Peroneal neuropathy is the most common mononeuropathy of the lower limbs. The causes of peroneal neuropathy include trauma, tumors of the nerve and nerve sheath, entrapment, and others such as perineurioma, fibromatosis, lymphoma, and intraneural and extraneural ganglia. The diagnosis is based on clinical manifestations and electrophysiological studies. Nowadays, however, magnetic resonance (MR) neurography is a complementary diagnostic technique that can help determine the location and cause of peroneal neuropathy. In this article, we describe the MR anatomy of the peroneal nerve, its relations, and the muscles it innervates. We also discuss the clinical and electrophysiological manifestations of peroneal neuropathy, describe the technical parameters used at our institution, and illustrate the MR appearance of various diseases that involve the peroneal nerve.
La neuropatía del nervio peroneo es la mononeuropatía más común de los miembros inferiores. Entre las causas se incluyen el traumatismo, los tumores del nervio y de la vaina, el atrapamiento, y otras como el perineuroma, la fibromatosis, el linfoma y el ganglión intraneural y extraneural. El diagnóstico se basa en las manifestaciones clínicas y los estudios electrofisiológicos. Actualmente, sin embargo, el complemento diagnóstico con neurografía por resonancia magnética (RM) permite aproximarse al lugar y la causa de esta neuropatía. El objetivo de este trabajo es describir con la RM la anatomía del nervio peroneo, sus relaciones y los músculos que inerva; mencionar las manifestaciones clínicas y electrofisiológicas de sus lesiones; describir los parámetros técnicos que se emplean en nuestra institución; y mostrar la apariencia en RM de las diversas enfermedades que afectan al nervio peroneo.
The peroneal nerve (PN) neuropathy is the most common mononeuropathy of the lower limbs.1 PN can host polyneuropathies or mononeuropathies. In the first group we find metabolic and inflammatory systemic diseases such as diabetes, Charcot-Marie-Tooth disease, chronic inflammatory demyelinating polyneuropathy, or amyloidosis.2–4 In the group of mononeuropathies we find trauma mononeuropathies, nerve and sheath tumors, entrapment and other mononeuropathies such as perineuroma, lymphoma, endometriosis, radiotherapy, and intraneural ganglion.5–11 Historically the study of neuropathies has been based on clinical evaluation and electrophysiological studies. But during the last years with the development of magnetic resonance (MR) sequences we have been able to evaluate the peripheral nerves. The assessment is more precise if 3T resonance equipments are used. Also thanks to Dixon sequences we have been able to do MR neurographies, obtain 3D sequences and see plexuses and peripheral nerves, identify the nerve fascicular appearance, and determine the relation to adjacent structures which in turn increases the diagnostic capacity of neuropathies seen through MR, and the location and cause of anomalies12–16 too. The goal of this review was to briefly summarize the clinical manifestations and electrophysiological methods to diagnose PN neuropathy, discuss the causes of PN neuropathy, and show both the normal and pathological appearances of PN through newly available MR sequences.
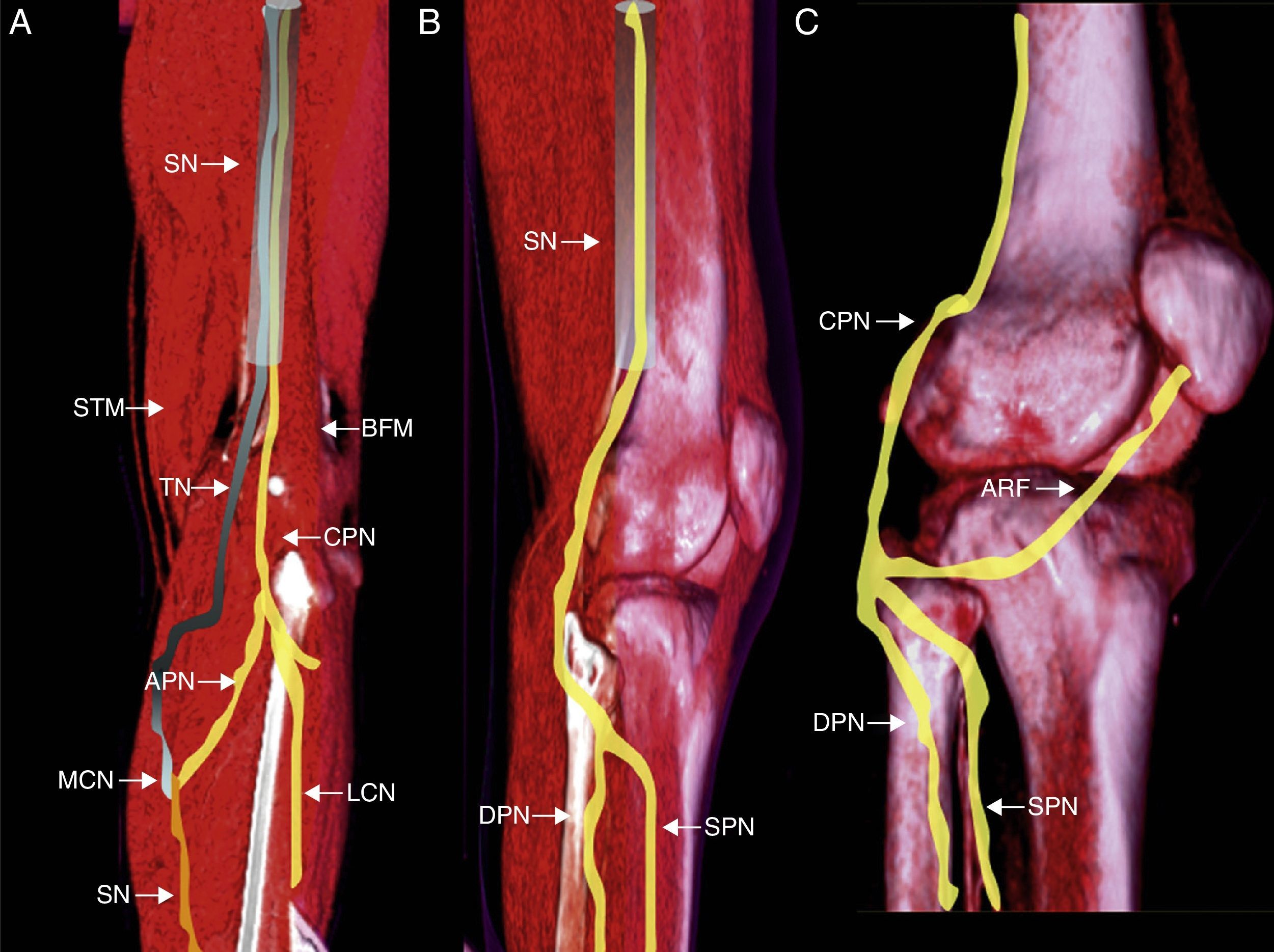
Anatomy: origin, course, relations and innervated muscles by peroneal nerveThe common peroneal nerve (CPN) and the tibial nerve (TN) make up a common trunk at muscle level, yet they are independent components of the sciatic nerve. At this level the CPN innervates the short head of the biceps femoris muscle.17 In general at the proximal sector of the popliteal fossa (though it can also be before this) both components are separated into one medial fasciculus, the TN and another lateral one (CPN).18 Two (2) fasciculi emerge from the latter one: the lateral cutaneous calf nerve and the peroneal anastomotic nerve which when linked to its medial counterpart (originated in the TN) makes up the sural nerve.19
CPN can be found in a fat plane separating the lateral gastrocnemius muscle from the biceps femoris muscle. At this level we can see a thin fibrous-adipose band separating its anterior and superficial (posterior) divisions18,20 (Fig. 1). All along the lateral sector of the popliteal fossa, the nerve descends to the posterior end of the peroneal head, incurvates itself to surround the external cortical and introduces itself into the long peroneal muscle21. Before penetrating into the peroneal conduit the CPN is covered with skin and subcutaneous skin only which makes it vulnerable to trauma and compression injuries.22 Caudally to the peroneal nerve the nerve trifurcates originating the articulate recurrent fasciculus (ARF), the superficial peroneal nerve (SPN) and deep peroneal nerve (DPN) in over 80% of the overall population in a space spanning 3cm distal to the outer side of the knee joint.23,24
Structure, divisions and anatomical relations of the CPN. (A) Posterior view of left leg. Origin of CPN from the common trunk and sciatic nerve (SN). Primary subdivisions of CPN in the posterior side of the lateral cutaneous nerve (LCN) and anastomotic peroneal nerve (APN) in its connection with the medial cutaneous nerve (MCN) to make up the sural nerve (SN). (B) Lateral view showing the main motion divisions of peroneal nerve into superficial peroneal nerve (SPN) and deep peroneal nerve (DPN) behind the head of fibula. (C) Lateral view showing peroneal nerve trifurcation–most common in the general population with the articulate recurrent fasciculus (ARF) toward the articular capsule. BFM: biceps femoris muscle; STM: semi-tendinous muscle.
