Magnetic resonance neurography is a technique that complements clinical and electrophysiological study of the peripheral nerves and brachial and lumbosacral plexuses. Numerous focal processes (inflammatory, traumatic, primary tumors, secondary tumors) and diffuse processes (diabetic polyneuropathy, chronic idiopathic demyelinating polyneuropathy due to amyloidosis or Charcot–Marie–Tooth disease) can involve the lumbosacral plexus. This article reviews the anatomy of the lumbosacral plexus, describes the technique for neurography of the plexus at our institution, and shows the diverse diseases that affect it.
La neurografía por resonancia magnética se ha convertido en una técnica de imagen complementaria al estudio clínico y electrofisiológico para explorar los nervios periféricos y los plexos braquial y lumbosacro. Este último es asiento de numerosos procesos focales (inflamatorios, traumáticos, tumorales primarios o secundarios) y difusos (polineuropatía diabética, la desmielinizante crónica idiopática, por amiloidosis o la enfermedad de Charcot-Marie-Tooth). El objetivo de este artículo es revisar la anatomía del plexo lumbosacro, describir la técnica de la neurografía del plexo en nuestra institución y mostrar las diversas enfermedades que lo afectan.
Magnetic resonance neurography (MRN) has become essential for the study of peripheral neuropathies and brachial plexus and lumbosacral plexus (LSP). The LSP is the cause of several conditions: traumatic, entrapment, inflammatory, metabolic, toxic, radiation and tumor diseases1–5 assessed clinically during decades and with electrophysiological modalities highly sensitive for the detection of conduction abnormalities.6,7 From the appearance of neurographic sequences in the 1990s the MRN has become part of the diagnostic armamentarium of plexopathies.8 Today 3T MR equipments and high-resolution dedicated images allow us to see with excellent definition the course, thickness, fascicles, and the intensity of signal of both the LSP roots and perineural tissue also adding to electrophysiological studies the exact location of the lesion and its nature.9–13
Lastly MRN gives us data on the muscles which indirectly helps us find the nerve involved.14,15
The goal of this study is to review the anatomy of LSP, the MRN modality in our institution and illustrate with various examples of the different conditions affecting such anatomy.
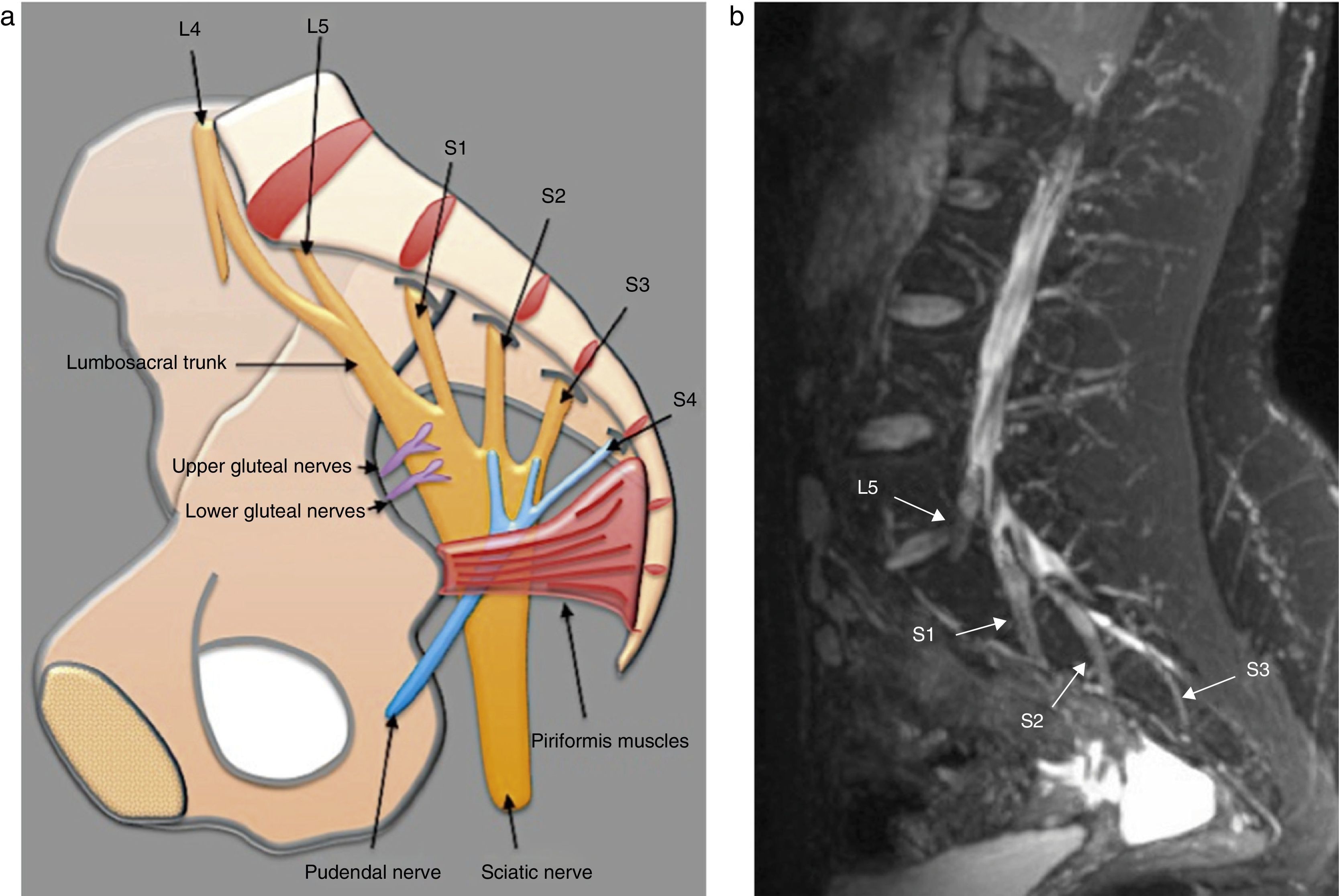
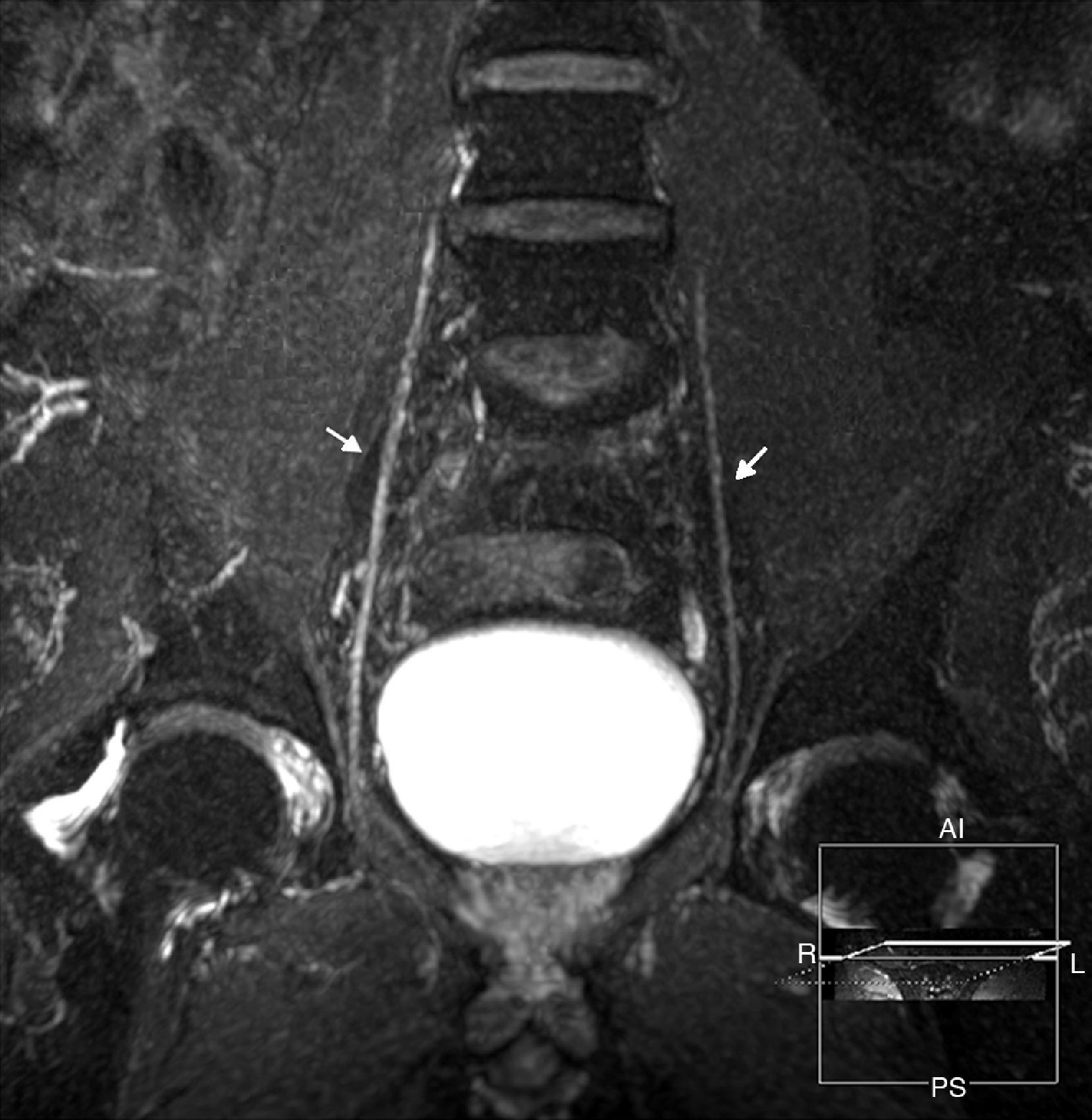
Anatomy of the lumbosacral plexusLumbosacral plexus stems from the ventral branches of nerves L1 to L4 usually with a contribution from nerve T12. It descends dorsally or inside the psoas muscle and branches that arise (a) from the muscle lateral edge: iliohypogastric, ilioinguinal, genitofemoral, lateral femoral-cutaneous and femoral nerves, and (b) from the medial edge: obturator nerve and lumbosacral trunk16 (Fig. 1).
