To determine the degree of tumor necrosis in surgical specimens of hepatocellular carcinomas treated with microspheres preloaded with doxorubicin and to analyze the relationship between the degree of necrosis and (a) morphologic factors and (b) imaging biomarkers.
Material and methodsWe studied the livers of 21 patients who had undergone selective arterial chemoembolization with DC beads (Biocompatibles, UK) before receiving liver transplants.
ResultsImaging techniques detected 43 nodules (mean size, 25mm). Angiography showed 25 hypervascularized nodules, 12 slightly vascularized nodules, and 6 avascular nodules. A total of 81 hepatocellular carcinomas (mean size, 15mm) were detected in the specimens: two were capsular and two had vascular infiltration. The mean degree of necrosis after chemoembolization was 39%; necrosis was greater than 60% in 28 hepatocellular carcinomas and less than 60% in 52. The degree of necrosis correlated significantly with the time elapsed between the last chemoembolization treatment and liver transplantation (the degree of necrosis decreased as time increased), with the number of nodules in the specimen, and with capsular infiltration. When imaging techniques detected 1 or 2 nodules, there was a greater probability of achieving greater than 90% necrosis. No relation with the degree of necrosis achieved was found for the size of the nodules detected at imaging, the enhancement pattern, or the number of chemoembolization treatments.
ConclusionThe degree of necrosis achieved depends on the time spent on the waiting list, on the number of nodules in the specimen, and on whether capsular infiltration is present.
Determinar el grado de necrosis tumoral en la pieza quirúrgica de hepatocarcinomas tratados con microesferas precargadas con doxorrubicina (QETA-DEB) y su relación con factores morfológicos y biomarcadores de imagen.
Material y métodos21 pacientes a quienes se realizó quimioembolización arterial selectiva con partículas DC-Beads (Biocompatibles, UK) fueron posteriormente trasplantados y analizados los hígados histológicamente.
ResultadosSe detectaron por diferentes técnicas de imagen 43 nódulos con un tamaño medio de 25mm. Angiográficamente eran 25 nódulos hipervascularizados, 12 discretamente vascularizados y 6 avasculares. En la pieza se encontraron 81 HCCs con un tamaño medio de 15mm.; dos presentaron infiltración vascular y otros dos infiltración capsular. La necrosis media conseguida tras QETA-DEB fue del 39% siendo >60% en 28 HCCs y <60% en 52. Encontramos correlación estadística entre el índice de necrosis conseguida con el tiempo que transcurre desde la última QETA-DEB hasta el trasplante (siendo significativamente menor la necrosis conforme aumenta el tiempo), con el número de nódulos encontrados en la pieza y con la infiltración capsular. Asimismo, podemos predecir que cuando por técnicas de imagen detectamos 1 ò 2 nódulos tendremos más probabilidad de conseguir de manera significativa índices de necrosis >90%. Sin embargo, ni el tamaño de los nódulos detectados por técnicas de imagen, ni el patrón de realce post-contraste ni el número de QETA-DEBs realizadas influye significativamente en la necrosis conseguida.
ConclusiónLa necrosis producida depende del tiempo en lista de espera, del número de nódulos en la pieza y de la infiltración capsular del tumor.
Transarterial chemoembolization (TACE) is a palliative treatment for patients with asymptomatic hepatocellular carcinoma (HCC), preserved liver function and lack of extrahepatic dissemination or vascular invasion (intermediate stage-B–based on the criteria from the Barcelona Clinic Liver Diseases–BCLC). Indications now include patients with early-stage HCC (Barcelona Clinic Liver Diseases–A) without surgical indication or local ablative therapies, to patients waiting for liver transplantation (LT) (in an effort to attain local control in the meantime) or to downstage the tumor in patients who exceed the criteria for LT.1,2
Not too long ago TACE started being used with preloaded doxorubicin hydrogel beads (DEB-TACE) that are better tolerated than conventional TACE (cTACE) with lipiodol+a chemotherapeutic agent+embolization particles (gelfoam or polyvinyl alcohol) since they release the drug in the liver blood stream more slowly and continually at the same time they reduce its passage into the systemic circulation (yet despite the fact that they increases the dose).3,4 Also it is more effective than conventional TACE in patients with recurrent and bilobar disease, although it is not been confirmed to improve survival or reduce local relapse.5,6 Nonetheless it does improve local response with respect to soft embolization–only embolization particles only.7
The effect of TACE in HCC is necrosis that is evaluated through the imaging criteria of the European Association for the Study of the Liver.8–10 An acceptable anatomo-pathologic correlation has been observed11–14 in studies performed on transplanted livers after cTACE.15–17 However, the studies of anatomo-pathologic correlation of the antitumor effect of DEB-TACE18 and the driving factors are scarce.
Our goal is to assess the degree of actual tumor necrosis caused by DEB-TACE and those factors it possibly correlates to based on the anatomo-pathologic examination of the livers explanted in the LT.