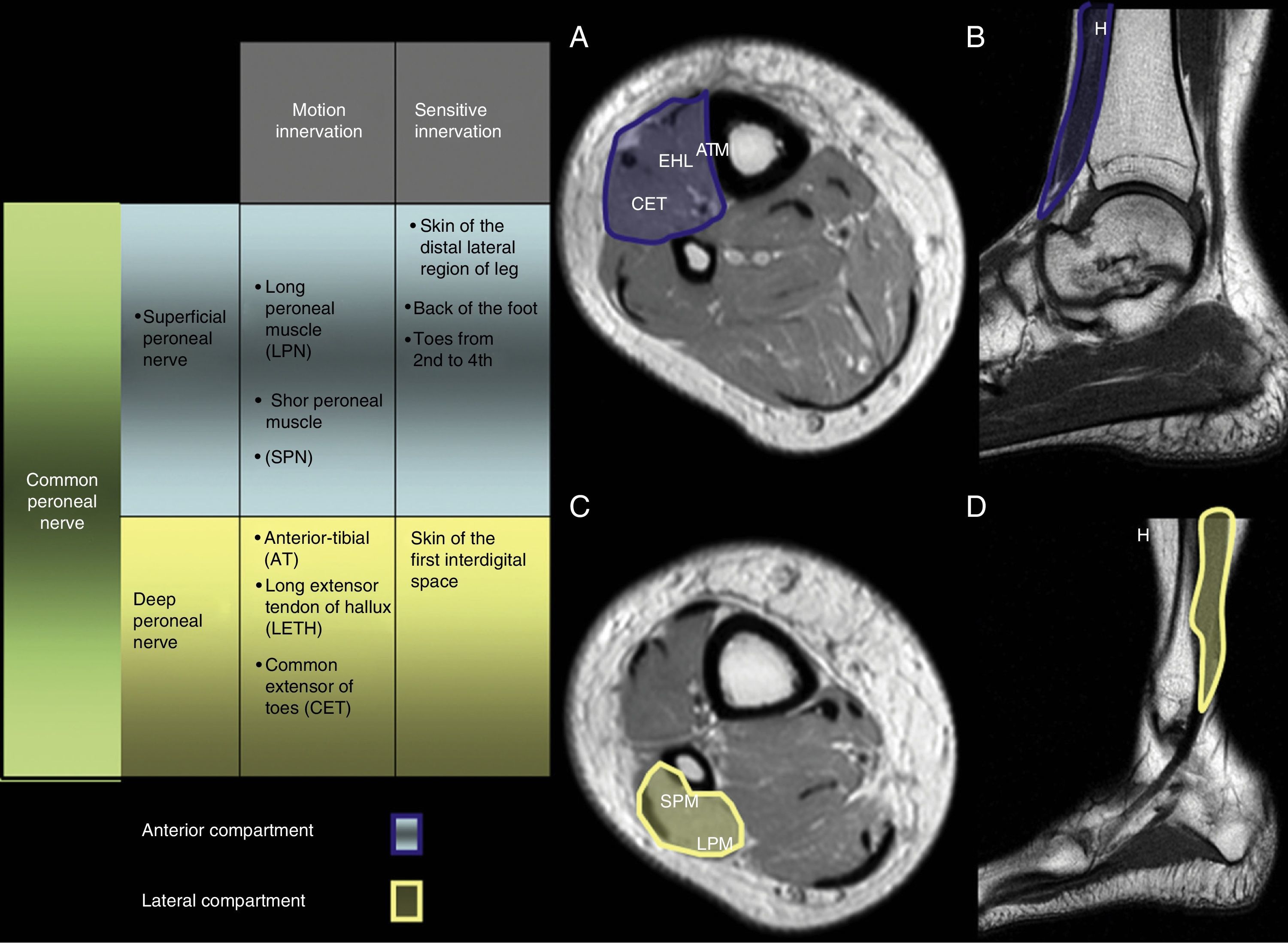
The DPN descends through the anterior compartment of the leg near the peroneal periosteum and together with the tibial artery runs through the intermuscular septum between the common extensor tendon of toes and the long extensor tendon of hallux. At the articular level the tibio-peroneal astragalin runs through the anterior tarsal tunnel and under the extensor retinaculum.21
The DPN is particularly a nerve responsible for motion that innervates the anterior muscular compartment including the anterior tibial compartment, the long extensor of hallux, the common extensor of toes and the anterior peroneal muscle.25
Superficial peroneal nerveThe SPN descends like the most posterior branch following the trifurcation of PN, runs through the crural fascia at the lateral sector of the leg to eventually become superficial. The nerve distal branches (superficial too) can be found some 6cm over the peroneal malleolus.21
The SPN is responsible for the motion innervation of the long and short peroneal muscles, and of the sensitivity of the back of the foot22 (Fig. 2).
Clinical manifestationsInjuries of the CPN present with paralysis of the dorsiflexion of foot and toes, and reduction or absence of sensibility in the outer side of leg and back of the foot.
In most cases the injury is located at the peroneal neck level involving both deep and superficial branches. Other than these symptoms if located inside the popliteal hollow on hypoesthesia on the outer side of leg can occur with involvement of the lateral cutaneous branch of the knee originating from the CPN main trunk before its bifurcation. Occasionally the injury can selectively compromise the deep branch causing paralysis of the dorsiflexion of foot and toes and an area of hypoesthesia at the back of the foot, resulting in paralysis of the eversion of the foot and hypoesthesia of the outer side of leg and back of the foot.
In the neurological screening of a patient presenting with these symptoms we must pay special attention to signs indicative of injury near the CPN (i.e. at the major sciatic nerve, the lumbosacral plexus or the L5 root). Paralysis of the inversion of foot is not usually conspicuous in injuries of CPN and suggests an injury of the major sciatic nerve or L5. Hypoesthesia of the outer side and the sole of foot exceeds the territory of CPN and is suggestive of injury of the major sciatic nerve and lumbosacral plexus. Finally the deficit of extension, abduction or rotation of the hip indicates that the injury affects the major sciatic nerve or the lower components of lumbosacral plexus.26,27
Electrophysiological screeningThe study of nerve conduction and electromyography data here brings topographic, evolutive, physiopathological, and prognostic information to clinical screening.
