Magnetic resonance (MR) neurography refers to a set of techniques that enable the structure of the peripheral nerves and nerve plexuses to be evaluated optimally. New two-dimensional and three-dimensional neurographic sequences, in particular in 3T scanners, achieve excellent contrast between the nerve and perineural structures. MR neurography makes it possible to distinguish between the normal fascicular pattern of the nerve and anomalies like inflammation, trauma, and tumor that can affect nerves. In this article, we describe the structure of the sciatic nerve, its characteristics on MR neurography, and the most common diseases that affect it.
La neurografía por resonancia magnética (RM) hace referencia a un conjunto de técnicas con capacidad para valorar óptimamente la estructura de los nervios periféricos y de los plexos nerviosos. Las nuevas secuencias neurográficas 2D y 3D, en particular en equipos de 3Tesla, consiguen un contraste excelente entre el nervio y las estructuras perineurales. La neurografía por RM permite distinguir el patrón fascicular normal del nervio y diferenciarlo de las anomalías que lo afectan, como inflamaciones, traumas y tumores. En este artículo se describe la estructura del nervio ciático, sus características en la neurografía por RM y las dolencias que lo afectan con mayor frecuencia.
The study of peripheral neuropathies (PN) has been historically the responsibility of neurophysiologists, who contribute functional and qualitative-quantitative data of the lesion's location, the conduction properties and neuronal distribution. The most commonly used technique is the electromyogram (EMG).1,2 However, the EMG is a time-consuming study, it is not comfortable for the patient, it does not locate the exact spot where the lesion is, or differentiate perineural fibrosis from compressive masses. Clinical electrophysiologic evaluation does not usually determine the cause of sciatic neuropathy.3 The advent of MR neurographic techniques has allowed for a more accurate diagnostic approach.4 At present, with 3T MR equipment and high resolution sequences, it is possible to study the sciatic nerve structure in detail, its course, its relationship with the adjacent structures which may contribute to trap it and compress it.3,5 This article reviews the sciatic nerve's anatomy, the RM technical parameters for its study and the most frequent processes that may affect it.
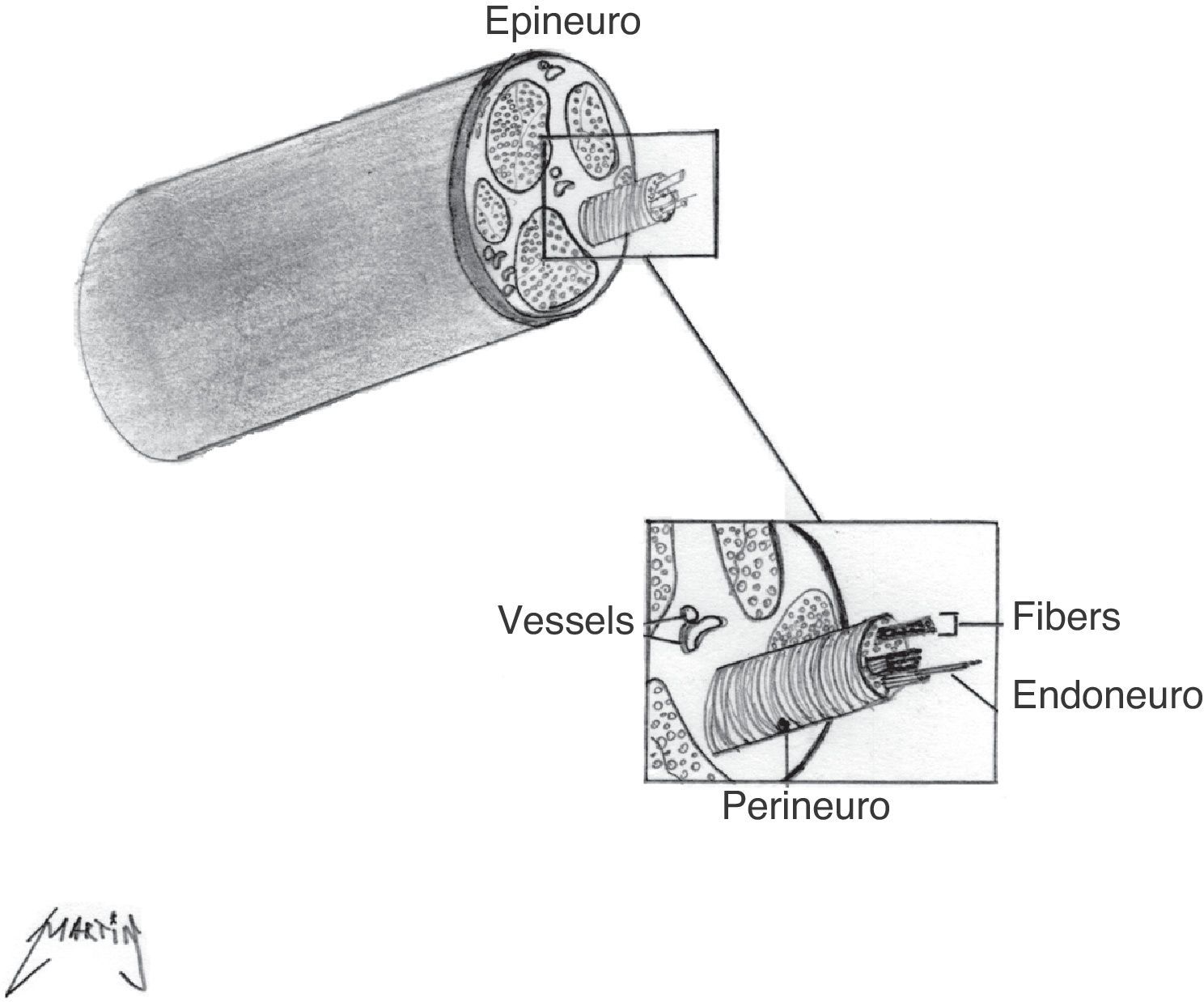
