This article aims to describe the imaging findings for mucinous tumors of the abdomen and pelvis, which have a similar appearance on imaging tests regardless of the organ in which they develop. Due to the high water content of mucus, the appearance of these tumors is generally similar to that of water on ultrasonography, computed tomography, and magnetic resonance imaging. Another common feature of mucin-producing tumors is that calcifications are often present. The rupture of these lesions and accumulation of mucinous material in the peritoneal cavity gives rise to pseudomyxoma peritonei. It is important to identify mucinous tumors because they have a different prognosis and clinical course than non-mucinous tumors and require different management. Depending on their anatomic location and their imaging characteristics, the treatment approach varies from follow-up to radical surgery together with chemotherapy or radiotherapy or both.
El objetivo de este artículo es describir los hallazgos en imagen de los tumores mucinosos de abdomen y pelvis que, por su composición, comparten características radiológicas independientemente del órgano de origen. Debido al alto contenido hídrico del moco, generalmente muestran un aspecto similar al agua en la ecografía, la tomografía computarizada y la resonancia magnética. Otra característica común de los tumores productores de mucina es la frecuente presencia de calcificaciones. La rotura de estas lesiones con acumulación de material mucinoso en la cavidad peritoneal da lugar al pseudomixoma peritoneal. La importancia de la identificación de las neoplasias mucinosas radica en el diferente manejo, pronóstico y evolución clínica con respecto a las no mucinosas; en función de su localización anatómica y las características de imagen, la actitud varía desde el seguimiento a la cirugía radical en combinación con quimio y/o radioterapia.
The epithelial cells of the digestive and urogenital tract express mucins in the abdominal-pelvic region. Mucins are a family of glycoproteins formed by oligosaccharides bound to a protein core. Their ability to form gels makes them a key component in mucous secretions, fulfilling functions related to lubrication, immune protection and cell signalling.
Many tumors, particularly adenocarcinomas, produce variable amounts of mucins that arise as a consequence of the deregulation of mucin core protein expression. In these cases, mucins contribute to carcinogenesis and tumor invasion.1
As a result of its high water content (95%), mucin has a similar appearance to water on ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI). However, the radiological appearance of mucin-producing neoplasms varies depending on the organ and the density or protein content of the mucin: when it is dense and proteinaceous, it tends to be echogenic on ultrasound, hyperdense on CT, hyperintense on MRI T1-weighted sequences, and hypointense on MRI T2-weighted sequences.1 Another common feature of these tumors is the frequent presence of calcifications, possibly due to the similarity of mucin with a glycoprotein present in the growth cartilage.2
Mucin-producing neoplasms can be classified according to their morphological characteristics into cystic neoplasms lining mucin-secreting epithelium: pancreatic mucinous cystic neoplasms (MCN-P), biliary mucinous cystic neoplasms (MCN-B), ovarian mucinous neoplasms (OMN) and appendiceal mucinous neoplasms (AMN); tumors containing more than 50% of extracellular mucin: mucinous gastric carcinoma (MGC), colorectal (MRC), perianal (MPAC), urachal, cervical; tumors with abundant intracellular mucin: gastric signet ring cell carcinoma (GSRC), colonic, pancreatic, bladder; and intraductal papillary mucinous neoplasm (IPMN) of the bile duct (IPMN-B), of the pancreas (IPMN-P), and of the bladder.1 The aim of this article is to describe, by organ, the radiological findings that characterise mucinous neoplasms of the abdomen and pelvis that allow the radiologist to suggest a diagnosis for the purpose of establishing a prognosis, management and clinical evolution (Table 1). Gastric and colorectal mucinous neoplasms have a poorer prognosis compared to non-mucinous neoplasms, possibly due to their greater tendency to recurrence and late presentation3,4; treatment is surgical with or without chemoradiotherapy. In the liver and pancreas, treatment is usually surgical, although management is controversial due to the different malignant potential of each lesion.5 In the kidney, prognosis is usually favourable, and conservative surgery is recommended, unlike the aggressive treatment required for mucinous tumors of the bladder and urachus that are resistant to chemoradiotherapy.6,7 In the ovary and appendix, treatment is mainly surgical and varies depending on the histological grade of the tumor.
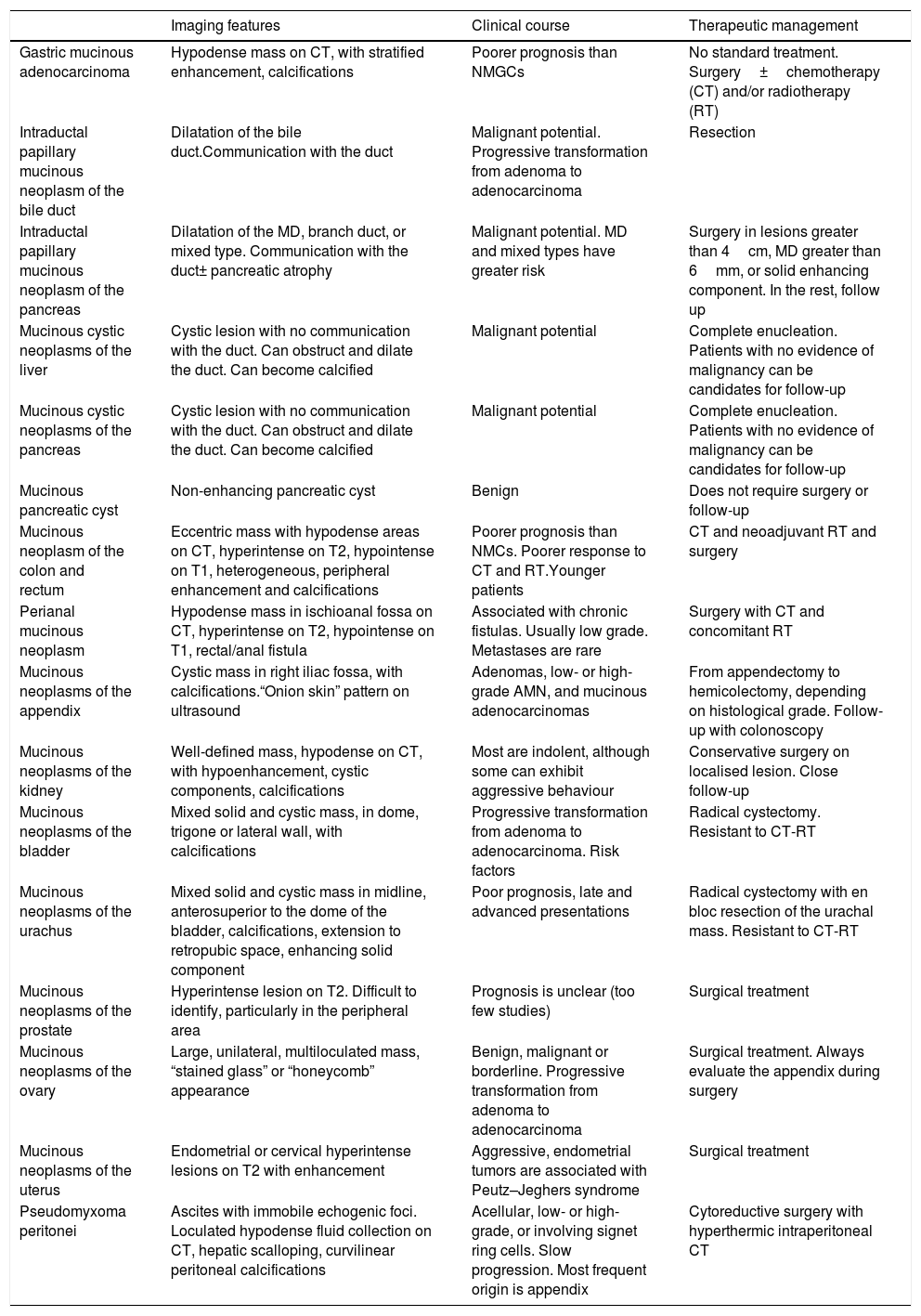
Mucinous neoplasms of the abdomen and pelvis.