Material and methodsPatientsFrom 2008 until April 2012, we performed DEB-TACE in 120 cirrhotic patients with HCC of whom 21 were subsequently transplanted and then made up the sample of this retrospective study approved by the hospital ethics committee with the signing of the written informed consent to undergo the procedure. Given that for the LT, the Milan1 criteria were followed, for the DEB-TACE the following criteria were included: (1) single tumor ≤5cm that cannot be treated through local ablative therapies; (2) up to three tumors none of them >3cm; (3) lack of vascular invasion or extrahepatic dissemination through imaging methods; (4) preserved liver function (child A–B); (5) acceptable coagulation condition (prothrombin activity>55%; platelets>60,000/mm3); (6) adequate kidney function (creatinine<1.5mg/dl); and (7) patients exceeding the Milan criteria (n=6) who were not initial candidates to undergo LT underwent DEB-TACE which led to tumor regression or downstaging were included in the waiting list.
The exclusion criteria were: (1) portal thrombosis (total or partial); (2) significant hepatofugous portal flow in the angiography; (3) contraindication of doxorubicin; and (4) biliary obstruction.
The diagnosis of HCC was achieved through different image modalitiesand pieces of equipment (because of our patients’ heterogeneity) and based on the criteria set by the clinical guidelines of the American Association for the Study of the Liver Diseases8,19 so the nodes were not biopsied yet six of them with atypical radiological behavior were tagged as HCC and then confirmed in an anatomo-pathological analysis.
Angiographic proceedingThe right femoral artery was canalized, after anesthetizing the area locally and with the patient under sedation (1cc of fentanyl and 2cc of midazolam) so that a standard introducer (5F and 10cm long) could be introduced. We selectively catheterized the celiac trunk and the superior mesenteric artery with multipurpose or cobra-type of catheters (4–5F) and after analyzing the arterial anatomic variables and verifying portal patency and its preferential hepatic flow, the segmental branches of each tumor were supraselectively catheterized with microcatheters (Progreat. Terumo, Leuven or Rapid Transit. Cordis, Miami, USA) to inject the hydrogel beads (DC Bead. Biocompatibles, UK) mixed with iodized contrast in a 1:1 ratio. These beads were preloaded with doxorubicin (75mg per vial) 12h before the procedure at the Pharmacy Service.
We considered that the DEB-TACE was adequate when fluoroscopically we started seeing the reflux of the embolization material–even if we had not administered the entire particle load available. This is why we always start the procedure with a 300–500mμ vial followed by another of 500–700mμ and when tumor devascularization was not adequate yet we completed it with 400–500mμ beads (Embozene, Celonova Biosciences, Newman, USA).
When the disease was bilobar, the supraselective procedure was performed in a single session.
No antibiotics or anti-inflammatories were administered in a prophylactic, routine fashion and post-embolization syndrome–abdominal pain, low-grade fever, vomiting, asthenia, etc. was managed individually and symptom-based.
Clinical-radiologic follow-upA strict clinical and analytical control was carried out (hemogram and hepatorenal function test: transaminase, bilirubin, alkaline phosphatase, gamma-GT, creatinine, urea) during the first week, and then a first dynamic liver CT was performed 1 month after the DEB-TACE with a 64-crown multidetector CT, VCT Light Speed, (GE Health Care, Milwaukee, Wi, USA). The images were interpreted by consensus among the radiologists involved in the study who measured the largest axis of the nodes detected and intra-tumor enhancement. The nodes detected in the explanted liver but remained undetected though images before the LT were considered «new onset» nodes. Applying the criteria from the European Association for the Study of the Liver,8,10 we did not perform a new DEB-TACE session when the response was complete but we did when it was partial. When in presence of disease progression the patient was removed from the LT waiting list as the possibility of performing a new session or administering Sorafenib was individually evaluated for every patient.
After this first control and given the retrospective character of our study the follow-up of patients on the waiting list was very variable and not constant (with different image modalities and equipment since they came from different hospitals) and in some of them we did not have any image modalities after the initial CT a month after the DEB-TACE yet no patient was lost during the follow-up.