Structure of the peripheral nerveIn order to understand better the characteristics of normal and pathological nerves in RM, it is necessary to review briefly the structure of the peripheral nerve. The axon is the peripheral nerve's functional unit, and it is contained in the sheath formed by the Schwann cells. A layer of connective tissue, the endoneuro, surrounds each axon. Multiple axons form a fasciculus that is covered by a fibrous stroma called perineuro. Finally, a group of fasciculi is covered by a layer of connective tissue, the epineuro, which contains the vessels6 (Fig. 1).
Anatomy of the sciatic nerveFormed by the junction of roots L4–S3, the sciatic nerve is the longest nerve of the body. It emerges through the greater sciatic notch where its two divisions can already be distinguished: the tibial and the fibular divisions, but they are both encased in a common nerve sheath. In its descent along the pelvis, it presents anatomical variations in its relations with the piriform muscle, usually going through underneath. However, the nerve or one of its divisions, usually the fibular division, may go through the muscle. The nerve continues to descend along the thigh, behind the adductor magna in front of the greater gluteus. In the distal third of the thigh, the two divisions separate physically into tibial nerve and common fibular nerve.7
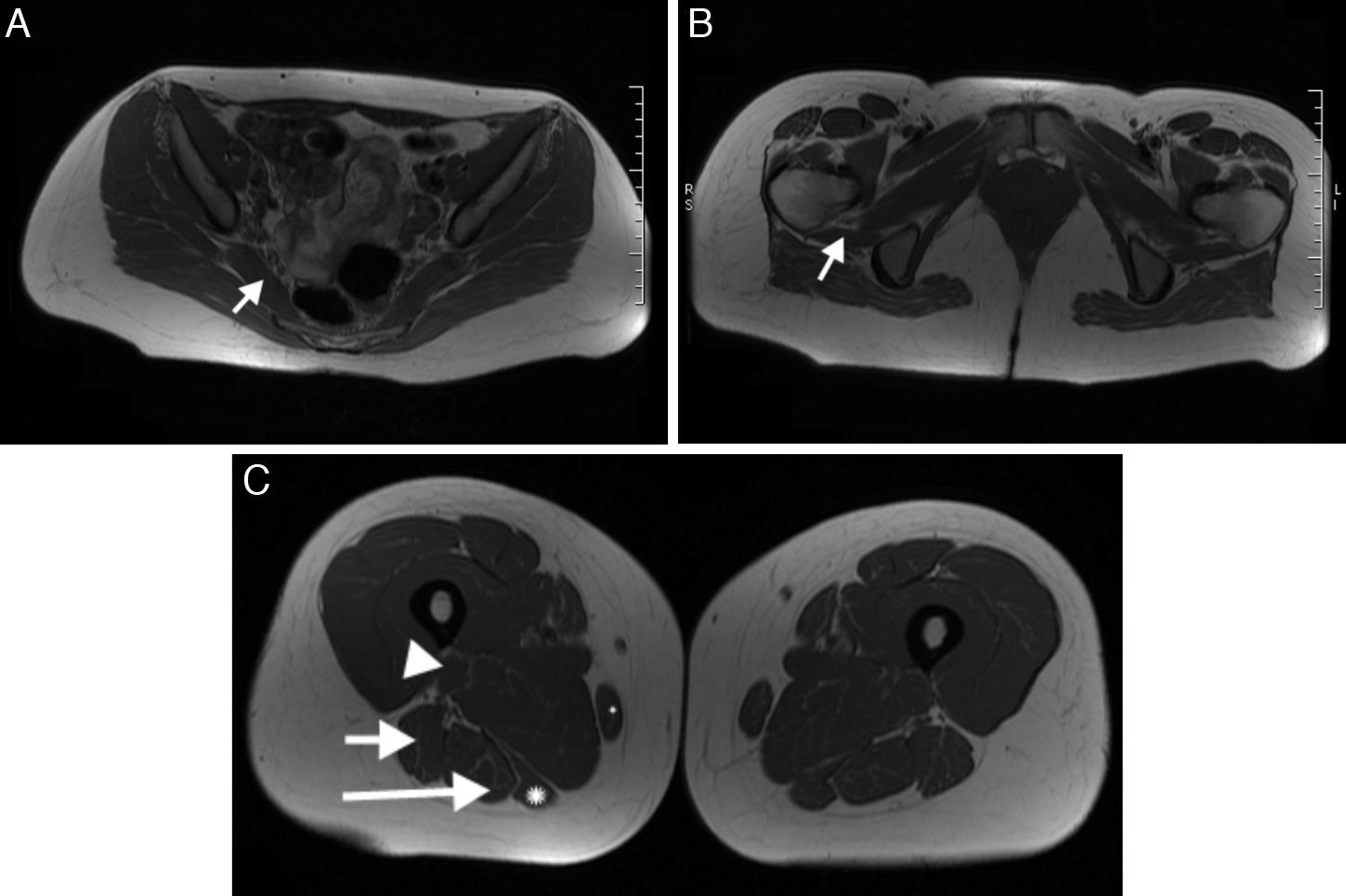
Muscles innervated by the sciatic nerveIn the pelvis, the sciatic nerve innervates the piriform muscle and the quadriceps femoris. In the thigh, the tibial division of the sciatic nerve innervates the long head of the biceps femoris muscle and the semitendinosus, semimembranosus muscle and the adductor magna. The fibular division innervates the short head of the biceps femoris8 (Fig. 2).
Muscles innervated by the sciatic nerve. (A) Piriform muscle (arrow). (B) Quadriceps femoris (arrow). (C) Gracile muscles (small asterisk), short head of the biceps femoris (arrow head), long head of the biceps (short arrow), semimembranous (large asterisk) and semitendinous (long arrow).
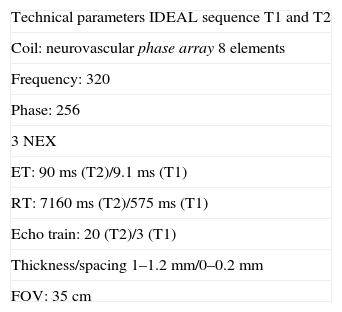