Injuries of the CPN located at the peroneal neck correlate with one or more of the following nerve conduction abnormalities: motor conduction block at peroneal neck level, reduced composed muscular action potential in the CPN territory and reduced amplitude of the SPN potential sensibilities. In the concentric-electrode electromyography (EMG) performed at rest we can see abnormalities in the extensor hallucis longus muscle, the long peroneal and the short peroneal. When the injury is located at the popliteal hollow level EMG abnormalities in the short portion of the biceps femoris muscle can be seen. Finally when it selectively compromises the CPN terminal branches the abnormalities are limited to anterior tibia and the extensor hallucis longus muscle when the DPN is damaged and to the long lateral and short peroneal muscles when the SPN is damaged.
According to the evolution and physiopathological characteristics of the injury the EGM shows signs of denervation (fibrillation and acute positive waves) and reduction of enrollment of motor units in the acute stage with axonal damage; and motor unit potentials and augmented amplitude and duration, excess of polyphasic units and reduced enrollment of motor units at the chronic stage with reinnervation. In the screening of conduction we can see block and reduction of the speed of conduction with no denervation when it comes to demyelinating injuries.
The electrophysiological screening provides us with prognostic data. Overall demyelinating injuries are associated with a complete fast recovery. Conversely axonal injuries are associated with a slow recovery and prospective residual deficit.28,29
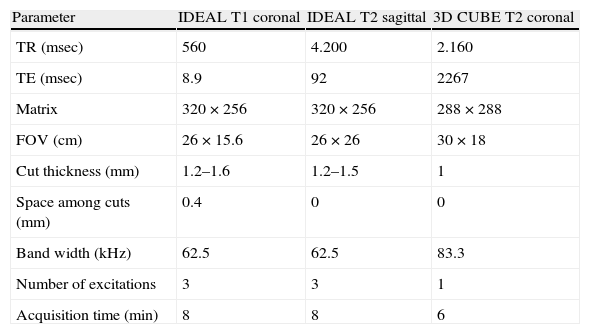
Technical parameters of high definition neurographyIn our institution we have an 8-channel knee or surface coil (Torso PA SIGNA–GE Healthcare, Milwaukee, WI, USA) 3T equipment (Signa HDxt 3T, 8ch, GE Healthcare, Milwaukee, WI, USA). According to the protocol we do a T1 and T2 fast spin-echo (FSE)-weighed thin multiplanar reconstructions (MPR) with the IDEAL modality (iterative decomposition of water and fat with echo asymmetry and least-squares estimation); T2 CUBE and eventually 3D T1-weighed FSPGR images with a gadolinium-based contrast agent, diffusion-weighed image (DWI), or diffusion tensor imaging (DTI) (Table 1).
Acquisition parameters of IDEAl and CUBE sequences for the evaluation of peroneal nerve at knee level.
| Parameter | IDEAL T1 coronal | IDEAL T2 sagittal | 3D CUBE T2 coronal |
| TR (msec) | 560 | 4.200 | 2.160 |
| TE (msec) | 8.9 | 92 | 2267 |
| Matrix | 320×256 | 320×256 | 288×288 |
| FOV (cm) | 26×15.6 | 26×26 | 30×18 |
| Cut thickness (mm) | 1.2–1.6 | 1.2–1.5 | 1 |
| Space among cuts (mm) | 0.4 | 0 | 0 |
| Band width (kHz) | 62.5 | 62.5 | 83.3 |
| Number of excitations | 3 | 3 | 1 |
| Acquisition time (min) | 8 | 8 | 6 |
CUBE: Fast spin-echo (FSE) sequence with flip angle variable; IDEAL: iterative decomposition of water and fat with echo asymmetry and least-squares estimation.
IDEAL modality–General Electric equipments (DIXON for Siemens and Phillips equipments) is a sequence derived from the principles set forth by Dixon12 that allows us to decompose the signals of water protons and liquid according to their different resonance frequencies (or chemical shift) by isolating them to obtain different images. Starting from these two (2) basic images with a pure signal of fat or a pure signal of water we can obtain other two (2) images derived: in phase–with the sum of water+fat signals; and out-phase–where the fat signal is subtracted from the liquid13,14 (Table 1).
CUBE modality–General Electric equipments (SPACE for Siemens equipments and VISTA for Phillips equipments) is a 3D FSE sequence of variable angle with thin cuts and isotropic resolution capable of reducing artifacts through partial volume. The possibility of reformatting the MPR sequence eliminates the need to re-do it while decreasing the time of screening.30,31
When a paramagnetic contrast agent is infused we normally use the volumetric T1-weighed FSPGR image capable of obtaining a good contrast of T1 signal with thin cuts and MPR in a short time. Sequence is done with a contrast-less mask after the IV infusion of 10ml gadolinium-based contrast agent through an infusion pump every 2ml/seg.
In selected cases DWI and DTI sequences are used to look into the restrictive effects in the diffusion of hydrogen molecules across the PN, the fractional segmental anisotropy and through additional post-processing (FiberTrak-GE Healthcare, Milwaukee, WI, USA) in order to perform tractographies both for the qualitative and quantitative evaluation of fiber bundles.32 Neurographic sequences show that more serious the injury is the more up-front the neural affectation can be. The opposite happens with DTI that seem more sensitive for the assessment of neural affectation which can make it a marker not only of diagnostic but also prognostic information. It allows us to mark out nerve fibers with greater accuracy than neurographic sequences which in turn can predict the recovery or not of fibers damaged by ischemia, trauma or inflammation.33 DWI sequences with values ranging from b 0 and 200 aimed at the peripheral nervous system to determine the anatomy and caliber of nerve. In our institution DTI is added especially when neurographic sequences do not show signal abnormalities.
Even though we recommend neurographic sequences in 3T equipments due to clinical suspicion of PN damage most injuries can be found with MR-conventional sequences like those used in knee screening.34 However it is important to know that because the definition of images is low such definition needs to be completed with neurographic sequences to be able to reach diagnosis.
Appearance of peroneal nerve mononeuropathy in the magnetic resonance neurographyTrauma neuropathyTrauma has been traditionally categorized following Seddon’ criteria35 who divided the degrees of neuronal injury in neuropraxia; axonotmesis; and neurotmesis according to the severity of damage.
Anatomopathological changes in neuropraxia–the lowest degree of damage deteriorate the myelin sheath surrounding the axon only while causing functional transient loss with an excellent prognosis with even better restitution ad integrum. MR shows mild widening of the nerve with hyperintensity in T2-weighed FSE sequences. We are usually dealing with damage caused by mild entrapment, mild stretching or compressions due to space-occupying processes (Fig. 3).