Anatomopathologic studyOnce the LT was performed the anatomo-pathologist made 3–5mm-thick coronal cuts and then transversal cuts of the explanted liver and the sections were fixed with 4% formaldehyde (buffered at pH 7 and stabilized in methanol). One hepato-pathologist with more than 20 years of experience selected 2.2–0.3cm sections and the nodes in order to process soak them in a paraffin solution, cut them and dye them. In her report, she recorded macroscopic data of the explanted piece (number and size of the nodes, anatomo-pathologic type and capsular or vascular infiltration). She also performed the microscopic study of each HCC subjectively assessing the percentage of necrosis in each one. Based on former studies three groups were separated based on this percentage: >90%, 60–90% and <60%.13,16
Statistical analysisFor the statistical analysis, the IBM software, PASW Statistics 18 (version in Spanish language) was used and the quantitative and qualitative variables were described. We compared the quantitative variables (pre- and post-DEB-TACE node size, number of nodes pre- and post-DEB-TACE and time elapsed from the last chemoembolization until the LT was performed) through the Kruskal–Wallis test and the Mann–Whitney U test. Qualitative variables (angiographic pattern, number of DEB-TACE and capsular/vascular infiltration of the HCC) with the Chi square test. The existing correlation between the nodes detected in the image and those found in the piece was established with Spearman's correlation ratio. Values were considered statistically significant when their level of reliability was beyond 95% (p<0.05).
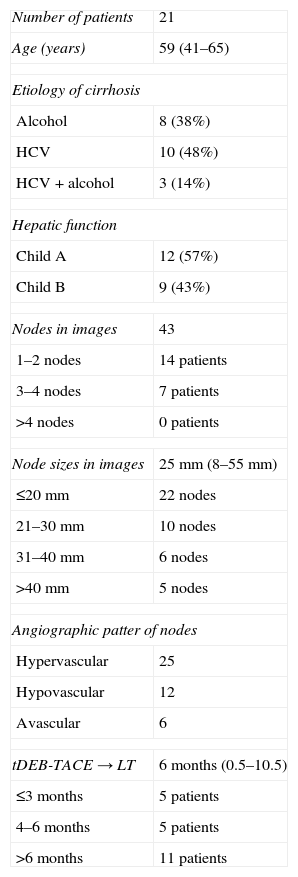
ResultsAmong the 21 patients studied, 18 were male with a mean age of 56 years (41–65 years). The cause of the cirrhosis was alcoholic (38%), hepatitis C virus (48%) or both (14%). Liver function was at a Child A (57%) and Child B (43%) stages. A DEB-TACE session was performed in 16 patients, two sessions in 3 patients and three sessions in two patients (mean: 1.3 sessions/patient). The mean time elapsed from the last session to the LT was 6 months (0.5–10.5 months) (Table 1).
Demographic characteristics of patients.
| Number of patients | 21 |
| Age (years) | 59 (41–65) |
| Etiology of cirrhosis | |
| Alcohol | 8 (38%) |
| HCV | 10 (48%) |
| HCV+alcohol | 3 (14%) |
| Hepatic function | |
| Child A | 12 (57%) |
| Child B | 9 (43%) |
| Nodes in images | 43 |
| 1–2 nodes | 14 patients |
| 3–4 nodes | 7 patients |
| >4 nodes | 0 patients |
| Node sizes in images | 25mm (8–55mm) |
| ≤20mm | 22 nodes |
| 21–30mm | 10 nodes |
| 31–40mm | 6 nodes |
| >40mm | 5 nodes |
| Angiographic patter of nodes | |
| Hypervascular | 25 |
| Hypovascular | 12 |
| Avascular | 6 |
| tDEB-TACE→LT | 6 months (0.5–10.5) |
| ≤3 months | 5 patients |
| 4–6 months | 5 patients |
| >6 months | 11 patients |
tDEB-TACE→LT: time (in months) from the last session of transarterial chemoembolizations with preloaded selective doxorubicin hydrogel beads (DEB-TACE) elapsed up to liver transplantation (LT).
HCV: Hepatitis C virus.
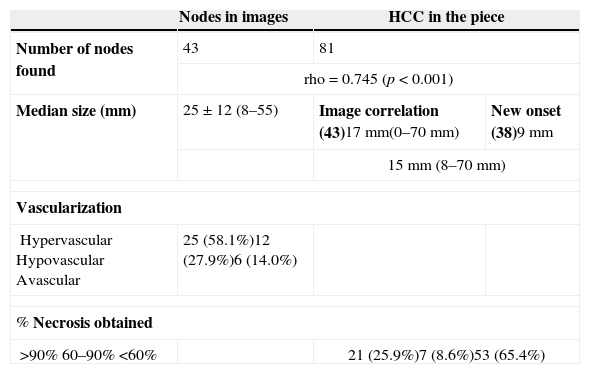
We detected (with a minimum of 2 coincidental image modalities) and treated 43 nodes with an average size of 25mm (8–55mm). Among them, 25 were classified subjectively as hypervascularized through image modalities, 12 were discreetly vascularized and 6 were avascular (Table 2). In the piece 81 HCCs were found whose size was significantly reduced after the DEB-TACE (Table 2), with a overall average size of 15mm (8–70mm) (p<0.001), 17mm for the 43 detected through image modalities and 9mm for the 38 new-onset HCCs. Three (3) of the nodes disappeared, two (2) presented with vascular infiltration and two others with capsular infiltration. The median necrosis achieved after DEB-TACE was 39%, >90% in 21 HCCs, 60–90% in 7 and <60% in the remaining 53 (Table 3).