Historically, the techniques used to evaluate peripheral nerves prioritized the weighted sequences in T2.4 Nevertheless, nowadays there are high resolution techniques available which, by using 1–1.2mm fine cuts, without spacing, and by combining weighted sequences in T1 for anatomical studies and weighted sequences in T2 with fat suppression for the pathological, make it possible for better contrast among the fasciculi, the perifascicular and perineural fat to be obtained. 3T equipment is not a requirement to perform these studies, since other field intensities also permit it. Byun et al., in a study with 24 patients, proved that with a 3D sequence echo gradient with millimetric planes (Proset sequence) in a 1.5T equipment and reconstructions in the work station, it is possible to study with a high degree of accuracy the relation between foraminal and extraforaminal hernias with diseased nerve root.9 However, the advent of 3T RM and high definition neurographic sequences equipment made it possible to obtain images with excellent signal/noise relation and to perform 3D examinations. There are different ways to generate saturation of a given tissue, among them, the most commonly used is fat saturation. One of these saturation techniques is based on signal decomposition taking advantage of fat proton precession frequency. This method, described by Dixon, is the basis for in-phase/out-of-phase images, which are broadly used in daily practice.10 At present, this sequence allows us to work simultaneously with T1 and T2 images and 4 combinations of saturation pulses (water suppression, fat suppression and combined suppression of water and fat or in-phase/out-of-phase images), by means of a modification of Dixon's technique (Table 1). This development corresponds to the IDEAL sequence (iterative decomposition of water and fat with echo asymmetry and least-squares estimation) by General Electric (GE) Healthcare11,12 and to 3D T2 SPACE (sampling perfection with application optimized contrast), by Siemens Healthcare.13,14 The sum of 3D T2 Cube images (GE Healthcare) acquired in the coronal plane and proton density with fat saturation allows for an optimal study of the region and it is reserved for when there is suspicion of tumors and infections.
Technical parameters. IDEAL sequence.
| Technical parameters IDEAL sequence T1 and T2 |
| Coil: neurovascular phase array 8 elements |
| Frequency: 320 |
| Phase: 256 |
| 3 NEX |
| ET: 90ms (T2)/9.1ms (T1) |
| RT: 7160ms (T2)/575ms (T1) |
| Echo train: 20 (T2)/3 (T1) |
| Thickness/spacing 1–1.2mm/0–0.2mm |
| FOV: 35cm |
FOV: Field of vision; IDEAL: iterative decomposition of water and fat with echo asymmetry and least-squares estimation; NEX: number of excitations; ET: Echo time; RT: repetition time.
