The study of the structures that make up the brachial plexus has benefited particularly from the high resolution images provided by 3T magnetic resonance scanners. The brachial plexus can have mononeuropathies or polyneuropathies. The mononeuropathies include traumatic injuries and trapping, such as occurs in thoracic outlet syndrome due to cervical ribs, prominent transverse apophyses, or tumors. The polyneuropathies include inflammatory processes, in particular chronic inflammatory demyelinating polyneuropathy, Parsonage–Turner syndrome, granulomatous diseases, and radiation neuropathy. Vascular processes affecting the brachial plexus include diabetic polyneuropathy and the vasculitides.
This article reviews the anatomy of the brachial plexus and describes the technique for magnetic resonance neurography and the most common pathologic conditions that can affect the brachial plexus.
El estudio de las estructuras que conforman el plexo braquial se ha visto particularmente beneficiado con las imágenes de alta resolución que brindan los equipos de resonancia 3T. El plexo braquial puede presentar mononeuropatías o polineuropatías. Entre las primeras se distinguen los traumatismos, el atrapamiento, como el síndrome de la abertura torácica por costillas cervicales, apófisis transversas prominentes o tumores. En el grupo de las polineuropatías se encuentran los procesos inflamatorios, entre los que destacan la polineuropatía desmielinizante inflamatoria crónica, la plexitis autoinmunitaria (síndrome de Parsonage Turner), enfermedades granulomatosas y la neuropatía por radiación. Entre los procesos vasculares se mencionan la polineuropatía diabética y las vasculitis.
En esta revisión se repasa la anatomía del plexo braquial y se describe la técnica de estudio de la neurografía por resonancia magnética y las principales patologías que pueden afectar al plexo braquial.
The study of the Peripheral Nervous System (PNS) through magnetic resonance imaging (MRI) dates back to the 1990's.1,2 MR neurography (MRN) has coined the term thanks to high-resolution techniques, in particular those provided by 3T equipment.3,4 These sequences have revolutionized the study of plexuses and peripheral nerves because they provide us with a significant improvement in the signal/noise relation in shorter acquisition times, and they convey high resolution and contrast images.5–7 Diagnosis of PNS disorders was traditionally based on three pillars: medical history, physical examination and electrophysiological studies.8 The complex anatomy of the brachial plexus makes clinical and electrophysiological assessment difficult; therefore, MRN can be placed as the fourth pillar among diagnostic studies. Firstly, MRN images distinguish a normal plexus from a pathological one; in addition, they determine accurately the location of the lesion (root, trunk, division or cord), and whether it affects one or several of them (mononeuropathy vs polyneuropathy). On the other hand, MRN images outline the extension of the lesion and it often specifies the etiology of the plexus disorder.8–11 Electrophysiological studies, though very useful to determine the location and degree of nerve compromise, do not define the exact location or etiology of brachial plexus lesions.12
The goals of this review are to study brachial plexus anatomy, describe the study protocol for MRN and the main diseases that can affect it.
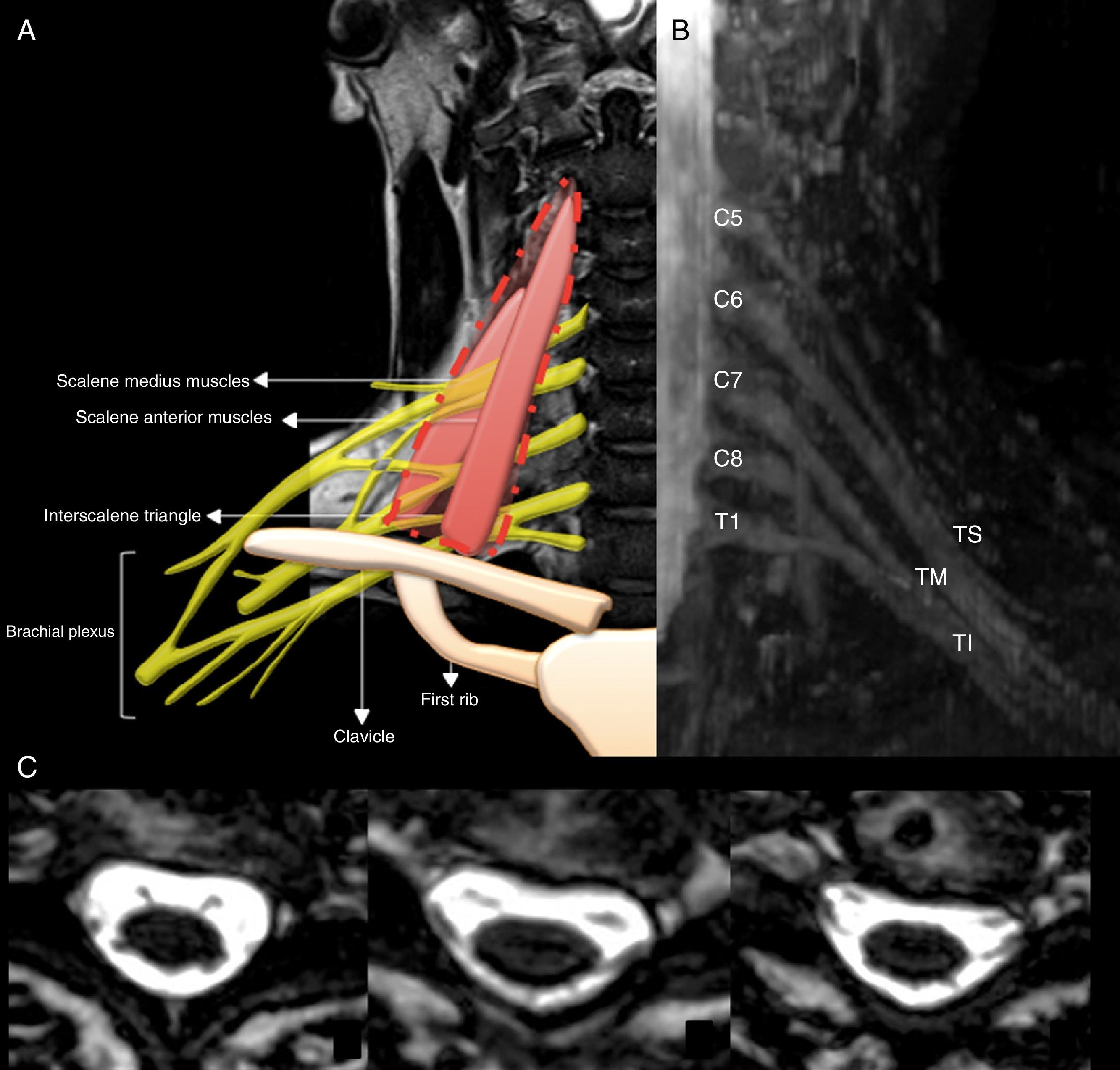
Anatomic analysis of the brachial plexus through MRIsMRI anatomic analysis of the brachial plexus benefited significantly from neurographic sequences. The anatomical points used as reference to assess MRN images are the clavicle, the first rib, the subclavian artery and vein and the scalene anterior and medius muscles. The brachial plexus is formed from the primary ventral branches of the spinal nerves that originate in the cervical (C) 5, 6, 7, 8 and thoracic (T) segments 1. In some individuals smaller ventral braches C4 and T2 participate.13
Topographically, the brachial plexus is divided in five segments: roots, trunks, divisions, cords and terminal branches (Fig. 1).
(A) Diagram of the topographic division of the brachial plexus. (B) IDEAL Sequence water-weighted and with fat suppression; maximum intensity reconstruction of projection on coronal plane where we can see all the elements that make up the brachial plexus (UT: Upper trunk; MT: Medial trunk; LT: lower trunk). (C) 3D GRE sequence in axial slice where we can see the exit of ventral and dorsal roots until the entry of the intervertebral foramen.
The ventral roots exit through the intervertebral foramen of the cervical spine and they are divided into pre- and post-ganglionic with respect to the ganglion annexed to the dorsal root.
Roots C5-C6 make up the upper trunk, root C7 continues as the medial trunk and roots C8-T1 make up the lower trunk. The three trunks go through the scalene anterior and medius muscles (interscalene triangle).13,14
The subclavian and suprascapular nerves come from the upper trunk. The phrenic nerve originates from roots C3-C5, the dorsal scapular nerve stems from root C5 and the long thoracic nerve from roots C5 to C7.