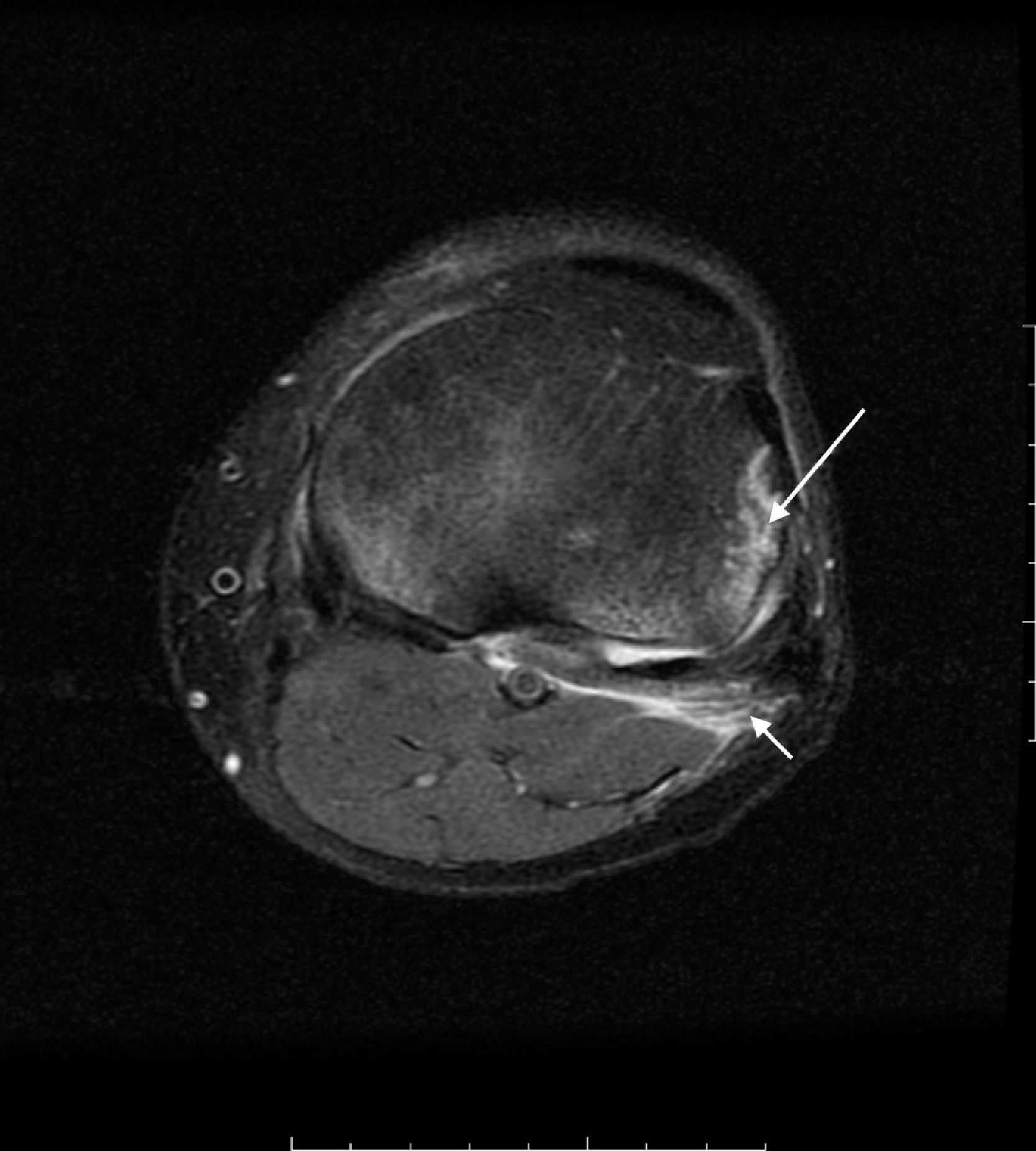
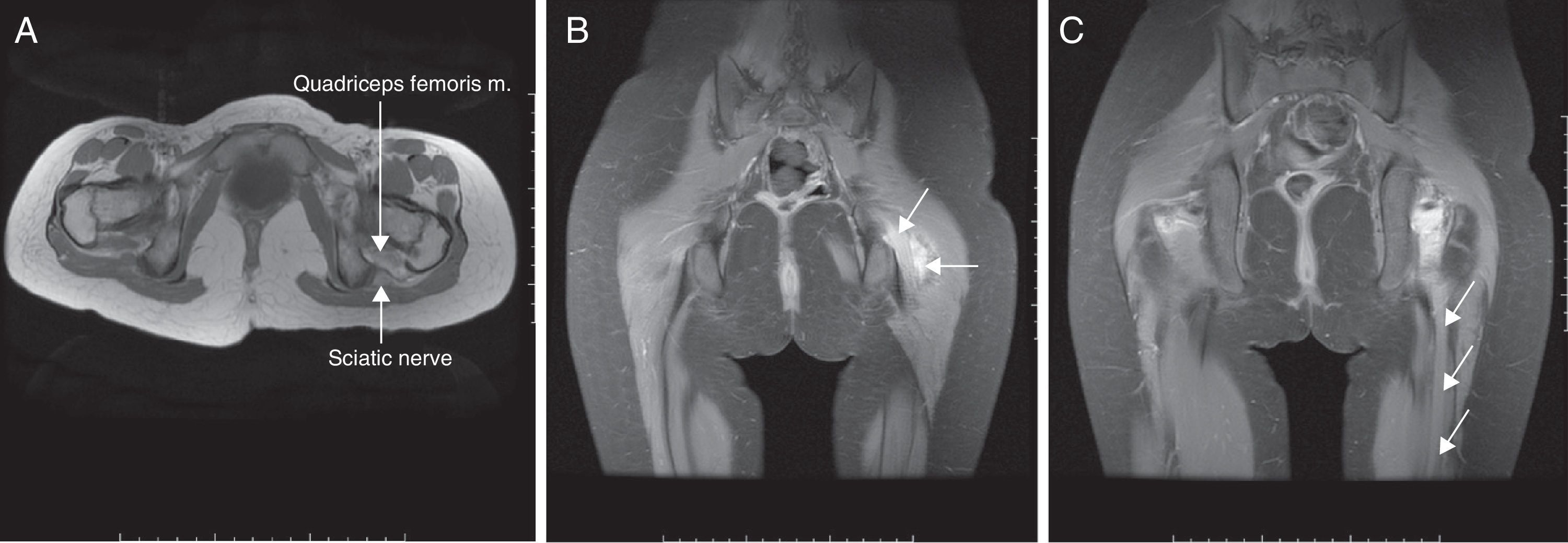
In the axonotmesis the axon is completely ruptured and as a result Wallerian deterioration of the distal segment occurs. Nevertheless support structures like perineurium and endoneurium remain unscathed.36,37 Prognosis is good but recovery time is slow taking into consideration that neuronal regeneration advances at a pace of 1mm/day.32 On the MR other than the findings seen in the neuropraxia fascicular pattern is also lost. These findings can be seen in cases of moderate entrapment or long-term serious entrapment (Fig. 4).