The after process of the work station is an essential part of the study. Multiple plane reconstruction (MPR), reconstructions with maximum projection intensity (MPI) and curve reconstruction techniques are performed. The latter makes it possible for the nerve course to be unfolded and followed along its length, thus providing information about the nerve with the adjacent structures.
In addition, the technique has been developed based on water molecule diffusion, such as diffusion boosted sequences (DWI) and diffusion tensor (DTI) with tractography, to use them routinely in the evaluation of the peripheral nervous system.15,16 It is predictable that, in the future, these techniques might contribute information about nervous regeneration. DTI provides data about the nerve's microstructure and function, in addition to information about its trajectory. Diffusion along the nerve is usually three times greater than through the nerve (anisotropy), due to the restrictions generated by the myelin sheath. Thus, DTI makes it possible to for a tractography to be performed as well as the quantitative estimation of parameters such as the apparent diffusion coefficient (map ADC), which translates the degree of diffusion and fractional anisotropy. The application of high b values, of approximately 1000–1200s/mm2, is essential for an optimal study of peripheral nerves.17
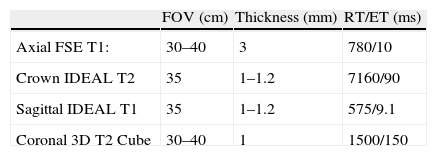
Although it is necessary to protocolize the study of the sciatic nerve, on occasion, the selection of a technique for an optimal examination must be agreed among the physician referring the patient, the radiology doctor and technician that will perform it. Table 2 summarizes the protocol used in our laboratory.
Sciatic nerve neurography protocol.
| FOV (cm) | Thickness (mm) | RT/ET (ms) | |
| Axial FSE T1: | 30–40 | 3 | 780/10 |
| Crown IDEAL T2 | 35 | 1–1.2 | 7160/90 |
| Sagittal IDEAL T1 | 35 | 1–1.2 | 575/9.1 |
| Coronal 3D T2 Cube | 30–40 | 1 | 1500/150 |
All the sequences were performed with high resolution matrix 256×320.
FOV: field of vision; FSE: fast spin echo; IDEAL: iterative decomposition of water and fat with echo asymmetry and least-squares estimation; ET: echo time; RT: repetition time.
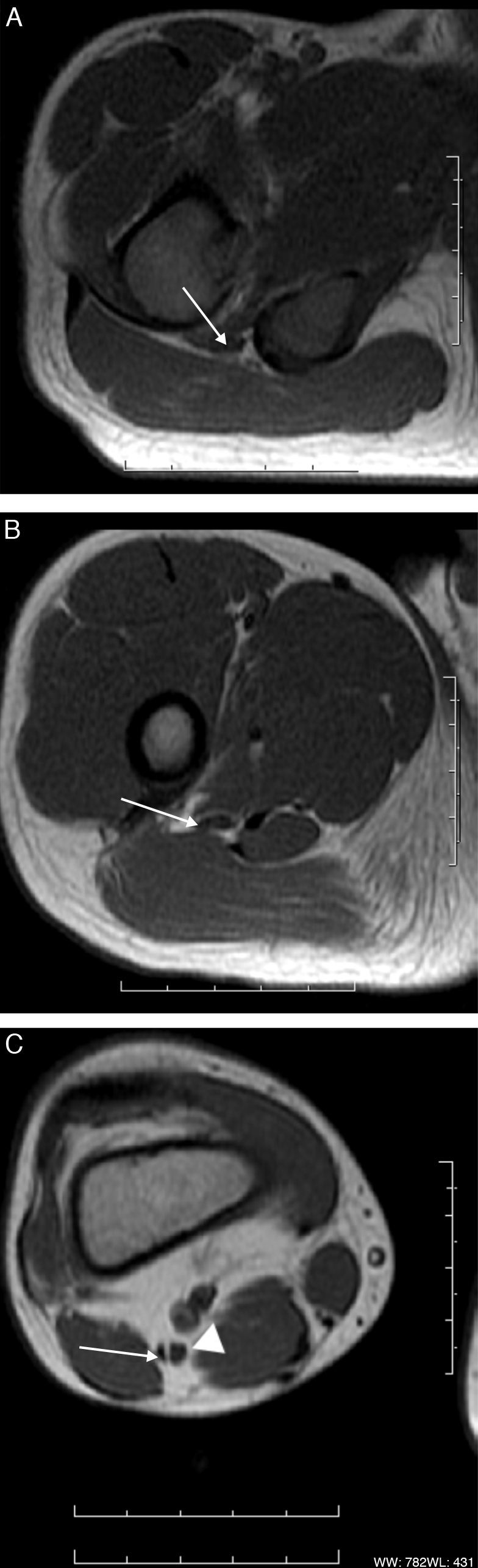
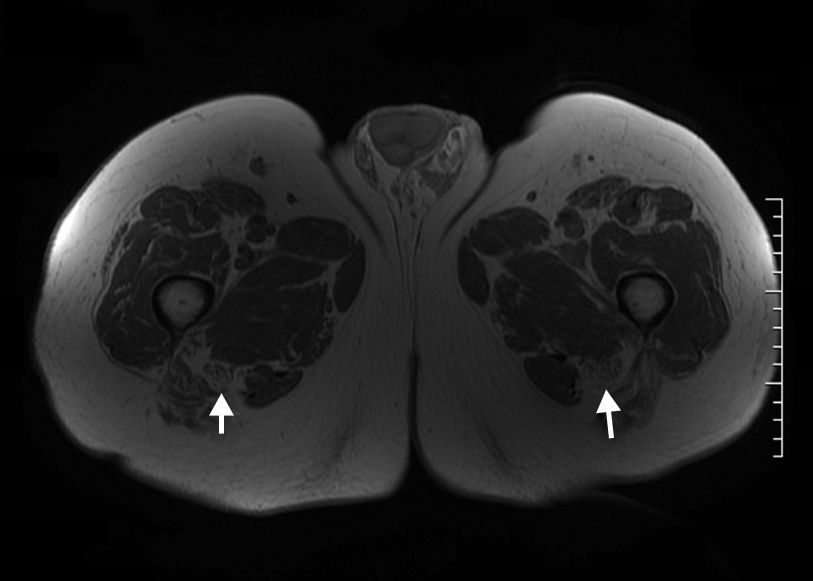