The trunks split to form three anterior and three posterior divisions, which in turn join to form three distal cords, located on the lateral edge of the first rib. When it comes to their relation with the axillary artery, cords are classified as lateral, posterior and medial. The lateral cord is formed by anterior divisions of the upper and medial trunks, gives rise to the lateral pectoral nerve (C5-7) and contributes to the formation of the musculocutaneous and median nerves. The posterior cord is made up of the posterior divisions of the three trunks giving rise to the subscapular nerve. The lower trunk continues as the medial cord and originates the medial pectoral nerve (C8-T1), the medial cutaneous brachial nerve (T1) and the medial cutaneous nerve of the forearm (C8-T1). The cords end in five branches: the axillary, median, ulnar, musculocutaneous and radial nerves.14
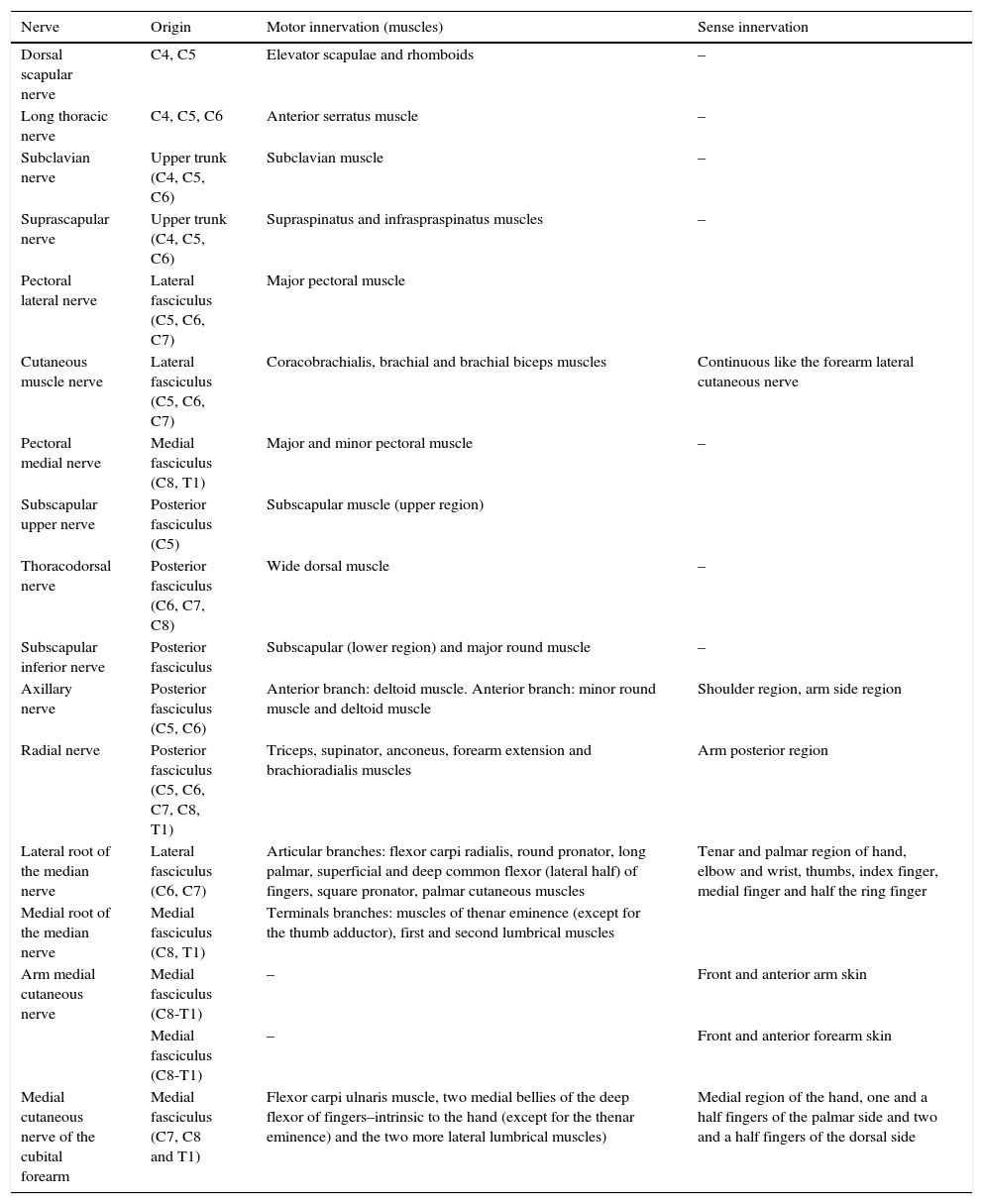
The brachial plexus innervates the muscles of the shoulder girdle, and its terminal branches, the muscles of the upper limbs. Table 1 describes the motor and sense innervations of the brachial plexus.14,15
Motor and sense innervation of the brachial plexus.
| Nerve | Origin | Motor innervation (muscles) | Sense innervation |
|---|---|---|---|
| Dorsal scapular nerve | C4, C5 | Elevator scapulae and rhomboids | – |
| Long thoracic nerve | C4, C5, C6 | Anterior serratus muscle | – |
| Subclavian nerve | Upper trunk (C4, C5, C6) | Subclavian muscle | – |
| Suprascapular nerve | Upper trunk (C4, C5, C6) | Supraspinatus and infraspraspinatus muscles | – |
| Pectoral lateral nerve | Lateral fasciculus (C5, C6, C7) | Major pectoral muscle | |
| Cutaneous muscle nerve | Lateral fasciculus (C5, C6, C7) | Coracobrachialis, brachial and brachial biceps muscles | Continuous like the forearm lateral cutaneous nerve |
| Pectoral medial nerve | Medial fasciculus (C8, T1) | Major and minor pectoral muscle | – |
| Subscapular upper nerve | Posterior fasciculus (C5) | Subscapular muscle (upper region) | |
| Thoracodorsal nerve | Posterior fasciculus (C6, C7, C8) | Wide dorsal muscle | – |
| Subscapular inferior nerve | Posterior fasciculus | Subscapular (lower region) and major round muscle | – |
| Axillary nerve | Posterior fasciculus (C5, C6) | Anterior branch: deltoid muscle. Anterior branch: minor round muscle and deltoid muscle | Shoulder region, arm side region |
| Radial nerve | Posterior fasciculus (C5, C6, C7, C8, T1) | Triceps, supinator, anconeus, forearm extension and brachioradialis muscles | Arm posterior region |
| Lateral root of the median nerve | Lateral fasciculus (C6, C7) | Articular branches: flexor carpi radialis, round pronator, long palmar, superficial and deep common flexor (lateral half) of fingers, square pronator, palmar cutaneous muscles | Tenar and palmar region of hand, elbow and wrist, thumbs, index finger, medial finger and half the ring finger |
| Medial root of the median nerve | Medial fasciculus (C8, T1) | Terminals branches: muscles of thenar eminence (except for the thumb adductor), first and second lumbrical muscles | |
| Arm medial cutaneous nerve | Medial fasciculus (C8-T1) | – | Front and anterior arm skin |
| Medial fasciculus (C8-T1) | – | Front and anterior forearm skin | |
| Medial cutaneous nerve of the cubital forearm | Medial fasciculus (C7, C8 and T1) | Flexor carpi ulnaris muscle, two medial bellies of the deep flexor of fingers–intrinsic to the hand (except for the thenar eminence) and the two more lateral lumbrical muscles) | Medial region of the hand, one and a half fingers of the palmar side and two and a half fingers of the dorsal side |
In MRIs the normal brachial plexus has some sort of a fascicular appearance and a continuous course. In T1-weighted sequences, the signal is isointense to that of the muscle and hyperintense with respect to it in the T2-weighed sequences. In T1 sequences, a thin fat plane is observed surrounding every plexus structure. The ganglion annexed to the dorsal root is observed as a pseudo-nodular image thicker than the root and it shows enhancement after the injection of IV contrast. The three brachial plexus trunks usually have a similar diameter, they are symmetric between both sides and do not present enhancement after the injection of IV contrast.16,17
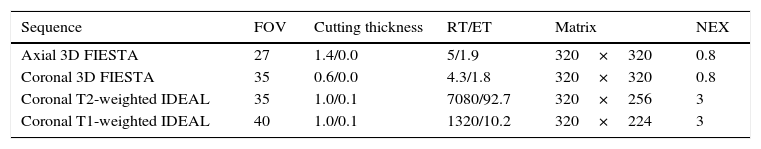
Protocol for the study of the brachial plexus through MRIsThe study protocol of the brachial plexus in our center is done using a Signa HDxt 3.0T machine (GE, Milwaukee, Wi), with an eight-channel neurovascular coil (HDNV Array) simultaneously including both sides of the brachial plexus. The sequences used are T1- and T2-weighted coronal IDEAL (Iterative Decomposition of water and fat with Echo Asymmetry and Least-squares estimation), with a thickness of 1–0.1mm, coronal 3D FIESTA with a thickness of 0.6mm, axial 3D FIESTA with a thickness of 1.4mm, and T1-weighted coronal sequences 3D IDEAL after the injection of IV contrast (Table 2). The IDEAL sequence developed by General Electric (GE) Healthcare, or 3D SPACE (Sampling Perfection with Application Optimized Contrast), developed by Siemens Healthcare, takes three different echo times that separate the image of the water from that of the fat. Finally, T1- and T2-weighted images are obtained simultaneously with four saturation pulse combinations: fat and water decomposition, and in-phase and out-of-phase images. This sequence has the advantage of generating an even fat saturation yet despite the geometric variations presented by the area of study. The drawback is in the temporary cost of the acquisition.18
Protocol for the study of the brachial plexus neuropathy.