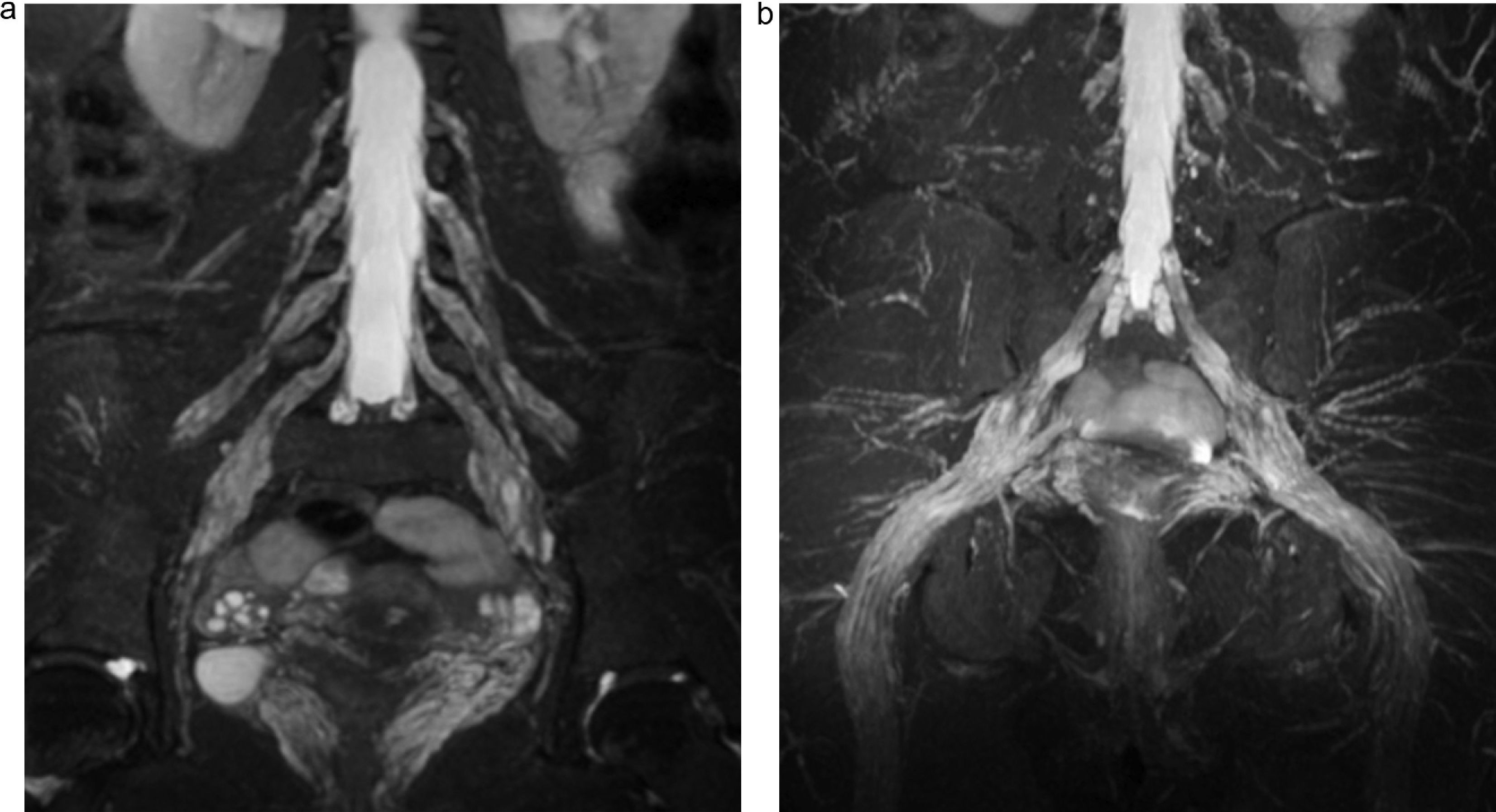
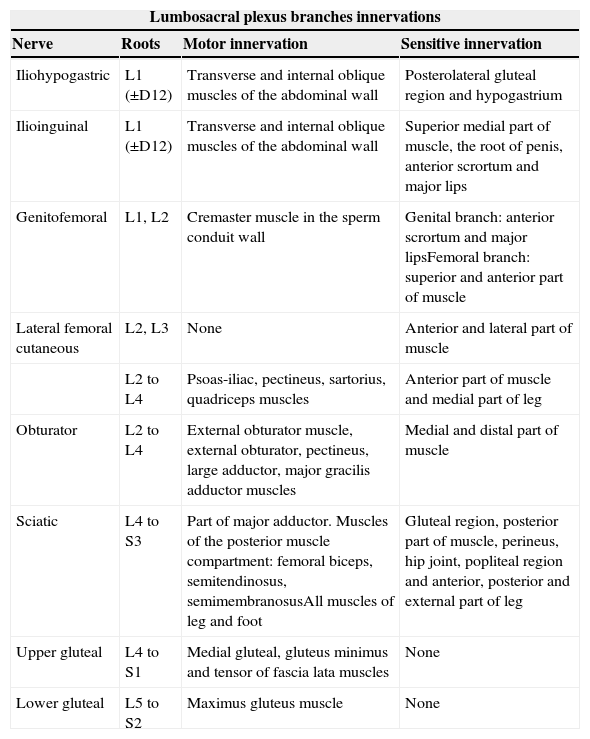
The lumbosacral trunk consisting of a lesser L4 branch and an L5 ventral branch descends on the sacral wing and connects to the roots S1 to S3 in the anterior side of the piriformis muscles to make up the sacral plexus from which the sciatic, pudendal, and upper and lower gluteal nerves are born17 (Figs. 2 and 3). The lumbar plexus innervates the muscles of the anteromedial region of the muscle while the sacral plexus does so with the muscles of the gluteal region, the anterior side of the muscle and with the muscles distal to the knee18 (Table 1).
(a) Representative scheme of sacral plexus. (b) Fat saturated T2 IDEAL sagittal sequence. MIP reconstruction identifying the L5 to S3 roots, of normal appearance. In the sagittal acquisition and reconstruction the T2 IDEAL sequence allows us to thoroughly identify the components of sacral plexus.
Motor and sensitive innervation of lumbosacral plexus.
| Lumbosacral plexus branches innervations | |||
|---|---|---|---|
| Nerve | Roots | Motor innervation | Sensitive innervation |
| Iliohypogastric | L1 (±D12) | Transverse and internal oblique muscles of the abdominal wall | Posterolateral gluteal region and hypogastrium |
| Ilioinguinal | L1 (±D12) | Transverse and internal oblique muscles of the abdominal wall | Superior medial part of muscle, the root of penis, anterior scrortum and major lips |
| Genitofemoral | L1, L2 | Cremaster muscle in the sperm conduit wall | Genital branch: anterior scrortum and major lipsFemoral branch: superior and anterior part of muscle |
| Lateral femoral cutaneous | L2, L3 | None | Anterior and lateral part of muscle |
| L2 to L4 | Psoas-iliac, pectineus, sartorius, quadriceps muscles | Anterior part of muscle and medial part of leg | |
| Obturator | L2 to L4 | External obturator muscle, external obturator, pectineus, large adductor, major gracilis adductor muscles | Medial and distal part of muscle |