The normal nerve shows a fascicular appearance, with signal intensity from isointense to minimally hyperintense with respect of the muscle in the sequences potentiated in T1 and T2.17–19 The perineural fat tissue has a homogeneous signal and a separation plane with the adjacent structures. The perineural tissue around the sciatic nerve is especially abundant, which makes it possible for it to be distinguished clearly from the surrounding structures with the present MR sequences. The nerve's course is rather straight, without acute angles. Thanks to high resolution sequences, nowadays it is possible to distinguish the sciatic nerve divisions, the thicker, inner one is the tibial division and the thinner, lateral one is the fibular division (Fig. 3).
The abnormal nerve loses its fascicular pattern, it thickens either focally or diffusely and it presents deviations, interruptions or compressions in its course. In the sequences weighted in T2, it is hyperintense with respect of its contralateral and it usually enhances with the gadolinium injection. In addition, it is accompanied by signal changes or perineural fat stratification, which is easier to see in potentiated sequences in T1, it is also accompanied by adjacent linear images and in contact with the nerve, characteristics of fibrosis.19–21
Classification of neuropathiesThe major etiological forms of NPs were described by Seddon in 1943,22 who highlighted those in which traction and/or extrinsic compression damage occurs. The nerve undergoes the effect of physical forces whether by friction, elongation or compression, which generate three lesion mechanisms: neuropraxia, axonotmesis and neurotmesis.
Neuropraxia is the smallest degree of lesion. The nerve shows suffering without sheath or axon discontinuity. It is generally a transitory disorder, one with complete restitution.
In axonotmesis the noxa generates axonal discontinuity with integrity of the connective wrapping (perineuro, endoneuro and epineuro). The complete rupture of the axon produces a Wallerian degeneration of the second distal. Prognosis in these lesions continues to be good, but recovery is slow (axon regeneration of approximately 1mm per day).
Neurotmesis is the greatest degree of lesion. Both the axon and the perineural sheaths are affected. Functional loss is complete and, unless it is surgically operated early, granulation tissue and subsequently, fibrosis appear producing posttraumatic neuromas and/or intraneural fibrosis.22
