Acute pelvic pain is a common condition in emergency. The sources of acute pelvic pain are multifactorial, so it is important to be familiar with this type of pathologies. The purpose of this article is to review the main causes of gynecological acute pelvic pain and their radiologic appearances to be able to make an accurate diagnosis and provide objective criteria for patient management.
El dolor pélvico agudo es una causa frecuente de consulta en los servicios de urgencias. Los síntomas inespecíficos hacen que a menudo se encuentre patología ginecológica en pacientes remitidas por otras sospechas diagnósticas. Por eso es importante familiarizarse con este tipo de patología y ser capaces de identificar sus hallazgos más representativos. El objetivo de este artículo es revisar las principales causas del dolor pélvico agudo de origen ginecológico, aportando datos clave que permitan establecer un diagnóstico acertado y orientar el tratamiento adecuado.
Acute pelvic pain in females is a common symptom of medical consultation at the emergency room.1 The abdominal ultrasound is considered the initial assessment modality.2 Before conducting any image studies it is essential to obtain the most significant clinical data that together with data from the lab are important to orient diagnosis.3,4 The first thing that needs to be assessed is the possibility of ectopic pregnancy that in turn may turn into life-threatening situations.
We will go over the physiology of the ovarian cycle and the application of different image modalities in the assessment of acute pelvic pain of gynecologic origin.
With this article we intend to review the main causes of acute pelvic pain: functional cysts, ectopic pregnancies, acute pelvic disease, ovarian torsions, endometriosis and complicated myomas by providing a practical approach that will allow the radiologist to identify the different conditions, make the appropriate differential diagnosis and orient therapy in the right way5 (Table 1).
Acute pelvic pain of gynecologic origin: key points in diagnosis and management.
| Diagnostic keys | Differential diagnosis | Attitude | |
|---|---|---|---|
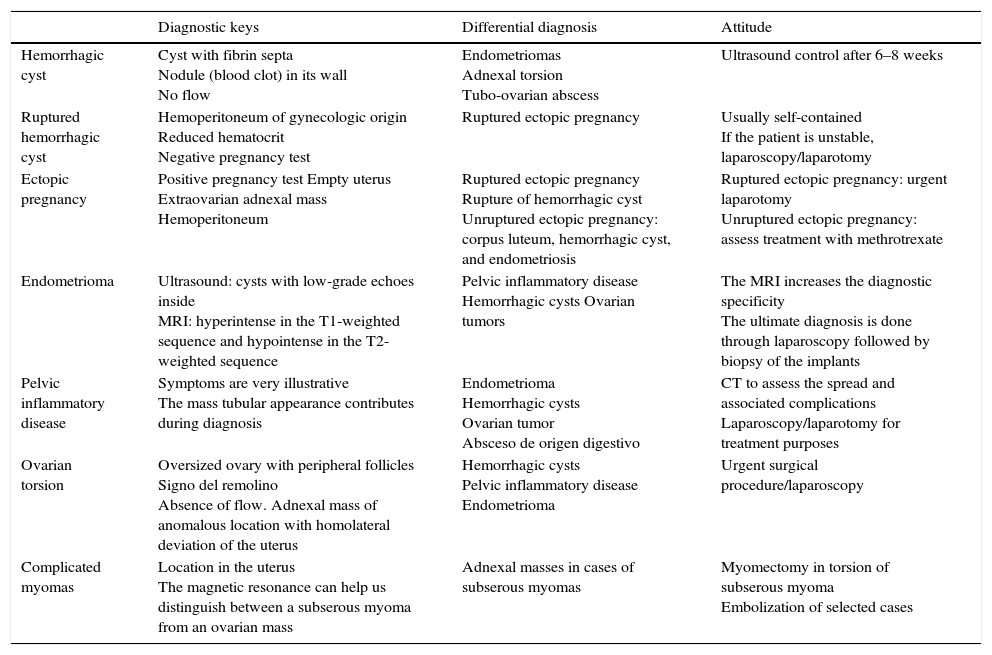
| Hemorrhagic cyst | Cyst with fibrin septa Nodule (blood clot) in its wall No flow | Endometriomas Adnexal torsion Tubo-ovarian abscess | Ultrasound control after 6–8 weeks |
| Ruptured hemorrhagic cyst | Hemoperitoneum of gynecologic origin Reduced hematocrit Negative pregnancy test | Ruptured ectopic pregnancy | Usually self-contained If the patient is unstable, laparoscopy/laparotomy |
| Ectopic pregnancy | Positive pregnancy test Empty uterus Extraovarian adnexal mass Hemoperitoneum | Ruptured ectopic pregnancy Rupture of hemorrhagic cyst Unruptured ectopic pregnancy: corpus luteum, hemorrhagic cyst, and endometriosis | Ruptured ectopic pregnancy: urgent laparotomy Unruptured ectopic pregnancy: assess treatment with methrotrexate |
| Endometrioma | Ultrasound: cysts with low-grade echoes inside MRI: hyperintense in the T1-weighted sequence and hypointense in the T2-weighted sequence | Pelvic inflammatory disease Hemorrhagic cysts Ovarian tumors | The MRI increases the diagnostic specificity The ultimate diagnosis is done through laparoscopy followed by biopsy of the implants |
| Pelvic inflammatory disease | Symptoms are very illustrative The mass tubular appearance contributes during diagnosis | Endometrioma Hemorrhagic cysts Ovarian tumor Absceso de origen digestivo | CT to assess the spread and associated complications Laparoscopy/laparotomy for treatment purposes |
| Ovarian torsion | Oversized ovary with peripheral follicles Signo del remolino Absence of flow. Adnexal mass of anomalous location with homolateral deviation of the uterus | Hemorrhagic cysts Pelvic inflammatory disease Endometrioma | Urgent surgical procedure/laparoscopy |
| Complicated myomas | Location in the uterus The magnetic resonance can help us distinguish between a subserous myoma from an ovarian mass | Adnexal masses in cases of subserous myomas | Myomectomy in torsion of subserous myoma Embolization of selected cases |
Abdominal ultrasound is considered the initial assessment modality. It allows the overall assessment and identification of gynecological and non-gynecological causes of acute pelvic pain and has proven to be highly accurate for the detection and characterization of most causes of gynecological emergencies.4
Transvaginal ultrasoundIt is conducted with an empty bladder. The transducer is introduced into the vaginal cavity while protected by a prophylactic soaked up in a special gel to be able to perform the ultrasound. It provides greater detail of the ovaries and the uterus internal structure but it has some limitations when trying to study large size masses. Its use is not recommended in virgin females and older women with vulvar dystrophy or other disorders that make the vaginal approach not feasible.
Computed tomography–CTThis acute gynecological disease can be useful if there are diagnostic doubts or to be able to assess the transmission process or any possible complications.6 The standard protocol includes axial slices from the diaphragatic dome toward the pubic synphysis with 5mm-slice acquisitions and posterior reconstruction. It is performed with IV contrast (between 100ml and 3ml/s) in venous phase (60–70s).
Magnetic resonance imaging–MRIIt has been the greatest advancement in the exploration of the female pelvis thanks to its excellent tissue characterization and multiplanar capacity. It is very useful for the characterization of adnexal masses. It is not a modality commonly used in the emergency room.7
Ultimately if the patient's situation of clinical instability does not allow the conduction of any image studies or if these studies do not provide accurate information for diagnosis, the surgical option will have both diagnostic and therapeutical purposes.4
The physiology of the ovarian cycleThe appearance of normal ovaries in a woman of reproductive age varies based on her menstrual cycle.8
In the follicular stage we can see many small follicles in the vaginal cortex. By the 8th or 9th days one follicle becomes dominant, destined to ovulate and the rest become atresic. Through the ultrasound the follicles look like thin-walled cystic, anechoic, round or oval structures and without flow through Doppler color flow mapping. The follicles or cysts of up to 3cm in diameter should be considered normal physiological findings.
In the luteal phase that follows ovulation is when the corpus luteum develops.8 The corpus luteum is usually a thick, irregular echogenic-walled cyst of a diffuse shape and prone to bleeding due to significant neoangiogenesis of granular layer. It is under 3cm in diameter. Generally speaking it has internal echoes and the Doppler color flow mapping shows one particular ring of peripheral vascularization (“ring of fire”)3,9 (Fig. 1).