Description of nodes studied through images (n=43) and in the surgical piece (n=81).
| Nodes in images | HCC in the piece | ||
|---|---|---|---|
| Number of nodes found | 43 | 81 | |
| rho=0.745 (p<0.001) | |||
| Median size (mm) | 25±12 (8–55) | Image correlation (43)17mm(0–70mm) | New onset (38)9mm |
| 15mm (8–70mm) | |||
| Vascularization | |||
| HypervascularHypovascularAvascular | 25 (58.1%)12 (27.9%)6 (14.0%) | ||
| % Necrosis obtained | |||
| >90%60–90%<60% | 21 (25.9%)7 (8.6%)53 (65.4%) | ||
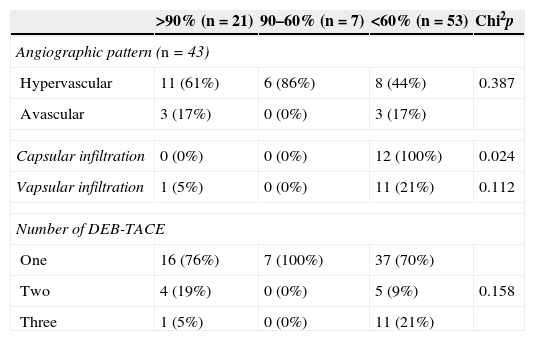
Three (3) possible factors associated with the percentage of necrosis obtained in the nodes after DEB-TACE.
| >90% (n=21) | 90–60% (n=7) | <60% (n=53) | Chi2p | |
|---|---|---|---|---|
| Angiographic pattern (n=43) | ||||
| Hypervascular | 11 (61%) | 6 (86%) | 8 (44%) | 0.387 |
| Avascular | 3 (17%) | 0 (0%) | 3 (17%) | |
| Capsular infiltration | 0 (0%) | 0 (0%) | 12 (100%) | 0.024 |
| Vapsular infiltration | 1 (5%) | 0 (0%) | 11 (21%) | 0.112 |
| Number of DEB-TACE | ||||
| One | 16 (76%) | 7 (100%) | 37 (70%) | |
| Two | 4 (19%) | 0 (0%) | 5 (9%) | 0.158 |
| Three | 1 (5%) | 0 (0%) | 11 (21%) | |
| Median | Median | Median | K–Wallis p | |
|---|---|---|---|---|
| Time DEB-TACE-LT (months) | 5 | 6 | 8 | <0.001 |
| Number of nodes in image (n) | 2 | 3 | 3 | <0.001 |
| Size pre-DEB-TACE (mm) | 28.17 | 22.71 | 22.67 | 0.285 |
| Number of nodes in the piece (n) | 3 | 5 | 7 | <0.001 |
| Size post-DEB-TACE (mm) | 15.76 | 18.86 | 13.96 | 0.029 |
DEB-TACE: transarterial chemoembolizations with preloaded selective doxorubicin hydrogel beads.
In 31 HCCs of 7 patients of the explanted piece, necrosis was nonexistent. Its mean size was 11mm, with an average 5 tumors/patient and a median time elapsed from the last session to the LT of 7.7 months. Among the 28 HCCs of the 16 patients where necrosis was >60%, the average size of the tumors was 20.5mm with an average 3.6 tumors/patient and a median time elapsed from the last session to the LT of 5.7 months.
The statistical correlation between the index of necrosis and the time elapsed from the last DEB-TACE until the LT was high (p<0.001) and it became significantly smaller with the passing of time. This factor was related with the number of nodes found in the piece that also drove the necrosis in such a way that more tumors the smaller the necrosis (p<0.001) (Table 3). In 13 patients with ≤4 HCCs in the piece (23 nodes, 1.7 tumors/patient) the mean necrosis was 78%, while in 8 patients with >5 HCCs (56 tumors, 6.6 tumors/patient) it was just 20%. When we detected one or two nodes through image modalities the odds of having indexes of necrosis beyond 90% was greater (Table 3).
Neither the size of the nodes before the DEB-TACE (p=0.285) nor the number of procedures performed (p=0.158) influenced necrosis significantly (Table 3) (Figs. 1 and 2).