| Imaging features | Clinical course | Therapeutic management | |
|---|---|---|---|
| Gastric mucinous adenocarcinoma | Hypodense mass on CT, with stratified enhancement, calcifications | Poorer prognosis than NMGCs | No standard treatment. Surgery±chemotherapy (CT) and/or radiotherapy (RT) |
| Intraductal papillary mucinous neoplasm of the bile duct | Dilatation of the bile duct.Communication with the duct | Malignant potential. Progressive transformation from adenoma to adenocarcinoma | Resection |
| Intraductal papillary mucinous neoplasm of the pancreas | Dilatation of the MD, branch duct, or mixed type. Communication with the duct± pancreatic atrophy | Malignant potential. MD and mixed types have greater risk | Surgery in lesions greater than 4cm, MD greater than 6mm, or solid enhancing component. In the rest, follow up |
| Mucinous cystic neoplasms of the liver | Cystic lesion with no communication with the duct. Can obstruct and dilate the duct. Can become calcified | Malignant potential | Complete enucleation. Patients with no evidence of malignancy can be candidates for follow-up |
| Mucinous cystic neoplasms of the pancreas | Cystic lesion with no communication with the duct. Can obstruct and dilate the duct. Can become calcified | Malignant potential | Complete enucleation. Patients with no evidence of malignancy can be candidates for follow-up |
| Mucinous pancreatic cyst | Non-enhancing pancreatic cyst | Benign | Does not require surgery or follow-up |
| Mucinous neoplasm of the colon and rectum | Eccentric mass with hypodense areas on CT, hyperintense on T2, hypointense on T1, heterogeneous, peripheral enhancement and calcifications | Poorer prognosis than NMCs. Poorer response to CT and RT.Younger patients | CT and neoadjuvant RT and surgery |
| Perianal mucinous neoplasm | Hypodense mass in ischioanal fossa on CT, hyperintense on T2, hypointense on T1, rectal/anal fistula | Associated with chronic fistulas. Usually low grade. Metastases are rare | Surgery with CT and concomitant RT |
| Mucinous neoplasms of the appendix | Cystic mass in right iliac fossa, with calcifications.“Onion skin” pattern on ultrasound | Adenomas, low- or high-grade AMN, and mucinous adenocarcinomas | From appendectomy to hemicolectomy, depending on histological grade. Follow-up with colonoscopy |
| Mucinous neoplasms of the kidney | Well-defined mass, hypodense on CT, with hypoenhancement, cystic components, calcifications | Most are indolent, although some can exhibit aggressive behaviour | Conservative surgery on localised lesion. Close follow-up |
| Mucinous neoplasms of the bladder | Mixed solid and cystic mass, in dome, trigone or lateral wall, with calcifications | Progressive transformation from adenoma to adenocarcinoma. Risk factors | Radical cystectomy. Resistant to CT-RT |
| Mucinous neoplasms of the urachus | Mixed solid and cystic mass in midline, anterosuperior to the dome of the bladder, calcifications, extension to retropubic space, enhancing solid component | Poor prognosis, late and advanced presentations | Radical cystectomy with en bloc resection of the urachal mass. Resistant to CT-RT |
| Mucinous neoplasms of the prostate | Hyperintense lesion on T2. Difficult to identify, particularly in the peripheral area | Prognosis is unclear (too few studies) | Surgical treatment |
| Mucinous neoplasms of the ovary | Large, unilateral, multiloculated mass, “stained glass” or “honeycomb” appearance | Benign, malignant or borderline. Progressive transformation from adenoma to adenocarcinoma | Surgical treatment. Always evaluate the appendix during surgery |
| Mucinous neoplasms of the uterus | Endometrial or cervical hyperintense lesions on T2 with enhancement | Aggressive, endometrial tumors are associated with Peutz–Jeghers syndrome | Surgical treatment |
| Pseudomyxoma peritonei | Ascites with immobile echogenic foci. Loculated hypodense fluid collection on CT, hepatic scalloping, curvilinear peritoneal calcifications | Acellular, low- or high-grade, or involving signet ring cells. Slow progression. Most frequent origin is appendix | Cytoreductive surgery with hyperthermic intraperitoneal CT |
AMN: appendiceal mucinous neoplasm; CT: chemotherapy; CT scan: computed tomography; MD: main duct; NMC: non-mucinous adenocarcinoma; RT: radiotherapy.
Gastric adenocarcinoma is the most common primary neoplasm of the stomach (95%). The World Health Organization (WHO) classifies it into four types: papillary, tubular, mucinous, and signet ring cell.4,8 MGC is a rare subtype (2.4%–4.9%)2 that predominates in men, in the lower third of the stomach. It is believed to arise as a typical adenocarcinoma that becomes mucinous with tumor progression.8 For unknown reasons, MGCs are usually diagnosed at more advanced stages, and have worse survival rates than non-mucinous gastric carcinomas (NMGC). Poor survival is usually due to serous and lymphatic invasion rather than the mucinous content in itself.4
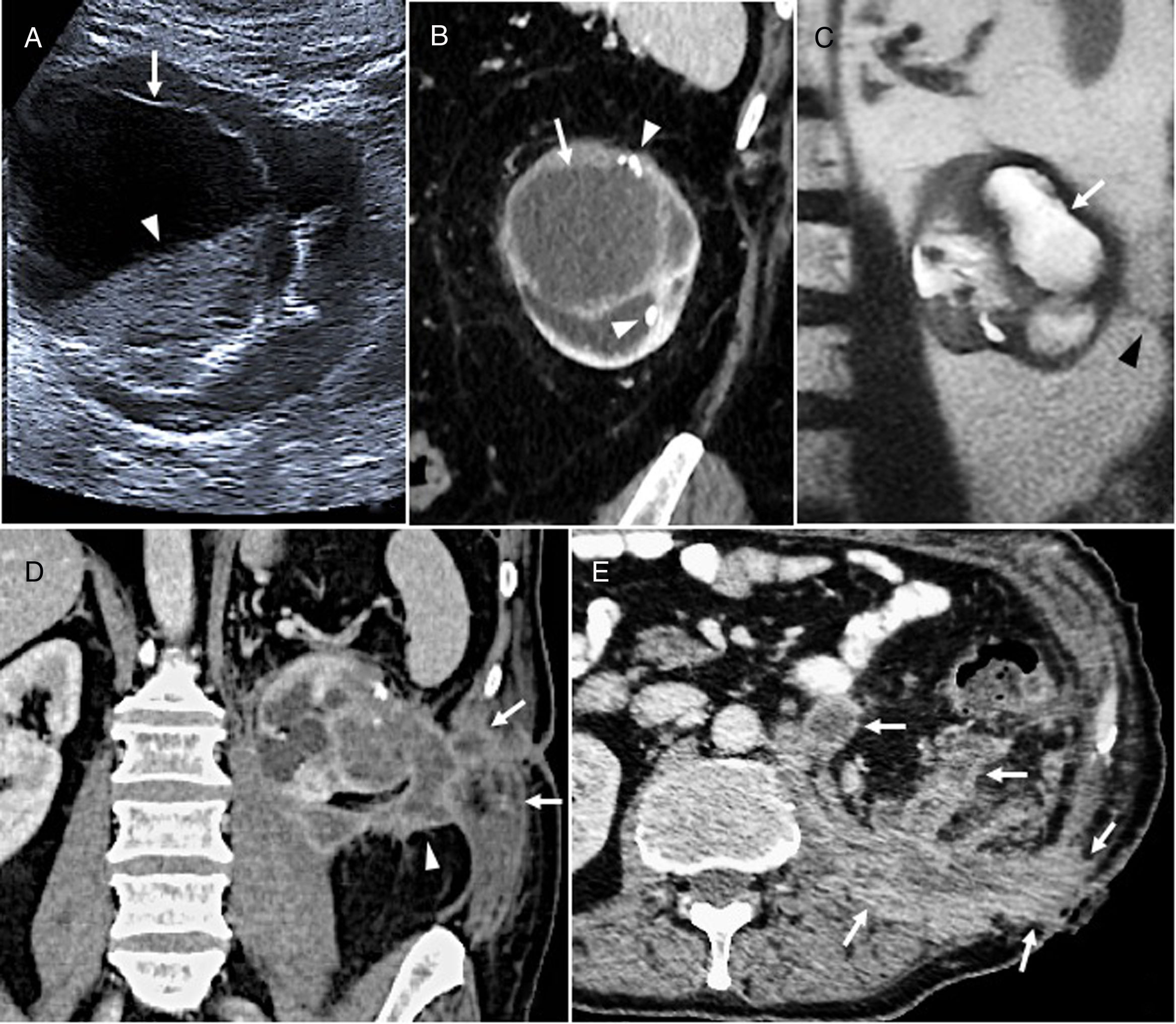
In MGCs, as in other mucous tumors, particularly of the rectum, biopsies often fail to confirm their mucinous nature, since they do not reach the submucosa. CT of the stomach with neutral or negative oral contrast agent to achieve adequate luminal distension9 is the main method used to evaluate local extension, and can also suggest a diagnosis of MGC. In MGCs, wall thickness is usually increased due to proliferation of extracellular mucin in the submucosal layer.10 In contrast to the homogeneous enhancement of NMGCs due to tumor invasion of all wall strata, MGCs show a characteristic stratified enhancement pattern due to the hypodensity of the submucosa and uptake of the overlying mucosa and of the malignant cells that line the mucin.4,9 (Fig. 1A). Finally, CT is very sensitive (33%) in the detection of the calcifications typically found in the middle and outer layers of MGCs, which are extremely rare in NMGCs.2,4,8,9
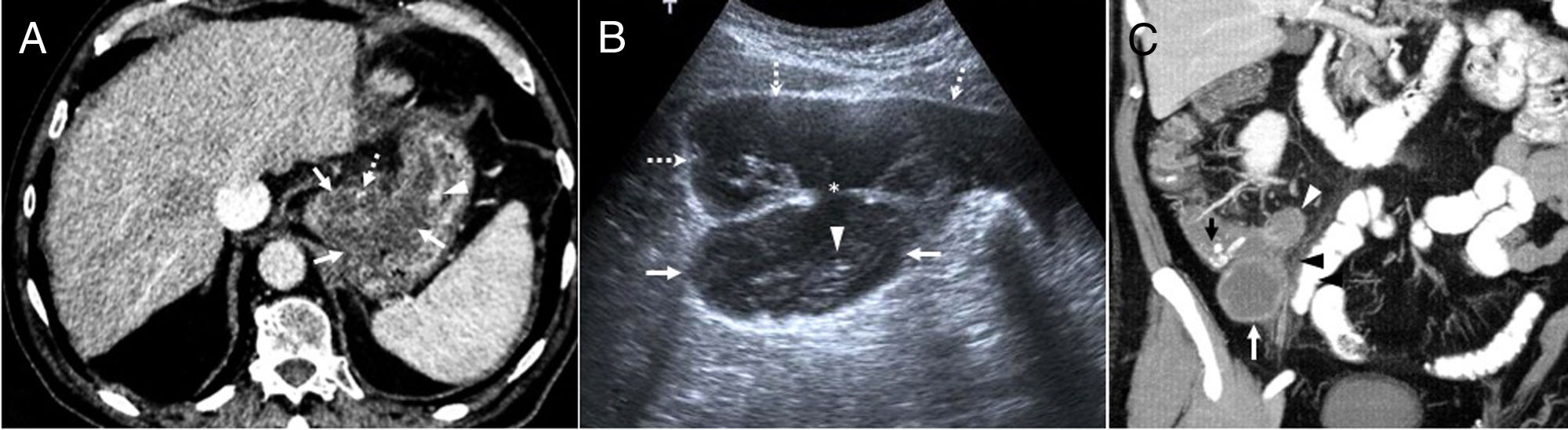
(A) Poorly differentiated mucinous gastric adenocarcinoma in an 80-year-old man. Computed axial tomography image with intravenous contrast showing a hypodense thickening of the gastric wall in the fundus and lesser curvature (arrows) with a small calcification (dotted arrow) and mucosal enhancement (arrowhead). After chemoradiotherapy with poor response, the tumor progressed with the appearance of peritoneal carcinomatosis and the patient died. (B) Appendiceal mucocele with pseudomyxoma peritonei in a 54-year-old man. Ultrasound image showing an oval lesion (arrows) with linear echogenic “onion skin” foci (arrowhead) compatible with mucocele. The mucocele is continuous with (asterisk) free fluid that shows echogenic foci that are immobile relative to mucinous ascites or pseudomyxoma (dotted arrows). Right hemicolectomy, peritonectomy and omentectomy were performed. After 10 years, the patient remains asymptomatic, with no signs of recurrence. (C) Appendiceal mucinous neoplasm. MIP coronal reconstruction of oral and intravenous contrast-enhanced CT scan. A lobulated hypodense lesion with peripheral enhancement is shown in the right iliac fossa (white arrow), measuring over 15mm, with calcifications (black arrow), compatible with an appendiceal mucocele. It has a small collection (white arrowhead) and adjacent fat hyperdensity due to inflammatory changes (black arrowhead) caused by acute appendicitis. The patient was a 75-year-old man with acute gangrenous appendicitis and a low-grade mucinous neoplasm that invaded the entire wall up to the mesenteric adipose tissue. Right hemicolectomy was performed, and the patient is currently asymptomatic.