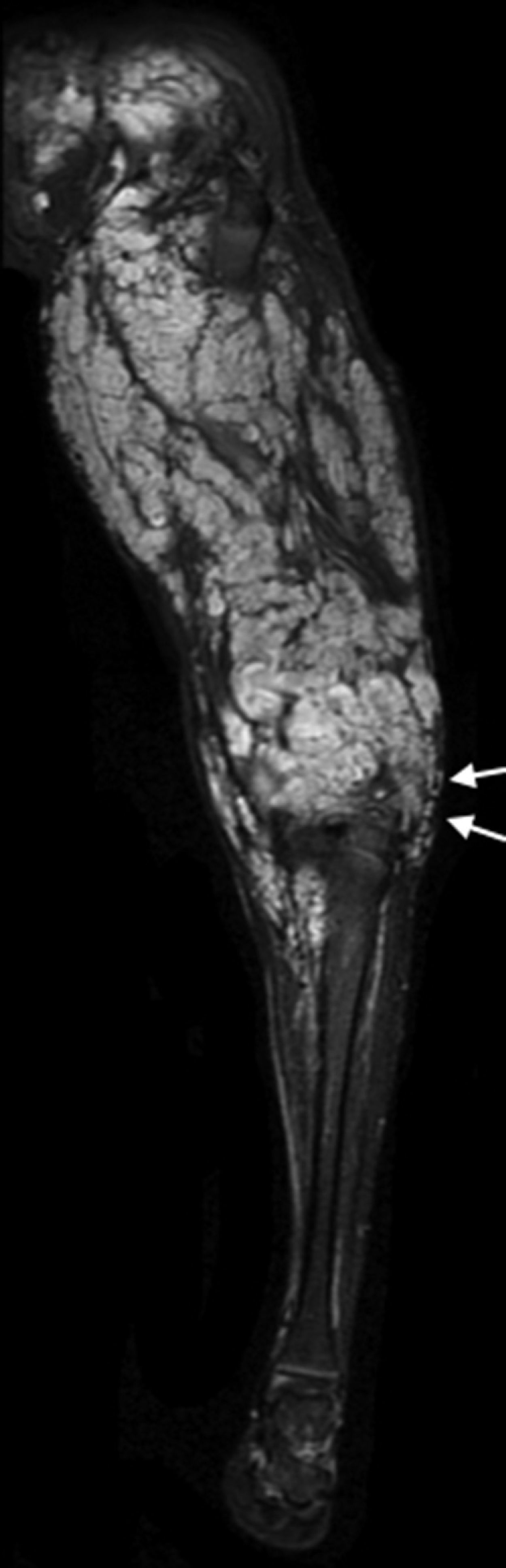
Axonotmesis of sciatic nerve. Patient with a history of lithotomy position during surgery. (A) T1-weighed FSE conventional sequences showing diffuse thickening of sciatic nerve (short arrow) of both the peroneal and tibial portions. Quadriceps femoris muscle edema (long arrow) can be seen too. (B) 3D IDEAL multiplanar reconstruction in the coronal plane showing hyperintense signal and nerve thickening (long arrow) accompanied by inflammatory changes of adjacent soft tissues (short arrow). (C) Same sequence seen in (B) with signs of Wallerian deterioration distal to the injury (arrows).
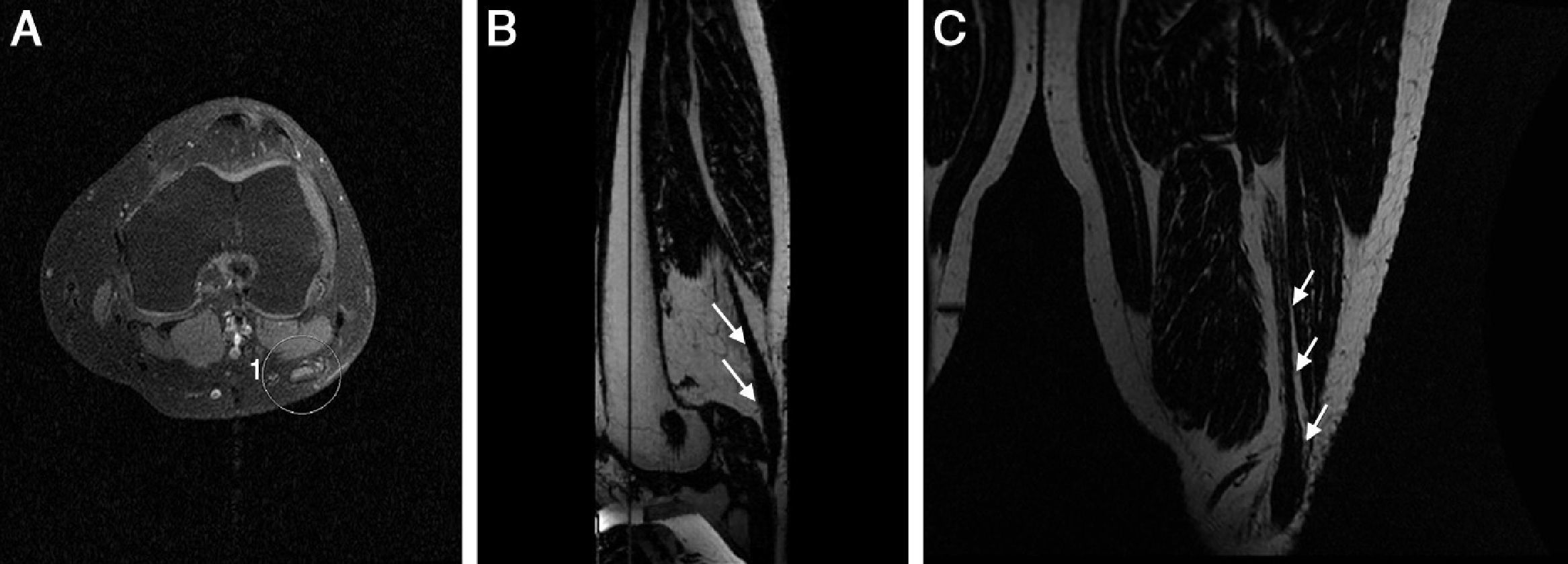
Neurotmesis is the most serious neuronal injury presenting with total functional loss unless rapid surgical repair takes place. In its acute phase discontinuation between both ends of the nerve occurs. This breach is occupied by liquid and granulation tissue evolving to fibrosis and forming the neuroma. On the MRI in the acute phase we can see a space between both ends of the nerve and the chronic phase–such space is occupied by a hypointense fibrous tissue seen on T2-weighed FSE sequences38 (Fig. 5).
Neurotmesis of PN. Knee trauma with ACL tear. (A) T2-weighed FSE conventional in the axial plane. Diameter of PN with hyperintense signal, distortion of fibers and perineurial thickening (circle). (B and C) 3D IDEAL T1-weighed water-saturation sequence in the axial (B) and coronal (C) planes. Nerve node thickening due to neuroma (arrow).