(a) Transverse slice ultrasound. Well-established thin walled-follicular cyst with anechoic content (arrow). (b) Transverse slice Doppler color ultrasound of corpus luteum. Thick-walled, echogenic, vascularized cystic image (arrow). (c) Computed tomography (CT) with contrast, axial slice. Typical image of a thick-walled, hyperdense, festooned-edged corpus luteum (arrow). (d) CT, VR: the corpus luteum vascular ring can be seen (arrow).
During the menopause folliculogenesis ceases and ovaries reduce their size. Simple small cysts of up to 1cm can be seen in 21 per cent of women,10–12 and they should be considered a significant clinical finding.3
In neonatal premenarcheal ovaries small follicles can be seen.
Ovaries can have variable positions. The ultrasound visualization of ovaries does not preclude the possibility of ovarian lesions.
Main causes of acute pelvic pain of gynecologic originFunctional cysts: follicular cystsFollicular cysts are the most common adnexal masses of all.1 They can be due to growth of dominant follicles that are not competent for ovule expulsion and be over 3cm in diameter.
The fast growth of these cysts can cause pelvic pain but they are usually incidental findings.
- •
Differential diagnosis:
Paraovarian cysts; hydrosalpinx; and benign cystic tumors.
- •
Management:
Simple adnexal cysts of up to 10cm in a female patient have a high probability of being benign regardless of her age.13–15 Cysts discovered incidentally are usually between 3 and 8cm in size and they usually disappear or recede or in a matter of one or two menstrual cycles. The best time to perform control ultrasounds and check the results is between days 5 and 10 (follicular stage) of a posterior menstrual cycle.1 The Society of Radiologists in Ultrasound Consensus Conference Statement3 establishes the following recommendations during follow-up:
- (a)
In women of reproductive age:
- –
≤3cm cysts: normal physiological findings that do not need to be written in the report and that do not require therapy either.
- –
>3 y ≤5cm cysts: described in the report as very probably benign and that do not require therapy
- –
>5 and ≤7cm cysts: described in the report as very probably benign but annual ultrasound follow-up is required.
- –
>7cm cysts: they can be difficult to assess completely through an ultrasound and this is why MRIs or surgeries are recommended.
- –
- (b)
In post-menopausal women:
≤1cm cysts: clinically non-significant–they do not need to be put down in the report and they do not require follow-up.
>1 and ≤7cm cysts: in the report they are usually described as very probably benign still annual follow-up is recommended at least at the beginning with the ultrasound. We can choose to increase the follow threshold from 1 to 3cm.
>7cm cysts: they can be difficult to assess completely through the ultrasound, hence they should be assessed through MRI or surgery.
- (a)
It is the most common cause of acute pelvic pain of gynecologic origin in young women and it usually presents clinically with sudden intense pain in the hypogastrium. The painful spot matches the cyst location. It is almost exclusive of women of reproductive age, post-menopausal women in hormone replacement therapy, and pregnant women.16