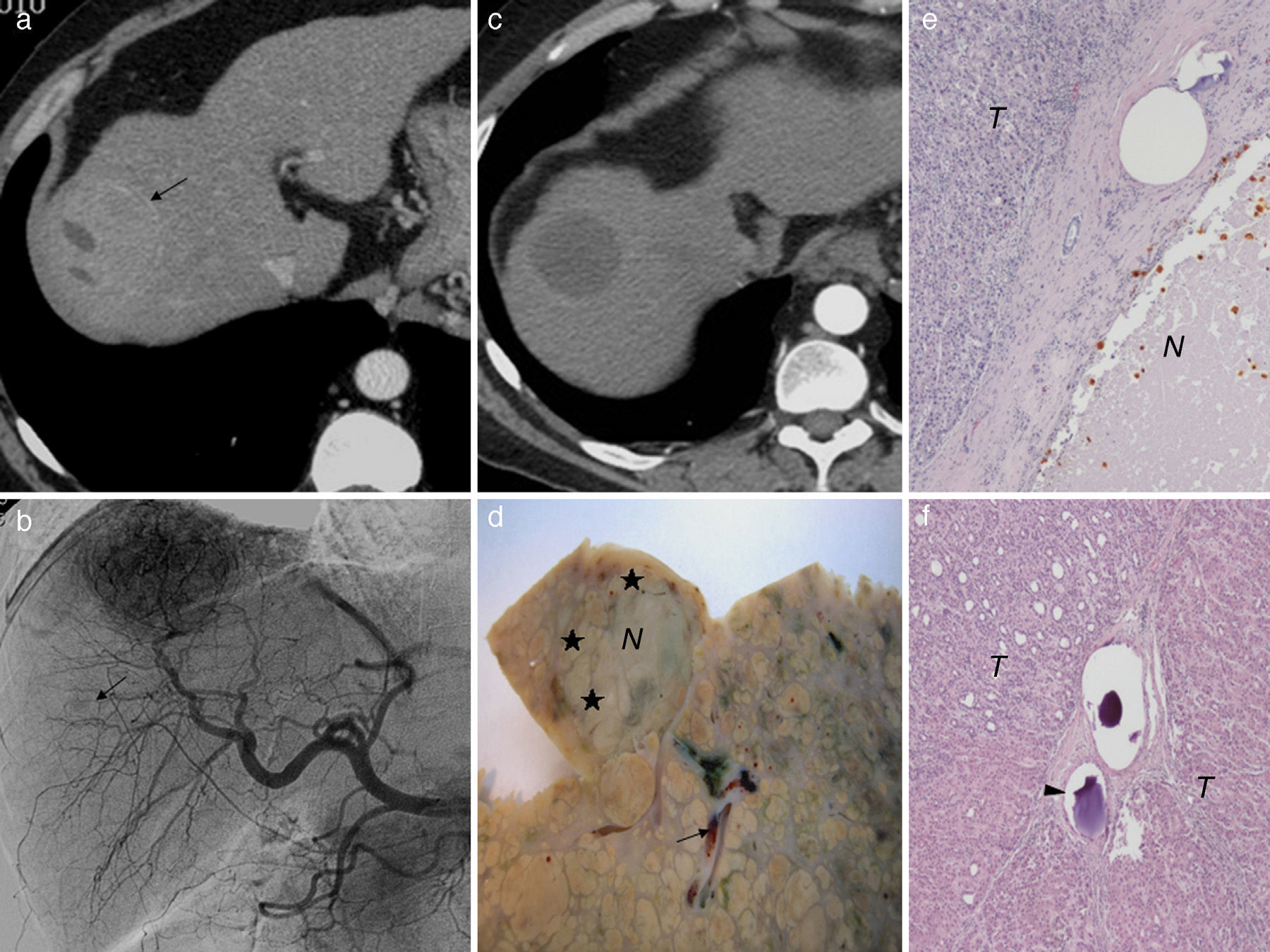
(a) Axial hepatic CT image in artery phase; 50mm-hepatocarcinoma in segment 8 and areas of necrosis (a). (b) Hepatic angiography confirming hypervascularized tumor and another adjacent 9mm-hypovascular one (arrow) not found through CT. (c) After two (2) sessions of selective transarterial chemoembolizations with hydrogel microspheres preloaded with selective doxorubicine (DEB-TACE) the tumor response is complete. (d) Liver explanted 9 months after the last session of DEB-TACE. Only partial necrosis (30%) of the tumor (N) can be seen with peripheral areas of feasible tumor (♦). See microspheres (arrow) in the vicinity of tumor. (e) Microscopic image (H&E 10×) of a bigger tumor where areas of necrosis (N) and tumor (T) feasibility can be seen. (f) Microscopic image of the smaller hypovascular turmor (H&E 10×). Complete tumor (T) feasibility yet despite the presence of microspheres in the tumor bed (arrow head).