| Sciatic | L4 to S3 | Part of major adductor. Muscles of the posterior muscle compartment: femoral biceps, semitendinosus, semimembranosusAll muscles of leg and foot | Gluteal region, posterior part of muscle, perineus, hip joint, popliteal region and anterior, posterior and external part of leg |
| Upper gluteal | L4 to S1 | Medial gluteal, gluteus minimus and tensor of fascia lata muscles | None |
| Lower gluteal | L5 to S2 | Maximus gluteus muscle | None |
Iliohypogastric and ilioinguial nerves are made up of the anterior division of the L1 root. Because of the nerves size being able to see them in the MRN is hard unless they are abnormally enlarged.17
Genitofemoral nerve originates at the anterior divisions of L1 and L2 roots runs through the psoas major muscle passing dorsally to the ureter and dividing itself into the medial and femoral lateral genital branches. In the MRN it has a relatively easy trajectory along the anterior edge of the psoas muscle toward the inguinal regions.18
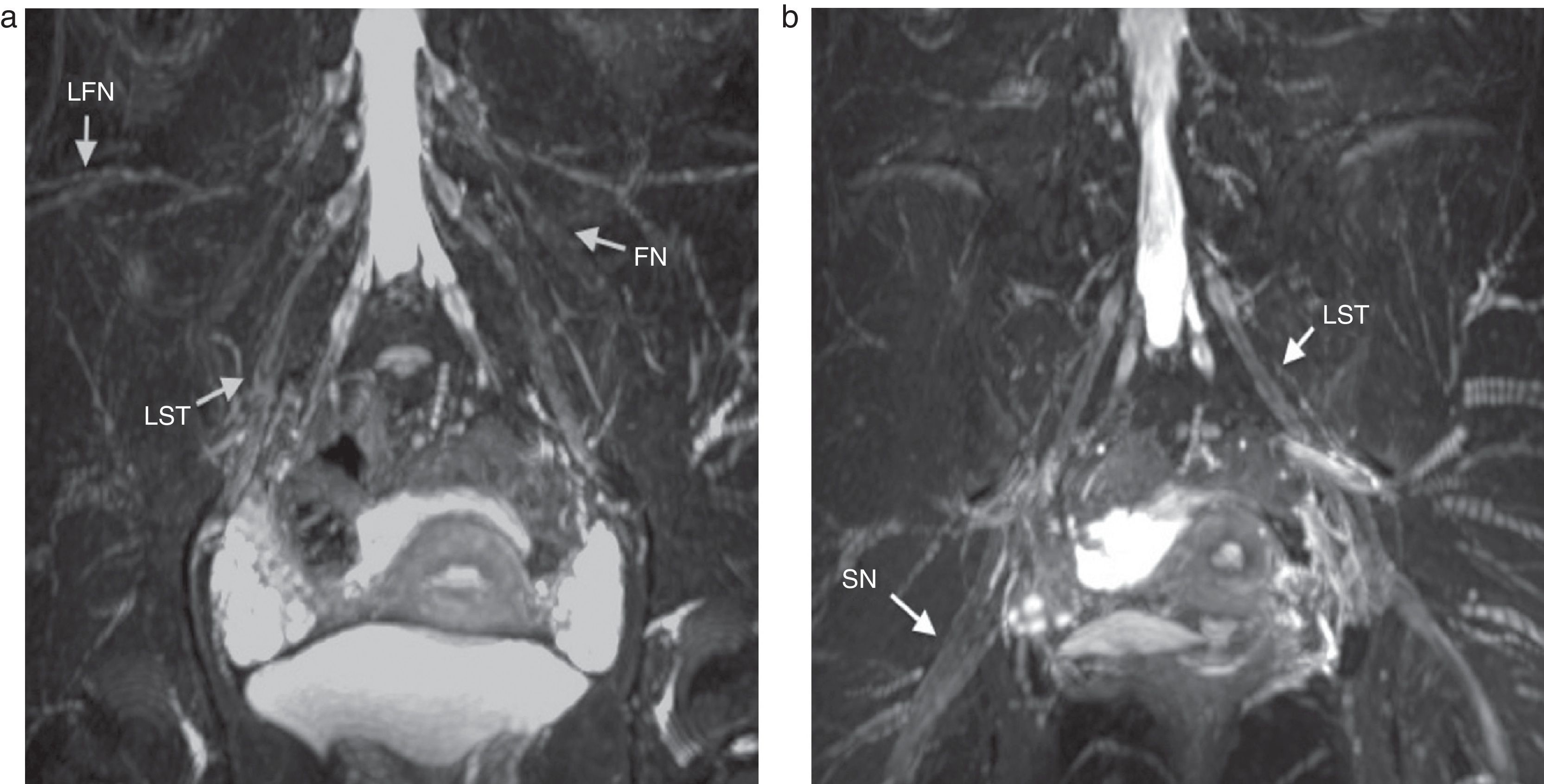
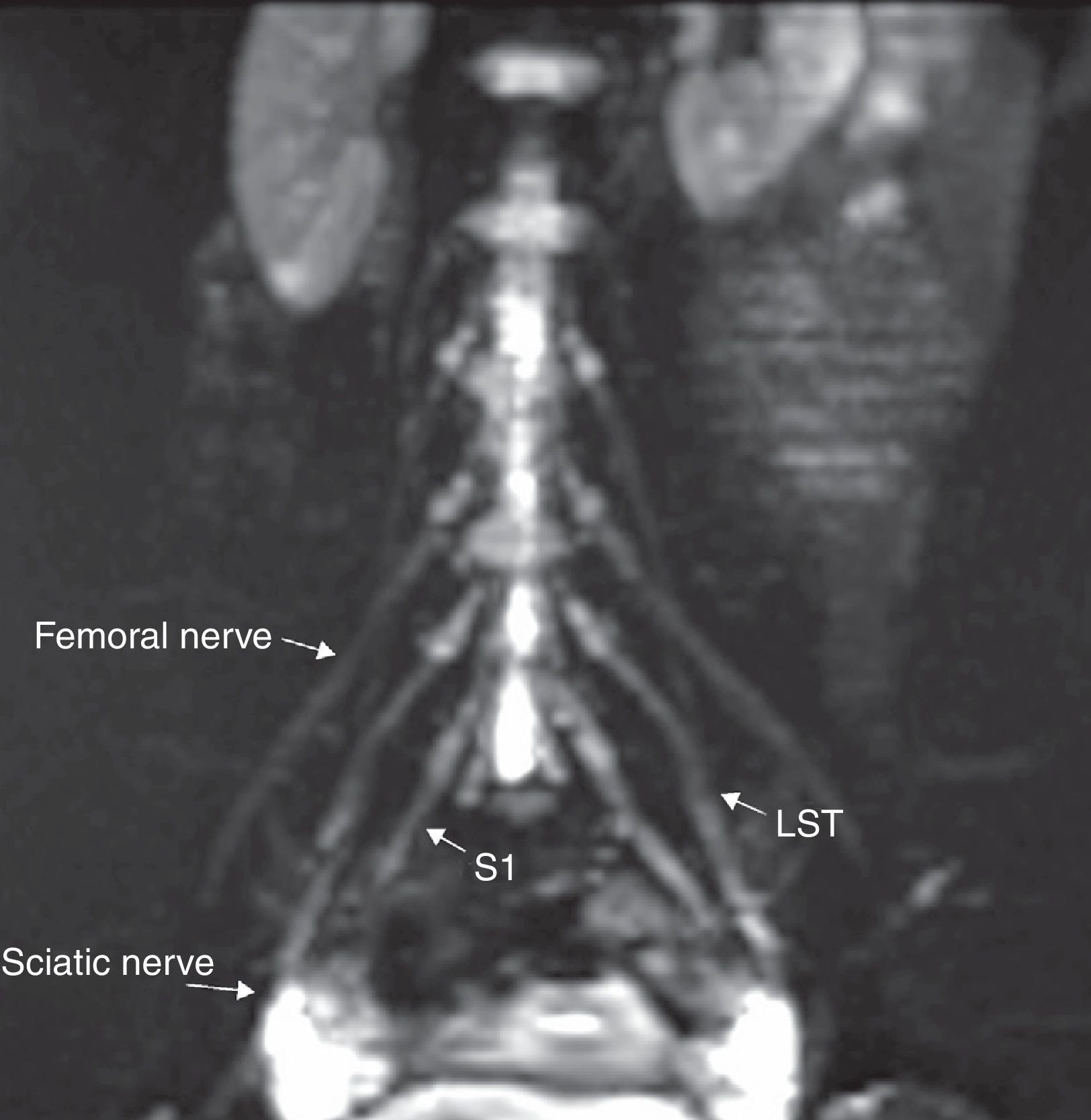
The lateral femorocutaneous nerve (Fig. 4) is a sensitive branch originated at the posterior divisions of the L2 and L3 roots and toward the anterior superior iliac spine.17
Coronal fat saturated T2 IDEAL sequence. MIP reconstruction. (a) Lateral femoral cutaneous nerve (LFN), femoral nerve (FN) and lumbosacral trunk (LST) all of normal appearance are identified bilaterally. (b) LST and sciatic nerve (SN) are also identified bilaterally and also with normal appearance.
The femoral nerve comes from the posterior roots of L2 to L4 (Figs. 4 and 5), descending across the psoas muscle, emerging from the inferior side of the lateral edge of the muscle and running inferior to the inguinal ligament to finally enter the muscle and reach one anterior branch that innervates the pectineal and sartorius muscles and one posterior division that innervates the quadriceps muscle. Femoral nerves are constantly seen in the MRN being symmetrical in hyperintensity and thickness.