GSRC is a variant (35%–45%) with abundant intracellular mucin, characterised by diffuse thickening of the wall and obliteration of the gastric folds (linitis plastica). This histologic subtype can also be found in other organs, such as the colon (0.1%–2.4%), where clinical presentation is often delayed and the tumor is highly aggressive.1 Treatment is the same in all types of gastric carcinoma.11
AppendixAMNs are a subtype of epithelial neoplasm that are more common in women in their forties.
The WHO classifies them according to histological criteria as adenomas, low- or high-grade AMNs, and mucinous adenocarcinomas.12 Rupture of any of these lesions can cause pseudomyxoma peritonei (PMP).12–14
They typically present as mucoceles, i.e. oval cystic masses, in the right iliac fossa, with concentric echogenic layers or “onion skin sign” on ultrasound, hypodense and homogeneous on CT, and hyperintense on T2 (Fig. 1B). Half (50%) present peripheral mural calcifications.
Shape, density, wall thickness, and presence of internal septa, mural calcifications, or free intraperitoneal fluid are not helpful in differentiating histological subtypes of AMNs. The presence of mural nodularity or irregular wall thickening and periappendiceal deposits suggest adenocarcinoma.13–15
AMNs may coincide with acute appendicitis, and are not a rare incidental histological finding. They should be suspected in patients with an appendix larger than 15mm or with mural calcifications (Fig. 1C).
AMNs have been associated with colorectal tumors, so follow-up with periodic colonoscopies is recommended.15
Treatment, according to the histological tumor grade and the presence of PMP, ranges from appendectomy with or without hemicolectomy to cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC).13,15
Colon and rectumMRC is a rare subtype of adenocarcinoma (5%–20%), which is more common in the proximal colon. Mean age of presentation is 42, which is lower than nonmucinous adenocarcinoma (NMC). Survival rates and prognosis are worse due to a higher incidence of adenopathies, venous and lymphatic invasion, local recurrence, metastasis, and more advanced stage at the time of diagnosis.16–19 This more aggressive behaviour could be explained by the ability of the mucin to separate the muscle fibres, increase intestinal wall pressure, form deposits in the perirectal fat, and spread to the peritoneal cavity and the lymph vessels. MRCs also respond poorly to preoperative chemoradiotherapy, probably due to limited drug transport in the tumor microvascular bed reduced by extracellular mucin.20–22 Like mucinous tumors in other locations, MRCs show hypodense areas on CT, heterogeneous peripheral enhancement, and presence of intratumoral calcifications that are usually small and punctate. They cause greater wall thickening, are more eccentric, and have a greater tendency to spread and infiltrate than NMCs, as shown by the frequent presence of extra-tumoral mucin deposits in the adjacent adipose tissue16,22 (Fig. 2). MRI can be superior to CT and even to biopsy18 in the diagnosis of MRCs due to its ability to detect mucin and differentiate it from the fibrous components of the tumor, both of which are hypodense on CT. However, it is less accurate in differentiating between the mucinous tumor deposits and the mesorectal fat which it frequently infiltrates, since both are hyperintense on T2-weighted images. It may be helpful, therefore, to perform a T1-weighted scan, in which the tumor is hypointense with respect to adipose tissue.17,20,21,23 In NMCs, chemoradiotherapy induces colloid changes that represent a measure of response to treatment.17 In MRCs, in contrast, colloid pools usually persist after treatment, making it difficult to estimate the degree of response given the possible persistence of tumor cells within them, and to differentiate recurrence from other lesions with liquid content, such as cysts, collections, or necrotic tumors. The standard treatment for MRCs is surgery, generally aggressive, with wide margins and lymph node dissection, preceded by chemoradiotherapy in patients with locally advanced rectal cancer.16,19