| Sequence | FOV | Cutting thickness | RT/ET | Matrix | NEX |
|---|---|---|---|---|---|
| Axial 3D FIESTA | 27 | 1.4/0.0 | 5/1.9 | 320×320 | 0.8 |
| Coronal 3D FIESTA | 35 | 0.6/0.0 | 4.3/1.8 | 320×320 | 0.8 |
| Coronal T2-weighted IDEAL | 35 | 1.0/0.1 | 7080/92.7 | 320×256 | 3 |
| Coronal T1-weighted IDEAL | 40 | 1.0/0.1 | 1320/10.2 | 320×224 | 3 |
SSFP (Steady State Free Precession) sequence, known by its different acronyms such as FIESTA (GE) or CISS (Siemens), is an echo gradient sequence that provides a high signal/noise relation. Though it has very few flow artifacts, it is sensitive to respiratory and deglutition movements. The clinical usefulness of this sequence derives from its capacity to generate an adequate tissue signal with a high T2/T1 relation.19 It provides a detailed analysis of the intradural tract of the roots since it creates a contrast with the signals of the cerebrospinal fluid (CSF). It is especially useful in cases of trauma lesions, where it is possible to visualize the alterations of the roots at the preganglionic level, even in the absence of pseudomeningocele. Notably this sequence generates poor soft-tissue contrast so it lacks usefulness in assessing the signal from the rest of the surrounding structures such as vertebral bodies and muscles.19
Through the use of multiplane reconstructions (MPR) and maximum intensity projection (MIP) images these sequences show the nerve tract and its anatomic relations. T1-weighted sequences along with fat signal suppression are useful in the assessment of nerve anatomy, they outline the perineural fat tissues and stud adjacent structures. In turn T2-weighted sequences are more suitable for the study of nerve course and to discard caliber and signal intensity abnormalities.
Also the combination of both sequence pulses allows us to observe denervation changes of the affected muscles. T2-weighted sequences allow us to find the presence of muscular edema/inflammation related to acute denervations. T1-weighted sequences show adipose infiltration–finding associated with chronic denervation.20,21
IV contrast sequences are reserved for the study of neoplastic or inflammatory diseases and during the late postoperative period to differentiate the presence of fibrous tissue.22
In cases of plexus brachial entrapment, it is necessary to evaluate vascular structures by angiographic sequences and 3D SPGR (Spoiled Gradient Recalled) T1 through an eight-channel coil CTL Array (Torso PA Signa–GE, Milwaukee, Wis), with IV contrast.23
Functional sequences are still in the pipeline.
Diffusion weighted images (DWI) assess both qualitative and quantitatively the movement of water in the tissue. High b values (1000 to 1400) are suggested for the study of diffusivity of peripheral nerve lesions, with quantification of the diffusion coefficient (ADC map).24 This sequence in particular obtains specific images of nerves with excellent fat and vascular signal suppression. Diffusion tensor images (DTI) derive from DWI and its 3D representation is tractography. These techniques assess the structure and disposition of neural tracts in normal situations, as well as their displacement, disruption, infiltration or disorganization of fibers due to tumors within or along the brachial plexus.25 Vargas et al. studied tractography images in patients with benign and malignant neural tumors. In benign tumors, they showed that the fibers surrounded or went through the tumor without any disruptions whatsoever. In malignant tumors, they found disruption/destruction and disorganization of nerve fibers.26 In our institutio, we have not had the results we were expecting with using these sequences and this is why we do not include them in the standard protocol. However we continue working in its optimization.
Diseases affecting the brachial plexusMononeuropathyNeuropathy due to traumaBrachial plexus traumatic pathology presents different etiologies based on the age group.
In pediatric populations, the most frequent cause is excessive traction during childbirth, that can cause proximal and/or distal paralysis of the brachial plexus.27,28
Erb-Duchenne palsy or proximal paralysis of the brachial plexus results from lesion of nerve roots C5-C6, sometimes C7 too, or of the entire upper trunk. In 25% of the cases, the phrenic nerve is also compromised.29,30
Klumpke's palsy or distal paralysis occurs due to the avulsion of roots C8 and T1; on some occasions it can affect root C7. It is less common than the proximal variant.31
In adults, trauma is the most common cause of brachial plexus injury usually due to traffic accidents, on motorcycle accidents in particular. Based on clinical and electrophysiological findings, it is difficult to determine the pre- or post-ganglionic origin of the lesions. The value of these distinctions is in the fact that postganglionic lesions can be repaired with nerve graft or transposition and prognosis is more favorable.32,33 Neurographic sequences have proven to be effective in this distinction.
In preganglionic lesions, the MRN shows the avulsion of the root or roots with end-retractions associated with the presence of pseudomeningocele (Fig. 2). These findings are usually accompanied by changes in T2-weighted signals of the spinal cord expressed by a hyperintense signal area due to edema in acute period and myelomalacia or syringomyelia in chronic periods. The spinal cord can also show changes of morphology due to displacement or traction. Associated with the preganglionic lesion, it is possible to find a lesion of the posterior roots which in the images it can be translated as the presence of edema in paravertebral muscles especially the multifidus muscle. In acute cases, the muscle can show enhancement after the injection of IV contrast.21,30–33
Trauma: 17-year-old male patient who had a motorcycle accident. He shows traumatic preganglionic lesion of the brachial plexus. (A) Water T2-weighted IDEAL sequence on the coronal plane. (B) 3D GRE Sequence on the axial plane. Pseudomeningoceles can be observed (arrows) at the level of the right intervertebral foramens C7-T1 and T1-T2 in relation to the avulsion of roots C8 and T1, respectively. Observe the changes of morphology and the displacement of the spinal cord in B. The patient did not undergo surgery since the MRN determined preganglionic lesion (data that electrophysiological studies cannot provide).
In postganglionic lesion, the MRN shows a wide spectrum of abnormalities ranging from root thickening to the complete separation of the fibers and end-retraction21,30,32,33 (Fig. 3).
Trauma: 21-year-old male patient who had a motorcycle accident. He shows traumatic postganglionic lesion of the brachial plexus. (A and B) Water-weighted IDEAL sequence. It is possible to observe avulsion of the roots and retraction of the ends (arrows). Notice hyperintensity and increase in root thickness with lesion due to stretching. The patient underwent surgery with nerve transposition and kinesic treatment. His evolution was not the one expected.