Even though the entrapment of PN can occur anywhere along the PN the most common location is the peroneal tunnel due to its fixed position between the peroneal bone and fascia. These injuries can be due to the pressure exerted by peroneal neck on the nerve during sleep as well as in anorexy and bariatric surgery–due to rapid loss of weight. Also the entrapment can be due to space-occupying extrinsic injuries including the intraneural ganglion, large Baker's cysts, tumors in soft tissues, osteochondromas and other bone tumors, non-consolidated fractures, injuries at the posterolateral angle of the knee, or post-surgical scars.17,39,40
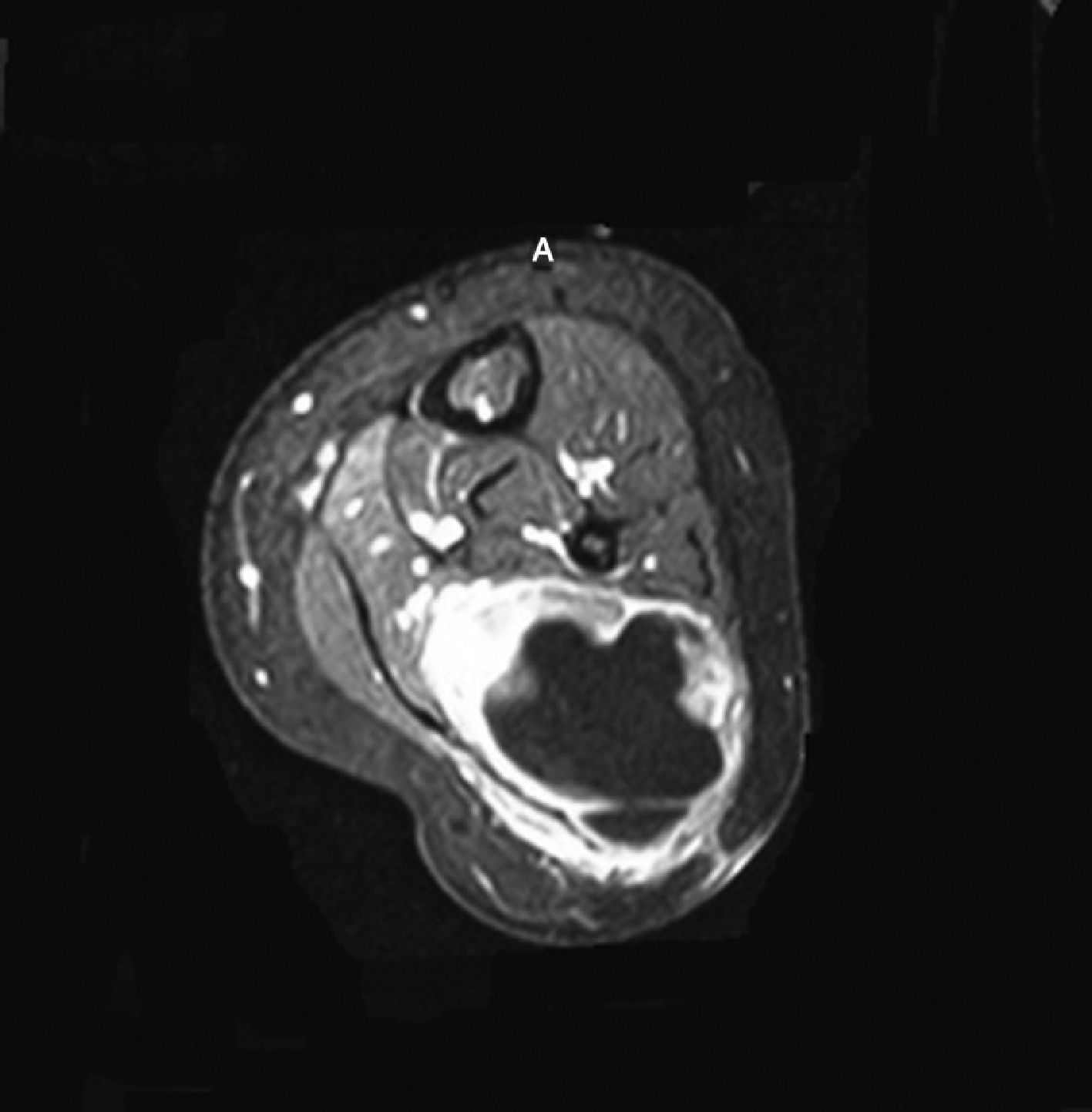
PN is the most common location of intraneural ganglion. Spinner et al. published 24 cases found through MR showing the relationship between cyst and the proximal tibio-peroneal articulation. Among its patients one was screened through 3T MR showing the CPN articular branch. During surgery the relationship between cyst and CPN articular branch was found.41
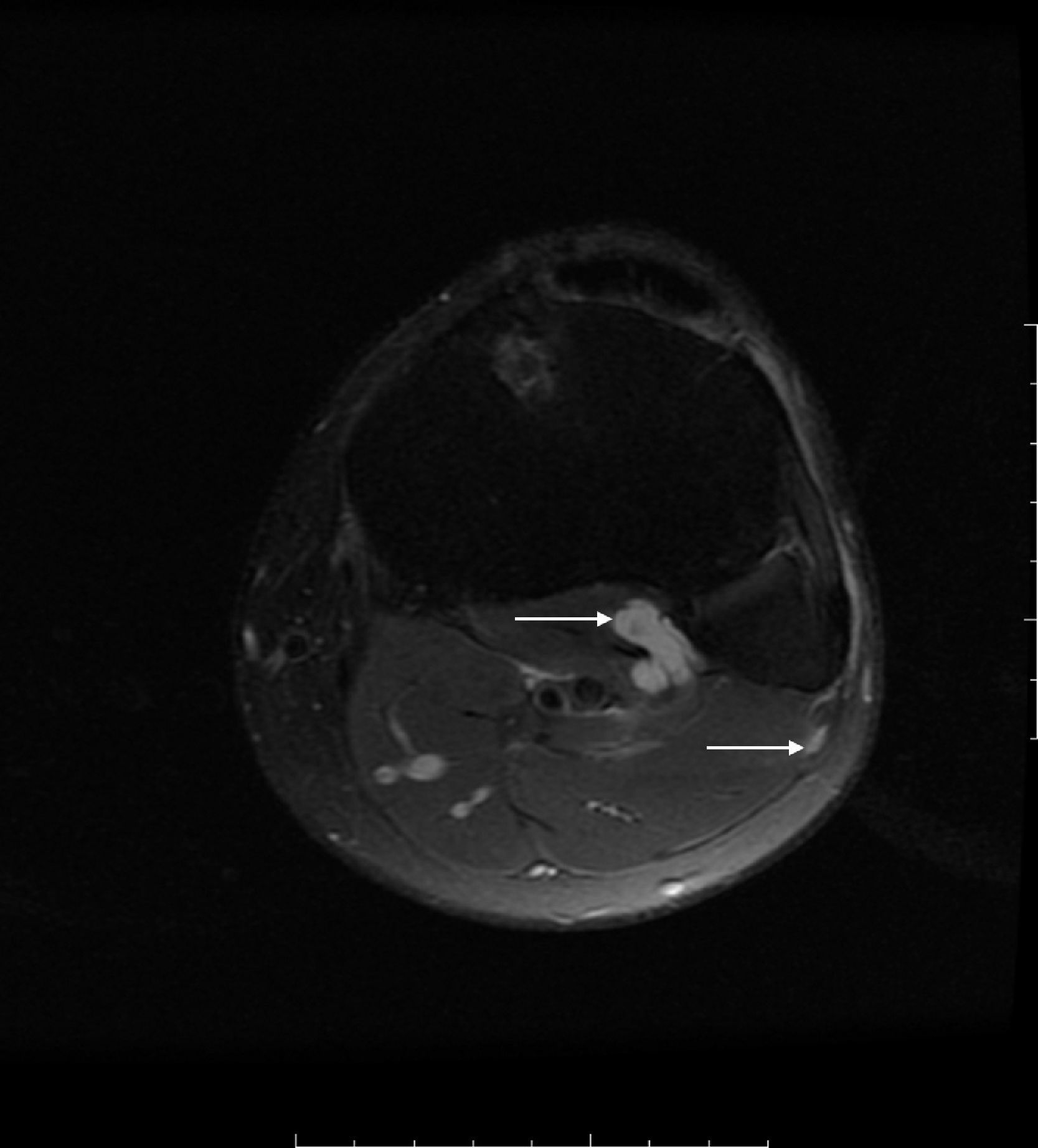
Unlike extraneural cysts that look like mass intraneural cysts are usually tubular and occur all across the nerve. Several signs let us distinguish one from the other: the “tail sign” shows articular connection but it does not distinguish intraneural from extraneural cysts; the “transverse limb” sign (cystic material inside the articular branch of peroneal nerve (PN) running through the anterior surface of fibula) can be seen in cases of intraneural cysts–never extraneural cysts; the “signet ring sign” (intrinsic displacement of fasciculi along the cyst inside the epineurium) has been found in intraneural cysts with a 86% specificity42 (Fig. 6).