(A–C) A 70-year-old woman with low-grade (moderately differentiated) mucinous adenocarcinoma of the proximal colon. Computed tomography (CT) axial image (A) with oral and intravenous contrast showing a mass on the posterior side of the right colon (arrows), predominantly hypodense, with slight peripheral and heterogeneous enhancement, eccentric, causing wall thickening, and slightly exceeding the muscle tissue in some areas. Magnetic resonance imaging (MRI) T2-weighted coronal image (B) showing the lobulated hyperintense mass (arrows), with a reticular, hypointense internal structure. T1-weighted coronal image (C) with fat suppression and intravenous contrast showing a hypointense mass, with internal septa and solid enhancing components (arrows). After surgery, with tumor-free resection margins and negative lymph nodes, the patient remains asymptomatic and is currently in follow-up. (D and E) A 77-year-old woman with well-differentiated mucinous adenocarcinoma of the rectum infiltrating the perirectal adipose tissue. CT axial image (D) showing a hypodense thickening of the circumference of the rectum (white arrow) with a mucosal calcification (black arrow) and invasion of the mesorectum (dotted arrow). The same lesion (E) in MRI T2-weighted sagittal image shows hyperintense areas (white arrow) and a hypointense reticular internal structure (black arrow). After chemoradiotherapy and abdominoperineal resection, the patient remains disease free.
MPAC is a rare entity that may originate de novo or, more frequently, as a complication of a chronic fistula,24 but rarely of abscesses, syphilis or diabetes.25 According to the prevailing hypothesis, chronic inflammatory changes induce epithelial regeneration that causes malignant transformation. MPACs are usually low-grade lesions and metastases are rare.26 Diagnosis is usually delayed due to their slow growth and nonspecific symptoms, except for MPACs associated with chronic fistula. Therefore, in patients with long-standing perianal fistulas (from 2 to 10 years), malignancy should be ruled out by biopsy and MRI. Biopsies often offer equivocal results, because superficial samples from the external orifices of the fistula may only reveal inflammation, and because tumor cells are scarce in mucin pools.27 On T2-weighted images, they appear as a hyperintense mass that may contain calcifications. The enhancement of peripheral structures, the presence of mucin pools with no fibrous capsule that show tumor invasion, and an internally heterogeneous, irregular enhancement pattern (Fig. 3) are useful for differentiating MPACs from complicated abscesses or fistulas. Nevertheless, areas of active inflammation in an abscess or fistula may be enhanced on MRI, so it is advisable to perform the study after resolution of the acute process.26,27 The presence of a fistula between the mass and the anus differentiates MPAC from a malignant transformation of a teratoma, a retrorectal cystic hamartoma, or a dermoid cyst.27 The combination of chemoradiotherapy with aggressive surgery, usually abdominoperineal resection, can improve survival.24,26
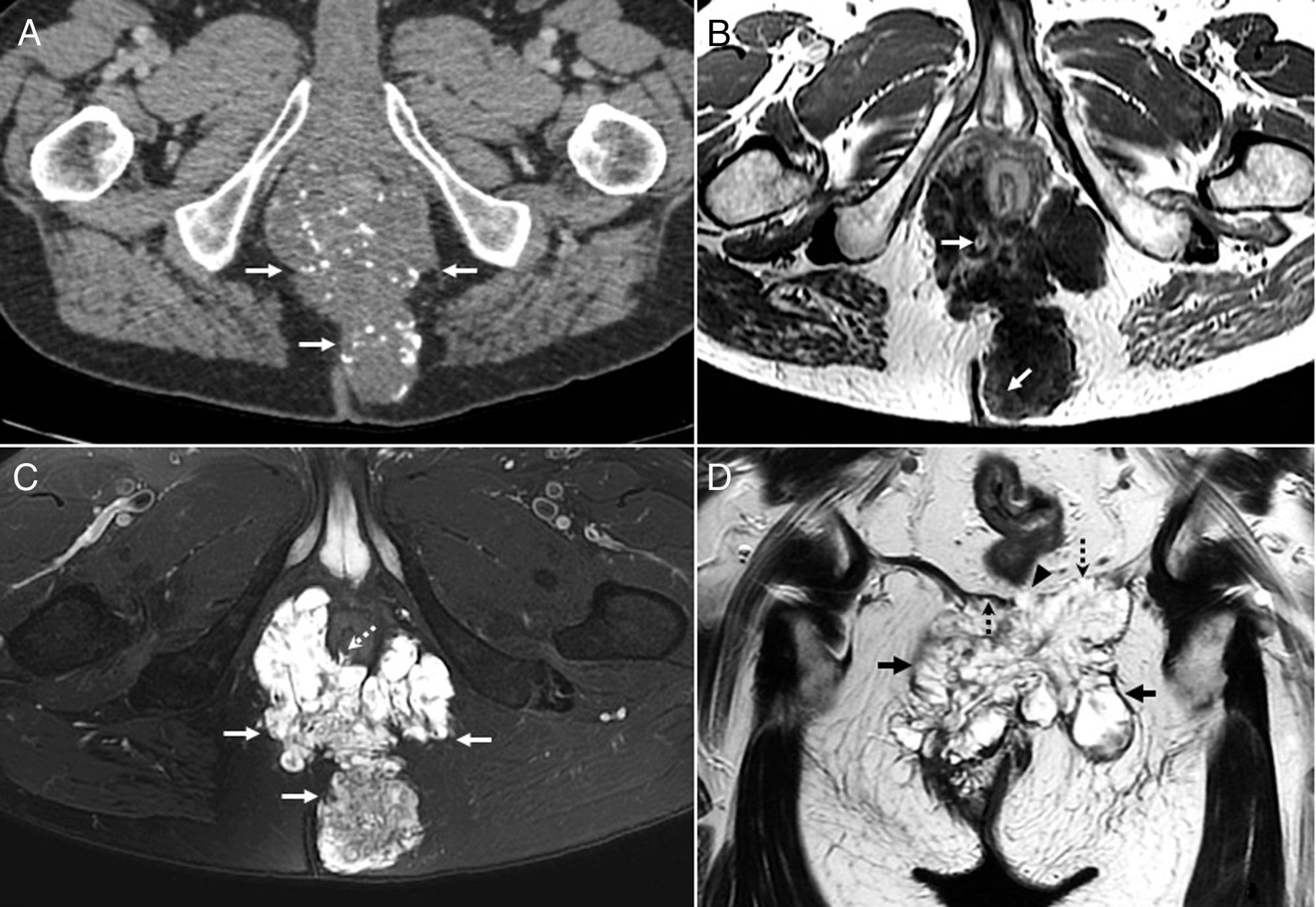
Well-differentiated perianal and peri-rectal mucinous adenocarcinoma at a locally advanced stage in a 68-year-old man with a long-standing history of fistula. (A) Contrast-enhanced computed tomography (CT) scan showing a large hypodense mass (arrows) in the ischioanal fossa with numerous gross, punctate calcifications. (B) T1-weighted axial image with intravenous contrast. The mass is hypointense and infiltrates the adipose tissue of the ischioanal fossae, with irregular internal foci with linear and nodular enhancement (arrows). (C) Axial T2-weighted sequence with fat suppression showing the high signal of the lesion (arrows) corresponding to the mucin contained in an irregular or reticular mesh-like solid internal structure, hypointense, formed by malignant cells and vessels. A rupture or fistula at the level of the sphincters (dotted arrow) is observed. (D) T2-weighted coronal image showing the mass (arrows), infiltration of the levator ani (dotted arrows) and a pool of mucin with no fibrous capsule (arrowhead), that is evidence of invasiveness. The patient received neoadjuvant chemoradiation therapy that reduced the tumor volume and expelled mucinous material through the different fistulous tracts. Later, extralevator abdominoperineal excision was performed, together with resection of the distal coccyx adjacent to the lesion. The patient still shows no signs of tumor recurrence after two years.