Microsurgery techniques have benefitted surgery of peripheral nerves in the last 20 years. Perineural fibrous tissue may be eliminated by neurolisis.23 In cases of severe axonotmesis or neurotmesis surgical repair is necessary. Direct suture of the nerve ends may generate tension. That is why the present criterion is to use auto- or alografts.20
Neuropathies specific of the sciatic nervePyramidal syndromeDescribed by Robinson in 1947,24 the pyramidal or piriform syndrome results from sciatic nerve compression as it passes through the piriform notch. The piriform muscle is the most active in running athletes and it is inserted in the pedicles of the third and fourth sacral vertebrae, it goes through the greater sciatic foramen and it inserts into the greater trochanter by means of a thick sinew. Pyramidal syndrome may result from modifications of the piriform muscle such as hypertrophies, contractures or spasms, direct traumas or from anatomical anomalies in the nerve's course as it goes through the sciatic notch. Therefore, it is included within the compression or entrapment neuropathies. Controversy about the origin of distal lumbosacral neuropathy to the neuroforamen has persisted for many years.25
Images in pyramidal syndrome have not been very helpful for years and the diagnosis has always been one of exclusion. For Stewart,26 piriform syndrome has been underdiagnosed for years, and he has suggested surgical examination for definite diagnosis. During intervention, it is possible to identify sciatic compression by the piriform muscle and/or association with fibrous bands.26 However, neurographic sequences may show today the sciatic nerve along all of its course and establish its relation with the piriform muscle.27,28
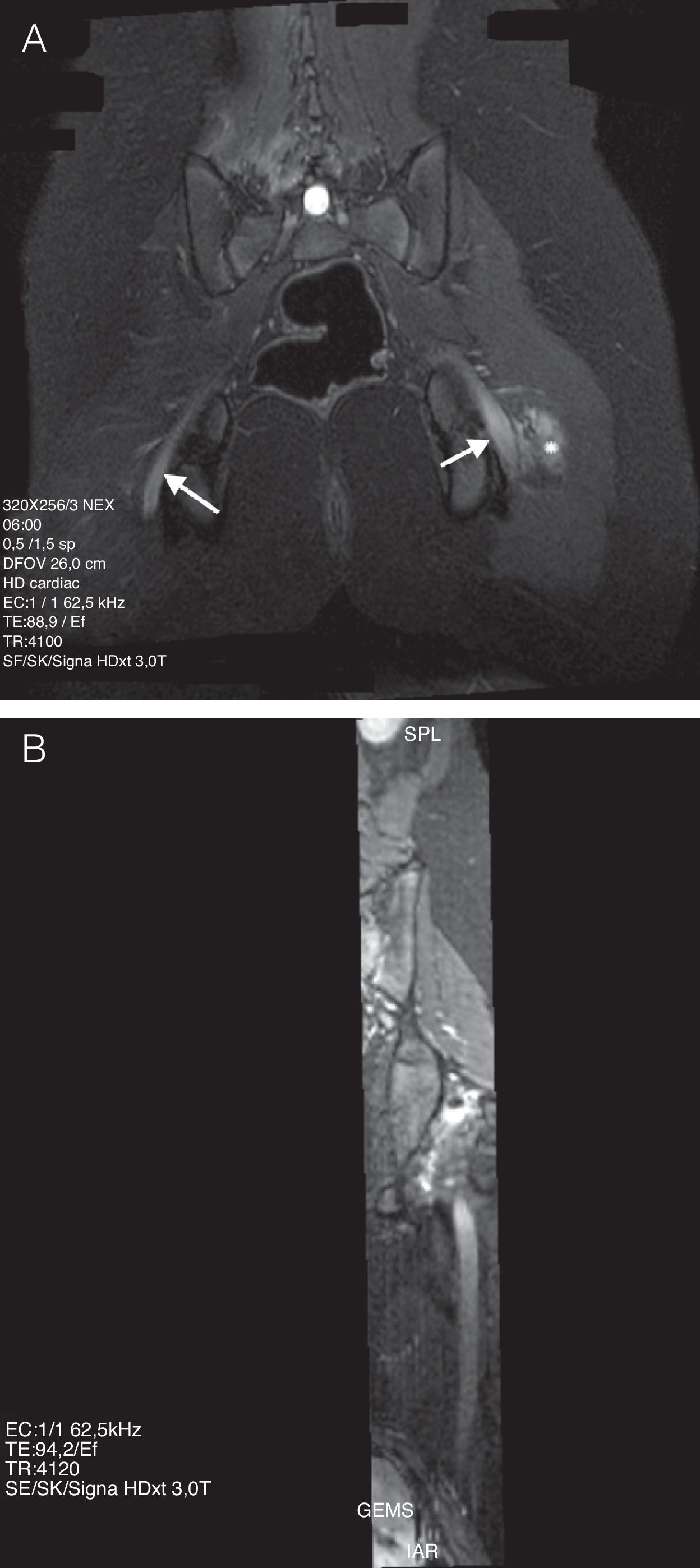
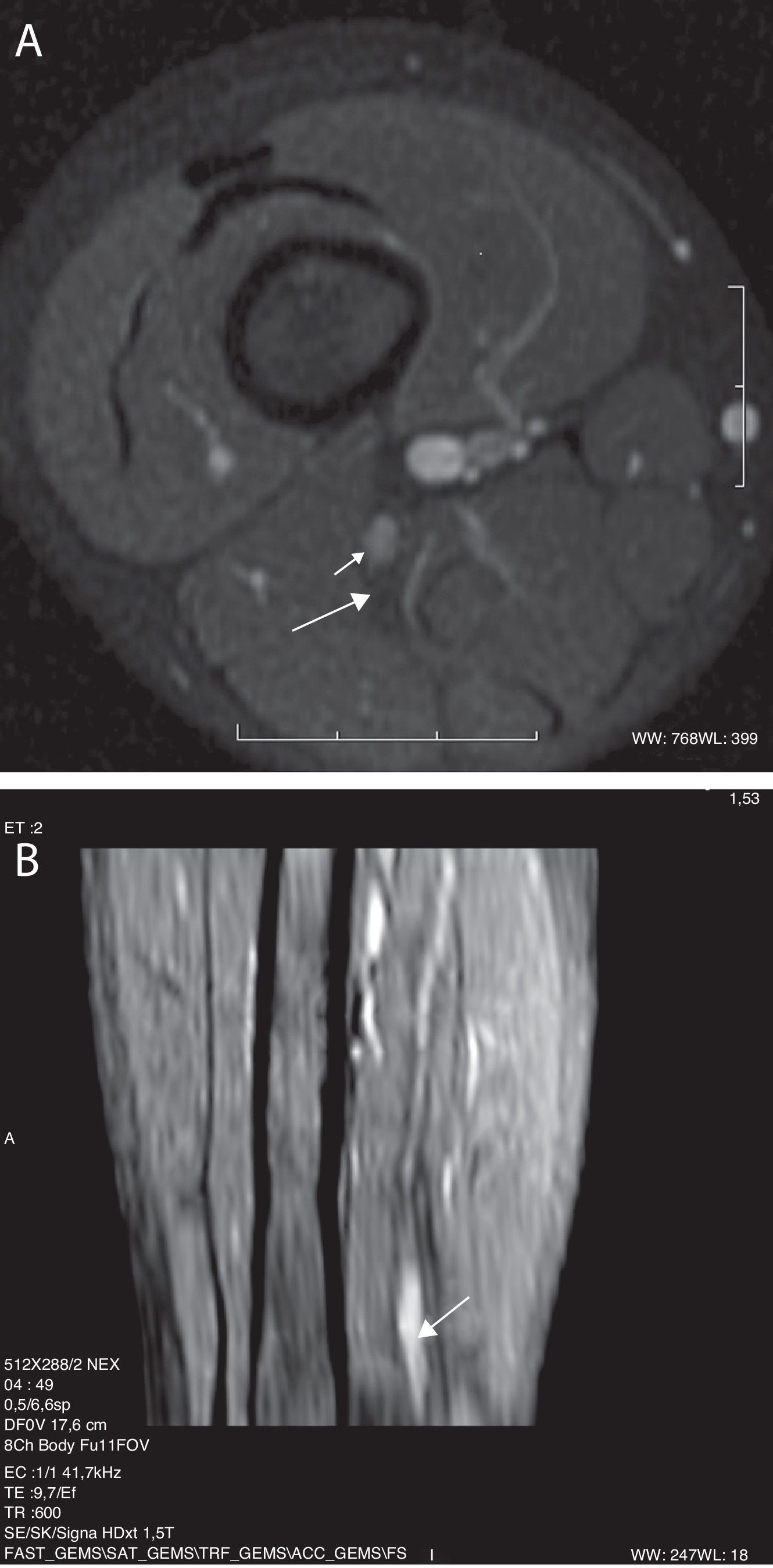
Among the lower limb neuropathies, postural neuropathies may occur in a variety of positions, are those of lithotomy and sitting. These causes for sciatic nerve lesions are preventable and they usually occur as a result of intraoperation carelessness, whether because the patient remains too long in the same posture or because of a positioning error. This has had a high legal-medical impact.29–31 In both positions, the same forces contribute to lesion by stretching of the ischiotibial