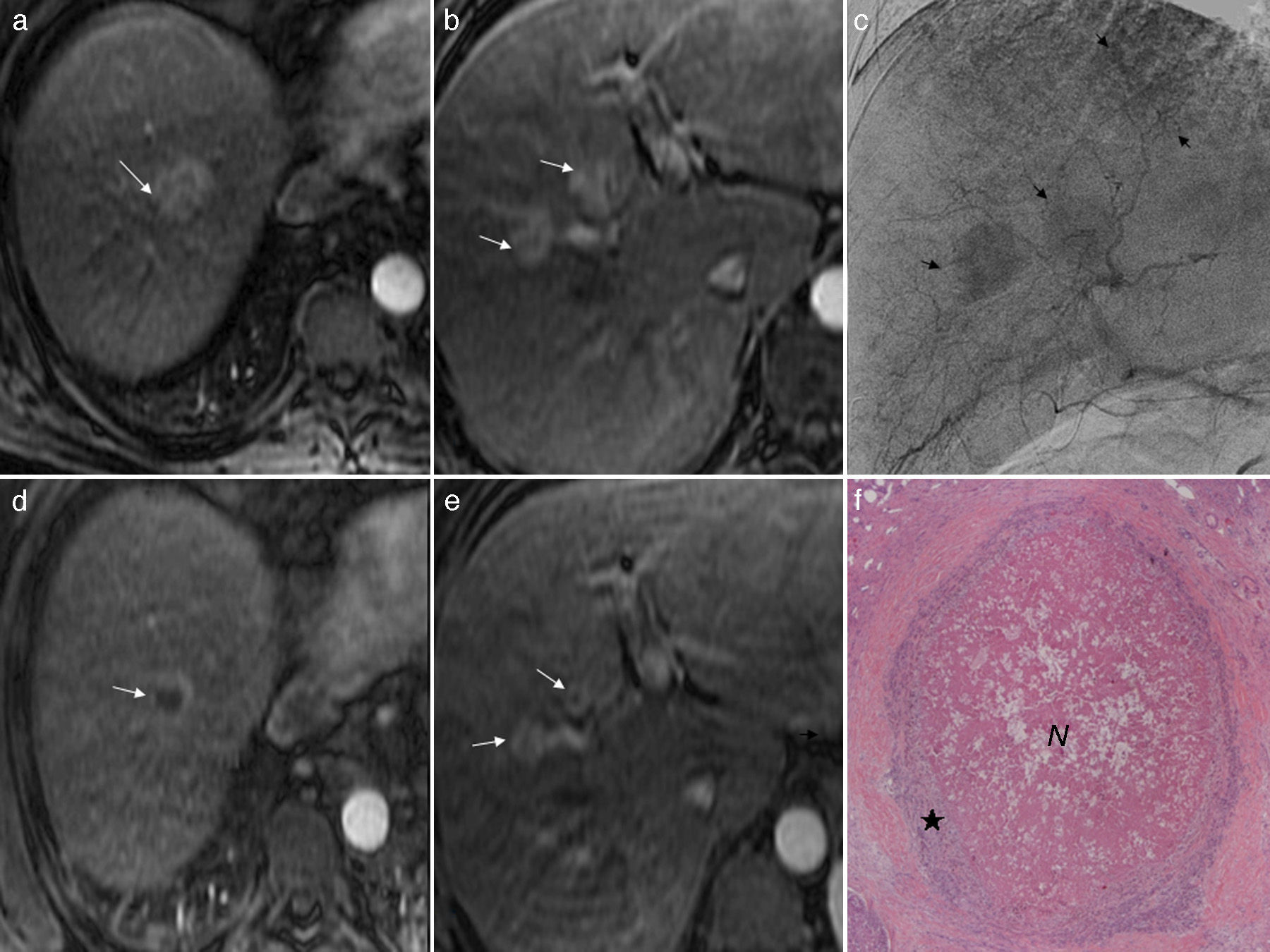
(a and b) Axial T1-weighted MRIs after the injection of gadolinium (artery phase). Three (3) 30, 20 and 20mm-hepatocarcinomas (arrows) can be seen. (c) Hepatic angiography (late phase) showing the tumors (arrows). (d) and (e) T1-weighted MRI axial images after the injection of gadolinium (artery phase). Partial response after one session of selective transarterial chemoembolizations with preloaded selective doxorubicin hydrogel beads (DEB-TACE) in the three (3) hepatocarcinomas (arrows) whose size has been reduced but have been enhanced peripherally. (f) Microscopic image (H&E 10×) of one of those tumors 10 months after DEB-TACE. Central necrosis (N) of 70% with a peripheral halo of tumor feasibility (♦).
In the 25 hypervascularized nodes (median size 25.6mm) the median necrosis was 70%, in the 12 hypervascularized tumors (median size 25.9mm), it as 46%, and in the 6 avascular tumors (median size 20.3mm), it was 50% but these differences were not statistically significant (p=0.387) (Table 3) (Figs. 1 and 2).
There was capsular and vascular infiltration in one patient in some of the nodes, in another patient just vascular infiltration and yet in another patient capsular infiltration only with an average 7.3 HCCs/patient. In the first two patients the median necrosis was nonexistent and in the third one it was 33%. Although tumor vascular infiltration did not significantly influence necrosis (p=0.112), the capsular infiltration did (p=0.024) (Fig. 3), yet these data are biased by the number of HCCs showing infiltration.