Perineural cyst. 42-year old patient presenting with pain in the posterior-external region of his knee and paresthesias in the antero-external region of his calf. Axial T2-weighed image showed cystic injury in the posterior view of the proximal tibio-peroneal articulation (long arrow). Peroneal nerve shows cystic injury surrounding fibers at fibula level (short arrow). Surgery showed the presence of perineural cyst.
MR can be useful to plan the surgical interventions of all those patients showing intraneural ganglion of PN since the success of such intervention depends on shutting down the aforementioned connection between the cyst and the tibio-peroneal articulation.10
Tumor neuropathyPN can be affected by tumors compromising the peroneal branch of sciatic nerve or even the very PN from its division in the popliteal hollow region and in some of its 3 terminal branches. The most common tumor is that of the peripheral neural sheath. It can occur in isolation (schwannoma or neurilemmoma or neurinoma) or it can be associated with neurofibromatoses (NF), including NF1, NF2 and schwannomatosis. Neurofibromatoses are hereditary syndromes predisposing to neural sheath neoplasms and are caused by mutations in the tumor suppressor genes (NF1, NF2, and SMARCB1, respectively). Its clinical criteria were established for NF1, and NF2 in the year 1987 and for schwannomatosis in 200543,44 (Fig. 7). Neurofibromas are categorized as isolated when they affect one fasciculum only and as plexiform when they affect multiple fasciculi. The isolated subtype occurs in 90% of all cases.45
The diagnosis of peripheral neural sheath tumors is based on clinical manifestations, the location of the injury, electrophysiological studies and MRIs. MRI allows us to determine the exact location of tumor, perform tumor volumetry and define the relation among the tumor, vascular structures and adjacent muscles. Both neurofibromas and schwannomas have common features on the MRI. The common appearance of peripheral neural sheath tumors is that of a round, oval or fusiform mass with signal of soft tissues and well-defined margins that rarely exceed 5cm. Most of them are isointense or mildly hyperintense with relation to the muscle in T1-weighed FSE sequences and highly hyperintense–similar to water in T2-weighed FSE sequences. The “target sign” of T2-weighed FSE sequences correlates to a hypointense center-image due to fibrous component and one hyperintense peripheral ring due to myxoid material.46 The atrophy of muscles of affected nerve is another finding of this type of tumors.47
Isolated neurofibromas occur at the center of the nerve and this is why in the surgical intervention it is very difficult to separate the fibers from the tumor tissue. The “target sign”, the central enhancement and a combination of both is a finding favoring neurofibromas.47 On the contrary neurinomas are usually excentrically located in the nerve and encapsulated by the perineurium so they can be easily resected without damaging the nerve. On the MRI the signs suggestive of neurilemmomas have some sort of fascicular appearance, a thin hyperintense ring on the T2-weighed FSE sequences, a combination of both and diffused enhancement after infusing the contrast agent.47 In long lasting injuries cystic areas, calcifications, hemorrhages and hyalinization can be seen which simulates sarcoma.48 Plexiform neurofibroma is almost pathognomonic of NF1. The risk of malignant transformation is 8–12%. Unlike the located neurofibroma the plexiform neurofibroma expands and distorts a long segment of nerve and branches giving him a characteristic radiological macroscopic “bunch grape” appearance. When it affects a whole limb it is called elephantiasis neuromatosa–condition hypertrophying the bone and soft tissues49 (Fig. 8). Malignant variant of neural sheath tumors–malignant schwannoma represents the most common type of sarcoma of soft tissues.48 Malignant schwannomas can be associated with NF–particularly type 1 even though they can appear in patients with history of radiotherapy and in some occasions with no history whatsoever. The complete resection of these tumors is hard and relapse is common. At first they are usually symptomatic, grow rapidly and on the MR show necrosis and enhancement50 (Fig. 9).
Other causes of PN mononeuropathy include perineuroma, neurolymphomatosis, endometriosis, radiation, vascular malformations and proceedings for the management of varicose veins.17
