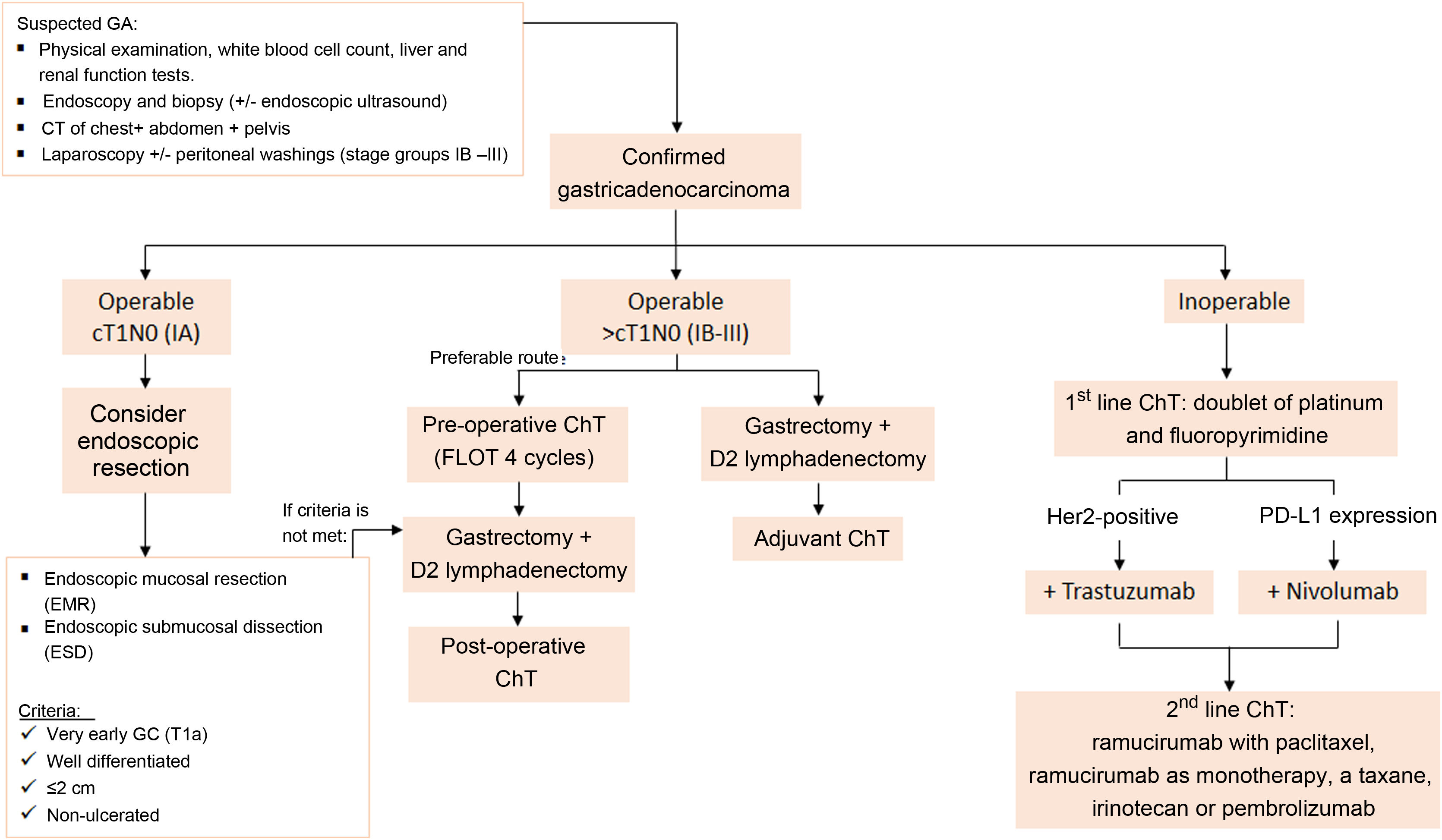
Fig. 10 is an algorithm for the management of GA. A physical examination, blood tests, endoscopy and CT of the chest, abdomen and pelvis should be performed in all suspected cases of GA. Confirmatory diagnosis should be carried out by gastroscopic or surgical biopsy3.