It is due to follicular cyst bleeding or more frequently to corpus luteum bleeding.
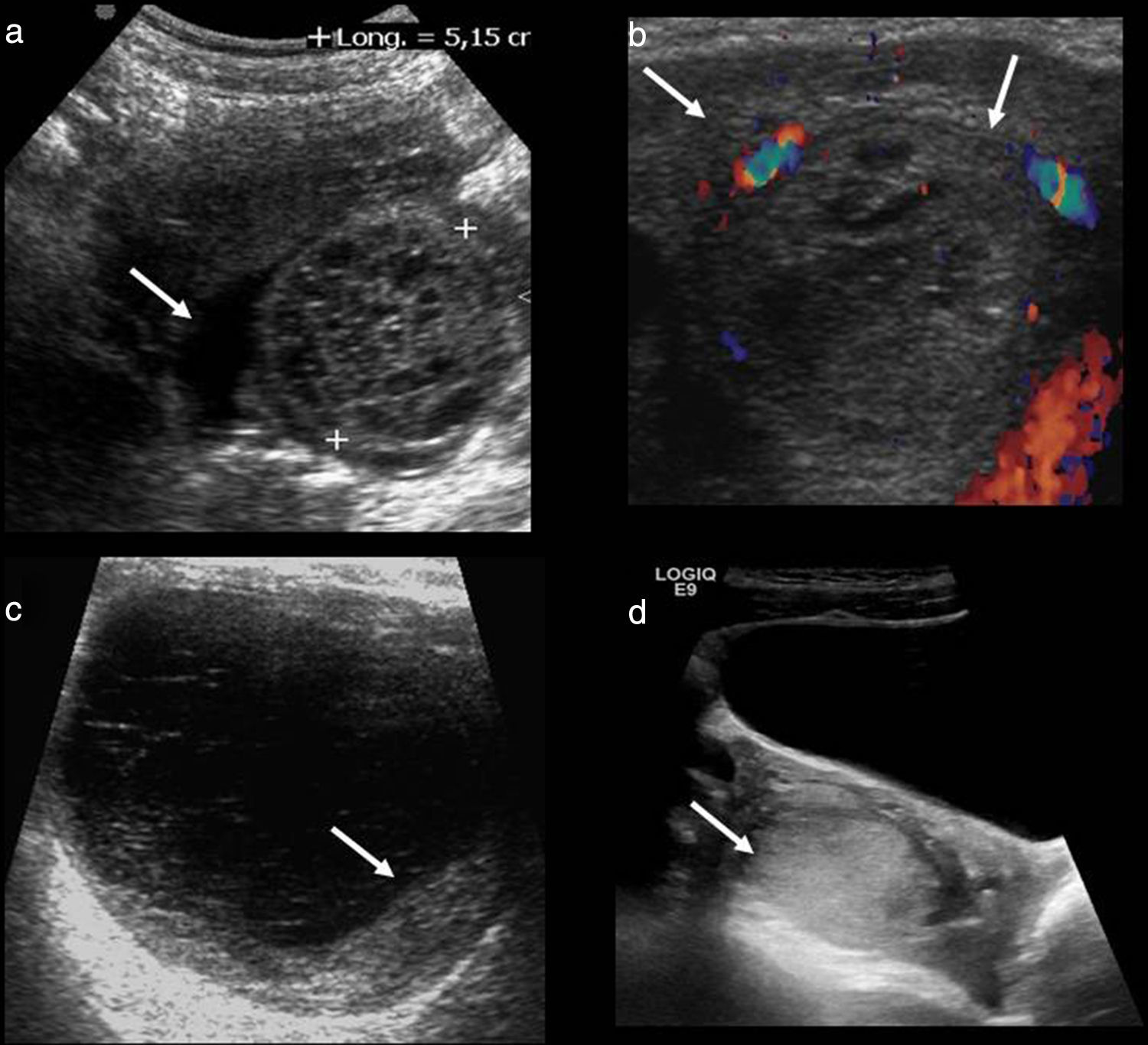
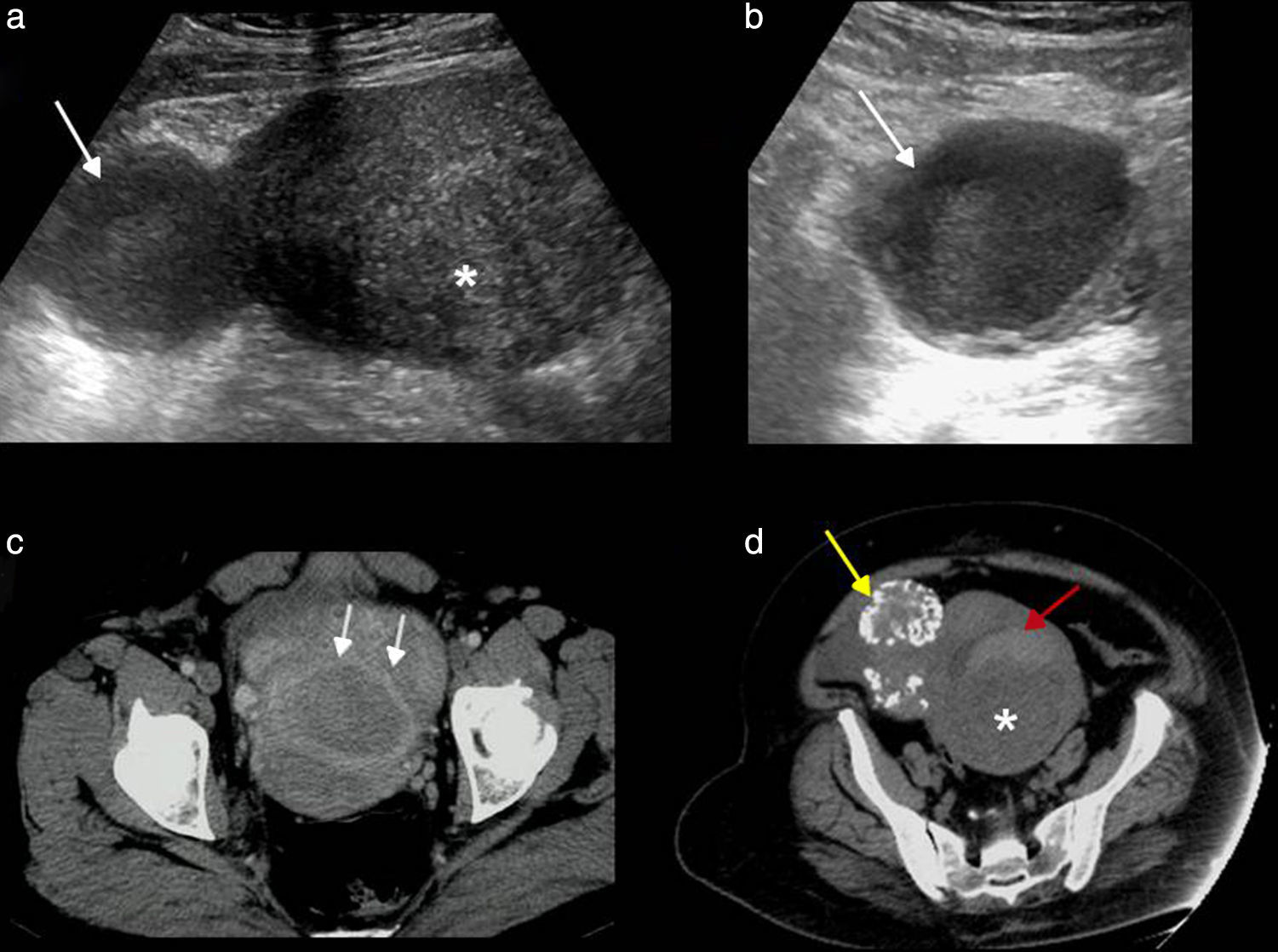
Its physical appearance varies based on the evolution of bleeding (Fig. 2). Initially when it comes to acute bleeding it can be hyperechogenic and simulate a solid mass that nevertheless shows the posterior acoustic reinforcement so typical of cystic lesions. The most typical appearance is that of a complex, cystic lesion (without Doppler color flow mapping) with posterior reinforcement and a thin reticular patter inside due to fibrin bands that give it a “fishing net” kind of appearance that is so typical of subacute phases.17
Different ultrasonic manifestations of hemorrhagic cysts. (a) “Fishing net” typical appearance with hyperechogenic linear bands (in between marks) and free fluid (arrow). (b) Bleeding inside the corpus luteum showing circumferential flow using Doppler color flow mapping in “ring of fire” (arrows). (c) Cyst with thin reticular pattern and blood clot attached to its wall (arrow). (d) Cyst with a solid appearance simulating an oversized ovary (arrow).
At times it can present with a fluid-fluid level. Another typical appearance is the existence of a blood clot stuck to the vessel wall simulating neoplastic mural nodule. The blood clot is usually hyperechogenic and characteristically showing concave avascular edges,13,18 while the neoplasic nodule shows convex edges and is isoechogenic and vascularized.
Wall thickness can be variable based on its origin. When the hemorrhagic cyst is due to corpus luteum bleeding the type of “ring of fire” produced can be identified thanks to the vascularization of its wall.
- •
Differential diagnosis:
Through endometriomas basically but also through adnexal torsion and tubo-ovarian abscesses.
- •
Management:
Ordinarily they resolve spontaneously in between six (6) and 8 (eight) weeks (from two to three menstrual cycles).16,19 If they are not gone during follow-up the possibility of cystic neoplasm should be considered.
Here are the follow-up recommendations from the Society of Radiologists in Ultrasound Consensus Conference Statement3:
- (a)
In women of reproductive age:
- –
≤3cm cysts: they do not need to be put down on paper and they do not require therapy.
- –
>3 and ≤5cm cysts: they are described in the report but do not need follow-up.
- –
>5cm cysts: they are described in the report and ultrasound follow-up is recommended in 6–12 weeks to assess its progression. If they do not go away an MRI is recommended.
- –
- (b)
In post-menopausal women:
- –
Early menopause: it is described in the report and ultrasound and follow-up is recommended in 6–12 weeks. If it does not go away assessment through MRI or surgery is recommended.
- –
Late menopause: any hemorrhagic cysts should be considered as possible neoplasms hence assessment through MRI or surgery is recommended.
- –
- (a)
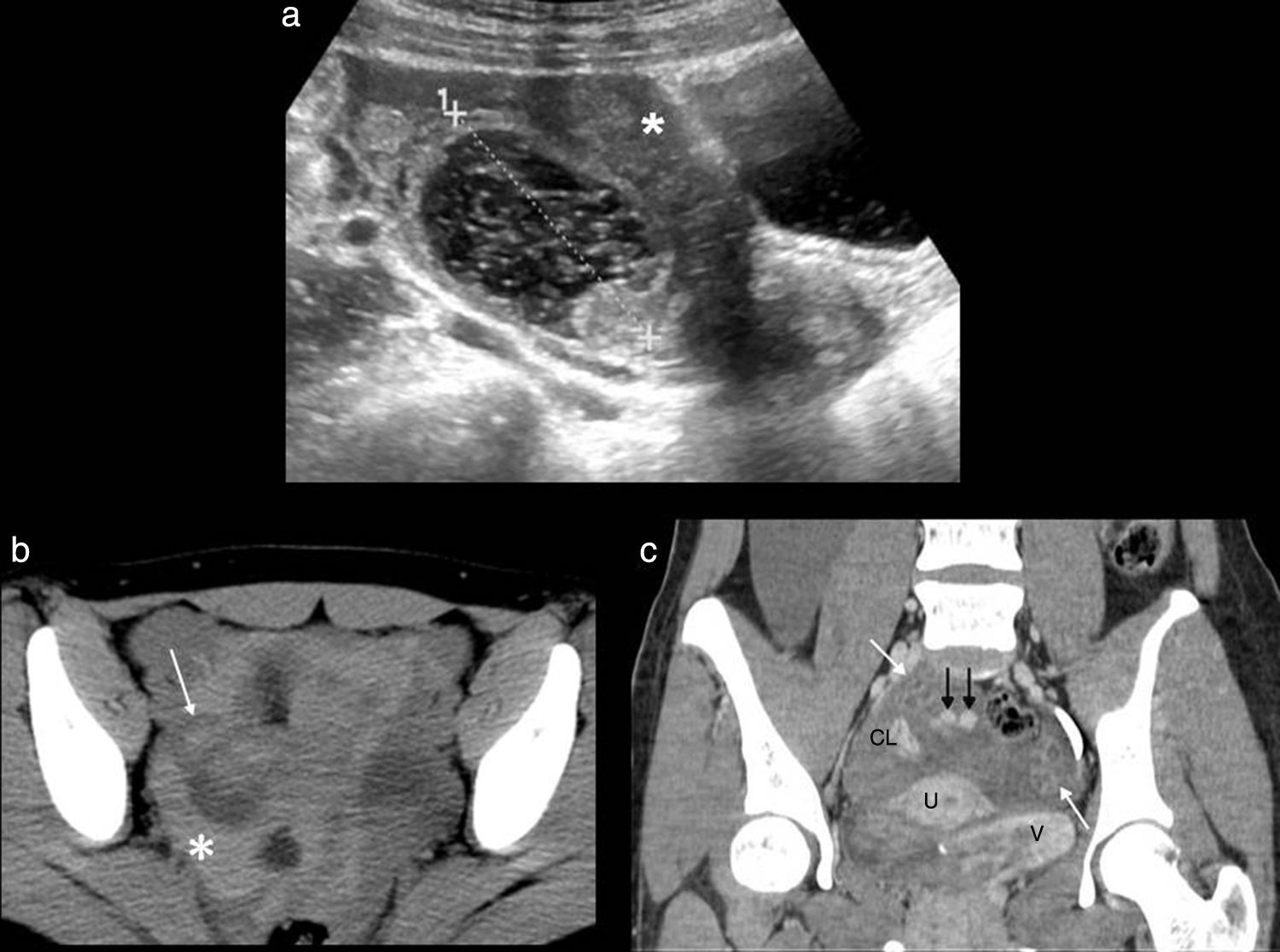
When a hemorrhagic cyst ruptures which is not uncommon this can be identified together with the loose fluid that can be limited to the pelvis or spread toward the abdominal cavity. The fluid based on the time of bleeding can be anechoic or complex with echogenic content (hemoperitoneum) (Fig. 3).