muscle group (for example, biceps femoris), they may generate sciatic nerve stretching. Due to the fact that the position affects both limbs at the same time, injury of sciatic nerve may be bilateral. Piriform muscle trauma generates muscle spasm or contracture, and secondarily, nerve lesion by compression and/or stretching.32 the MR findings, taking into account clinical antecedents, are very revealing. The images show a fusiform thickening of the nerve as it passes through the sciatic notch, a focal point of contusions in piriform, quadriceps femoris and gluteal muscles, as well as in the adjacent fat planes. Contrast uptake by these structures may be explained by the acute inflammatory component of the vascular-nervous package of the neural sheath, along with the adjacent muscular compression (Fig. 4).
13-year-old girl with an antecedent of surgery in a sitting position for 8h. (A) IDEAL sequence weighted in T2 with fat saturation in the coronal plane. It is possible to observe a signal increase and diffuse thickening of both sciatic nerves, with prevalence on the left side (arrow). It is accompanied by edema of the adjacent soft tissue on the left side (asterisk). (B) MPR curve reconstruction in the nerve's longitudinal axis.
The most common neoplasia described in the bibliography is the Peripheral Neural Sheath Tumor (PNST).33–35 The importance of being able to differentiate in images benign tumors such as neurofibroma and neurilemmoma or schwanoma lies in that the schwanoma may dry up without affecting the nerve, because it is contained within the same capsule, the epineuro, and it can usually be separated surgically. Contrariwise, neurofibroma must be resected with part of the nerve because the tumor cannot be separated from its fibers. Both tumors have been broadly studied and described in RM works. In a study of 52 patients analyzing the differences in the images weighted in T2, the target sign (58 vs 15%), central highlight (75 vs 8%) and a combination of both (63 vs 3%) were present mainly in the neurofibromas. Contrariwise, the findings suggesting neurilemmomas were the fascicular appearance, a thin hyperintense ring in T2, a combination of both (8% neurofibromas vs 46% neurilemmomas) and diffuse highlight. Large neurilemmomas often undergo degenerative changes that include cysts, hemorrhage and fibrosis36 (Fig. 5).