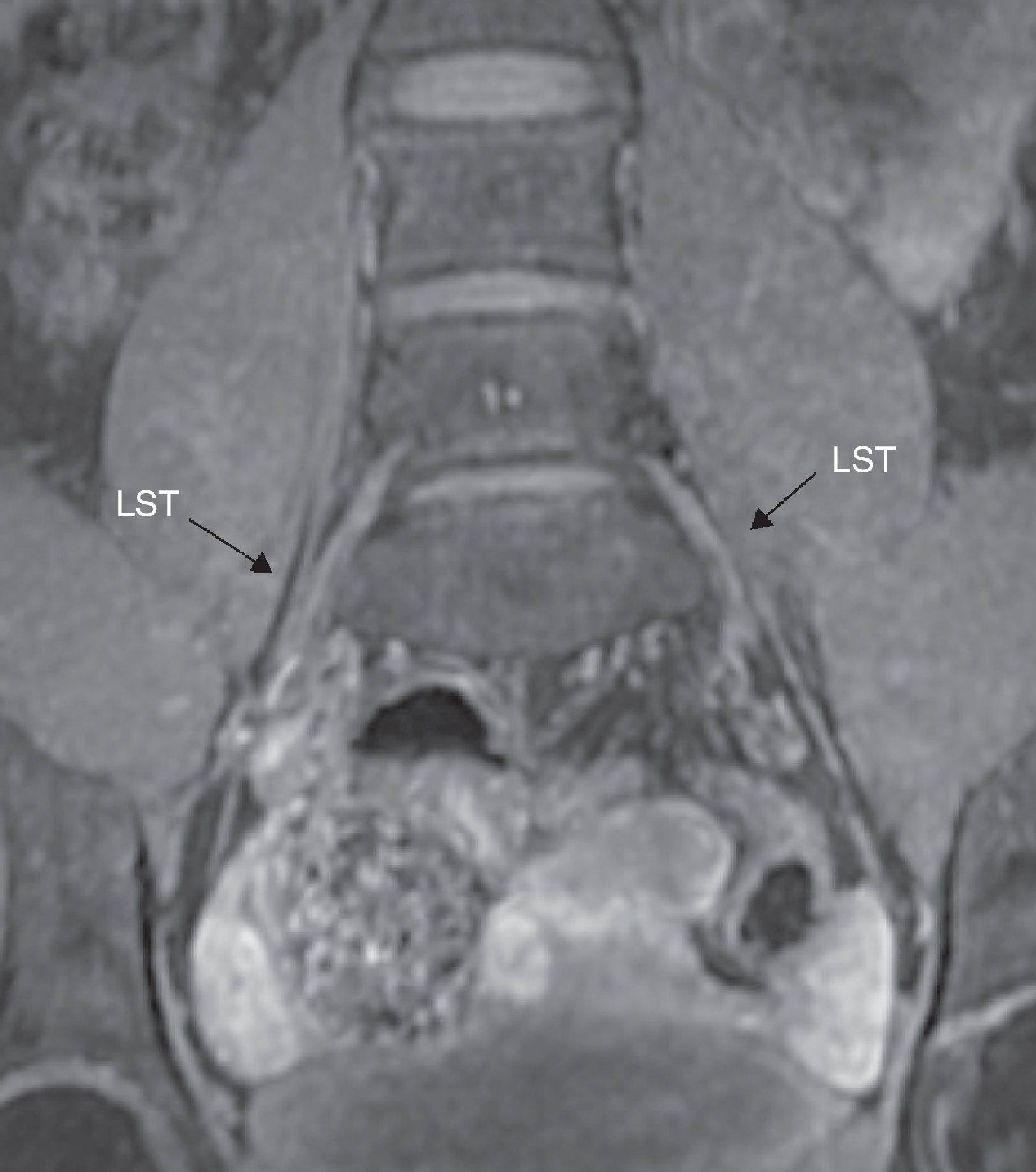
The lateral branches of L2 to L4 make up the obturator nerve that runs through one descending course to finally exit through the medial edge of the psoas muscle next to the iliac crest. Then it passes through the pelvic cavity through the obturator channel at the superior side of the obturator orifice.18 This nerve is surrounded by a huge amount of perineural fat that in turn facilitates its identification in all planes of MRN (Fig. 6).
The sciatic nerve (Fig. 4) is made up of the ventral branches of L4 to S3 roots descending anteriorly, cranially or inside the piriformis muscle. It exits the pelvis through the major sciatic orifice and descends between the major adductor muscle and the gluteus maximus muscle and in the distal tertium of the muscle two branches originate: the tibial nerve and the common peroneal nerve. Due to its huge size and abundant perineural fat the sciatic nerve is easy to evaluate in all planes and views.19
The pudendal nerve is made up of the union of the ventral branches of S2 and all branches of S3 and S4. It runs through the piriformis and coccigeous muscles exiting through the pelvis through the major sciatic orifice. Then it crosses the ischial spine running through the Alcock channel parallel to the walls of ischiorectal fossa. Its terminal branches are the inferior, perineal, and dorsal nerves of penis or clitoris.20
The gluteus maximus muscle originates from the L4 to S1 nerve roots emerging from the pelvis through the major sciatic orifice and above the piriformis muscle. The inferior gluteal nerve originates from the L5 to S2 nerves emerging from the pelvis across the major sciatic orifice and below the piriformis muscle. Unless its size is increased abnormally its small volume does not allow us to see it in the MRN.21
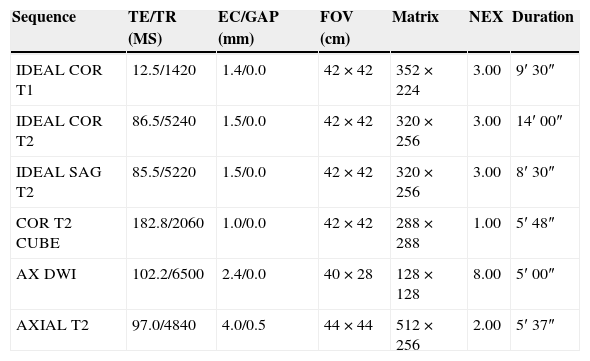
Protocol for the study of lumbosacral plexus through magnetic resonance imagesIn our institution the protocol for the study of the LSP is using the Signa HDxt 3.0 Tesla equipment (GE, Milwaukee, Wisconsin, U.S.A.) with an 8 channel-coil CTL Array (Torso PA Signa–GE, Milwaukee, Wisconsin, U.S.A.). The patient is placed in the decubitus dorsal position with limps in their fully extended position. The coil coverage spreads from the xiphoid appendix toward the root of inferior limbs. These are the sequences used: Coronal T1 and T2-weighted 3D IDEAL (Iterative Decomposition of water and fat with Echo Asymmetry and Least-squares estimation), sagittal T2-weighted IDEAL, coronal T2 CUBE, and axial e-DWI (Table 2). Volumetric sequences are processed through multiplanar reconstructions and reconstructions of maximum intensity projections (MIP).
Study protocol of lumbosacral plexus.
| Sequence | TE/TR (MS) | EC/GAP (mm) | FOV (cm) | Matrix | NEX | Duration |
|---|---|---|---|---|---|---|
| IDEAL COR T1 | 12.5/1420 | 1.4/0.0 | 42×42 | 352×224 | 3.00 | 9′ 30″ |
| IDEAL COR T2 | 86.5/5240 | 1.5/0.0 | 42×42 | 320×256 | 3.00 | 14′ 00″ |
| IDEAL SAG T2 | 85.5/5220 | 1.5/0.0 | 42×42 | 320×256 | 3.00 | 8′ 30″ |
| COR T2 CUBE | 182.8/2060 | 1.0/0.0 | 42×42 | 288×288 | 1.00 | 5′ 48″ |
| AX DWI | 102.2/6500 | 2.4/0.0 | 40×28 | 128×128 | 8.00 | 5′ 00″ |
| AXIAL T2 | 97.0/4840 | 4.0/0.5 | 44×44 | 512×256 | 2.00 | 5′ 37″ |
Through the IDEAL sequences developed by General Electric Healthcare, or 3D SPACE (Sampling Perfection with Application Optimized Contrast) developed by Siemens Healthcare13 the images separate the water signal from fat overcoming the limitations imposed by the traditional techniques of fat suppression. Inspired by the Dixon22 method it acquires three images with different echo time to obtain both fat and water signals with multiple phase differences allowing us to work simultaneously with T1 and T2-weighted images with four different combinations of saturation pulses (water suppression, fat suppression, phase images and out of phase).9 On T1 both thin cuts and fat suppression are optimal for the anatomical evaluation of nerves by adequately outlining perineural fat tissues and studying the adjacent structures (Fig. 3). On T2 both high resolution and fat suppression contrast with the signal of liquid which allows us to do adequate MPR and MIP reconstructions along the nerves (Figs. 2, 4 and 5) and evaluate the alterations of thickness and signal. With both sequences we can also study changes due to denervation in the affected muscles: hyperintensity in T2 in the acute phase, loss of muscle volume, and fat replacement in more chronic phases.15,23
CUBE sequence is one fast spin-echo (FSE) 3D sequence obtained from one only acquisition and allowing us to reconstruct the image in all planes with the same resolution. It is achieved using long echo trains to reduce the time of acquisition yet continuously modeling the angle of rotation to avoid the loss of signal that usually happens at the end of the train. This sequence high spatial resolution is great for the morphologic study of nerve roots, perineural fat tissue and its relation with adjacent structures (Fig. 5).
Other than showing nerves like hyperintense structures diffusion sequence (e-DWI) with b values between 0 and 100 also suppresses simultaneously the signal of blood vessels allowing one extensive MIP reconstruction of nerve structures without overlapping the vessels. With low b values (b 200) the image has less resolution but it is useful as a complement of IDEAL sequences for the evaluation of the extension and distribution of lesions. High B values (between 700 and 1.000) allow us to study diffusion in lesions by quantifying the diffusion coefficient (CDA map) especially useful for the study of tumors24,25 (Fig. 7).
Pathologic conditions affecting the lumbosacral plexusTrauma lesionsUnlike brachial plexus–highly vulnerable–the LSP is not very exposed to trauma due to the protection of the axial skeleton. However, the direct trauma is uncommon, but indirect trauma is more frequent than in the brachial axial and is associated with lesions of the spine, hip fractures or luxations, pelvis fractures, surgical gynecologic iatrogenics, colorectal or inguinal hernia, aorta aneurysm, and lesions compromising the psoas muscle like hematomas and abscesses.18,26 In 1943, Seddon27 proposed one categorization based on the seriousness of trauma lesions of peripheral nerves. The lesser degree of lesion is neuropraxia produced by axonal dysfunction but without interruption of axons or nervous sheath. It is usually a transient disorder with complete resolution. The second degree is axonotmesis implying the discontinuity of axons preserving the integrity of connective tissue–perineurum, endoneurum, and epineurum. The greater degree of lesions is neurotmesis where the continuity of axons and the perineural sheath are lost. Prognosis depends on neuronal damage driven by the capacity of self-regeneration during the two first degrees and by the swiftness of surgical therapy in cases of serious neurotmesis and axonotmesis. Lack of recovery causes proximal neuroma.28,29
In 1951, Sunderland30 extended the categorization of axonotmesis. He divided trauma lesions into the following degrees: type I: equivalent to neuropraxia. Recovery is complete after weeks or months; type II: endoneurum and perineurum are intact but axons are physiologically interrupted. Since the endoneurum is intact axonal regeneration is directed along its original course, so a complete functional recovery can be expected; type III: endoneurum is interrupted and the perineurum is intact; functional recovery is intact; type IV: the integrity of the nerve course is due to scar tissue containing the interrupted nerve fascicles. Retrograde degeneration and interfascicular fibrosis is more extensive because recovery is minimum; type V: equivalent to neurotmesis. Since it happens in oper lesions surgical exploration is always indicated. Spontaneous recovery does not exist (Fig. 8).