Plexopathies and neuropathies due to radiotherapy evolve progressively years after therapy. These injuries are mainly due to radiotherapy of pelvic and lower limb tumors caused by sarcoma of soft tissues. Literature establishes the maximum tolerated dose in 60Gy. This neuropathy can grow worse due to associated conditions such as diabetes or vascular processes and associated therapies.51
Neural damage due to endometriosis is caused by cyclical inflammation of menstrual cycle. Once implanted in the nerve the ectopic uterine tissue advances progressively through the epineurium and perineurium. Diagnosis is based on associating the symptoms related to the menstrual cycle and the findings on the MRI. We find cystic or solid hypointense masses on the T2-weighed FSE sequences with variable signal according to the time of evolution of bleeding.52
Lymphoma affects occasionally the peripheral nervous system. Most complications are due to non-Hodgkin's lymphoma infiltrating the nerve and causing axonal damage. They can present as mononeuropathies or polyneuropathies. Diagnosis is very hard to achieve if we know nothing about the existence of lymphoma.53
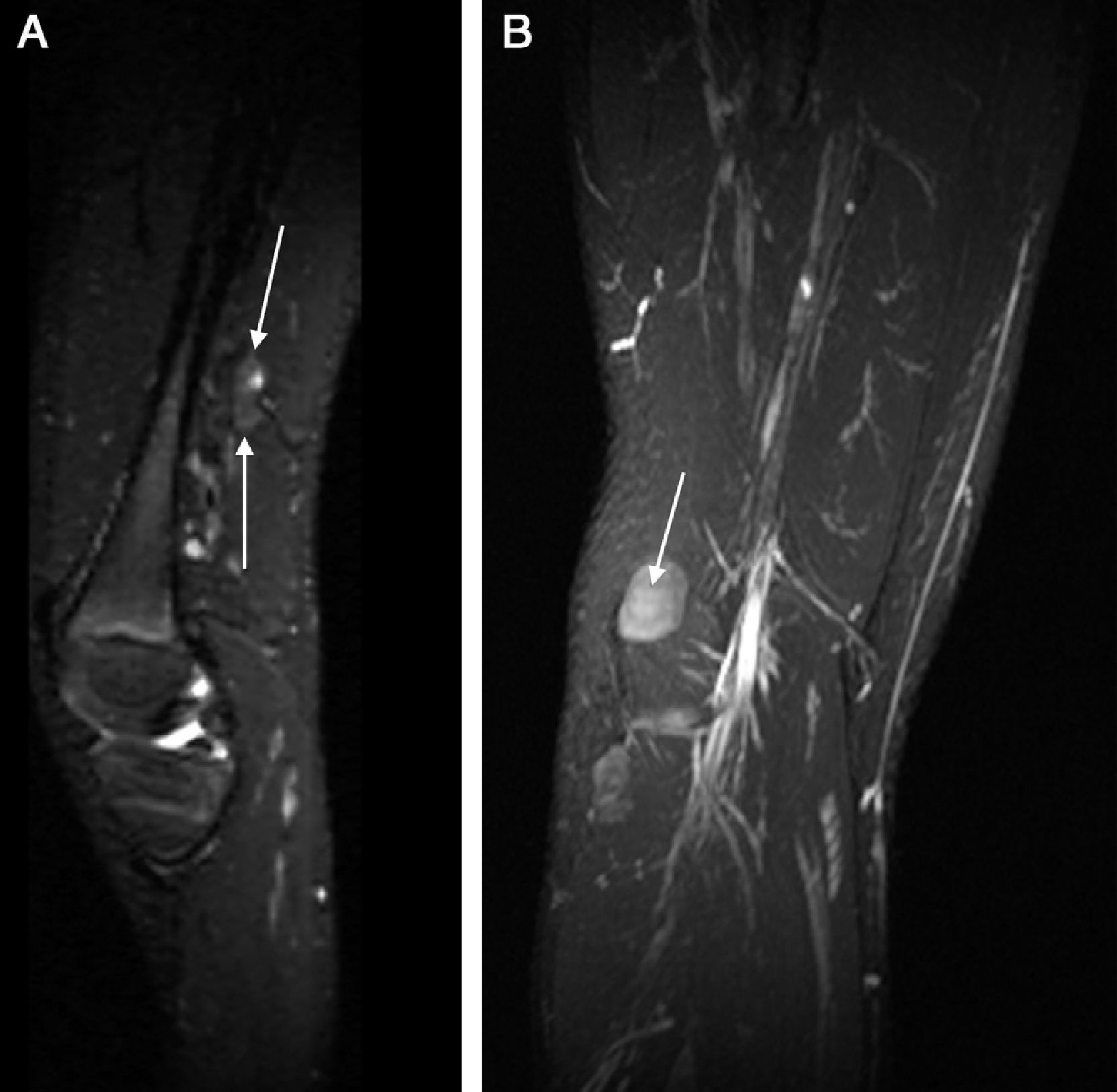
Sclerotherapy, if done adequately, it is an efficient modality to manage varicose veins located at the lower limbs, with low rate of complications. Drug applied is potentially neurotoxical but if the dose and concentrations used are right it does not lead to major complications. Irritation of superficial nerves usually occurring during therapy is well known. Persistent serious neuropathy is a rare complication depending on the concentration and quantity of drug applied and on the distance between the nerve and the site of infusion. Intravaricose, intraarterial, and around-the-vessel injections can cause neuronal damage. In these cases we need to take into consideration medico-legal issues of this complication. A similar situation can occur when using laser as the therapy to manage varicose veins54,55 (Fig. 10).
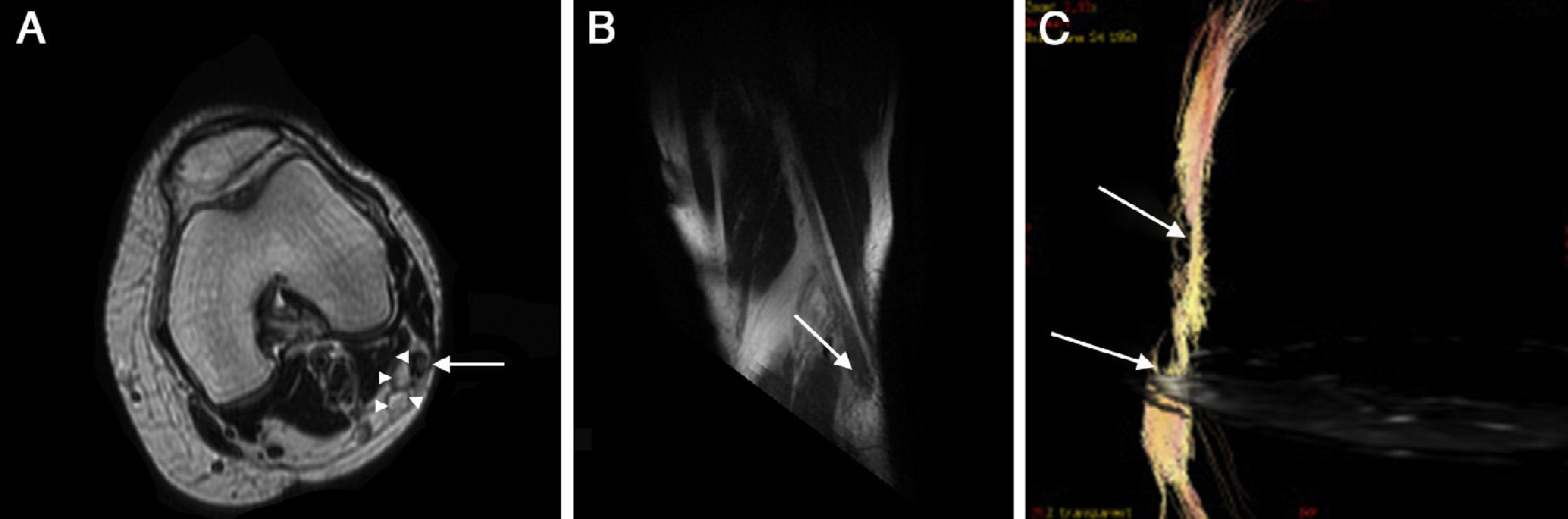
PN neuropathy due to sclerotherapy of varicose veins. (A) Strict axial plane showing a larger nerve diameter with hyperintense signal of fibers and thickening of perineurium (long arrow). Next to the nerve 2 varicose-like tubular images can be seen (arrow heads). (B) Reconstruction on the oblique sagittal plane showing diffuse thickening of PN with distal node (neuroma) (arrow). (C) Diffusion tensor image with tractography showing slimming of nerve and fiber distortion.
High resolution 3T MR neurography allows us to clearly identify the trajectory and relations of the PN. Diffusion images and diffusion tensor images are complementary to conventional images in an effort to improve the visualization of the processing affecting this nerve. Also conventional images provide us with data on denervated muscles. It is an excellent adjuvant bloodless diagnostic modality to electrophysiological screenings to determine injuries at nerve level, find the exact site of the injury and get to know the cause of peroneal neuropathy.
Ethical responsibilitiesProtection of humans and animalsThe authors confirm that no experiments have been done with humans or animals during this research.
Confidentiality of dataThe authors confirm to have followed the protocols of their institutions on the publication of data of patients and also confirm that all patients included in this study have been adequately informed and have given their written informed consent to participate in this study.
Right to privacy and informed consentThe authors have obtained the informed consent of patients and/or subjects of this study. This report is under the custody of the author for correspondence.
Authors- •
Authors of the study: DP, FB, HCH, CC
- •
Study idea: CC
- •
Study design: DP, FB, HCH, CC
- •
Data mining: DP, FB, HCH, CC
- •
Data analysis and presentation: DP, FB, HCH, CC
- •
Statistical analysis: Not applicable in this study.
- •
Reference search: DP, FB, HCH, CC
- •
Writing: DP, FB, HCH, CC
- •
Manuscript critical review with intellectually relevant contributions: DP, FB, HCH, CC
- •
Final version approval: PD, FB, HCH, CC
The authors reported no conflicts of interests.
Special thanks to radiologists Miriam López, Adriana López and Alberto Irala for optimizing MRIs and to physical therapist Dr. Jorge Calvar, MD for collaborating the reconstruction of the DTI sequences.
Please cite this article as: Pineda D, Barroso F, Cháves H, Cejas C. Neurografía de alta resolución del nervio peroneo en resonancia magnética 3T. Radiología. 2014;56:107–117.