Thoracic outlet syndrome is neuropathy due to compression in which the brachial plexus can show compressions at three specific anatomic levels: the interscalene triangle, medially located, whose base is made up by the subclavian artery, where the brachial plexus trunks are located posterior and superior to the artery; the costoclavicular space, of intermedial location, and the minor retro-pectoral space, lateral to the others. In the two latter spaces the subclavian vein is the most anterior structure, the homonymous artery is located posterior to the vein. The three brachial plexus cords are located above and posterior to the subclavian vessels. At these three levels, the brachial plexus can get trapped along with the subclavian artery and/or vein. The interscalene and costoclavicular triangles are the spaces that are usually compromised.34
Brachial plexus entrapment can be due to the presence of prominent transverse process of C7 vertebra, or that of cervical ribs or accessory scalene muscles. Other less common causes are post-traumatic fibrosis of scalene muscles, compression due to “backpack” activities or one massive bone callus due to the fracture of the clavicle. It can also be due to muscular hypertrophy associated with activities such as swimming, weight lifting, sports where the arm is the dominating limb such as tennis or even surgical positions.35 Other causes of entrapment are tumors from surrounding structures such as lipomas.34,35
Based on the entrapment location the MRN of the brachial plexus can show alteration in the roots, trunks or cords. The affected nerves can show displacement, thickening and increase of the T2-weighted signal intensity. Similarly the neurography allows us to determine the presence of anatomic variants or other lesions resulting from entrapment (Fig. 4), and provide additional data such as post-denervation changes in the adjacent muscles. In these patients, as it was explained in the section about the study techniques, the protocol must be completed with 3D SPGR sequences after the injection of IV contrast and with dynamic manoeuvers: one phase with the arms by both sides of the body and another phase with the arms raised. The MRN plays an important role in the entrapment syndromes of the brachial plexus because other than determining the cause and the exact location of the lesion it also allows the surgeon to improve the strategy of treatment.36–38
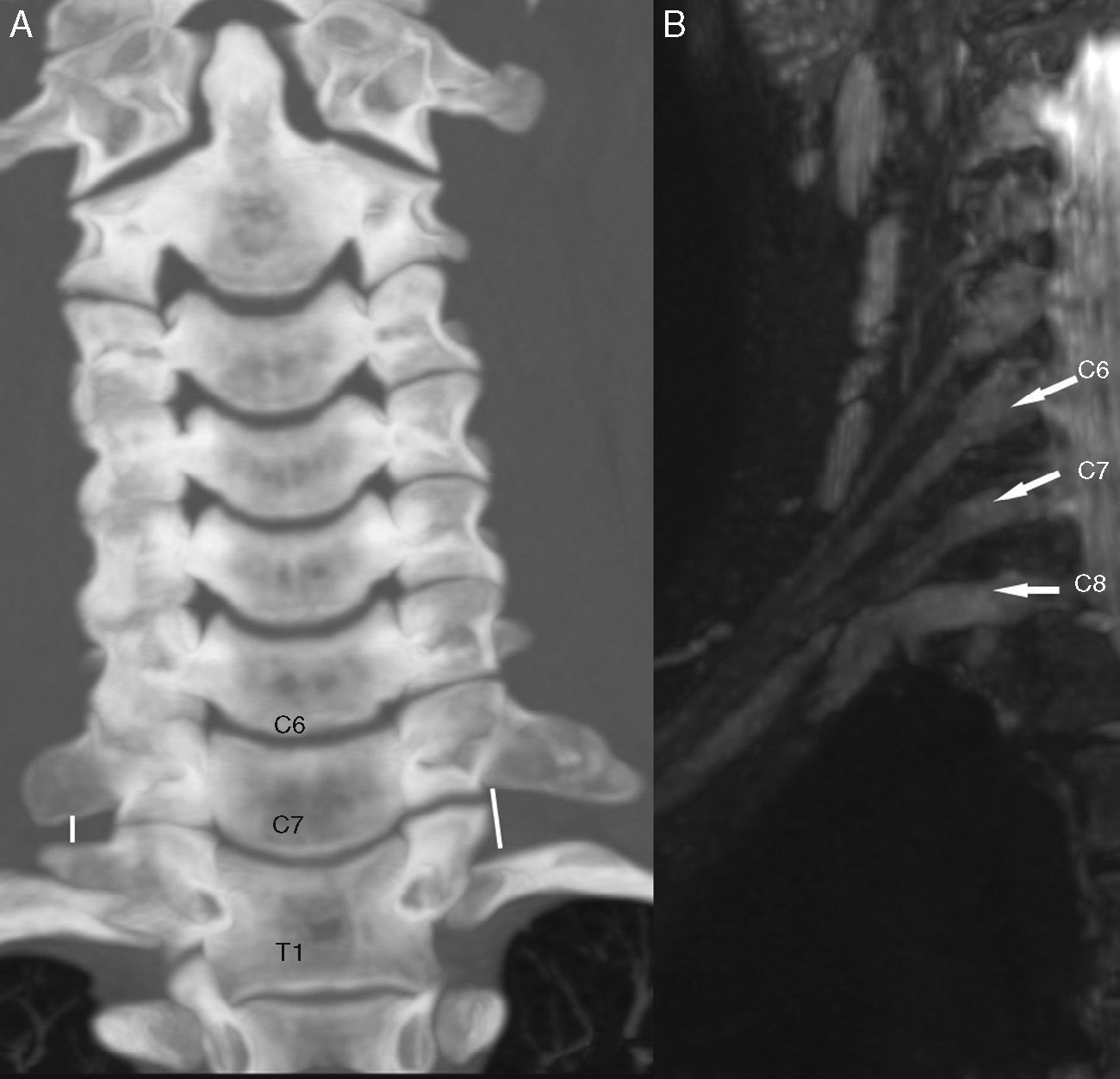
Lesion by entrapment. Thoracic outlet syndrome in a 9-year-old girl with paresthesia of the right upper limb. (A) Computed Tomography (CT) in coronal reconstruction showing prominent transverse bilateral apophysis and right rudimentary cervical rib with inferolateral inclination causing decrease of space for the lower trunk of the brachial plexus. (B) T2-weighted IDEAL sequence showing thickening and course deviation of the right root C8. The patient underwent surgery with resection of cervical rib, with excellent clinical evolution.
The most common primary tumors of the brachial plexus are benign tumors of the nerve sheath such as schwannomas, neurofibromas and their malignant variants. Neurofibromas are usually found in the context of neurofibromatosis.39