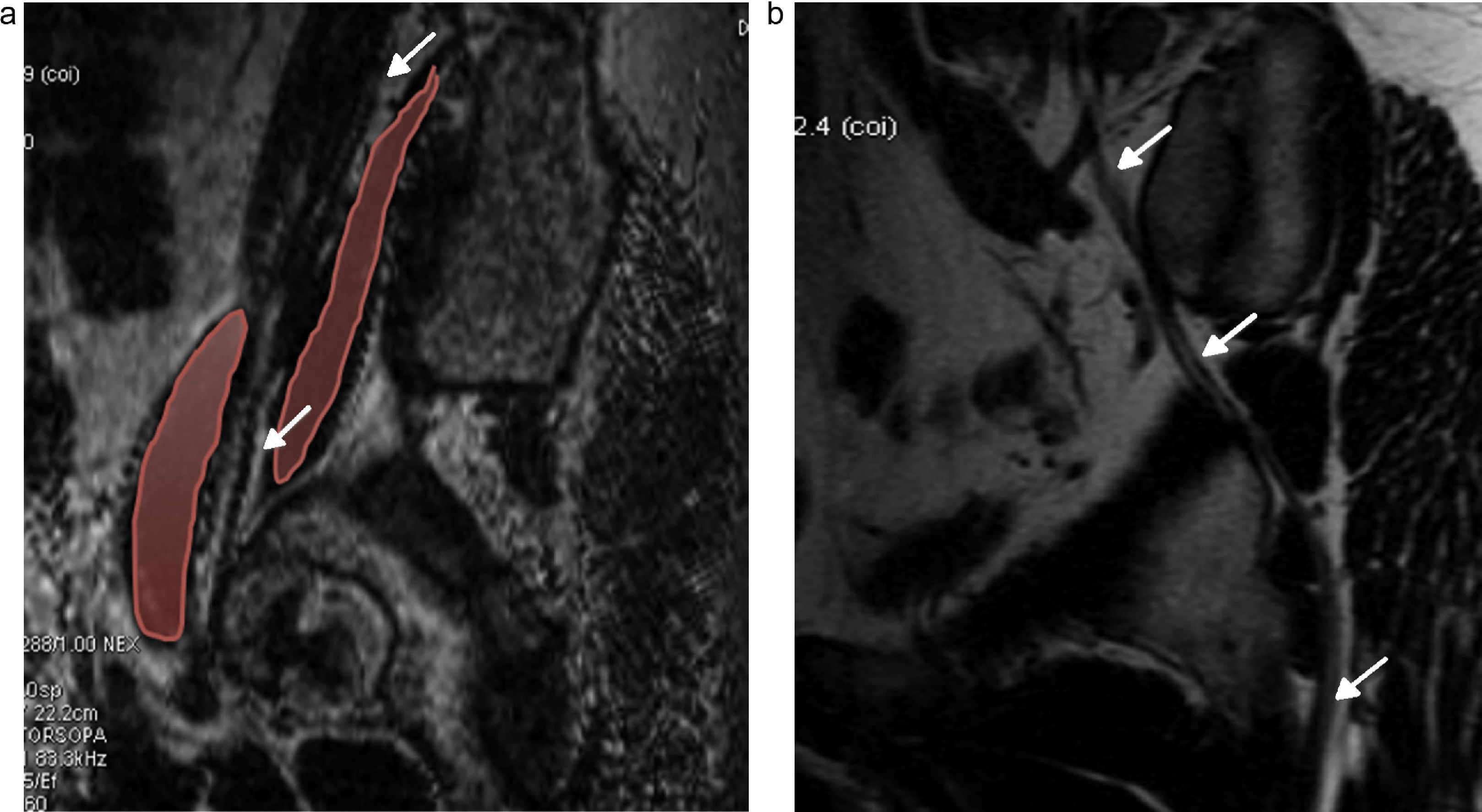
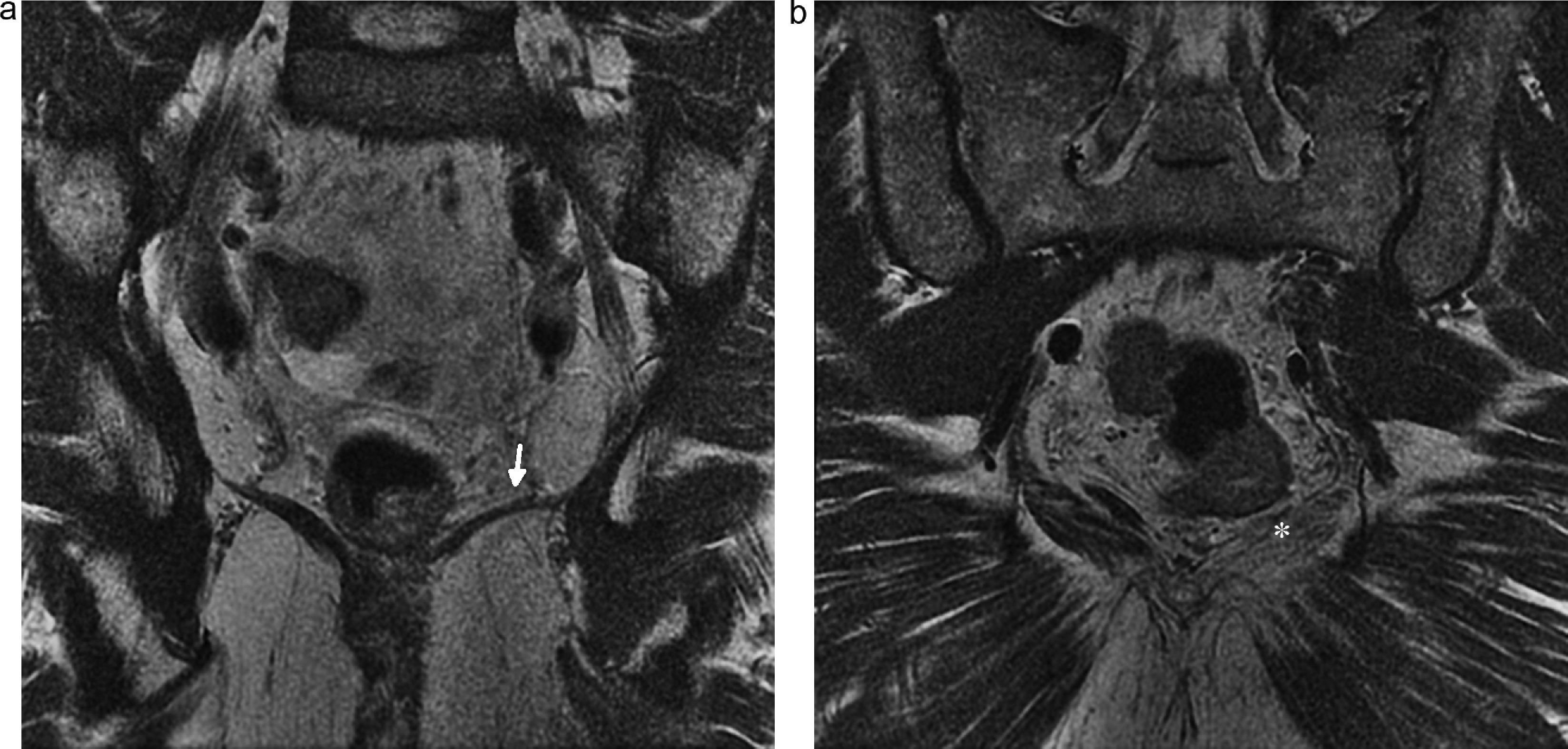
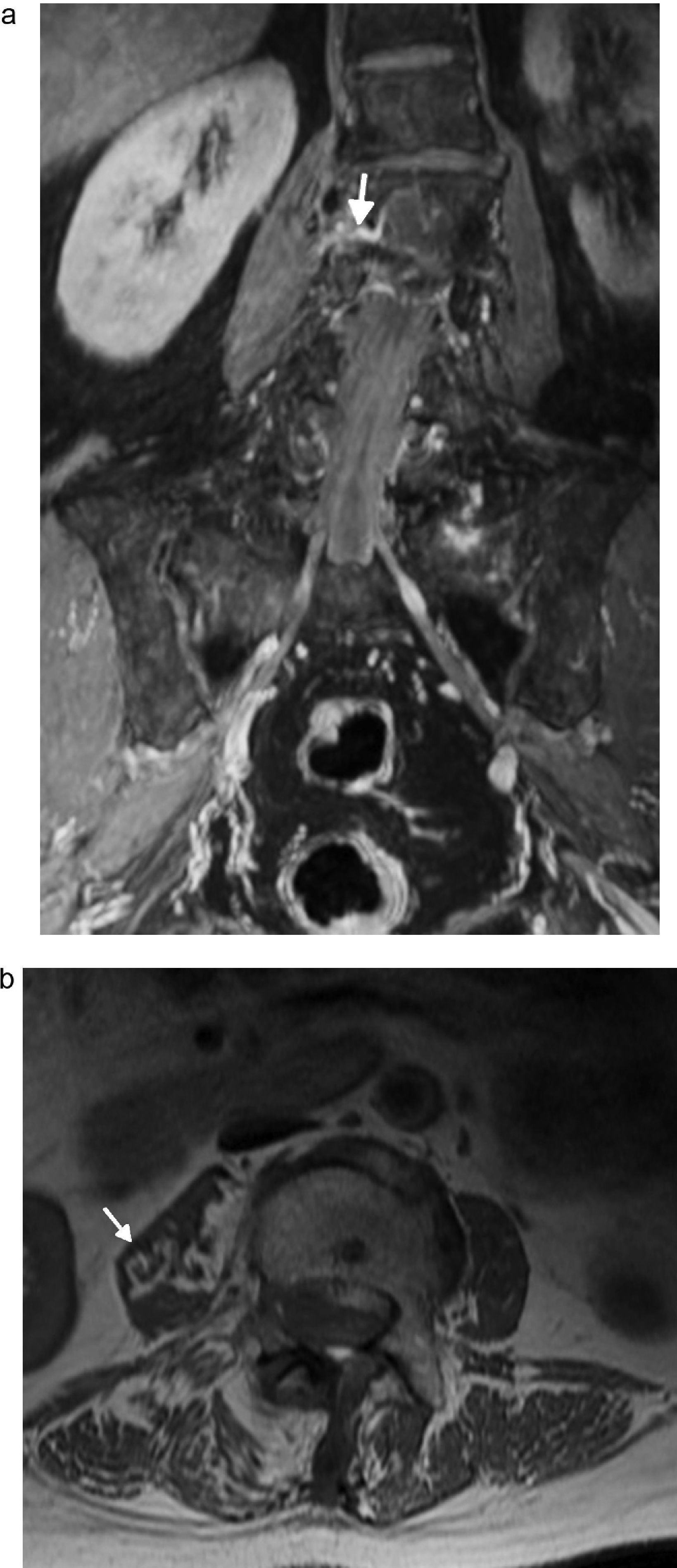
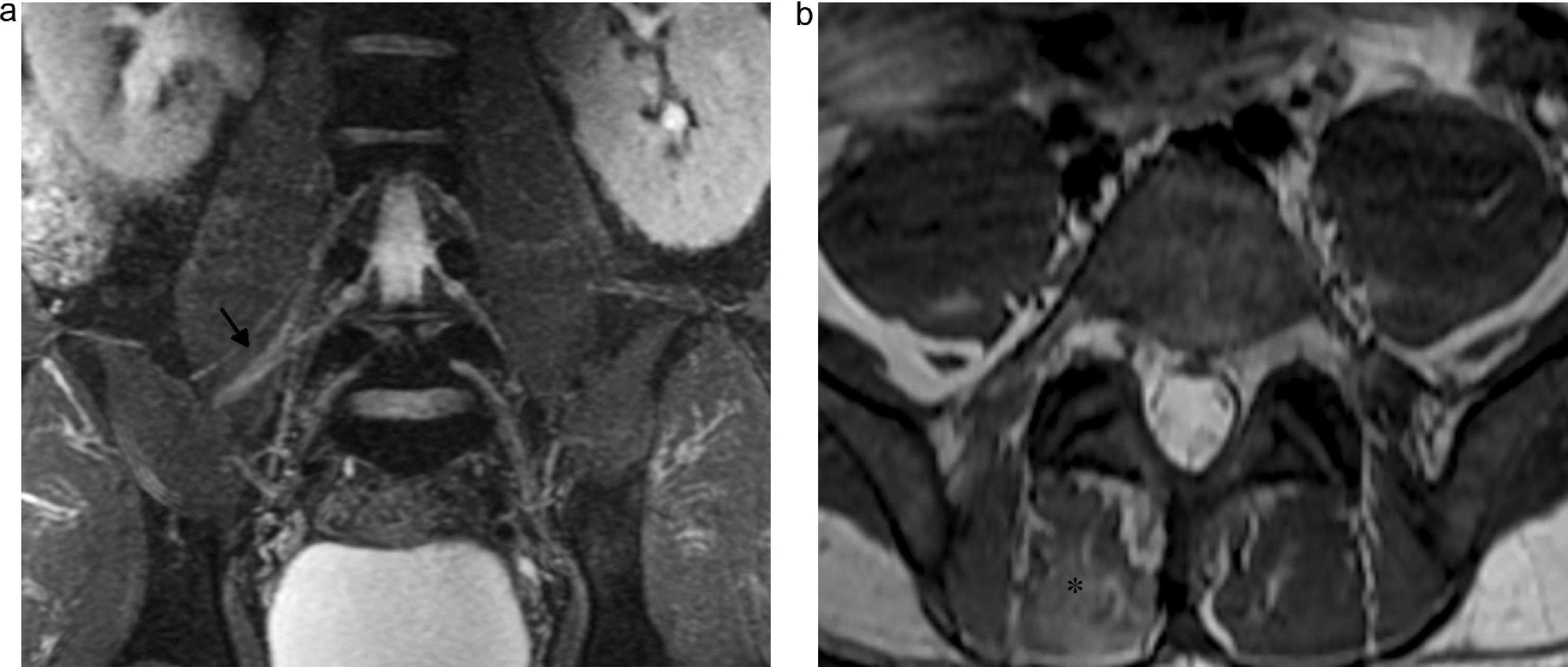
Lesion of pudendal nerve. Surgery on a 60-year-old male due to prostate carcinoma with dysesthesias in the left gluteal region and pain in the perineal region spreading toward the external genitala. (a and b) Coronal fat saturation T2 IDEAL image showing signs of atrophy and fat infiltration in the left elevator annus muscle (arrow) and homolateral ischioccigeus muscle (asterisk) due to chronic denervation of the pudendal nerve.
Lumbosacral plexus can be affected directly by extrinsic compression, diffuse infiltration or secondarily by systemic diseases or inflammatory processes. Peritoneal processes usually compromise the lumbar plexus while pelvic diseases usually compromise the sacral plexus. The lesions of the psoas muscles are one of the more common causes of compromise of the lumbar plexus: traumas