The term “biliary diseases and their pancreatic analogues” has recently been proposed to describe a group of biliary and pancreatic lesions with similar clinical, histopathological and radiological characteristics, possibly due to a common embryological origin. Thus, pancreatic ductal adenocarcinoma, IPMN-P and MCN-P are considered to be the pancreatic analogues of extrahepatic cholangiocarcinoma, IPMN-B and MCN-B, respectively.28–31
Intraductal papillary mucinous neoplasmIPMN-Bs and IPMN-Ps are characterised by papillary or exophytic proliferation of biliary or pancreatic epithelium, respectively, towards the ductal lumen, with no subepithelial ovarian-type stroma, and over-production of mucin that prevents biliary or pancreatic flow, giving rise to the main radiological characteristic of IPMN: dilatation and communication between the lesion and the bile duct.32–38 Due to its small size, the tumor is not usually identified, although tumor nodules can sometimes cause filling defects that, unlike defects caused by ductal mucin, are enhanced by radiological contrast media39 and restrict diffusion. IPMNs are potentially malignant lesions that extend superficially through the mucosa and can gradually transform from adenomas to infiltrating or invasive adenocarcinomas.29–40
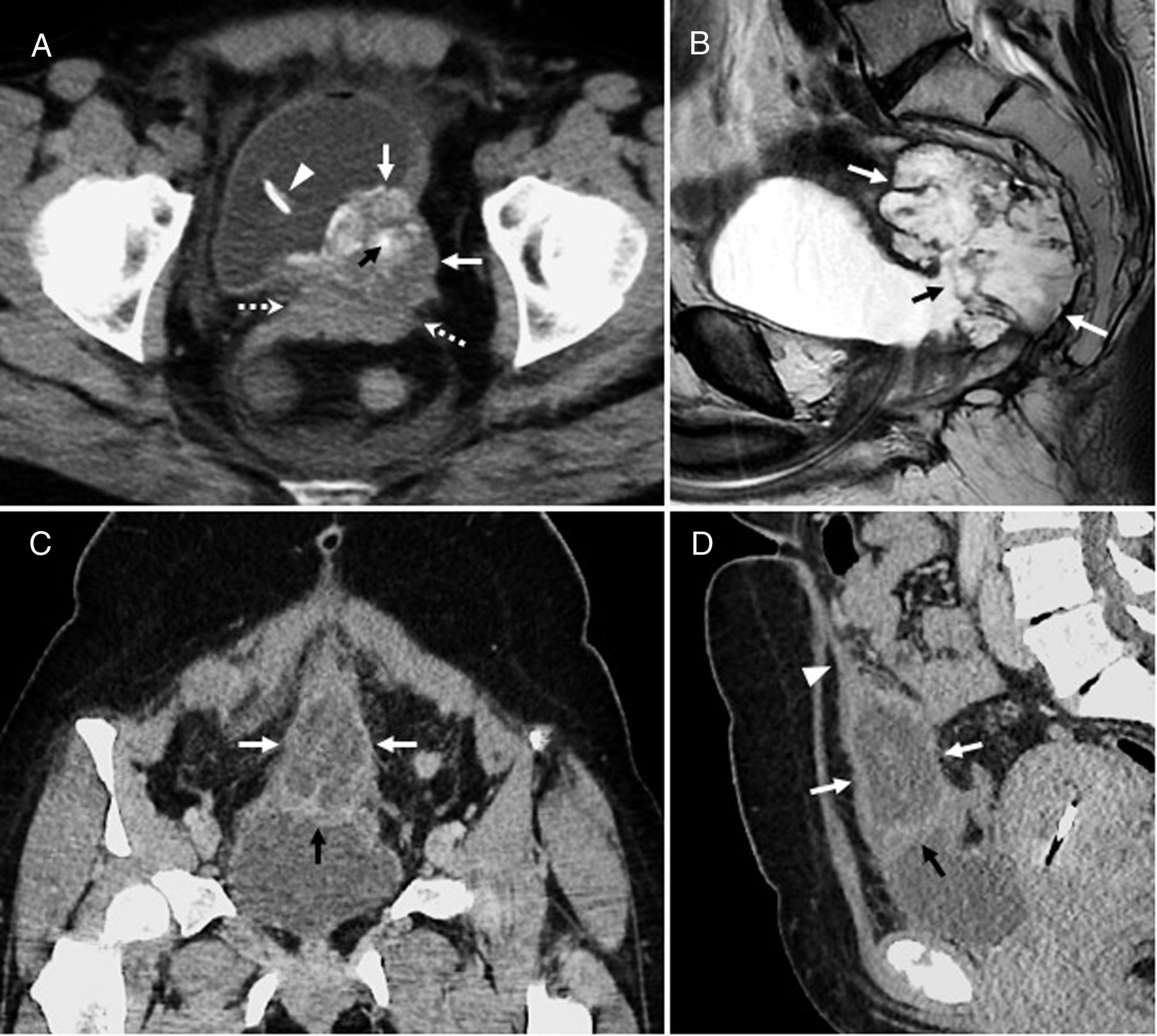
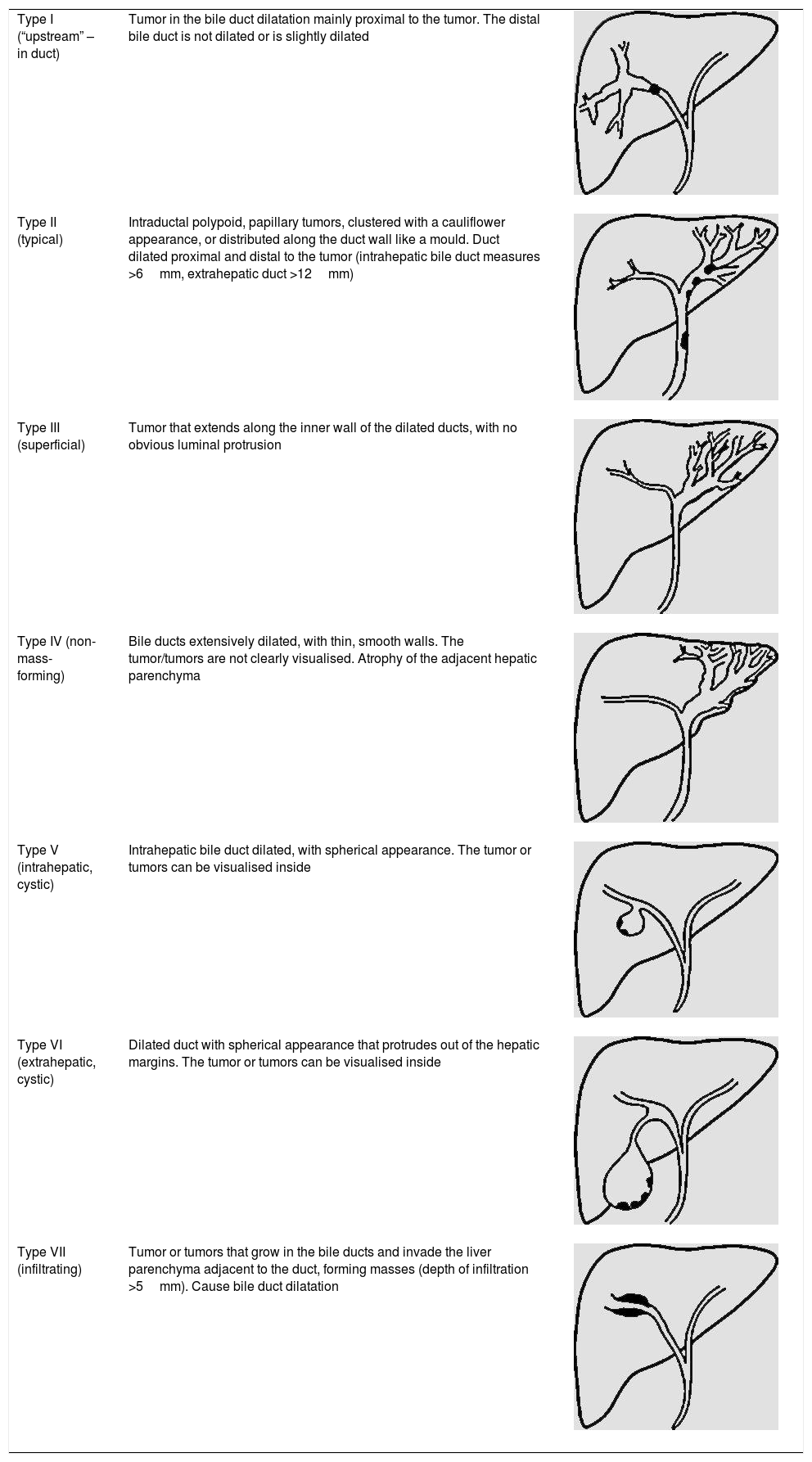
IPMN-BThere are numerous radiological classifications of IPMN-Bs.15,24,27,30 The recent classification published by Shihong Ying et al.33 includes up to seven types (Table 2), and for the first time establishes morphological and prognostic differences between a non-mucin producing subtype that usually manifests as proximal dilation of the bile duct (Type I), and IPMN-B, which generally has a better prognosis and shows diffuse dilatation or cystic variants (Types II–VII) (Fig. 4). Individually, the infiltrating type (VII) has the poorest prognosis, irrespective of whether it produces mucin or not. The differential diagnosis of IPMN-B is performed with intrahepatic cholangiocarcinoma, intrabiliary metastases, other cystic lesions, and with recurrent pyogenic cholangitis with stones that cause incomplete intermittent biliary obstruction and possible intraluminal filling defects.30
Morphological classification of intraductal papillary mucinous neoplasms of the bile duct.
| Type I (“upstream” – in duct) | Tumor in the bile duct dilatation mainly proximal to the tumor. The distal bile duct is not dilated or is slightly dilated | |
| Type II (typical) | Intraductal polypoid, papillary tumors, clustered with a cauliflower appearance, or distributed along the duct wall like a mould. Duct dilated proximal and distal to the tumor (intrahepatic bile duct measures >6mm, extrahepatic duct >12mm) | |
| Type III (superficial) | Tumor that extends along the inner wall of the dilated ducts, with no obvious luminal protrusion | |
| Type IV (non-mass-forming) | Bile ducts extensively dilated, with thin, smooth walls. The tumor/tumors are not clearly visualised. Atrophy of the adjacent hepatic parenchyma | |
| Type V (intrahepatic, cystic) | Intrahepatic bile duct dilated, with spherical appearance. The tumor or tumors can be visualised inside | |
| Type VI (extrahepatic, cystic) | Dilated duct with spherical appearance that protrudes out of the hepatic margins. The tumor or tumors can be visualised inside | |
| Type VII (infiltrating) | Tumor or tumors that grow in the bile ducts and invade the liver parenchyma adjacent to the duct, forming masses (depth of infiltration >5mm). Cause bile duct dilatation |
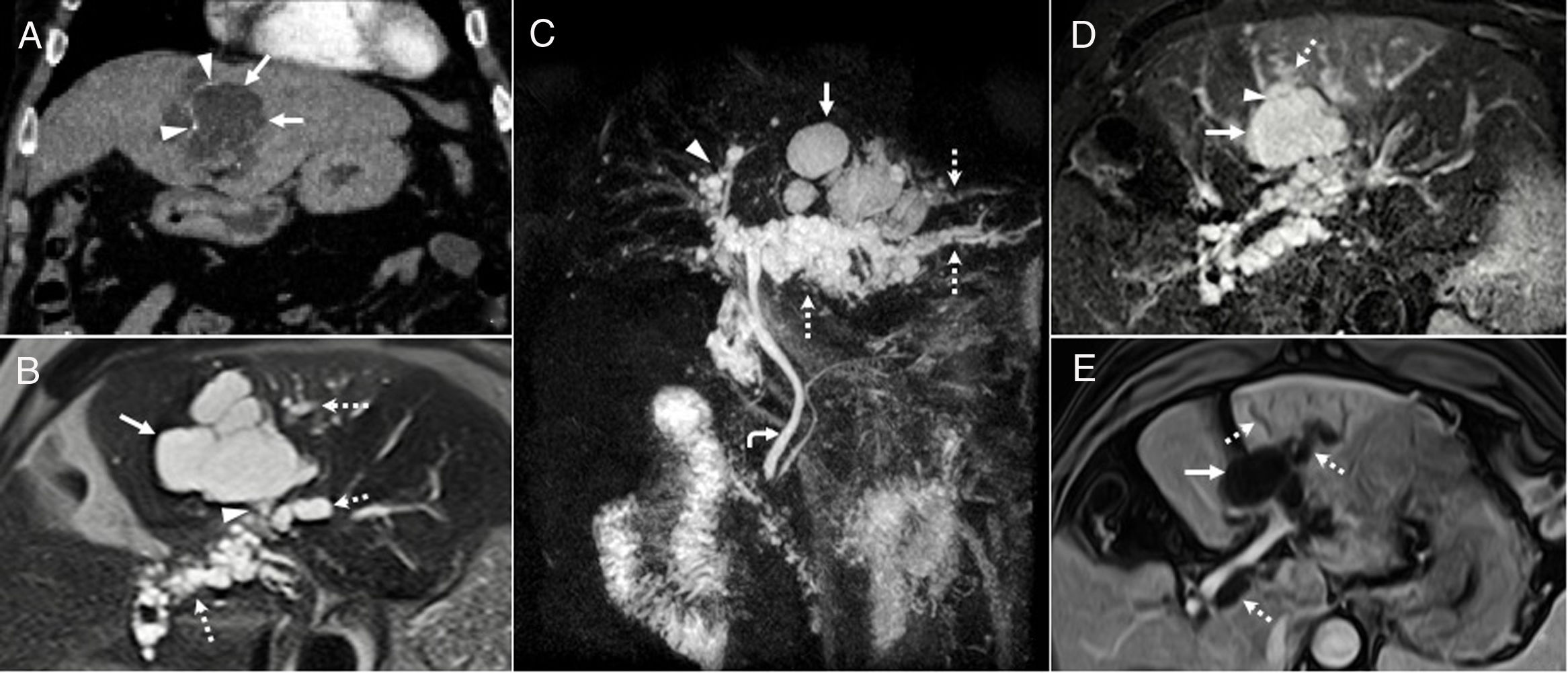
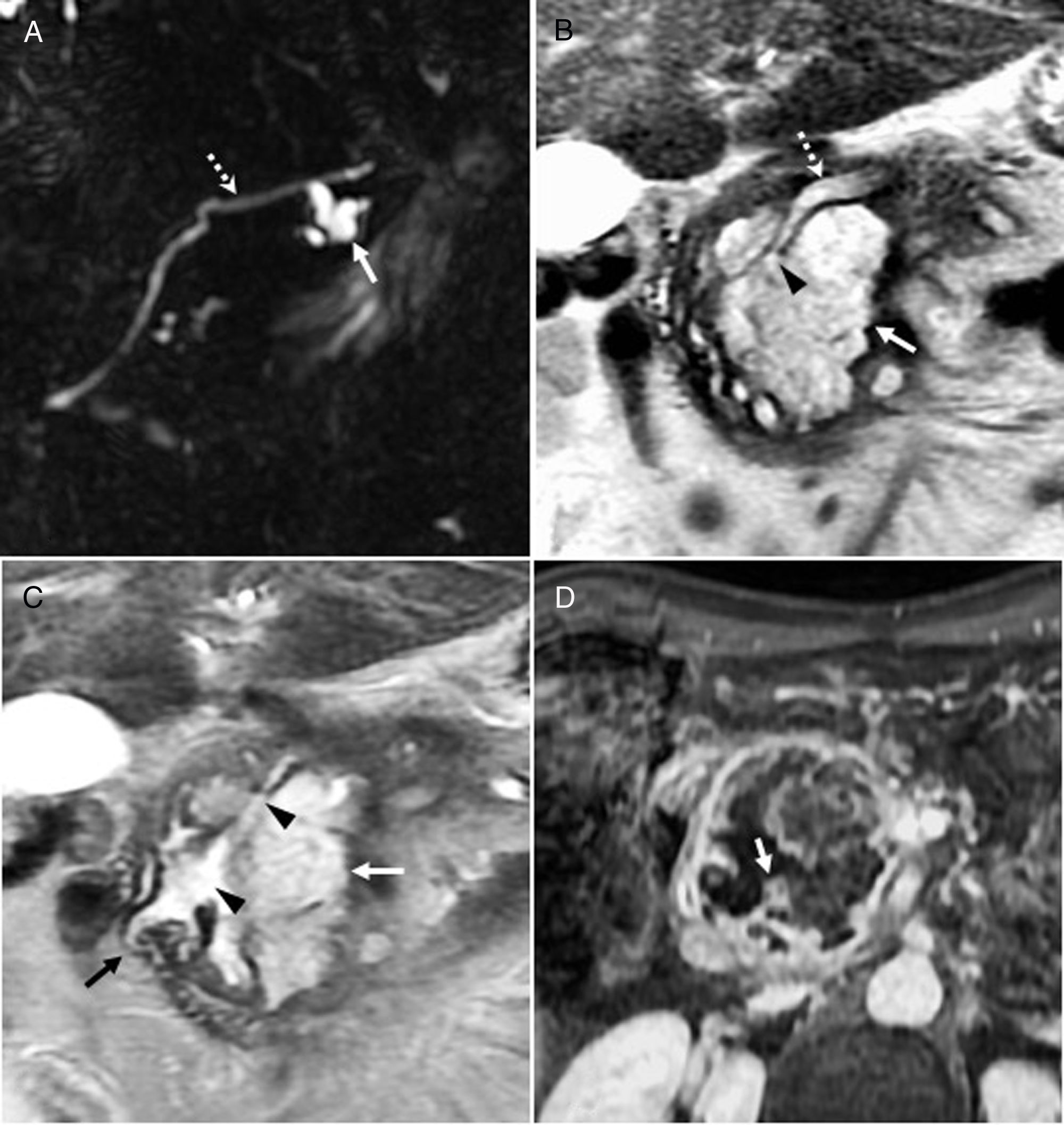