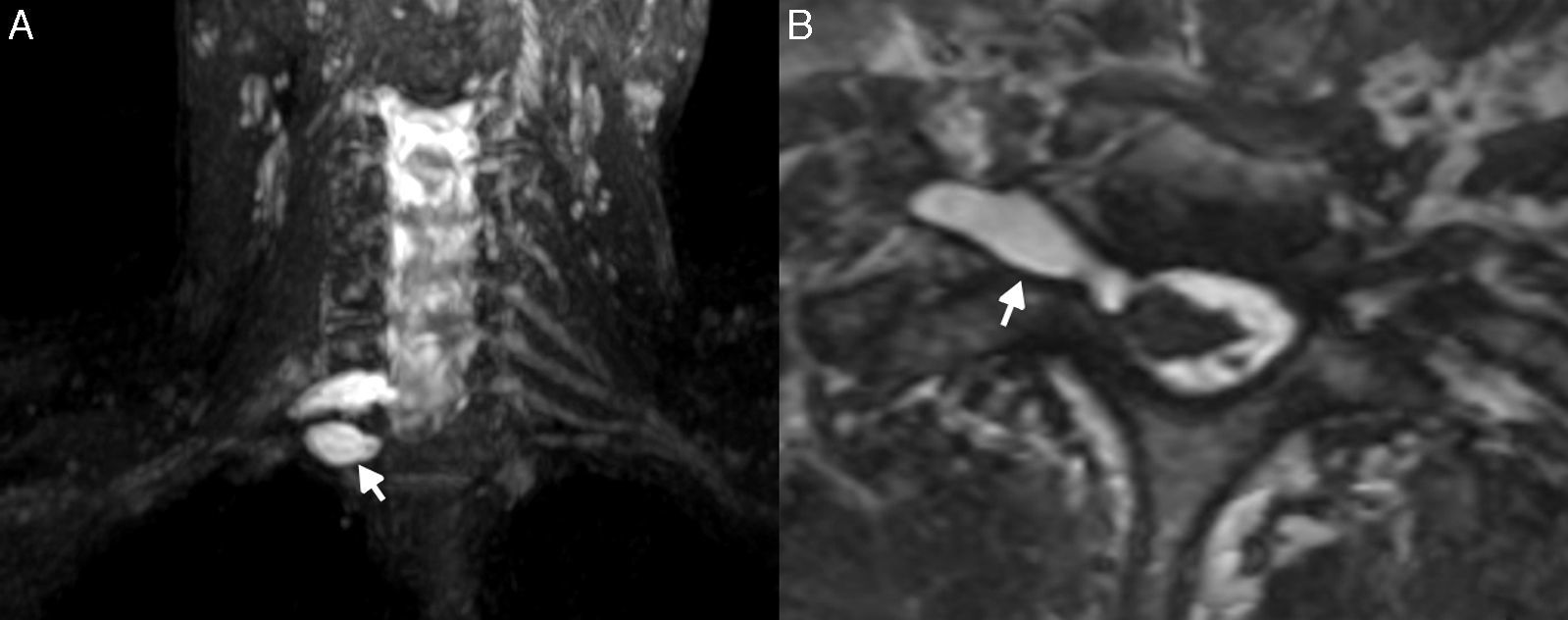
In the MRN, schwannomas and neurofibromas show characteristics in common. They are homogeneous lesions, isointense to the nerve and the muscle in T1-weighted sequences and hyperintense in T2-weighted sequences, with homogeneous enhancement after the injection of IV contrast. Several signs have been described that allow us to suspect the presence of neurogenic tumors. Presentation in “onion layers” describes a fascicular pattern of fusiform morphology, the “target sign” in T2-weighted sequences shows one hypointense center due to the presence of fibrosis and a peripheral ring associated with the presence of myxoid material. The maximum tumor diameter is not usually greater than 5cm39–41 (Fig. 5). However they can be heterogeneous due to the presence of necrosis, cystic degeneration or calcifications which makes differential diagnosis difficult with the malignant variant.42
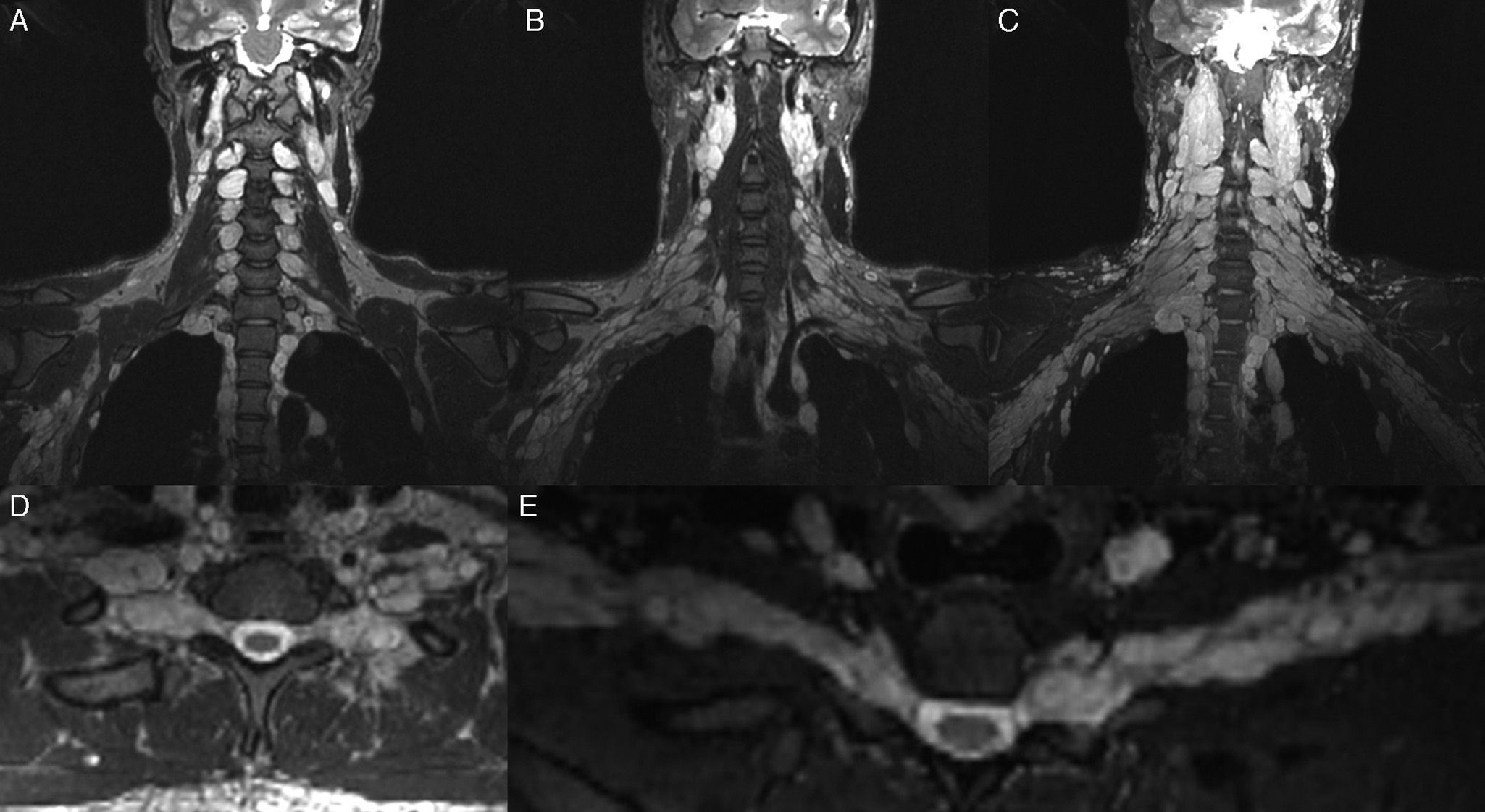
Neurofibromatosis; 24-year-old male patient in follow-up of his baseline disease. Study for the screening of malignant transformation of neurofibromas. T2-weighted IDEAL sequence. (A–C) Coronal cuts. (D) Axial cut. (E) Curvilinear reconstruction. It is possible to see “bundle” thickening of the brachial plexus roots, trunks, divisions and cords. In the present study no signs of malignant transformation were observed.
Half of malignant tumors originate de novo and the other half due to the malignant degeneration of a neurofibroma. Approximately 10% of neurofibromas show malignant transformation, hence the special interest of performing an image follow-up of patients with neurofibromatosis. Both malignant schwannomas and neurofibromas are usually greater than 5cm in its maximum diameter, they show irregular edges and a very heterogeneous enhancement after the injection of IV contrast due to the presence of necrotic areas.43,44
Lymphomas do not often affect the brachial plexus and are usually due to primary lymphomas of the central nervous system.45–47 Tumor lesions of lipoma origin even in their benign variants, can cause neuropathy due to extrinsic compression.48 The pancoast tumor is a tumor of the pulmonary apex that characteristically invades the brachial plexus, and on some occasions neuropathic pain makes the patient go to see the doctor49 (Fig. 6).
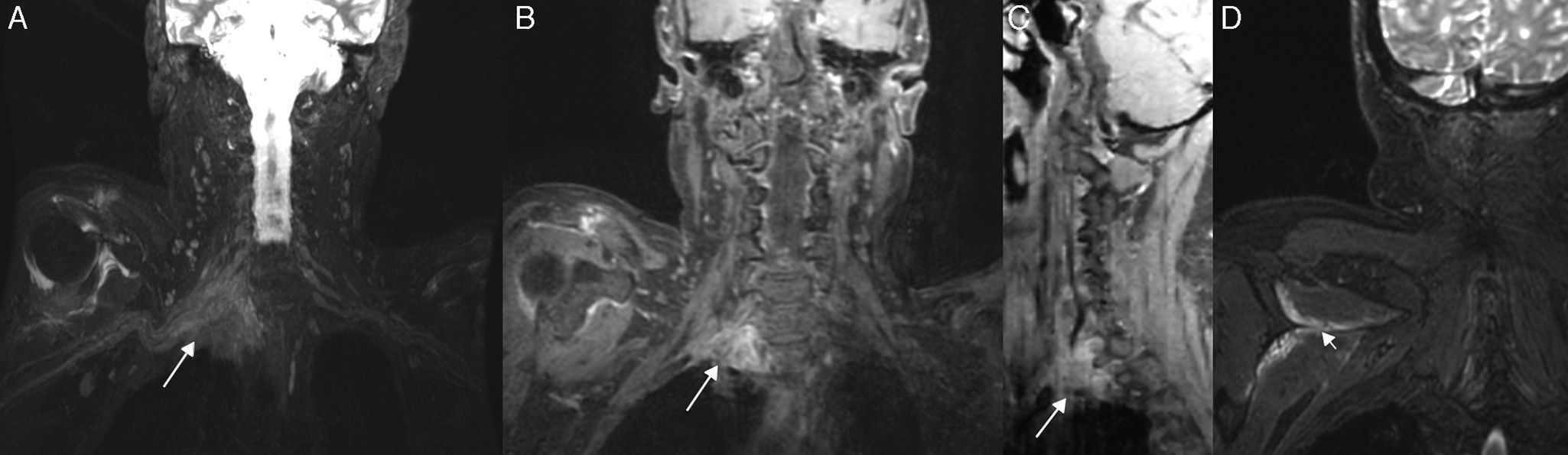
Pancoast Tumor; 55-year-old male patient with right three month old progressive omalgia and palpebral ptosis. An expansive, solid lesion is observed in the right supraclavicular fossa that contacts the pleural dome while compromising the homolateral roots from C6 to T1, and extension onto the lower trunk and divisions. (A) T2 IDEAL sequence on the coronal plane showing one hyperintense and heterogeneous neo-formation with irregular edges (long arrow). T1-weighted 3D IDEAL sequence after injection of contrast. (B) Coronal plane. (C) On the parasagittal plane we can see an intense heterogeneous enhancement of mass (long arrows). (D) T2 IDEAL sequence on the coronal plane. Notice the acute denervation signs of the muscles of the rotator cuff (short arrow).