Ruptured hemorrhagic cyst. (a) Longitudinal slice ultrasound. Hemorrhagic cyst with a typical “fishing net” appearance (in between marks) and complex free fluid (hemoperitoneum). (b) Computed tomography (CT) without contrast–axial slice. Cystic image with hyperdense content inside due to bleeding (white arrow) and hyperdense free fluid in the pelvis due to hemoperitoneum (asterisk). (c) CT with contrast–coronal slice. Hyperdense foci due to contrast extravasation due to active bleeding (black arrows). Uterus (U), ovaries (white arrows), bladder (V) and corpus luteum in right ovary (CL).
The computerized tomography (CT) scan with IV contrast can show active bleeding.
- •
Differential diagnosis:
A pregnancy test that tests negative is essential since the rupture of an ectopic pregnancy shows similar symptoms and findings.5
- •
Management:
Wait and see based on the clinical progression and analysis (falling hematocrit) since normally these are self-contained processes. If the hemoperitoneum is large and causes hemodynamic instability then performing laparoscopy/diagnostic and therapeutic laparotomy should be considered.20
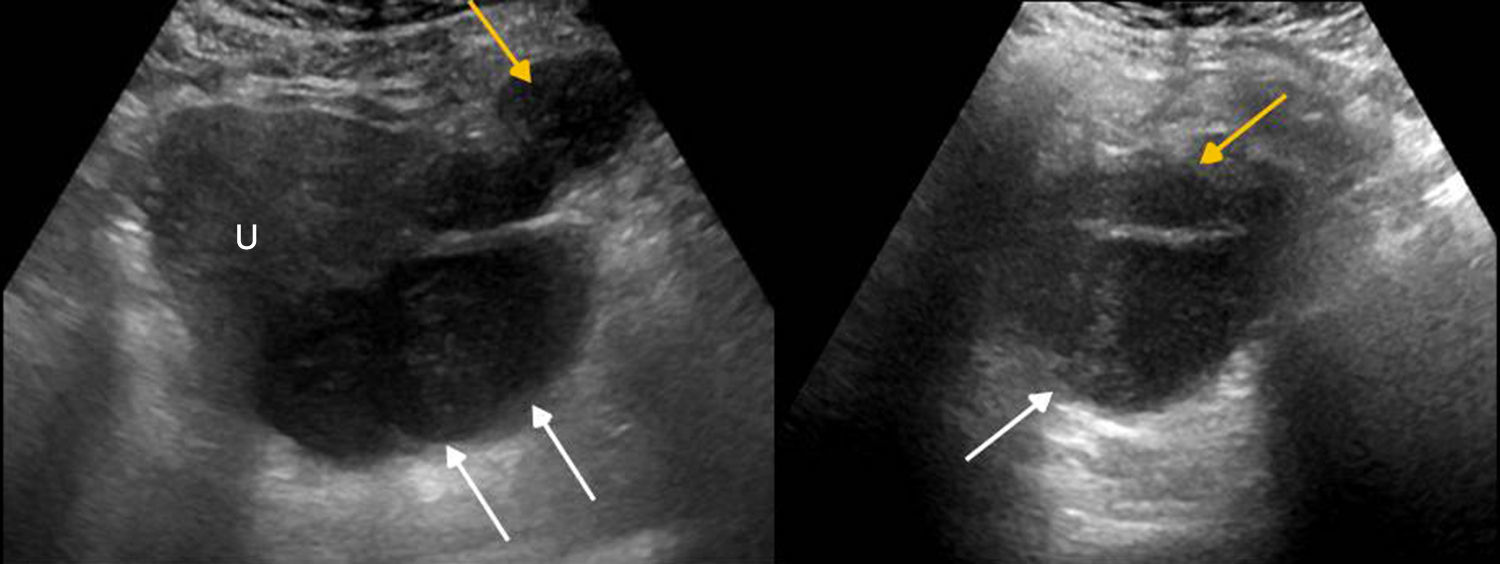
Ectopic pregnancy is the implantation of the gestational sac outside the endometrial cavity. In 95 per cent of all cases such implantation occurs in the fallopian tubes (more commonly in the ampullary region and the isthmus).21
In a female of reproductive age with an elevated human chorionic gonadotropin beta or a positive pregnancy test and absence of intrauterine pregnancy the identification through ultrasound of an adnexal, extraovaric mass should be considered ectopic pregnancy until proven otherwise21 (Fig. 4).
The presence of intrauterine pregnancy almost excludes the diagnosis of coexistent ectopic pregnancy except for high risk patients like those undergoing assisted fertilization techniques.20
The incidence of ectopic pregnancy is higher if there is a history of another ectopic pregnancy, pelvic inflammatory disease, endometriosis, surgery of the Fallopian tubes, use of intrauterine device and infertility therapy.22
When an ectopic pregnancy progresses, its risk of rupture grows accordingly. An ectopic pregnancy becomes an urgent matter when it ruptures. A ruptured ectopic pregnancy is uncommon but it is the cause of 10 per cent of maternal deaths.21 It usually presents with acute pain and vaginal bleeding. These are some of the ultrasound findings today:
- –
It is more reliable, has a 100 per cent specificity but it is uncommon; it shows the pregnancy sac with a live embryo in extrauterine location (Fig. 5).
- –
The second more specific sign is the presence of an adnexal, extraovaric, solid, cystic, hyperechogenic, thick walled-mass of trophoblastic tissue (“tubal ring”),18 with marked vascularization through Doppler color flow mapping (“ring of fire”),18 that may contain the yolk sac or embryo. If no sac or embryo is present it is important to confirm that it is independent from the ovary so it is not mistaken for a corpus luteum–ovarian ectopic pregnancies are rare.
- –
The free echogenic fluid due to hemoperitoneum in one patient with a positive human chorionic gonadotropin beta has an 86–93 per cent positive predictive value. In 15 per cent of all ectopic pregnancies the presence of free fluid may be the only ultrasound finding.16
- •
Differential diagnosis:
- –
Ruptured ectopic pregnancy: ruptured hemorrhagic cyst.
- –
Unruptured ectopic pregnancy: corpus luteum, hemorrhagic cyst and endometriosis.
- –
- •
Management:
- –
Ruptured ectopic pregnancy: it is a life-threatening situation that requires urgent care. If the patient is clinically unstable it is not significant to distinguish between a ruptured ectopic pregnancy and a ruptured hemorrhagic cyst since laparotomy is indicated in both cases.
- –
Unruptured ectopic pregnancy: assess medical therapy with methotrexate.