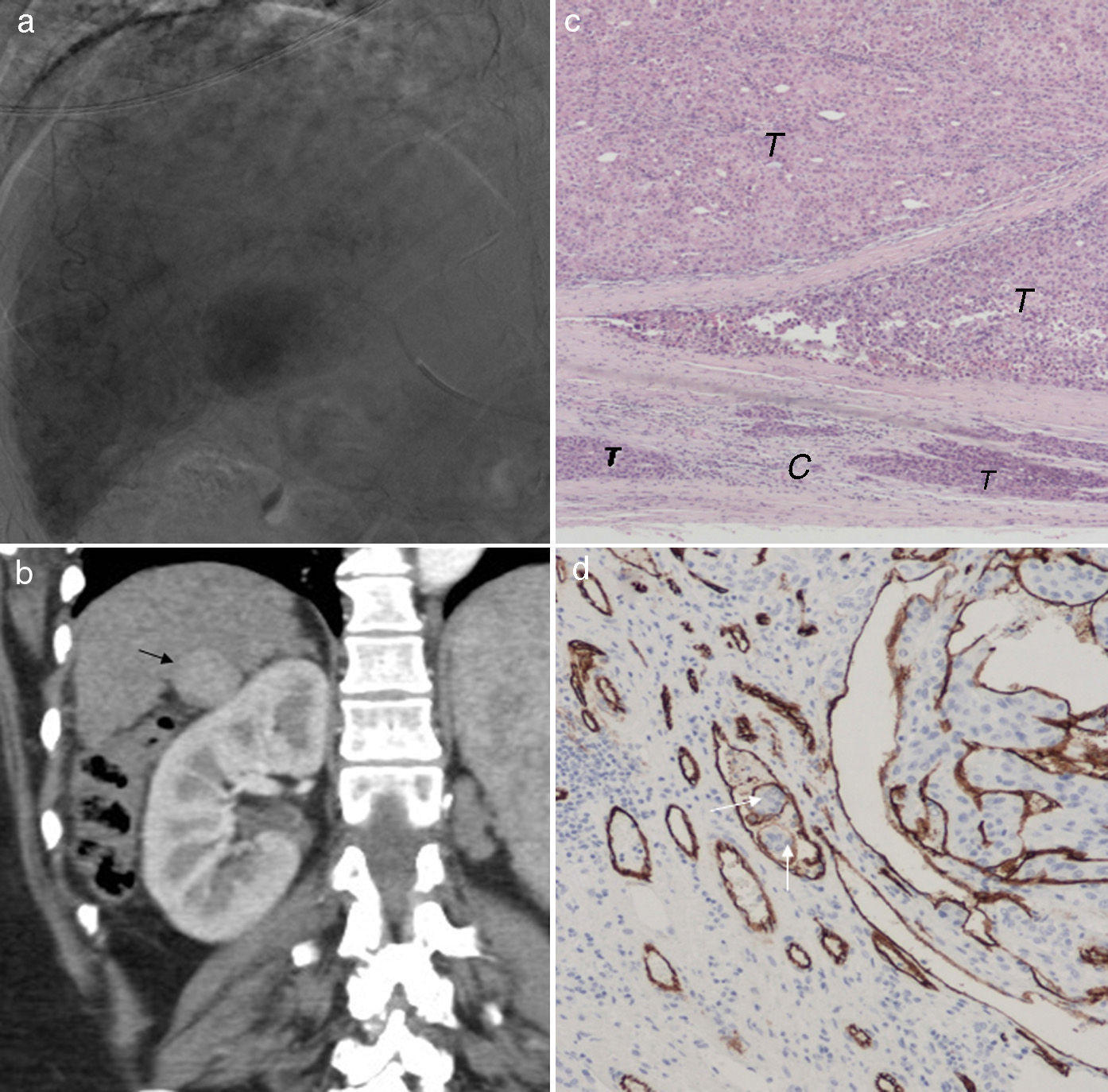
(a) Hepatic angiography (late phase) 30mm-hypervascularized tumor in segment 6. (b) Coronal hepatic CT image in artery phase after three (3) sessions of selective transarterial chemoembolizations with preloaded doxorubicin hydrogel beads. The effect has been nonexistent persisting the complete tumor hypervascularization (arrow). (c) Microscopic image (H&E 10×) of tumor 13 months after the last session of chemoembolization. Complete tumor (T) feasibility with tumor infiltration of the capsule (C). (d) Microscopic image (cd 10× stain specifically staining the vascular wall in brown color). Tumor cells inside a vessel (arrows). In this liver another 10mm-hepatocarcinoma was found too.