Intraductal papillary mucinous neoplasm of the bile duct. A 68-year-old man with a lesion radiologically compatible with papillary neoplasm with intraductal proliferation of mucin-producing cells (IPMN-B). (A) Intravenous contrast-enhanced CT coronal image showing a hypodense, cystic, lobulated lesion (arrows), with peripheral linear calcifications (arrowheads). (B) T2-weighted axial image confirming that the lobulated cystic lesion (arrow) communicates (arrowhead) with dilated biliary branch ducts with a beaded appearance (dotted arrows), and that, therefore, it is a focal dilatation of the bile duct, a radiological finding that is compatible with a cystic variant of IPMN-B. (C) 3D-MIP T2 coronal reconstruction showing the lobulated cystic lesion (arrow) associated with the beaded dilatation of the left (dotted arrows) and to a lesser extent the right (arrowhead) intrahepatic bile duct. The curved arrow indicates the common bile duct, which is within normal limits. (D) T2-weighted axial image with fat suppression showing how the cystic formation (arrow) communicates (arrowhead) with the proximal bile duct (“upstream”) (dotted arrow). (E) T1-weighted axial image with contrast and fat suppression showing the absence of solid enhancing mural nodules in the larger cystic focal dilatation (arrow) and the duct (dotted arrows). After six years of follow-up for the larger focal cystic lesion shown in the images, diffuse dilatation of the intrahepatic biliary tract was observed, which showed that the lesion communicated with bile duct, so it was finally diagnosed as a cystic variant of papillary neoplasia with intraductal proliferation of mucin-producing cells (IPMN-P). The patient remains asymptomatic, without treatment, with slightly elevated liver enzymes.
Curative resection is the treatment of choice for IPMN-Bs due to the risk of invasiveness and biliary obstruction.
IPMN-PIPMN-Ps affect patients from 60 to 80 years of age, with a slight male predominance. They are grouped into different clinically relevant classes, according to their prognosis5,37–42:
- •
Main duct (MD) IPMNs. These have the highest risk of malignancy. They show diffuse or segmental dilatation of the MD, and differential diagnosis with chronic pancreatitis is particularly difficult when calcifications are present.
- •
Branch duct IPMNs. These appear as a round or oval uni- or multilocular lesion with lobulated margins, most frequently located in the uncinate process (Fig. 5A). The differential diagnosis includes pseudocysts, oligocystic serous cystic neoplasm (SCN), and cystic neuroendocrine tumors.
Figure 5.Intraductal papillary mucinous neoplasm of the pancreas. (A) A 76-year-old woman with branch duct papillary mucinous neoplasm. T2-weighted image of the bile duct showing a lobulated cystic lesion (arrow) in communication with the pancreatic duct (dotted arrow). The lesion has remained stable after several years of follow-up. (B–D) Mixed-type intraductal papillary mucinous neoplasm, of the main and branch duct. T2-weighted coronal image (B) showing dilatation of the main duct (dotted arrow) and a large cystic lesion (arrow) in communication with the duct (arrowhead). T2-weighted coronal image (C) showing the cystic mass (white arrow) in communication with the duct (arrowheads) and protrusion of the papilla towards the duodenum (black arrow). T1-weighted axial image (D) with contrast and fat suppression showing peripheral and heterogeneous enhancement of the lesion (arrow). This is a 58-year-old patient with mixed-type intraductal papillary mucinous neoplasm that was unresectable due to portal vein and hepatic artery infiltration. After chemoradiotherapy treatment, tumor volume decreased significantly, and mild atrophy of the rest of the pancreas was observed. Five years later, the mass remains stable and the patient is asymptomatic except for occasional episodes of anorexia.
(0.31MB). - •
Mixed-type IPMNs. These affect both the MD and its branches. They can be diffuse, and can appear in the body or the tail of the pancreas. The differential diagnosis and prognosis are similar to MD IPMNs (Fig. 5B–D). They usually cause focal or diffuse pancreatic atrophy, and bulging duodenal papilla is a very specific characteristic.
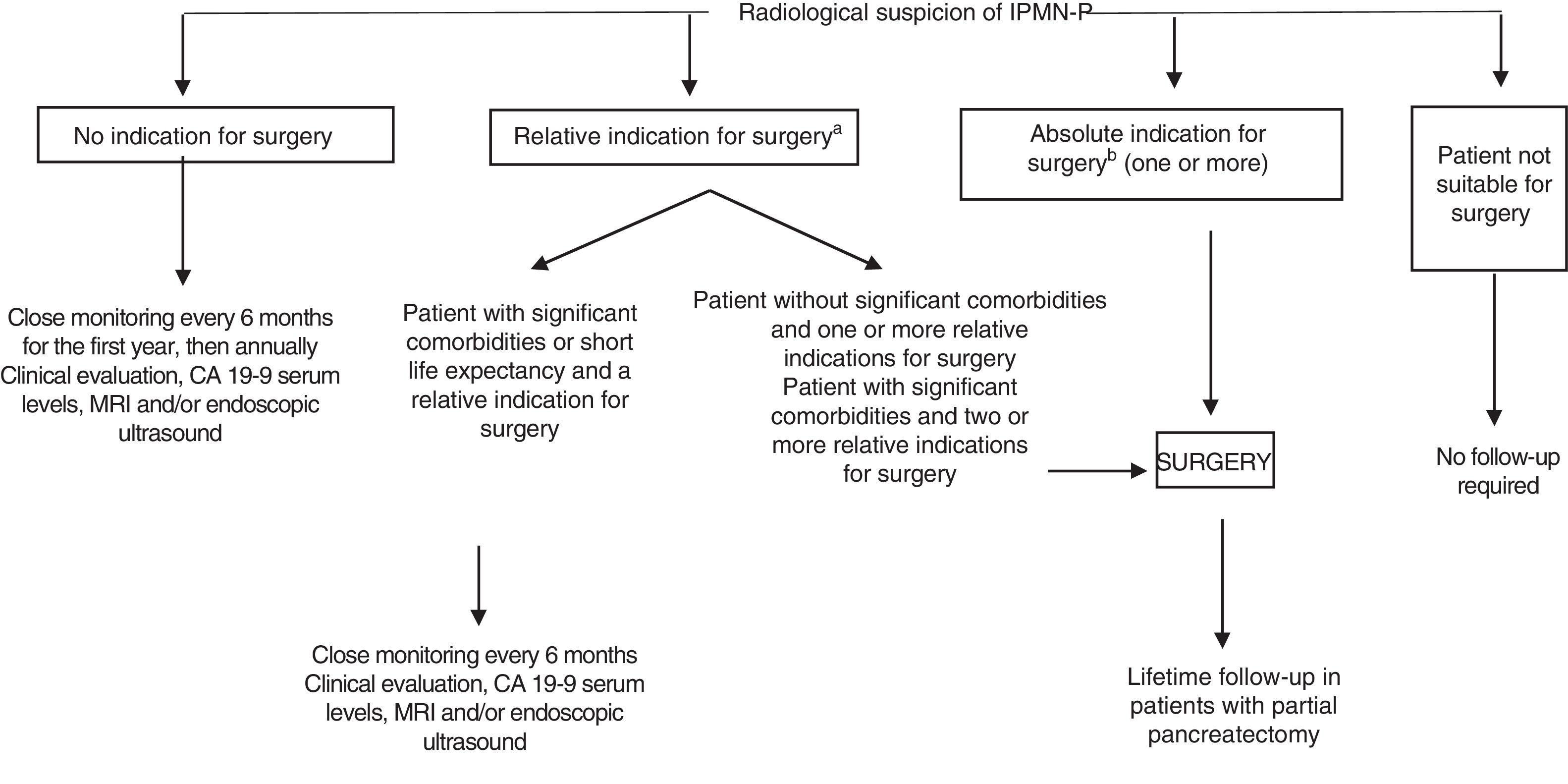
The therapeutic management of IPMN-Ps is controversial. A consensus statement from European experts in 2018 recommends surgery when the lesion is suspicious for malignancy43 (Fig. 6). Otherwise, follow-up is recommended.