- –
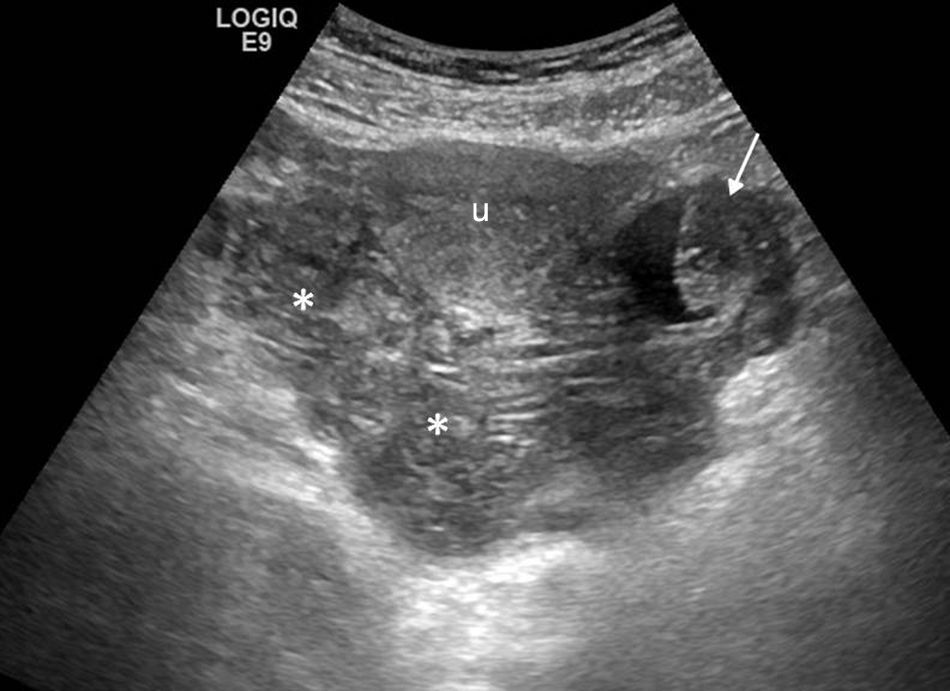
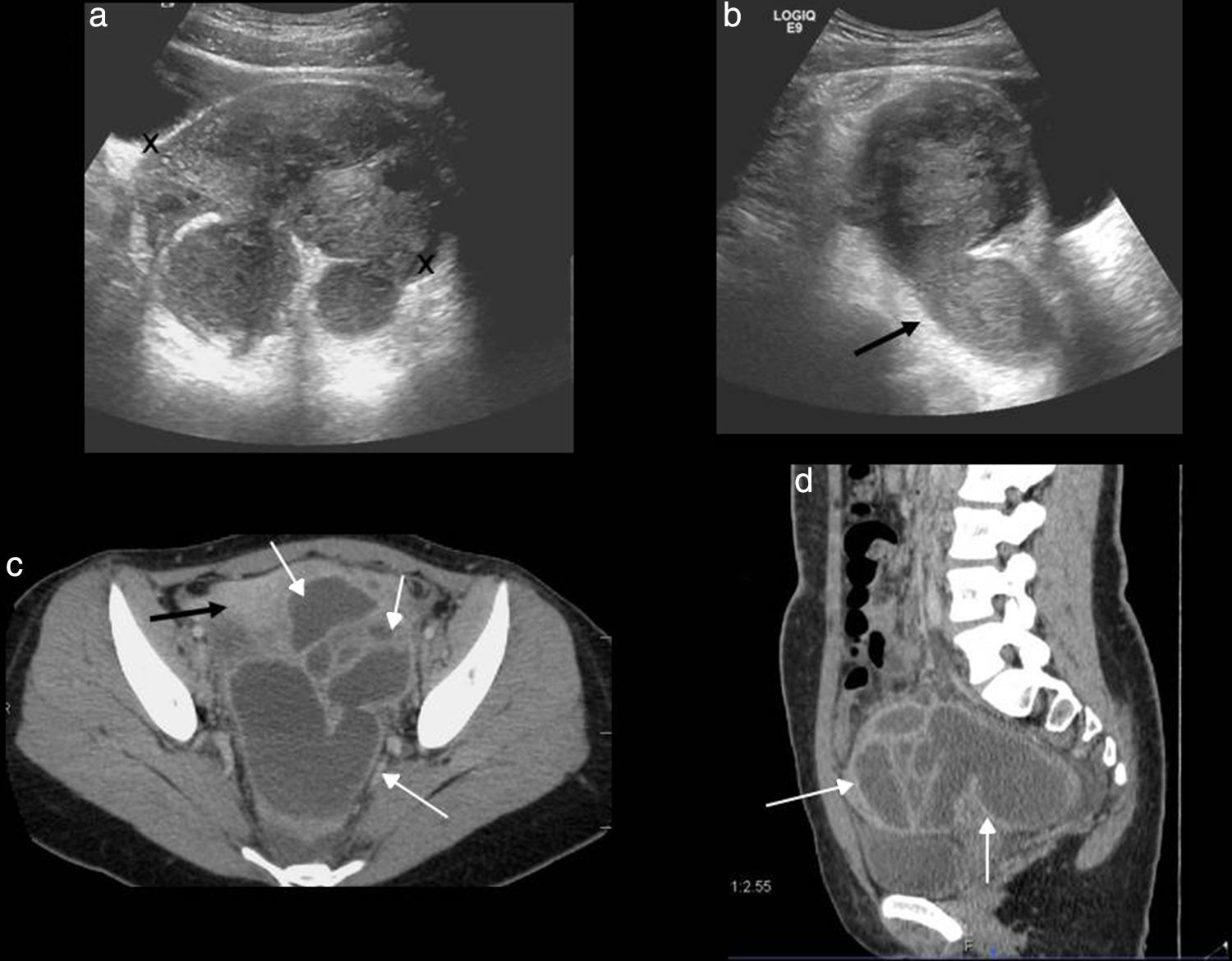
Up to 24 per cent of all consultations in the emergency room for gynecologic pain are due to pelvic inflammatory disease1–inflammatory-infectious disease of the upper genitourinary tract that is clinically characterized by acute pelvic pain, fever, vaginal secretions and pain to cervix movilization. It can be secondary to an ascending infection of the inferior genital apparatus and occur through sexual transmission though the hematogeneous dissemination and direct spread of an adjacent infection are possible too. Vaginal or cervical infections progress in an ascending way and cause endometritis followed by salpyngitis. Without the appropriate therapy the pelvic inflammatory disease can spread to the ovary and give rise to tubo-ovarian abscesses. Neisseria gonorrhoeae and Chlamydia trachomatis are the causative agents of two-thirds of all cases.1 Patients who are carriers of intrauterine devices are prone to one special type of pelvic inflammatory disease due to Actinomyces israelii that gives rise to one chronic suppurative infection that sometimes is hard to distinguish from pelvic neoplasms with carcinomatosis.6 In the early stages of infection there might not be any changes and the ultrasound and the CT are usually normal. Other times symptoms can be mild and nonspecific and an increased in size, poorly-established uterus or free fluid in the pelvis or endometrial fluid (nonspecific sign and common in young women) may be identified. It is important to identify the existence of small quantities of complex fluid in the pelvis suspicious of free pus (abscess).23 The identification of a dilated uterine tube is the most characteristic finding of all (Fig. 6).24 Adherences within the tubes causing tubarian obstruction with hydropsalpinx and pyosalpinx are common. In case of pyosalpinx a tubular-like, serpiginous structure can be identified containing echogenic material inside that is compatible with pus, fluid-fluid level or increased enhancement in the CT. In later stages tubo-ovarian abscesses can occur that are inflammatory conglomerates including the ovary and the uterine tube with complex fluid collections sometimes containing gas bubbles. In the ultrasound and the CT the appearance is variable and can present as purely cystic adnexal masses with multiple loculations, walls and thick walls enhanced in the CT and debris (Fig. 7). The Doppler ultrasound characteristically shows an increase of vascularization. There can be affectation of adjacent structures causing adynamic ileus of intestinal loops, uretheral dilation or hydronephrosis due to mechanical or functional obstruction of ureters or Fitz-Hugh-Curtis syndrome24 when the pelvic inflammatory exudates spread across the paracolic gutter toward the peritoneal surface anterior to the liver while causing perihepatitis associated to pelvic inflammatory disease.
- •
Differential:
Endometriomas, hemorrhagic cyst, ovarian tumor and appendicitis.
- •
Management:
The ultrasound is the initial image modality to conduct studies. The CT is used to assess the process spread and any possible associated complications. With the actual image modalities, the laparoscopy/laparotomy is reserved for complex or complicated cases with both diagnostic and therapeutic purposes.
Advanced pelvic inflammatory disease. (a) Ultrasound–transverse slice. Tubal ovarian abscess (in between marks) looking like a loculated mass of complex appearance affecting both the uterine tube and the ovary. (b) Ultrasound–longitudinal slice. Pyosalpinx with dilated uterine tube with complex fluid content inside (arrow). (c and d) Computed tomography with contrast–axial and sagittal slices. Loculated mass with multiple thick-walled serpiginous cystic structures compatible with abscesses (white arrows). Adjacent peritoneum enhancement can be seen (black arrow).