In this study we have analyzed the effect of DEB-TACE on HCCs with hydrogel beads loaded with 75mg of oral doxorubicin and saw that necrosis depends on the time elapsed until liver transplantation is performed, on the number of nodes in the piece and on the tumor capsular infiltration. The importance of these results is that there is one similar study published only18 yet despite the fact that in many centers chemoembolization preloaded with particle has replaced both soft chemoembolization due to a better local response and conventional chemoembolization due to a better tolerance and response in advanced disease.3,7
The local response (degree of necrosis) to chemoembolization in patients with HCC is usually analyzed based oto radiologic criteria (mainly those of the European Association for the Study of the Liver). With conventional TACE the radiopathologic correlation of tumoral necrosis has been variable, with slightly more sensitivity of MR than CT,11,12 but it has not been studied much with DEB-TACE. In our study, we cannot correlate the local response of this modality with image modalities since they are not available on the days close to the transplant for many patients. However we can analyze factors that can possibly condition the necrosis caused by the particles. In our case one of the factors that influenced the necrosis the most was the time elapsed from the last treatment to the LT that is somehow related with the time on the waiting list. The longer the time the smaller the index of necrosis. As it has been described with the cTACE it was also related with the discordance between the number of tumors in the piece and those detected through image modalities and then treated supraselectively.12,15,20 Probably this is due to the fact that our patients on the LT waiting list are not followed up according to a protocol after their first liver CT (in an attempt to detecting new-onset untreated tumors) so in addition to a strict follow-up through image modalities we believe that repeated chemoembolization based on a protocol should be considered regardless the imaging response or in a lobar instead of a supra-selective fashion in order to operate on these new-onset tumors that are altering the desired antitumor effect. Similarly and just as it happens with the cTACE15,21 the smaller the number of nodes detected through image modalities the greater the overall necrosis in the piece.
The correlation between the degree of necrosis attained with the cTACE and tumor size has been dissimilar. For some authors HCCs<2–3cm have better complete indexes of response12,22 but other results say otherwise23,24 and some other authors even defend the benefit of this modality to downstage tumors <5cm25. There is still little information about the DEB-TACE yet our results indicate that tumor size considered in isolation has little influence on necrosis somehow more driven by the other factors analyzed.
Hypervascularization has been, in some cases, a factor independent from tumor necrosis when treated with cTACE12,15,26 but not in others.27 Although we have not found any statistically significant relation there is a clear trend in our cases.
Finally the tumor vascular and capsular infiltration is negatively correlated with the necrosis in HCC treated through cTACE and they are known factors of poor prognosis.12,15 Our results with DEB-TACE confirm that there is little necrosis in cases with capsular infiltration since they are closely related with tumor multiplicity being this trend similar to vascular infiltration which by the way does not achieve statistical significance probably because of the few tumors presenting with capsular infiltration.
This study has its limitations especially those derived from its retrospective character and the small number of patients. Also the follow-up of the patients through image modalities has been irregular and inconsistent–non-protocolized due to the fact that our patients came from very different origins preventing the radio-pathologic correlation. Unlike other authors,28 it has not been a goal of this paper to analyze survival and all those factors determining it.
In sum the time elapsed from the last DEB-TACE session to the LV, the number and size of the tumors found in the piece and capsular infiltration are associated with the degree of necrosis, but neither the tumor vascularization pattern, the number of chemoembolization sessions or the size of tumors before the procedure had a strong influence. When one or two HCCs are found through image modalities, the odds of necrotizing are greater. Also the DEB-TACE can downstage the size and stage of HCC significantly.
Ethical responsibilitiesProtection of people and animalsThe authors declare that no experiments with human beings or animals have been performed while conducting this investigation.
Data confidentialityThe authors declare that in this article there are no data from patients.
Right to privacy and informed consentThe authors declare that in this article there are no data from patients.
Authors’ contributions- 1.
Manager of the integrity of the study: ZLJ, CM, MJL.
- 2.
Study Idea: ZLJ, E JJ, CM, MJL.
- 3.
Study Design: ZLJ, EJJ, CM, MJL, MMC.
- 4.
Data Mining: ZLJ, EJJ, LS, CM, MJL.
- 5.
Data Analysis and Interpretation: ZLJ, EJJ, LS, MT, MMC.
- 6.
Statistical Analysis: ZLJ, MT.
- 7.
Reference Search: ZLJ, EJJ, LS, MT, CM, MJL.
- 8.
Writing: ZLJ, EJJ, LS, MMC.
- 9.
Critical review of the manuscript with intellectually relevant remarks: ZLJ, EJJ, LS, MT, CM, MJL, MMC.
- 10.
Approval of final version: ZLJ, EJJ, LS, MT, CM, MJL, MMC.
The authors declare no conflict of interests.
Please cite this article as: Zurera LJ, Espejo JJ, Lombardo S, Marchal T, Muñoz MC, Canis M, et al. Estudio histológico sobre el efecto de la quimioembolización con partículas precargadas con doxorrubicina en el hepatocarcinoma. Radiología. 2015;57:419–27.