Management of lesions suspicious for intraductal papillary mucinous neoplasm of the pancreas (IPMN-P) (2018 guidelines of the European Study Group on Cystic Tumors of the Pancreas).
aRelative indications: growth ≥5mm/year, serum CA19-9 >37U/ml, MD=5–9.9mm, cyst >40mm, appearance of diabetes mellitus, acute pancreatitis, enhancing mural nodule <5mm.
bAbsolute indications: cytology+for malignancy or high-grade dysplasia, solid mass, jaundice, enhancing mural nodule ≥5mm, MD≥10mm.
MCN-Bs and MCN-Ps are cystic neoplasms arising from the mucin-producing biliary and pancreatic epithelium, respectively, associated with ovarian-type subepithelial stroma, a criterion that is necessary to establish diagnosis. Both occur almost exclusively in middle-aged women. They are usually asymptomatic, although large lesions can create mass effect and cause abdominal pain, biliary obstruction, nausea, and anorexia.28,32,35,36,44 Unlike IPMN-Bs, MCN-Bs and MCN-Ps do not communicate with the bile or pancreatic ducts, and do not cause dilatation of these ducts, although the presence of secreted mucin can cause distal dilatation due to a mass effect.28,38,43,45 They appear as uni- or multilocular masses, with cystic behaviour on T1- and T2-weighted images, although they may show hyperintensity on T1 if the content is haemorrhagic or proteinaceous,28,39,41 with a thick fibrous capsule that can become calcified (Fig. 7). The presence of internal septa, mural nodules with increased uptake that restricts diffusion, size greater than 4cm, eggshell calcifications, aged over 55, or growth of more than 3mm per year should suggest malignant transformation41,46 (Fig. 8). The differential diagnosis of MCN-Bs is established with cystic variants of intrahepatic IPMN-B, and with simple or solitary and hydatid cysts. The differential diagnosis of MCN-Ps includes pancreatic pseudocyst, mucinous non-neoplastic cysts (MNNC) and SCNs in the pancreatic body and tail, where MCN-P is almost exclusively found.35 MCN-Bs and MCN-Ps are considered lesions with malignant potential, so the treatment of choice is complete enucleation, in which case the prognosis is excellent. However, when surgery is not indicated, radiological follow-up is recommended, preferably with MRI, especially when there is no evidence of malignancy.5,44–48
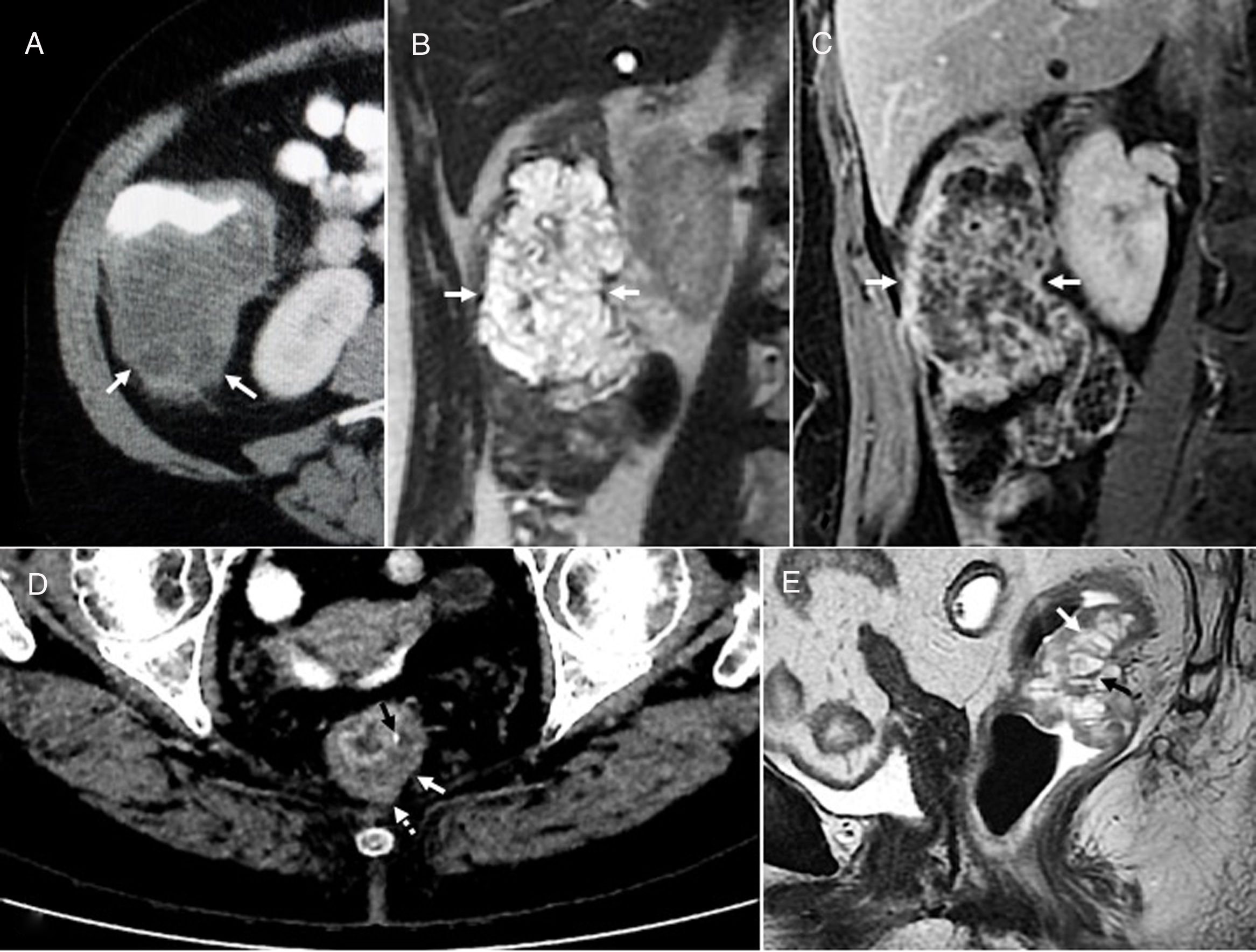
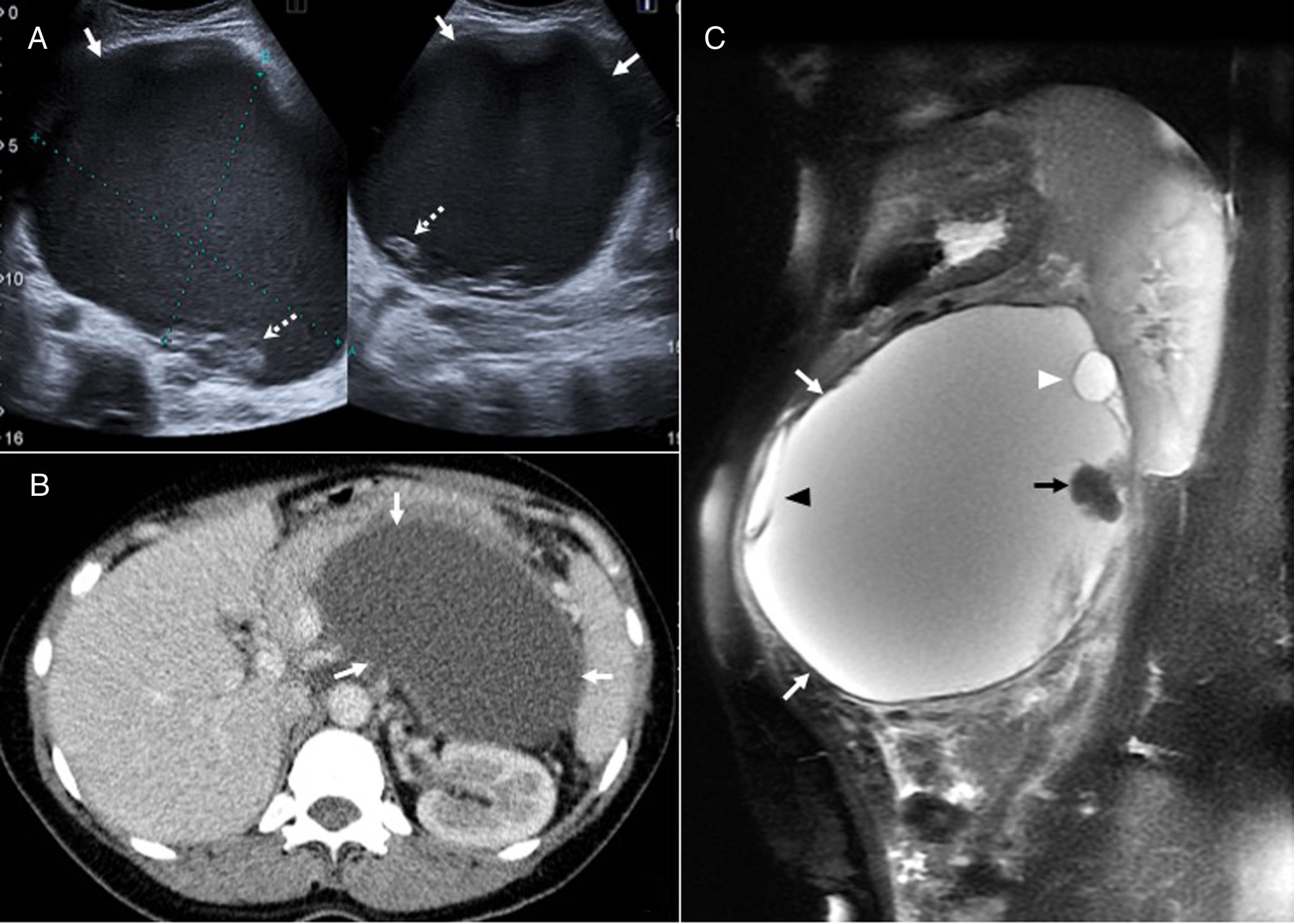
Mucinous cystic neoplasm of the bile duct in a 33-year-old woman. (A) Sagittal ultrasound image showing a well-defined lobulated hepatic cystic lesion (arrows). (B) Computed tomography with intravenous contrast reveals a hypodense lesion with peripheral calcification (arrow). (C) T2-weighted coronal sequence showing the cystic nature of the lesion, partially septated (black arrow), with a relatively thick wall (white arrow). (D) In the T1-weighted sequence without contrast, the lesion (black arrow) shows a slight internal hyperintensity that may be due to proteinaceous mucin or haemorrhagic content (white arrow). (E) Diffusion-weighted axial image (b=600) showing hyperintense lesion due to T2 effect (arrow). Fine-needle aspiration of the lesion was performed, which showed the presence of mucoid material and secretory cells, so the patient underwent surgery for complete tumor resection. No recurrence after four years of follow-up.