or surgical interventions, hematomas associated with anticoagulant therapies, abscesses and tumor infiltration. The serious osteoarthritic processes of the spine associated with scoliosis are also a cause of radicular compression (Fig. 9). The LSP can also be affected by primary tumors of colon, ovary, endometrium or uterine neck and secondarily by metastases of breast tumors, sarcomas, lymphomas and multiple myeloma.31
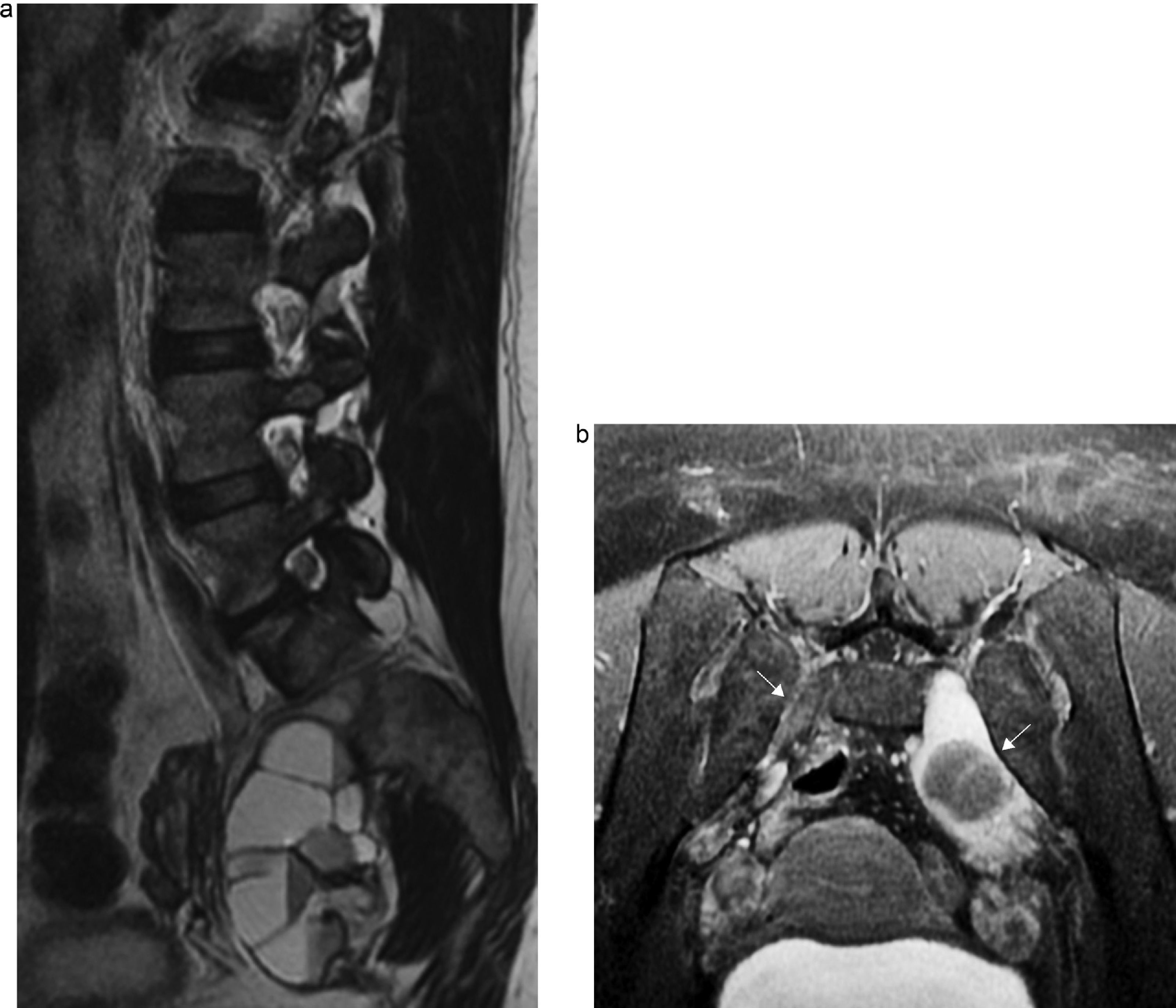
Compression radiculopathy. Sixty-six-year-old woman presenting with back pain and inner right thigh pain. (a) Coronal fat saturated T1 IDEAL sequence. Left convexity lumbar scoliosis associated with osteoarthritic changes causing thickening and post-contrast enhancement of the right postganglionar L2–L3 root with horizontalization of its course (arrow). (b) Axial fat saturation T1 IDEAL images. Atrophy and fat replacement from the psoas muscle (arrow) of the right side associated with changes due to denervation.