A 4 year-old boy with polyneuritic gait of a year of evolution, with EMG compatible with fibular nerve lesion. (A) SPGR sequence weighted in T1 with fat saturation and gadolinium injection. It shows a nodule in the sciatic nerve's fibular division (short arrow). Behind, it is possible to observe the tibial division with a normal fascicular pattern (long arrow). (B) MPR curve reconstruction where the fusiform thickening in the sciatic nerve distal third is observed (arrow).
The plexiform neurofibroma has a pathognomonic appearance. It may be observed as a diffuse nodularity along the nerve course and its branches, with a hyperintensity similar to water in the sequences weighted in T2. The large ones replace the adipose tissue creating a “hive-like” appearance. They manifest clinically by neuromatose elephantiasis 37–39 (Fig. 6).
14 year-old boy, with Type I neurofibromatosis antecedents. SSFSE sequence weighted in T2 in the thigh's coronal plane, in which a voluminous formation is observed in the soft part of the thigh, with high signal intensity, polylobulated, with multiple septs. The biopsy showed a sciatic nerve plexiform neurofibroma.
Neurofibroma or malign schwanoma, also known as malign PNST, account for 5–10% of the soft tissue sarcomas and it is associated to Type I neurofibromatosis in 25 to 70% of the cases. It has also been described 10–20 years after it has been radiated. The sciatic nerve is the one most often affected by these tumors.33,40 Neurography is the state of the art image study for diagnosis. It shows the characteristic fusiform morphology, sometimes areas of necrosis or hemorrhage and, occasionally, cartilage or bone heterotopic focuses. Local recurrence and metastasis are frequent.27,41,42
Sciatic nerve lipomatosis, also known as fibromatose hamartoma, is a rare pseudotumoral condition characterized by the fusiform thickening in a nerve by fibroadipose tissue anomalous hypertrophy. The appearance of this entity in the MR is pathognomonic. In the sequences weighted in T1 it shows the nerve's fascicular pattern, hypointense, hyperintense similar to the fat signal that is distributed evenly among the nerve's fibers. In the sequences weighted in T2, and in particular in those with fat saturation or STIR (short tau inversion recovery), the nerve appears homogeneously hypointense due to the suppression of the fat component and the low signal of the normal fascicular pattern. The amount of fat component is variable43,44 (Fig. 7).
A 73 year-old Paciente presenting mild paresthesias in the fibular area on both sides. The weighted T1 images show an increase in thickness of the sciatic nerve on both sides at the expense of an intraneural fat component hypertrophy, a finding compatible with sciatic nerve bilateral lipomatosis (arrow).
Moreover, perineural infiltration by lymphoma45 has been described. Other processes simulate tumors such as amyloidosis,46 the inflammatory pseudotumor.5,47 More rarely lipomas,48 lymphangiomas, neurofibrosarcomas and desmoid tumor have been described.49
Differential diagnosis between radiation and neoplastic plexopathy may be complex. On these occasions, Positron Emission Tomography (PET) with 18F-fluorodeoxyglucose may help differentiate them.50
ConclusionsThe current high resolution RM techniques improve visualization of normal and pathological peripheral nerves and provide complementary information for clinical and electrophysiological trials. It is a complement for lumbosacral spine MR in the study of lumbosciatalgias.
Ethical responsibilitiesAnimal and people protectionThe authors declare that no experiments were conducted either on people or on animals.
Data confidentialityThe authors declare that they have followed the protocols of their work place about publication of patients’ information and that all the patients included in the study have been informed enough and they have given their consent in writing to participate in said study.
Right to privacy and informed consentThe authors declare that no patient information appears in this article.
Authors- 1.
Person Responsible for the study's integrity: CC.
- 2.
Conception of the study: CC and MA.
- 3.
Design of the study: CC, MA, NC and LF.
- 4.
Data acquisition: MA, LF, NC and MCA.
- 5.
Data analysis and presentation: LF, NC and MCA.
- 6.
Statistical treatment: Not applicable.
- 7.
Bibliographic search: CC, MA and LF.
- 8.
Writing of the paper: CC, MA and NC.
- 9.
Critical revision of the manuscript with relevant intellectual contributions: CC, MA, LF, NC and MCA.
- 10.
Approval of the final version: CC, MA, LF, NC and MCA.
The authors declare that they have no conflict of interests.
Please cite this article as: Cejas C, et al. Neurografía por resonancia magnética de alta resolución (3Tesla) del nervio ciático. Radiología. 2013;55:195–202.