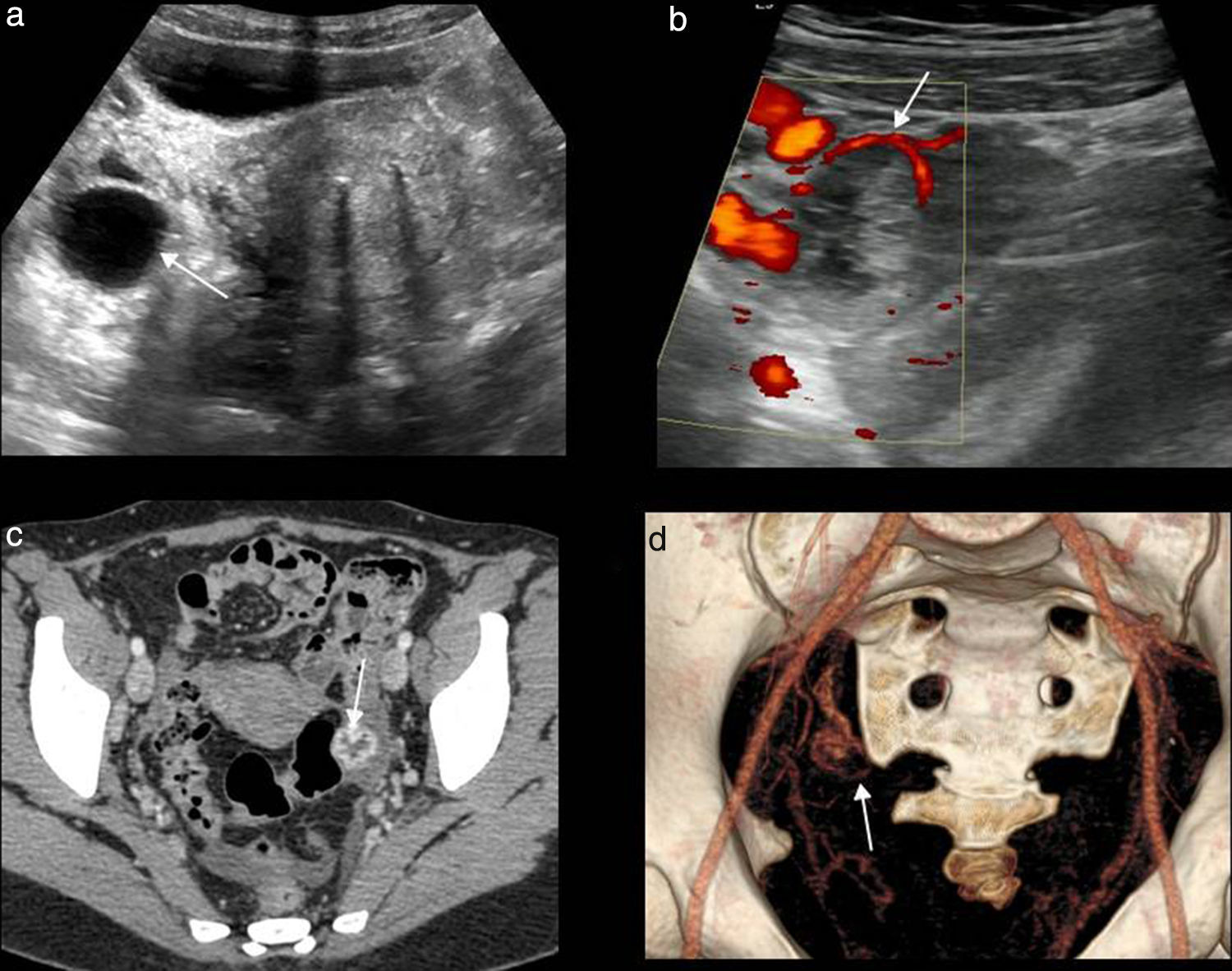
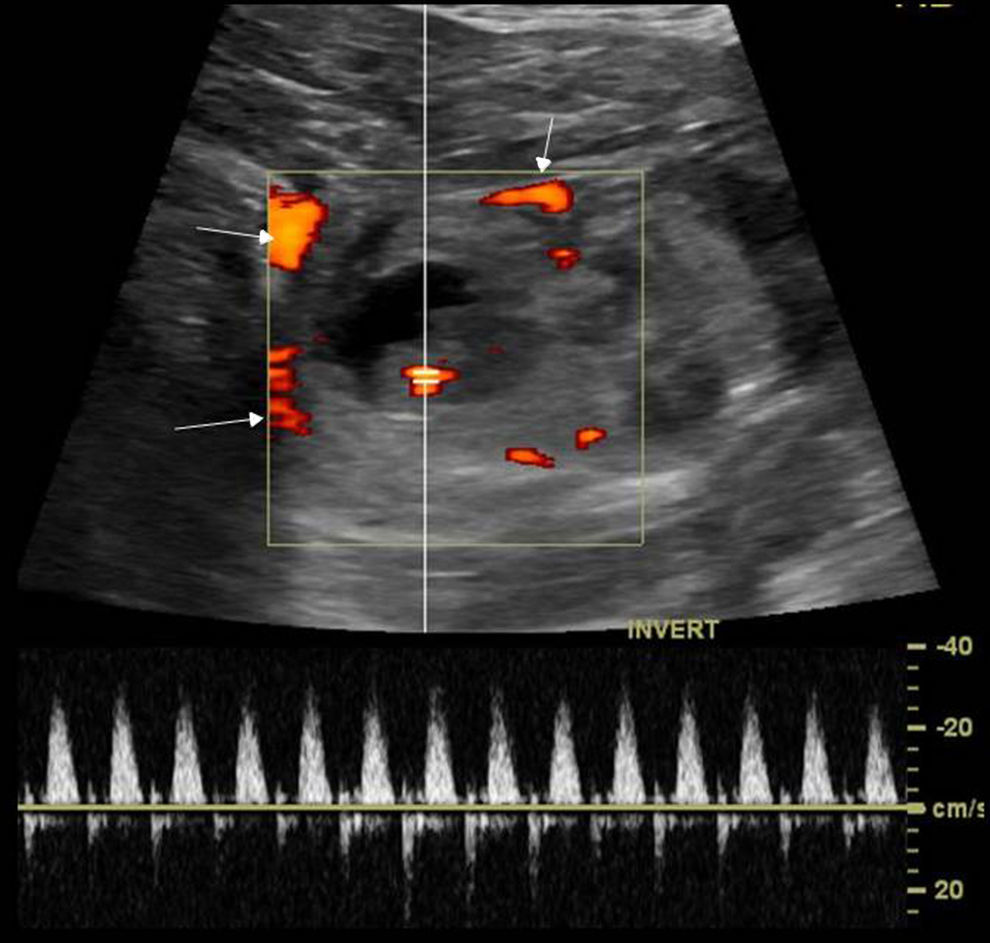
Adnexal torsion is a rare but serious cause of acute pelvic pain25 caused by the partial or complete rotation of the ovary or the tube and more commonly both over its suspensory ligament and vascular pedunculum which in turn causes the progressive affectation of lymphatic, arterial, and venous fluids causing parenchymal congestion and if maintained infarction and necrosis.26 It may occur in the ovaries and normal tubes (mainly in teenagers with suspensory ligaments laxity or tubarian hypermovility,27 or be secondary to cysts or masses (over 5cm) being the mature cystic teratoma or dermoid cyst28 the tumor that predisposes the most to torsion.
Overall dermoid cysts are asymptomatic and their finding happens at random. In the CT they show mass attenuation in over 90 per cent of all cases. When in the presence of acute pelvic pain and large sized-dermoid cysts we should consider the possibility of torsion. In these cases the wall thickening of the cyst29 or free fluid can be identified.
Adnexal torsion is uncommon in patients with a history of pelvic inflammatory disease, endometriosis or malignant neoplasms which may be due to the presence of adhesions partially immobilizing the ovary.26
The ultrasound findings in B mode are variable based on the time of progression, degree of vascular affectation and presence of one associated mass30 (Fig. 8). Here are some of these findings:
- •
The most common sign is the ovary size increase over 4cm–ovary that looks hyperechogenic due to venous congestion.
- •
The pearl necklace sign: multiple small follicles in the periphery of the increased sized-ovary displaced due to edema and venous congestion. It is the only relatively specific sign though it is not always present.
- •
The twisted ovary can be found in the medial position above the uterus while the latter is deviated toward the affected ovary; finding present in over 40 per cent of all cases.30
- –
Associated adnexa mass: those over 5cm have a greater risk of torsion.