The MRN allows us to determine the location and compromise of brachial plexus structures, and at the same time provides us with data about the characteristics of the tumor in a more accurate way than other modalities.50
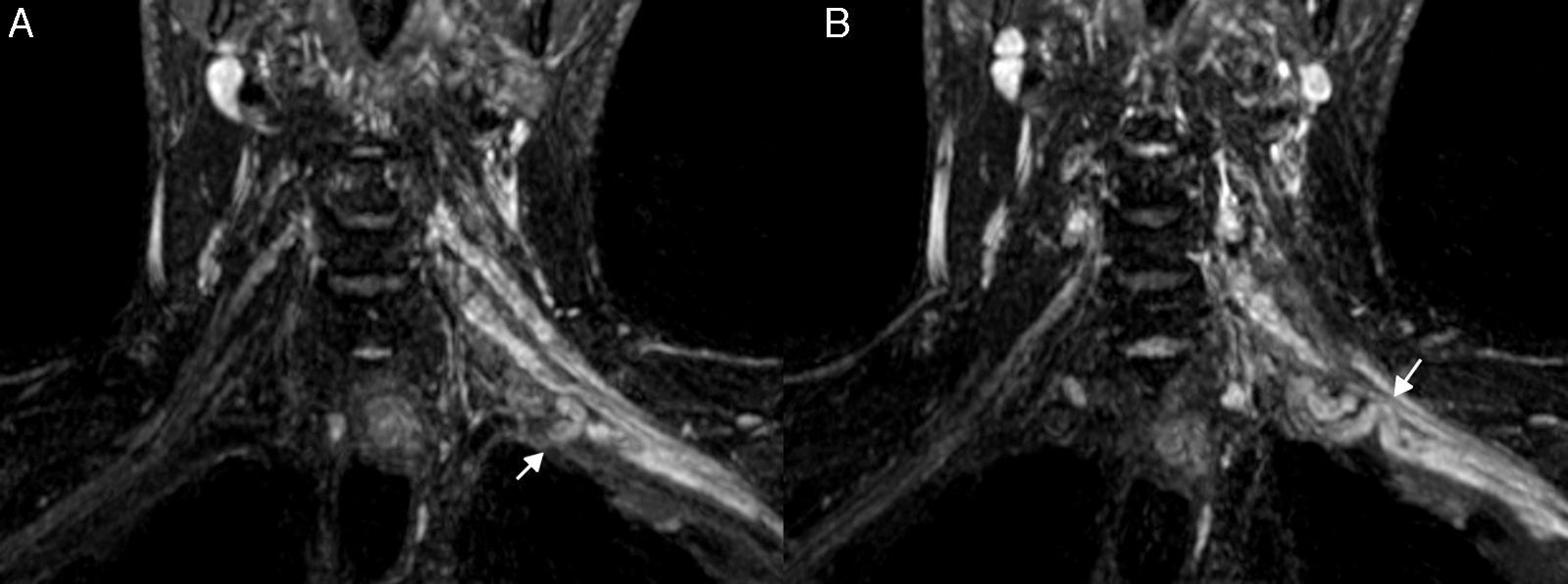
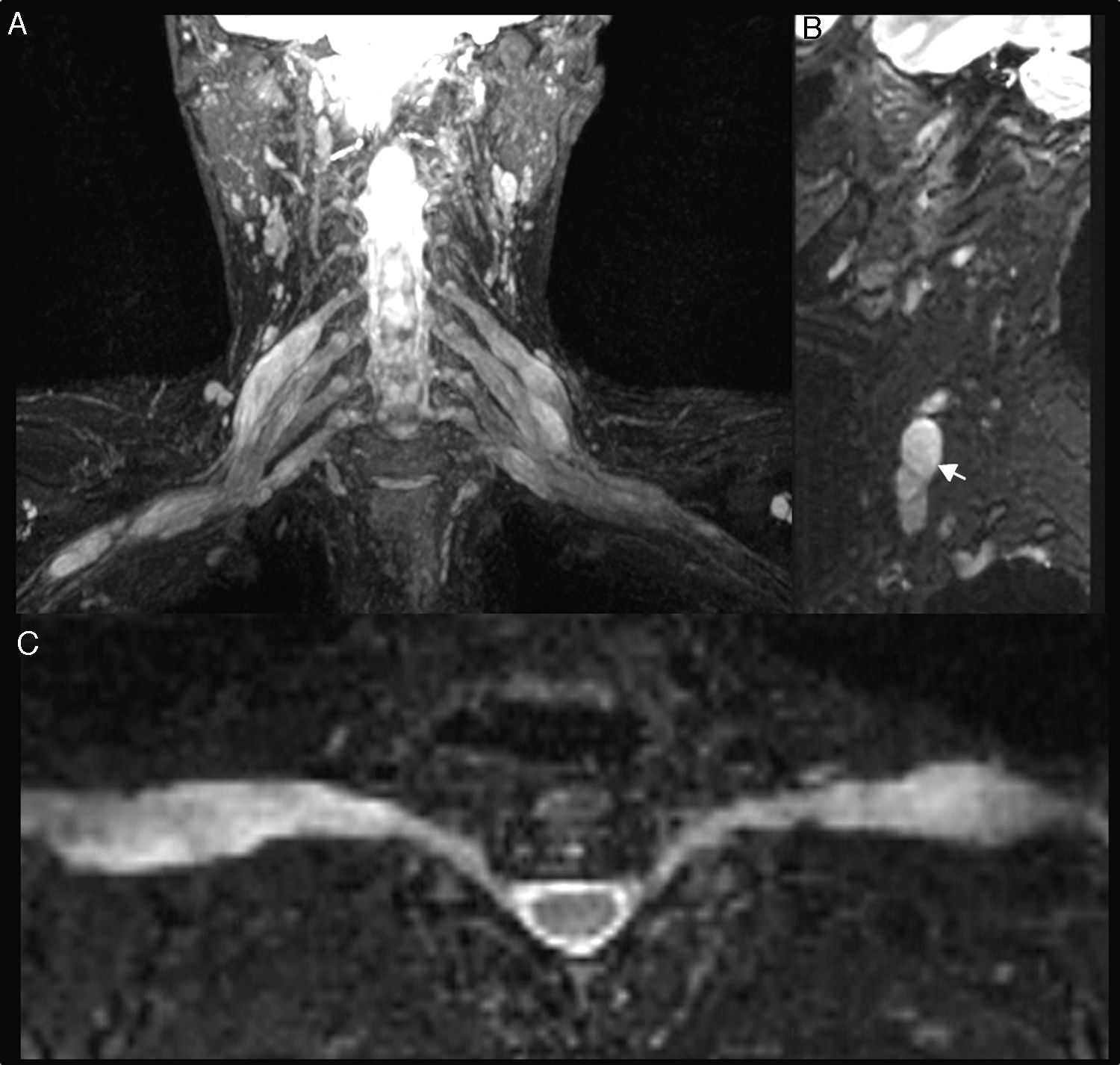
Brachial polyneuropathyPolineuropathies of inflammatory originParsonage TurnerParsonage Turner syndrome or amyotrophic neuralgia is of unknown etiology, although an inflammatory/autoimmune origin is presumed. It occurs more often in middle-aged men, with affectation of the right brachial plexus, and in nearly 30% of the cases it is bilateral. It debuts with severe, sudden-onset pain and muscular weakness. In most cases it resolves in two months; however, it can persist for up to 2 or 3 years.51 In its acute/subacute stage, the MRN shows diffuse thickening and an increase of signal intensity in the T2-weighted sequences of the affected nerves (Fig. 7). It also allows us to determine the extent of the lesions which is very often greater than the clinical one and that of the data provided by electrophysiological studies. It is also possible to confirm postdenervation changes that usually compromise the muscles of the shoulder girdle especially the serratus anterior, the supraspinatus and infraspraspinatus muscles.52 In its chronic stage, as in other polyneuropathies, a reduction of muscle trophism and adipose infiltration can be observed.20,21
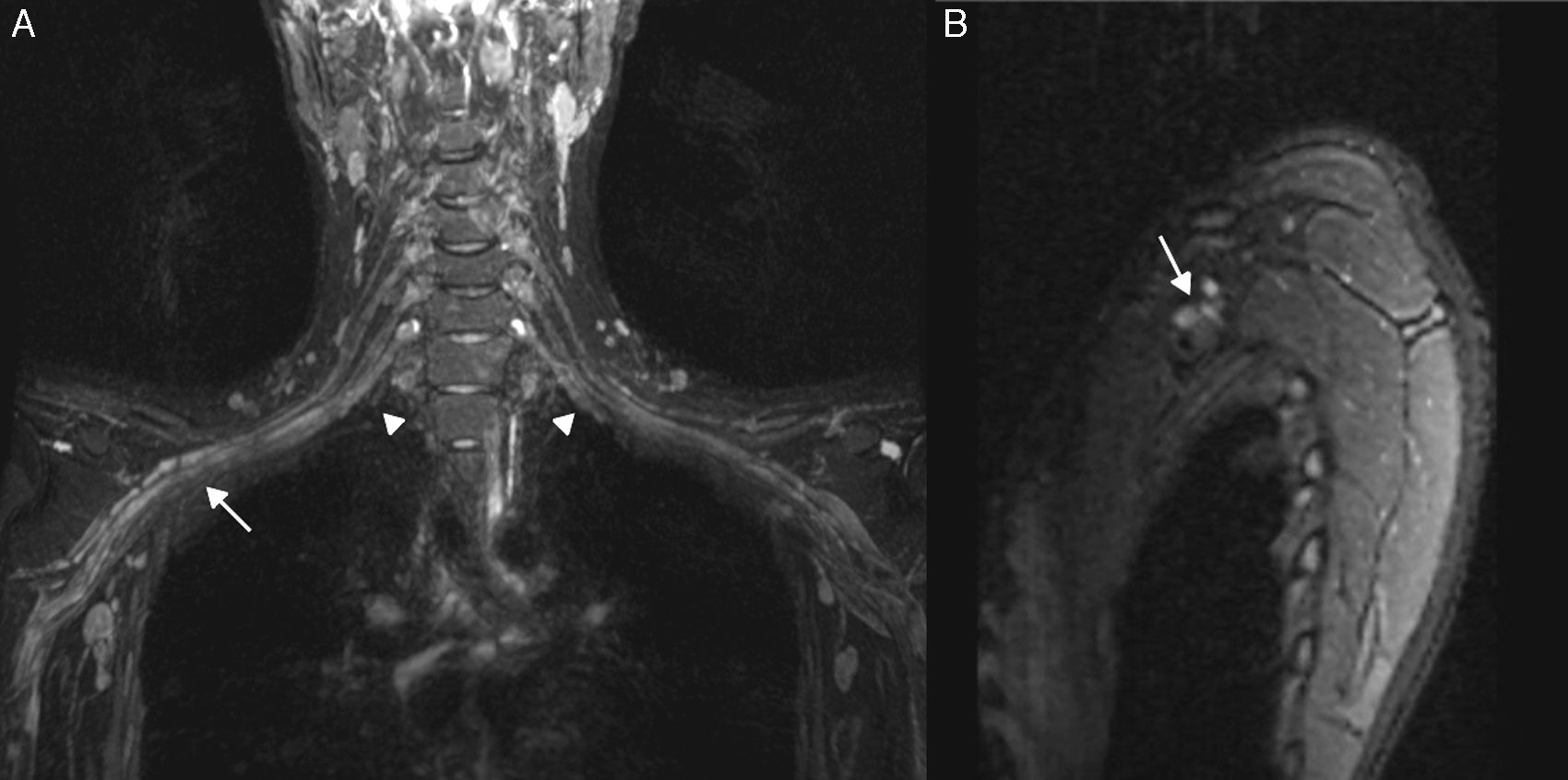
Inflammatory Plexitis. Parsonage Turner syndrome in a 22-year-old woman with 15 day old pain in shoulder and right upper limb. T2-weighted IDEAL sequence. (A) Coronal plane. (B) Right parasagittal plane. We can see the thickening of right roots C5, C6, C7, C8 and left roots C6, C7 and C8 (arrowheads), the upper, medial and lower trunks and the anterior and posterior cords in right predominance (arrow). Although the clinical manifestation was on the right side, the findings in the images were bilateral. She had a good evolution after therapy with corticoid and immunoglobulin pulses.