The lesion of sacral plexus on the other hand can be associated with infectious processes or osteoarthritic disease of sacroiliac joints, pelvis or hip fractures, surgical interventions, aneurysms of aorta and tumor infiltration due to colorectal and uterine neck cancer.
MRN shows thickening of plexus or the affected nerve, hyperintensity in T2 pulses and IV post-contrast enhancement. It is common to see denervatory changes in the paraspinal and the peripheral nerve-dependent muscles.21
Tumors of lumbosacral plexusBenign tumorsSchwannoma, neurilemmoma or neurinoma is a slow growth-benign tumor of Schwann cells of the neural sheath. This lesion usually evolves excentrically to the nerve and is contained by the perineurum. It corresponds to 5% of benign neoplasms of soft tissues and debuts at 20–50 years of age. The LSP is a common place for the development of these tumors. Its association with type I neurofibromatosis is rare.32–34
There are two subtypes of neurofibroma: solitary and plexiform. The solitary type is the most common of the two and affects peripheral nerves rather than the LSP. It represents 5% of neoplasms of soft tissues and affects young adults between 20 and 30 years of age. Since they are fusiform, central, not-encapsulated lesions they cannot be separated from the nerve35 (Fig. 10).
Neurofibroma. Thirty-four-year-old woman with a history of neurofibromatosis showing pain in sacral region, calf and left foot. (a) Sagittal T2-weighter FSE sequence of the lumbosacral spine. Heterogeneous expansive lesion and multi-cystic appearance running from the S1–S2 left conjunction hole toward the pelvic region. (b) The very same lesion in one axial fat saturated T2-weighted IDEAL image (thick arrow); check the normal contralateral root (thin arrow).
Schwannomas and neurofibromas share several characteristics in MRN like being fusiform, well defined and rarely over 5cm of diameter. In spinal lesions they enlarge the conjunction orifice and adopt the shape of a “sandwatch”. Frequently it is impossible to distinguish them due to the intraneural or perineural location. Those of intermuscular location are usually surrounded by a fat tissue-layer well seen in T1-weighted images and in cuts oriented in the axis longitudinal to the muscle (split fat sign) like a capsule. Most benign tumors of the neural sheath are isointense–hyperintense with respect to the muscle in T1 and highly hyperintense with respect to fat in T2. In STIR sequences they can show one hyperintense peripheral halo with one hypointense central area or of intermediate signal due to the presence of myxoid tissue in the periphery and fibrosis in the middle called “target sign”. Previously one pathognomic sign of neurofibroma it was considered; however, it can be seen in both and also occasionally in the malignant variable of the neural sheath tumor.36,37 The fascicular pattern is another finding reported in neurogenic tumors like “onion layer”-type of images hyperintense in T2.34
Long-term evolution schwannomas usually suffer degenerative phenomena and show one more heterogeneous pattern with calcifications, hemorrhage areas and cystic degeneration that can simulate one sarcoma. Contrast uptake is variable though it is common in small tumors highly avid for contrast–of homogeneous distribution while in big tumors the uptake can be central, peripheral or irregular nodular.38
The plexiform subtype is almost pathognomic of NFI, usually happens during childhood and has a malignant rate for 8–12%. These tumors expand and distort big segments of one or several nerves and in the MRN they are usually hyperintense in T2 sequences with a “worm sac” kind of look. Post-contrast enhancement is similar to that of solitary neurofibromas39,40 (Fig. 11).
Plexiform neurofibroma. Seven-year-old kid with a history of type I neurofibromatosis. Control MRIs. (a and b) axial STIR MRI showing multiple expansive heterogeneous images located in the lumbosacral plexus region with involvement of the femoral nerve (black arrow), lumbosacral trunk (long white arrow), sciatic nerve (short white arrow), internal obturator (asterisk), and pudendal nerve (dotted arrow) of the right side.
Malignant schwannoma, neurogenic sarcoma and neurofibrosarcoma are de novo tumors or come from the benign variable. They are 5–10% of soft tissue-sarcomas.33 As a matter of fact 25–50% of malignant tumors of the neural sheath occur in patients with NF1. Of these half are de novo and the other half due to malignant transformation of preexisting neurofibromas.32 Most occur like bulky and painful masses between 20 and 50 years of age. In patients with NF the mean age of occurrence is lower. Usually they are high-grade sarcomas so prognosis is usually poor with a trend toward recurrence and distance metastasis. Anatomopathologically they show areas of myxoid component, hemorrhage and necrosis (Fig. 12).
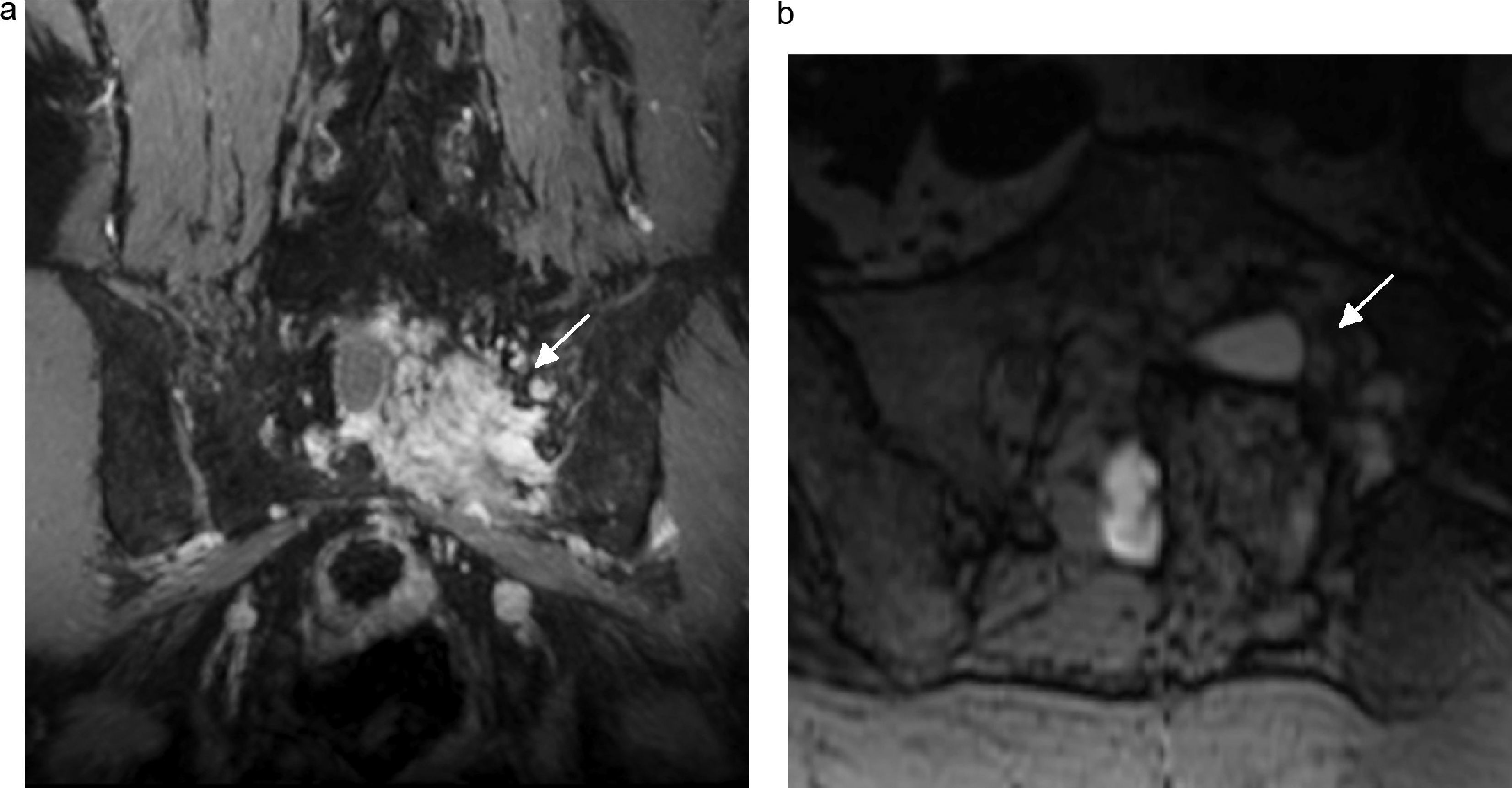
Malignant schwannoma. Seventy-one-year-old male whose malignant sacral schwannoma was removed now showing treatment-refractory cyatalgia. Tumor relapse confirmed on the control MRI. (a) Coronal fat and water saturated IDEAL T1-weighted sequence with contrast showing one heterogeneous lesion intensely enhanced after IV injection of contrast (arrows) based on L5–S1 and S1–S2 nerve roots. (b) The very same lesion in the axial fat saturated IDEAL T1-weighted sequence (arrow).
They typically evolve in major nervous trunks, in the segment more proximal to LSP and in major nerves of lower limbs like great masses affecting the whole thickness of the nerve, with proximal and distal extension.