- –
Free fluid in the pelvic: nonspecific.
- –
The twisted vascular pedunculum can be seen as a target image with the twisted vessels showing a circular or spiral morphology (“whirlwind sign”).30,31 This sign has been suggested as pathognomonic.
- –
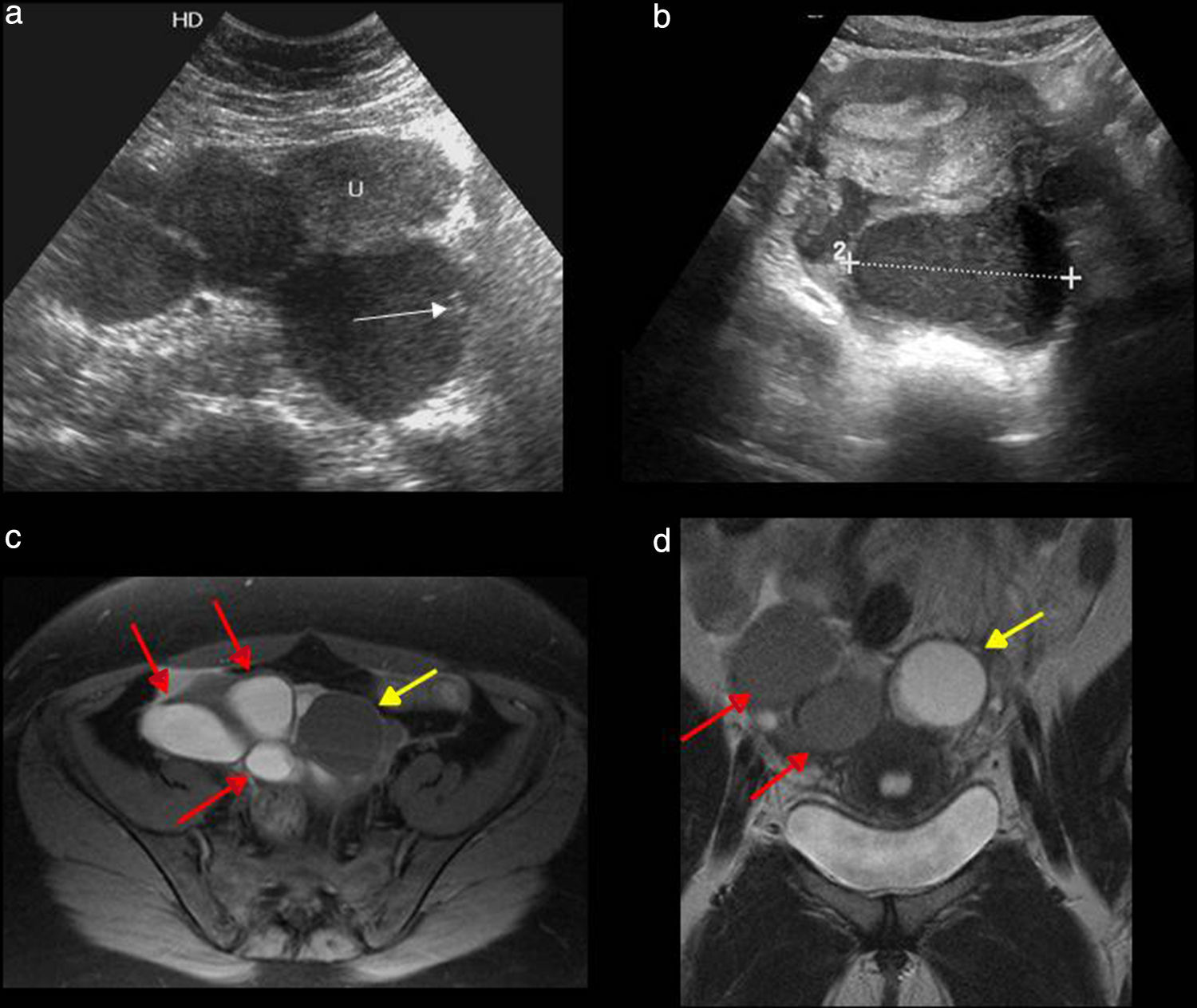
Ovarian torsion. (a) Ultrasound–longitudinal slice. Oversized, hyperechogenic ovary with peripheral follicles. (b) Ultrasound–longitudinal slice. Cystic image compatible with ovarian necrosis due to long-term torsion. (c) Computed tomography (CT) without contrast–coronal slice. Oversized left ovary in anomalous position with multiple peripheral follicles. Uterus (U) displaced toward the affected side, edematous thickened uterine tube (white arrow) and free fluid (yellow arrow). (d) CT without contrast–coronal slice. Dermoid tumor containing fat and calcium (asterisk) with mural thickening, free fluid (white arrows) and an increased density of adjacent fat (asterisk).
In the Doppler color flow mapping the findings look highly variable.30 The absence of Doppler color is not a reliable criterion for torsion since there can be an absence of normal ovaries and the presence of Doppler arterial sign does not preclude a diagnosis of torsion since the ovary receives double arterial vascularization and there is a possibility of incomplete torsion. However, the presence of high resistance coronary artery flow waveforms with an absence of venous fluid is highly suggestive of torsion in the appropriate clinical context. The Doppler color flow mapping is important because it determines the ovary preoperative viability though this can be hard to do. The existence of flow inside the ovary is suggestive that the ovary may be feasible especially if the flow is central. One piece of information about ovarian infeasability in the absence of twisted vascular pedunculum which corresponds to one necrotic or infarcted ovary during surgery. The overall accuracy of CT is lower that that of the ultrasound in the diagnosis of adnexal torsion.32
The complications associated to adnexal torsions are infarctions, hemorrhages, necrosis, infections and peritonitis.
- •
Differential diagnosis:
Hemorrhagic cysts, pelvic inflammatory disease and endometriomas.
- •
Management:
The suspicion of torsion requires urgent surgical care in order to be able to save as much ovarian tissue as possible.33
Endometriosis may be defined as the presence of functioning endometrial tissue outside the uterine cavity. The most common location is the ovary (80 per cent) followed by the uterine ligaments, the pouch of Douglas cul de sac, the pelvic peritoneum, the uterine tubes, the bladder and the rectum-sigmoid.34 Clinically it starts with pelvic pain of clinical characteristics since this ectopic endometrial tissue is sensitive to estrogens and synchronically proliferates with the endometrium.
The classical clinical triad is dyspareunia, dysmenorrhea and infertility.36 The typical findings of endometriosis are endometriosic implants, endometriomas or chocolate cysts (cystic masses due to repetitive cyclic bleeding) and adherences (due to bleeding and repeated inflammatory changes).35 The ultrasound findings may vary but the typical appearance of endometriomas is that of one adnexal mass with low level-echoes inside, and absence of internal vascularization in the Doppler color flow mapping.18 Sometimes they have a solid appearance, other times they can be multiple and show multiloculations of thin or thick septa (Fig. 9a and b). The appearance of “kissing” ovaries (linked behind the bottom of the uterine pouch)34 is an indirect sign of endometriosis especially the most serious manifestation of the disease when there is an important pelvic spread accompanied by dense adherences.
Endometriomas. (a and b) Ultrasounds–transverse slices. (a) Multiples cysts with low-grade echogenic content one of them with echogenic bottom due to the presence of calcium in its wall (arrow). (b) Typical image of endometrioma (dotted line in between marks) with low-grade internal echoes. (c) Magnetic resonance imaging (MRI), T1-weighted sequence with fat saturation–axial slice. Hyperintense cystic structures compatible with endometriomas (red arrows), and low-grade functional cyst of low intensity (yellow arrow). (d) MRI, T2-weighted sequence–coronal slice. Endometriomas with low intensity signal–shading effect (red arrows) and hyperintense functional cyst (yellow arrow).
The MRI has 90 per cent sensitivity and 98 per cent specificity in the finding of endometriomas.33 Findings in the MRI are hyperintense cysts in the T1-weighted images and hypointense cysts in the T2-weighted images. This shading phenomenon in the T2-weighted sequences is due to the viscosity of the recurring hemorrhage inside the cyst (Fig. 9c and d). On rare occasions it can present like an acute set of symptoms with rupture or infection of the endometrioma.1 In these cases when they cannot be distinguished from a tubo-ovarian abscess only the use of an MRI can confirm the existence of underlying endometriosis.36 Also we can have an acute presentation in the form of an intestinal occlusion or subocclusion due to intestinal subserous implants that infiltrate the wall or due to adherences associated with peritoneal implants.