Chronic idiopathic demyelinating polyneuropathy (CIDP) is the most common cause of demyelinating polyneuropathy. It shows chronic progressive or recurrent course, with predominance of motor affectation of the proximal and distal muscles of the limbs. Although there are clinical criteria for the diagnosis of CIDP set by the American Academy of Neurology, in practice diagnosis is usually difficult to confirm due to the heterogeneity of its clinical and electrophysiological presentation. It is often necessary to resort to the biopsy of the peripheral nerve that confirms the selective loss of the myelin sheath.53
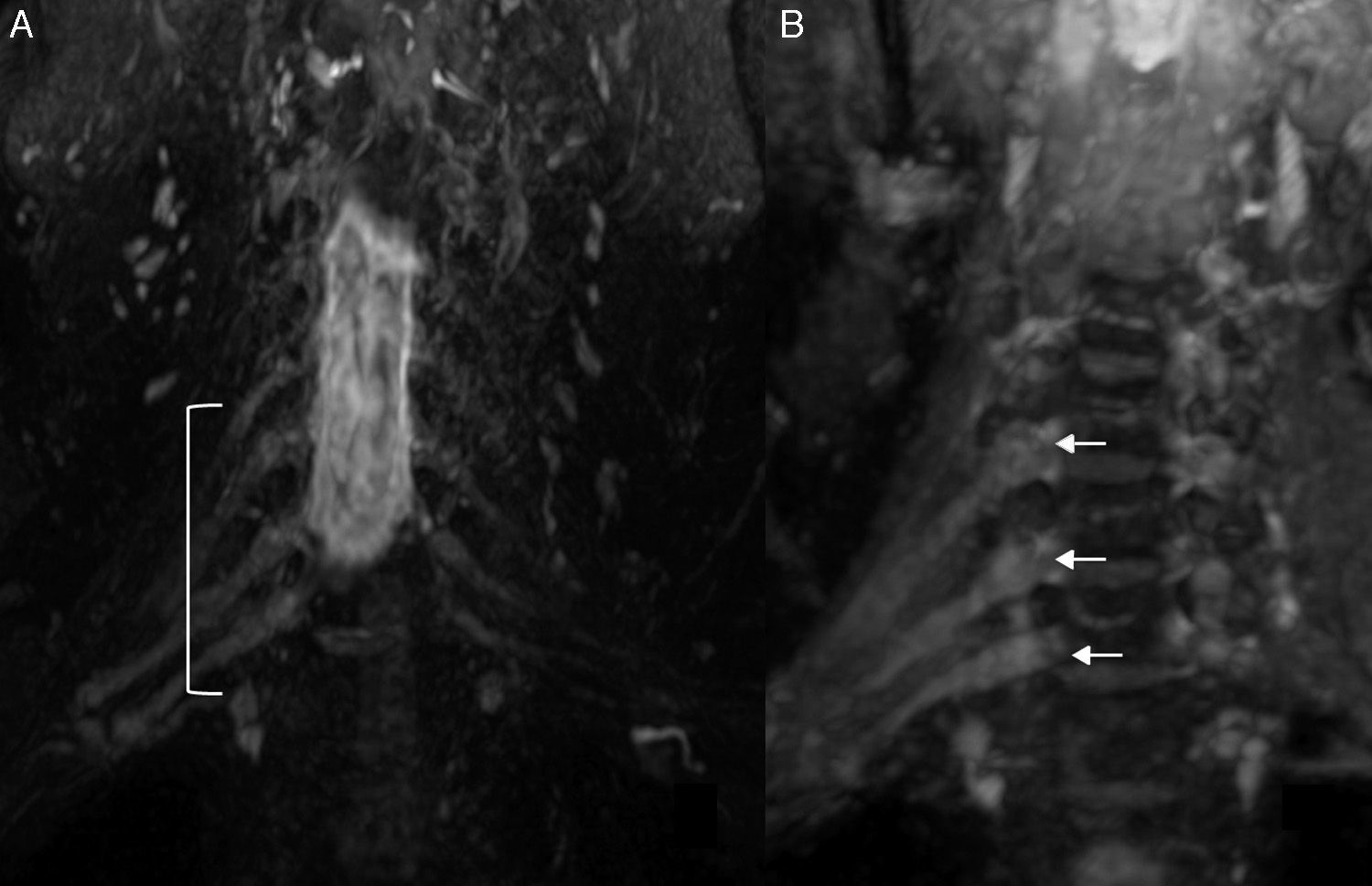
The MRN allows us to determine the extent of the disease at its onset and during the follow-up. It is possible to observe the diffuse thickening of the brachial plexus branches, which acquires a fusiform morphology that allows us to distinguish it from other plexopathies of inflammatory origin.54 Tazawa et al55 compared the diameter of roots C6 and C7 in CIDP patients and in normal subjects. They determined a 5mm cut-off point from which it can be defined as CIDP. Another finding made by the MRIs is the increase of signal intensity in T2-weighted sequences of compromised nerves. Also it is possible to observe focal or diffuse enhancement after the injection of IV contrast which translates into the rupture of the hematoneural barrier. Enhancement can persist after the treatment and remission of symptoms.54 It is usually accompanied by affectation of the lumbosacral plexus so its study is convenient56 (Fig. 8).
Chronic inflammatory demyelinating polyradiculoneuropathy. 32-year-old woman with symptoms of lumbosacral polyneuropathy who had been diagnosed with chronic idiopathic demyelinating polyneuropathy through clinical examination and neurography of lumbosacral plexus. She did not show any clinical manifestations of brachial plexus compromise. T2-weighted IDEAL sequence. (A) Coronal Plane. (B) Right parasagittal plane. (C) Curvilinear reconstruction. We can see significant hypertrophy and signal increase of the brachial plexus roots, trunks, divisions and cords from C4 to T1 both bilaterally and symmetrically.
Post-actinic neuropathy has an incidence of 10–20% and it occurs in patients who have received high doses of radiation (>60Gy).57 It is necessary to differentiate tumor recurrence from post-radiation inflammation when there is brachial plexus compromise in the context mentioned above.