With the MRN it is not possible to distinguish between benign and malignant tumors of the neural sheath.9 In general both the intensity of signal and post-contrast enhancement are inespecific in the malignant variable and indistinguishable from benign tumors. They are also not significantly different from soft tissues-sarcomas.40 The characteristics indicating the malignancy of MRN images are >5cm, irregular margins and highly heterogeneous signals.33,40,41
LymphomaExcept for neural sheath tumors the malignant tumors affecting the lumbosacral plexus are extremely rare. The primary involvement of LSP represents one extraganglionar manifestation of a high-grade B cell non-Hodgkin lymphoma. They debut as one diffuse thickening of a hyperintense neural segment in T2 and isointense to the muscle in T1. Enhancement is variable42,43 (Fig. 13).
Lymphoma. Seventy-two-year-old male with a history of rapidly advancing-B cell lymphoma and paraparesis with symptoms of sphincter disturbances. (a) Axial T1-weighted D12–L1 image showing occupation of the epidural space due to one lesion of intermediate signal running through the conjunction holes (arrows). (b) Axial T1-weighted sequence with contrast showing the homogeneous uptake of lesions.
Secondary affectation of LSP can be due to direct extension, contiguity or lymphatic dissemination. Melanomas can be due to LSP and not as commonly to breast and lung tumors. Image studies cannot distinguish between these types of tumors from primary tumors; this is why knowing the history of the underlying disease helps the diagnostic work-up–same thing happens with the presence of adjacent lymphatic ganglia, the affectation of bone tissue in the pelvis or spine, or the appearance of multiple infiltrating lesions.18
Lumbosacral polyneuropathyThe LSP can be affected by systemic and inflammatory causes like diabetes mellitus, the Guillain–Barré syndrome, vasculitis, chronic inflammatory demyelinating polyneuropathy, hereditary neuropathies like the Charcot–Marie–Tooth disease, radiation neuropathies, amyloidosis, sarcoidosis and connective tissue diseases.4,44
Diabetic plexopathyDiabetic lumbosacral polyradiculoneuropathy, also known as diabetic amyotrophy, usually has a subacute course while debuting with pain, loss of weight and multifocal uni- or bilateral45,46 affectation of LSP. Katz et al.47 confirmed the coexisting affectation of the brachial plexus in 15% of one series of 60 patients with lumbosacral plexopathy.
Massie et al.48 could confirm anatomopathologically ischemic lesion and microvasculitis in nerves affected by diabetes characterized by multifocal loss of fibers, perineural thickening, neovascularization and neuromas associated with perivascular inflammatory collections, vessel wall inflammation and hemosiderin-laden macrophages.
In the MRN we can see the hyperintense nerve on T2 with variable degrees, as well as root hypertrophy and post-contrast enhancement. Nerve or affected trunk-dependant muscles show changes in structure, T2 hyperintensity in cases of acute or subacute denervation and T1 hyperintensity with fat infiltration due to chronic denervation that might be associated with muscle atrophy48 (Fig. 14).
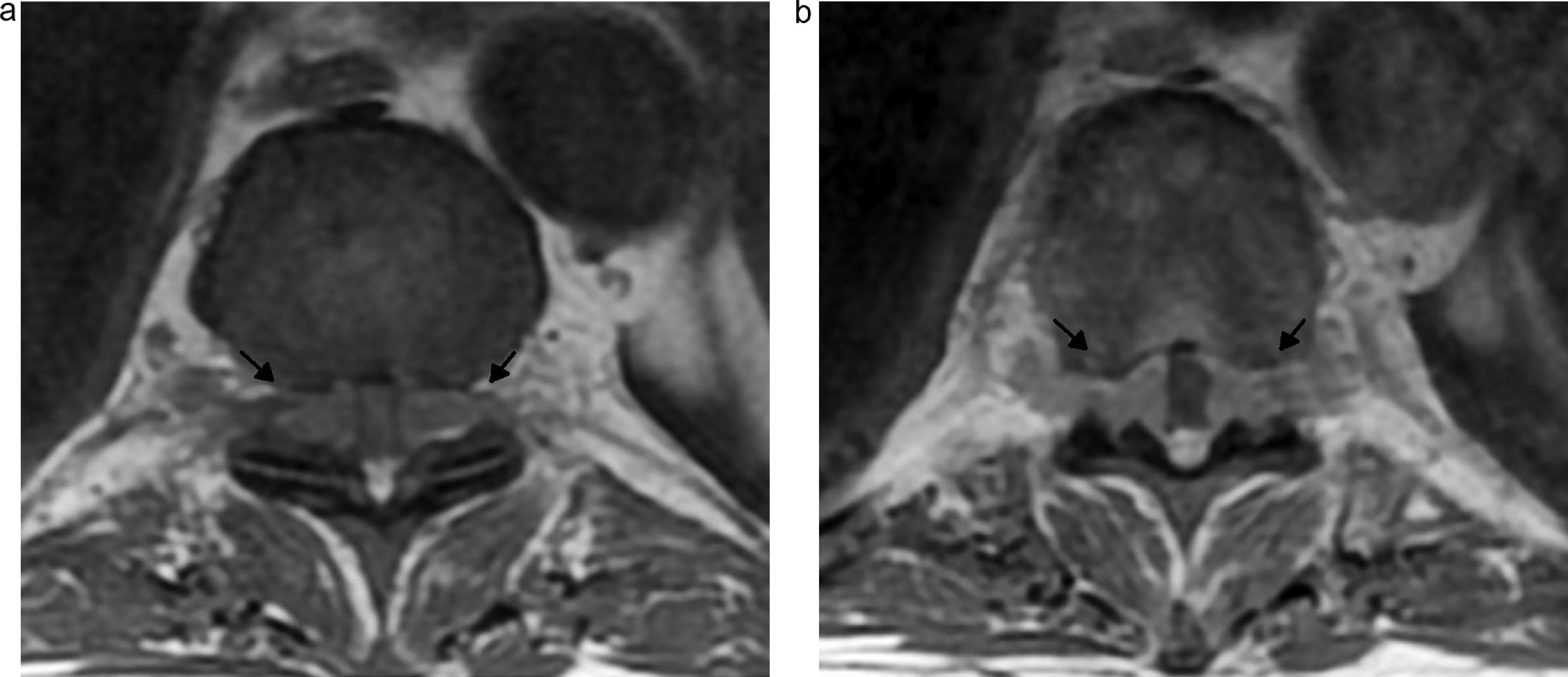
Diabetic polyneuropathy. Forty-three-year-old male with a history of uncontrolled diabetes mellitus type IIand back pain radiating the anterior side of the right thigh. (a) Coronal IDEAL fat saturation T2-weighted image showing the lumbosacral plexus slightly thickened with fascicular appearance and predominant at the right femoral nerve (arrow). (b) Axial T2-weighted image showing hyperintensity of the paraspinal musculature right medial tract at the level of L5–S1 as a sign of muscle edema (asterisk).
The acronym CIDP was proposed by Dyck et al.49 in 1982, meaning an autoimmune demyelinating neuropathy capable of responding to corticoid therapy. Symptoms are sensitive motor, mainly sensitive and affecting the upper and lower limbs and less commonly cranial pairs. It is usually subacute (between 4 and 8 weeks).
The diagnosis is usually based on demyelinating signs when studying nerve conduction and hyperproteinorrachy in the cephaloraquideal liquid. The electromyogram shows signs of demyelination. The biopsy is for inconclusive cases and segmental demyelination and re-demyelination only.50
Several studies have confirmed hypertrophic changes in the LSP branches of patients with CIDP.3,50–55 In the MRN fusiform hypertrophy of peripheral nerves and thickening of nerve roots in most patients have been reported. Exceptionally, hypertrophy can be seen in the roots of the cauda equina.56 These hypertrophic changes are not pathognomic of this chronic polyradiculoneuropathy since they can also be seen in lymphoma-associated infiltration or hereditary Charcot–Marie–Tooth-like polyneuropathies.57
In some cases post-contrast enhancement of ganglion annexed to the nerve root as a sign of disruption of the hemato-neural barrier has been reported. Enhancement persists even though symptoms have decreased with therapy58 (Fig. 15).
Unknown neuropathy or plexopathyIn cases where clinical exploration cannot reveal the cause of neuropathy it is possible that LSP shows local or diffuse abnormalities on the MRN indicative of structural damage that usually disappears in further controls but that can be associated with inflammatory, idiopathic or even viral conditions like those seen in brachial plexitis.19
ConclusionMRN, and in particular with high resolution-3T sequences, needs to be considered like one diagnostic method complementing clinical and electrophysiological data for the assessment of lumbosacral plexus. It allows us to find focal or diffuse lesions, locate its uni- or bilaterality, locate them precisely, make assumptions in the cause, and determine muscular changes due to denervation.
Ethical responsibilitiesRight to privacy and informed consentAuthors confirm that they have obtained the written informed prior consent from patients and/or subjects appearing in this article. This document is in the possession of the corresponding author.
Data confidentialityAuthors confirm that there are no personal data from patients in this article.
Protection of people and animalsAuthors confirm that no experiments have been performed on human beings or animals.
Authors/collaborators- 1.
Manager of the integrity of the study: CC and FB.
- 2.
Original idea of the study: CC and FB.
- 3.
Study design: CC, MS, IE, and FB.
- 4.
Data mining: CC, MS IE, and FB.
- 5.
Data analysis and interpretation: CC, MS, IE, and FB.
- 6.
Statistical analysis: N/A.
- 7.
Reference search: CC, MS, and IE.
- 8.
Reference search: CC, MS, IE, and FB.
- 9.
Manuscript critical review with intellectually relevant contributions: CC, MS, IE, and FB.
- 10.
Final version approval: CC, MS, IE, and FB.
The authors reported no conflict of interest.
Please cite this article as: Cejas C, Escobar I, Serra M, Barroso F. Neurografía de alta resolución del plexo lumbosacro en resonancia magnética 3T. Radiología. 2015;57:22–34.