- •
Differential diagnosis:
Pelvic inflammatory disease, hemorrhagic cysts, and ovarian tumors.
- •
Management:
The initial study is done through ultrasounds. If not removed the cystic masses with endometrioma-like clinical characteristics should be followed through ultrasound. The follow-up frequency is variable3 but in general at least it should be annual to make sure they do not grow in size and do not show any changes in the internal architecture since approximately 1 per cent of endometriomas (usually larger than 9cm) may have a malignant transformation that is usually endometrioid or clear cell carcinoma.37,38
The ultimate diagnosis of endometriosis is through laparoscopy and biopsy.33
The MRI is key if we want to be able to determine the degree of spread and depth of the infiltrated ones especially when the laparoscopic inspection is limited by the adhesions.
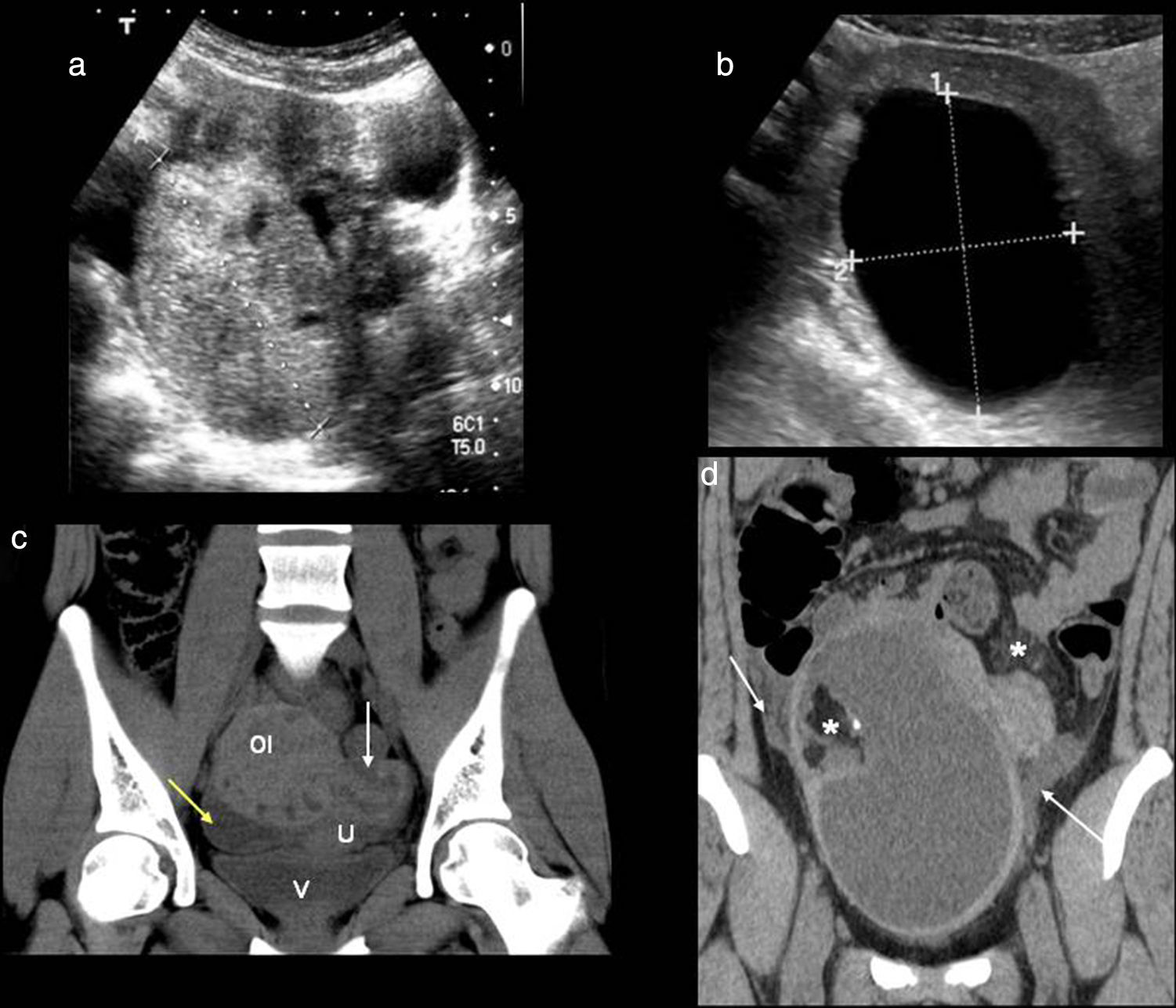
Torsion or acute degeneration of myomasBenign smooth muscle tumors (leiomyomas) are the most common tumors of the uterus and can be found in more than one-fifth of women over 30 years of age.13 They are estrogen-dependent. Their location is usually intramural, subserosal and then submucosal. In the ultrasound they look like solid uterine masses both homogeneous and heterogeneous (due to internal degeneration) that may also have calcifications inside (present only in 10 per cent of all cases).
These tumors can compress neighboring structures, become twisted or degenerate (cystic degeneration, myxoid, hemorrhagic) (Fig. 10) causing pelvic pain in approximately 30 per cent of patients. On top from pain, vaginal exudates or bleeding can occur as well as low-grade fever and leukocytosis. The phenomena of degeneration occur when the growth of the myoma tops the capacity of vascular supply as it happens during the first ten (10) weeks of pregnancy.39 Radiologically one large and irregular focus of fluid content can be identified within a solid tumor that if prior studies have been conducted will show that it grew in a short span of time. Pedunculated myomas are predisposed to torsión.40 Whenever this happens and the become necrosated they show an absence of Doppler flow inside or CT enhancement with IV contrast.
- •
Differential diagnosis:
Adnexal masses in the case of subserous myomas.
- •
Management:
Myomectomy in the case of torsion. Embolization of myoma in selected cases.
Myomas. (a and b) Ultrasound–transverse slice. Intramural myoma (asterisk) and subserous myoma with internal anechoic area representing a focus of hyaline degeneration (arrows). (c) Computed tomography (CT) with contrast–axial slice. Intramural myoma with hyaline degeneration looking like a hypodense mass unenhanced with contrast (arrows). (d) CT without contrast–axial slice. Intramural myoma (asterisk) with hyperdense semilunar image due to hemorrhagic content. Calcified subserous myoma (yellow arrow).
Gynecological pathologies should always be taken into consideration in the differential diagnosis of acute pelvic pain in women. Numerous affections of gynecologic origin and even normal physiologic changes can be the cause of pain. The ultrasound is the default diagnostic imaging modality because it is not invasive, it is highly available, is cost-efficient and provides accurate results. The CT is reserved for cases of diagnostic doubts and the assessment of the process spread or any possible complications. The clinical data together with the radiologic findings will allow us to adequately carry out the appropriate differential diagnoses for the appropriate management and care.
Authors- 1.
Manager of the integrity of the study: ARD, AMJ.
- 2.
Study Idea: ARD, AGO, DAM.
- 3.
Study Design: ARD, AMJ.
- 4.
Data Mining: ARD, AMJ, AGO, DAM, LCA.
- 5.
Data Analysis and Interpretation: ARD, AMJ, AGO, DAM.
- 6.
Statistical Analysis: N/A.
- 7.
Reference: AMJ, ARD.
- 8.
Writing: ARD, AMJ.
- 9.
Critical review of the manuscript with intellectually relevant remarks: ARD, AMJ, AGO, DAM, LCA.
- 10.
Approval of final version: ARD, AMJ, AGO, DAM, LCA.
The authors declare that no experiments with human beings or animals have been performed while conducting this investigation.
Confidentiality of dataThe authors confirm that in this article there are no data from patients.
Right to privacy and informed consentThe authors confirm that in this article there are no data from patients.
Conflict of interestThe authors declare no conflict of interests associated with this article whatsoever.
Please cite this article as: Rivera Domínguez A, Mora Jurado A, García de la Oliva A, de Araujo Martins-Romeo D, Cueto Álvarez L. Dolor pélvico de origen ginecológico como patología urgente. Radiología. 2017;59:115–127.