Post-actinic neuropathy occurs between 30 months and 20 years after radiotherapy, and in usually compromises the infraclavicular region. In turn, tumor recurrence occurs within the first year of treatment and it usually affects the supraclavicular region.58
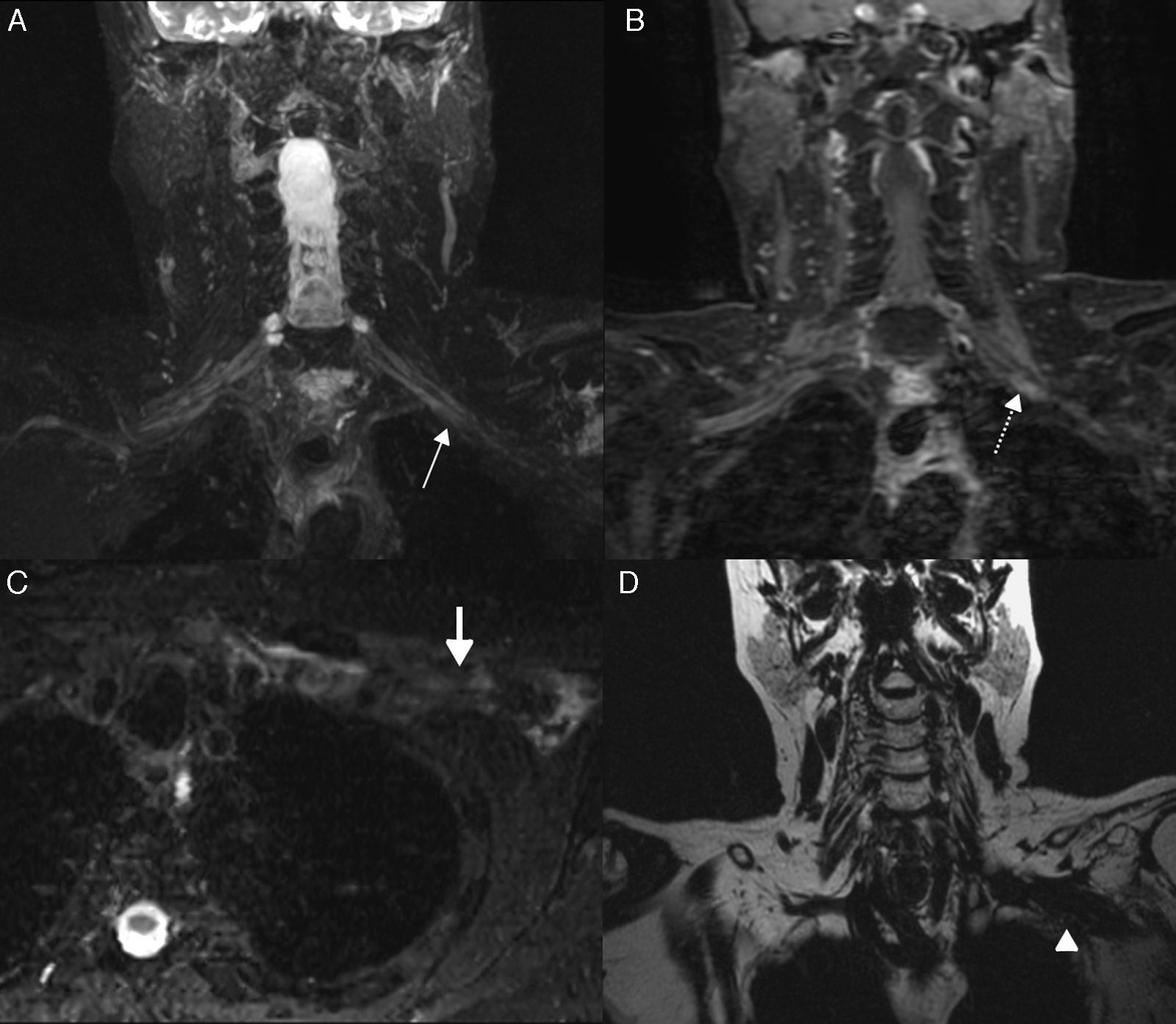
The MRN is the modality of choice to differentiate both entities. In post-radiation neuropathy it is common to observe diffuse, symmetric thickening of the plexus, which acquires a geographic disposition in relation to the irradiation field with an absence of tumor lesions. It is not common to observe enhancement after the injection of IV contrast. In chronic periods, it is possible to observe tortuosities of plexus branches with stratification of perineural fat due to the presence of fibrosis59 (Fig. 9).
Postradiotherapy Plexopathy. 74-year-old woman with a history of breast cancer who had been undergone surgery and radiotherapy 15 years ago. (A) T2-weighted IDEAL sequence shows distal nodular thickening of left roots C8 and T1 (arrow). (B) T1-weighted 3D IDEAL sequences with contrast, where nodular reinforcement is observed (discontinued arrow). (C) T2-weighted IDEAL sequence shows signs of pectoral muscle edema (thick arrow). (D) T2-weighted IDEAL sequence with water suppression. Infraclavicular soft tissue fibrosis can be seen (arrowhead).
Diabetic polyneuropathy is the most common neuropathy in diabetic patients, with a prevalence as high as 54% in type I diabetes and 45% in type II diabetes.60 It occurs in a diffuse or focal/multifocal manner and it can be classified as having a typical or an atypical presentation.
The typical diabetic polyneuropathy is usually of chronic, symmetric evolution, with sensitive-motor affectation and it is the most common form of presentation. The atypical variant can occurs any time during the course of the disease; its evolution is acute or chronic, it is usually asymmetric, monophasic and it can alternate nerve structure in time.61 Compromise of the brachial plexus is usually less frequent than that of the lumbosacral plexus, but when it is present, it usually coexists with lumbosacral polyradiculopathy.62
The MRN is particularly useful for the atypical forms, where an accurate diagnosis of neuropathy has not been obtained yet. The most common findings are mild to moderate increase of signal intensity in T2-weighted sequences, as well as variable thickening of the roots, trunks and cords compromised. It is common to find asymmetric distribution, even when the electromyographic examination shows neuropathy of one nerve only. As it happens in other plexopathies, postdenervation changes will be observed in the muscles of the region63 (Fig. 10).
Diabetic Neuropathy. 64-year-old male patient with a 10 year old poorly controlled type II diabetic polyneuropathy. He showed sensorimotor signs in the right upper limb. T2-weighted IDEAL sequence. (A) We can see an irregular thickening of the right spinal nerves C6 to T1 from its exit from the intervertebral foramens and in distal direction. (B) Multiplane reconstruction and magnification of image A.
Isolated vasculitis of the peripheral nervous system is an uncommon cause of brachial plexopathy. This polyneuropathy shows an asymmetric, progressive pattern of distribution.64 During the anatomopathological examination the multifocal neural ischemic lesion due to inflammation of the small- and mid-caliber epineural blood vessels is described, and less frequently the lesions located in the perineurium and endoneurium. The clinical, electromyographic and pathological findings are similar both in the neuropathy due to vasculitis and in the diabetic neuropathy. The status of non-diabetic patient is the way to differentiate both entities.65 In the MRN, the findings have similar characteristics to those of other inflammatory neuropathies. As it happens to be the case with other polyneuropathies, the MRN is capable of showing a greater compromise to both the clinical presentation and electrophysiological studies.66
ConclusionThe brachial plexus is an anatomically complex region home of mononeuropathies and polyneuropathies of different etiologies. The MRN in high-field equipment accompanied by high-resolution sequences has become one of the pillars together with clinical examination and electrophysiological studies for the diagnosis and follow-up of brachial plexus lesions.
Ethical responsibilitiesProtection of people and animalsThe authors declare that no experiments with human beings or animals have been performed while conducting this investigation.
Data confidentialityThe authors confirm that in this article there are no data from patients.
Right to privacy and informed consentThe authors confirm that in this article there are no data from patients.
Author contributions- 1.
Manager of the integrity of the study: CC and MN.
- 2.
Study Idea: CC, CR, GM and MN.
- 3.
Study Design: CC, CR, GM and MN.
- 4.
Data Mining: CC, CR and GM.
- 5.
Data Analysis and Interpretation: CC, CR and GM.
- 6.
Statistical Analysis: NA.
- 7.
Reference Search: CC, CR and GM.
- 8.
Writing: CC, CR, GM and MN.
- 9.
Critical review of the manuscript with intellectually relevant remarks: CC, CR, GM and MN.
- 10.
Approval of final version: CC, CR, GM and MN.
The authors declare no conflict of interests associated with this article whatsoever.
Thanks to Ms. Inés Escobar, M.D., for her design of the brachial plexus scheme shown in Fig. 1.
Please cite this article as: Cejas C, Rollán C, Michelin G, Nogués M. Neurografía de alta resolución en resonancia magnética 3Tesla del plexo braquial. Radiología. 2016;58:88–100.