The algorithm used at our centre for managing GA according to the clinical stage assigned. It is based on the latest clinical practice guidelines for gastric cancer published by the European Society for Medical Oncology (ESMO) and the National Comprehensive Cancer Network (NCCN). D2 lymphadenectomy: resection of perigastric lymph nodes and those adjacent to the left gastric, common hepatic, splenic and coeliac arteries. Source: Lordick et al.3 and Ajani et al.48.
Once GA is confirmed, management is adjusted according to the TNM stage of the tumour. If the patient is operable with stage IA (cT1N0), endoscopic resection may be considered (see indication criteria in Fig. 10)54.
If the patient is operable with stage IB-III (>cT1N0), total gastrectomy with D2 lymphadenectomy and perioperative ChT (pChT) (pre- and post-surgery) is recommended3. The combined use of pChT and surgery has been demonstrated to significantly increase survival compared to surgery alone or surgery with adjuvant ChT55,56. Patients with stage>IB cancer who have already undergone surgery due to pre-surgical staging problems or in emergency cases should be followed up with adjuvant ChT57. Radiation therapy is not currently recommended for patients who undergo radical resection (R0) and there is no evidence to suggest that its combination with adjuvant ChT is beneficial58.
In patients with inoperable advanced or metastatic GA (stage IV), the recommended first-line Ch treatment is a doublet combination of platinum and fluoropyrimidine. The combination of ChT with trastuzumab for patients with HER2 overexpression positive disease and the combination of ChT with nivolumab for patients with PD-L1 expression (combined positive score [CPS] > 5) have both achieved a longer survival rate than ChT alone59,60. In these patients, CT scans of the chest, abdomen and pelvis are recommended every 6−12 weeks3. Recently, several studies have been published showing increased overall survival and disease-free survival in patients with limited peritoneal carcinomatosis treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal ChT (HIPEC)61–63. This has led various groups—including the Chicago Consensus Working Group—to establish criteria for selecting patients for CRS and HIPEC with curative intent64. In our centre, it is currently advised for patients with GA if they also have a positive peritoneal cytology or macroscopic peritoneal implants with PCI≤6 points, and where it is feasible to achieve complete cytoreduction and there is an absence of extraperitoneal disease.
ConclusionIn order to achieve correct clinical staging of GA by imaging, it is necessary for professionals to be familiar with the latest version of the TNM staging system and the potential pathways of disease spread. Radiological findings that are relevant to clinical staging, treatment and the prognosis for patients with GA include perigastric fat involvement, lymph node involvement, perigastric ligament involvement, the direct invasion of adjacent organs and metastases.
Key points- •
There are three significant changes to the eighth edition of the TNM staging system compared to the seventh edition.
- •
Invasion of the perigastric ligaments implies subserosal spread of the tumour (at least cT3) and precludes laparoscopic gastrectomy.
- •
The clinical N category is based on the presence or absence of lymph node involvement, irrespective of the number of nodes involved. Non-regional lymph node involvement is considered M1.
- •
There are six pathways of disease spread: lymphatic, subperitoneal, direct invasion, transperitoneal, haematogenous and extramural venous invasion.
- 1
Research coordinators: PLS, MLE, EII, AAR, MIAO and MZE.
- 2
Development of study concept: PLS, MLE, EII, AAR, MIAO and MZE.
- 3
Study design: PLS, MLE, EII, AAR, MIAO and MZE.
- 4
Data collection: PLS, MLE, EII, AAR, MIAO and MZE.
- 5
Data analysis and interpretation: PLS, MLE, EII, AAR, MIAO and MZE.
- 6
Statistical processing: not applicable.
- 7
Literature search: PLS, MLE, EII, AAR, MIAO and MZE.
- 8
Article authors: PLS.
- 9
Critical review of the manuscript with intellectually relevant contributions: MLE, EII, AAR, MIAO and MZE.
- 10
Approval of the final version: PLS, MLE, EII, AAR, MIAO and MZE.
The authors declare that they have no conflicts of interest.