Mucinous cystic neoplasm of the pancreas in a 40-year-old woman. (A) Ultrasound image showing a cystic lesion (arrows) containing echogenic foci and solid mural components (dotted arrows). (B) Intravenous contrast-enhanced computed tomography shows a hypodense lesion (arrows) with mass effect on the adjacent structures. Due to their size, it is impossible to determine which is associated with the lesion. (C) T2-weighted sagittal sequence with fat suppression confirming a thick-walled cystic lesion (white arrows), loculated (white arrowhead), septated (black arrowhead), and with solid mural nodules (black arrow). Histopathology confirmed the presence of a non-invasive mucinous cystic neoplasm of the pancreas with necrosis and abscess. Despite surgical treatment (distal pancreatectomy and splenectomy) and chemotherapy, hepatic metastasis and peritoneal carcinomatosis appeared, with implants in the mesentery, the gut and in the rectus abdominis muscles of the abdomen. The patient died 20 months after diagnosis.
MNNCs are rare benign lesions that may be suggested in certain circumstances (male patient with pancreatic head cyst, no enhancement). However, preoperative diagnosis of MNNC, macrocystic SCN, MCN-P or branch duct IPMN-P may be impossible. In these cases, follow-up can be performed if the lesion measures less than 4cm.49 Another option is fine-needle aspiration to collect samples for cyst fluid analysis. This technique is not routinely performed because of its poor diagnostic yield, which is due to the low cellularity of the samples obtained. Only if it changes clinical management43,46 can MCN be suggested when carcinoembryonic antigen (CEA) levels are higher than 800ng/ml, without distinguishing the degree of invasiveness, while amylase levels greater than 250U will rule out pseudocysts.46
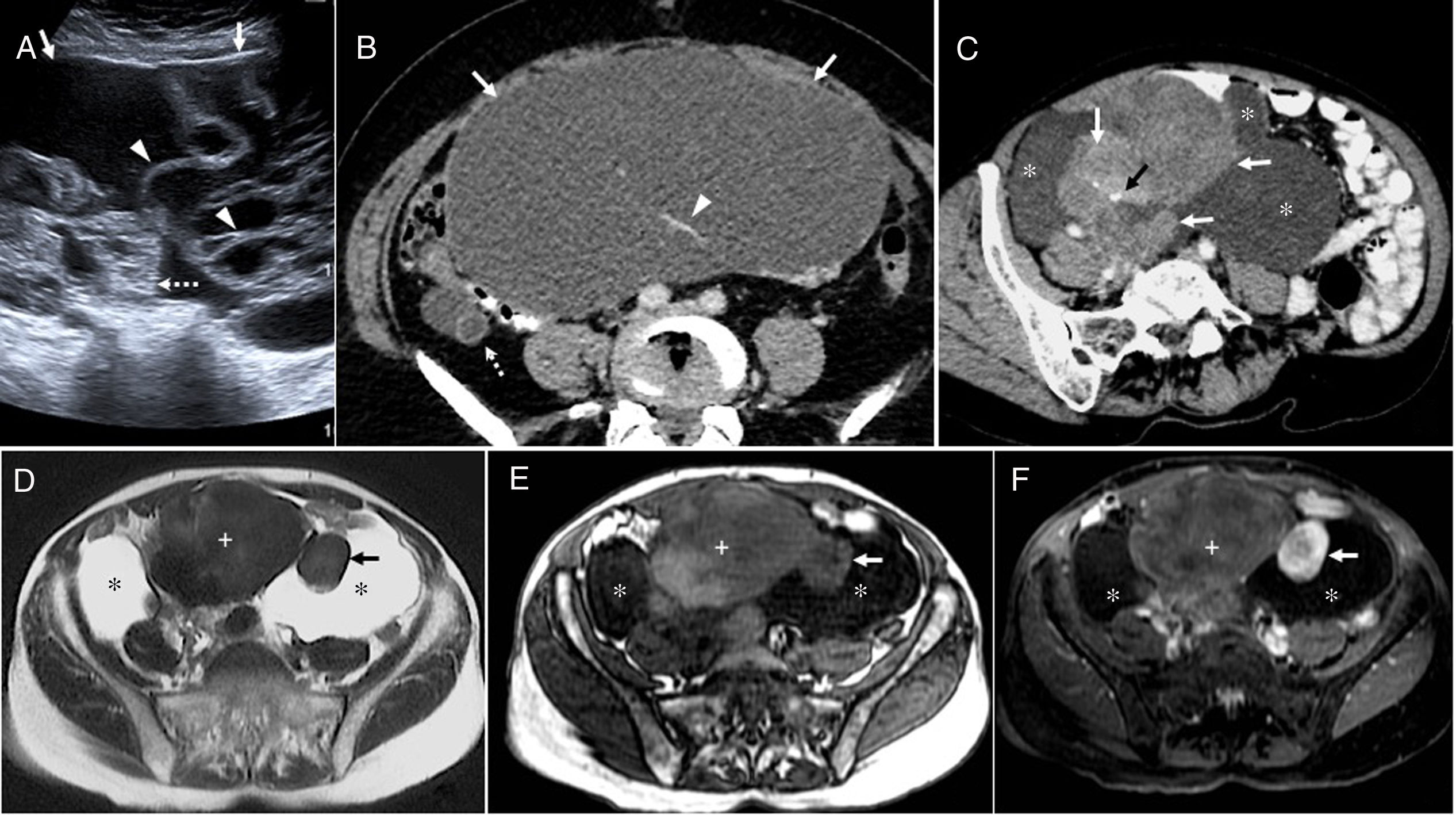
KidneyMucinous tubular and spindle cell carcinoma (MTSCC) of the kidney is a rare neoplasm that was recognised by the WHO in 2004 as a subtype of squamous cell carcinoma. It is more common in women, and the age of presentation varies.50–54 Most are asymptomatic, indolent tumors. However, following reports of cases with metastasis, aggressive behaviour or sarcomatoid changes, the WHO no longer classes it as a low-grade lesion in their 2016 classification.55 Histologically, it is characterised by tubules lined by cuboidal epithelium and fascicles of spindle cells separated by a mucinous stroma.52–56 MTSCCs are well-demarcated exophytic or parenchymal masses of variable size, with an expansive growth pattern. They are hypodense in the baseline study, generally homogeneous, although they can show intralesional bleeding, calcifications, and cystic components.50,53 In T2-weighted images, they vary from hypo- to hyperintense, depending on their mucinous content. They do not contain fat, and restrict diffusion.50,53 They show size-dependent, homogeneous or heterogeneous contrast enhancement, but this is always faint, gradual and progressive and less than the cortex in all phases, with maximum enhancement in the nephrographic phase54 (Fig. 9). Given their favourable prognosis, conservative surgery is usually recommended in cases of localised disease. Close follow-up is needed, as these tumors can metastasise, even years after successful surgery.15,51
Mucinous tubular and spindle cell carcinoma of the kidney. A 71-year-old man in follow-up for 6 years for a cystic lesion in the kidney (Bosniak IIF-III). (A) Ultrasound image showing a complex cystic lesion with a thick hyperechoic wall (arrow), calcifications, and an air-fluid level (arrowhead). (B) Intravenous contrast-enhanced computed tomography (CT) coronal image that confirms the presence of an intrarenal, hypodense lesion (arrow), with punctate linear mural calcifications (arrowheads). (C) Magnetic resonance T2-weighted coronal image after the first surgical intervention confirming a cystic lesion with thick hypointense walls (arrow). The arrowhead points to a postoperative fibrous tract. (D) Contrast-enhanced CT coronal image after conservative surgery that resulted in tumor seeding, with metastases in the lumbar (arrows) and retroperitoneum wall (arrowhead). (E) After rescue surgery with nephrectomy, CT shows greater dissemination, with metastases in the psoas, retroperitoneum, muscle wall and subcutaneous cellular tissue (arrows). Despite concomitant chemotherapy, the tumor progressed and liver metastases developed. In addition, the tumor metastases became superinfected. The patient remained in hospital and died of sepsis a few weeks after the last intervention and 10 months after the first surgery. Although these tumors usually have a favourable prognosis, histology confirmed the presence of a mucinous tubular and spindle cell carcinoma of the kidney with a malignant fibrous component with a Ki-67 of 40%.
Mucinous adenocarcinoma of the bladder is a rare subtype of bladder tumor (2%) that presents with haematuria, irritative symptoms, or mucosuria.3,57 Hypotheses to explain the origin of this tumor include persistent urachus, which is considered a risk factor, the presence of vestigial embryonic glands in the transitional epithelium, or even the malignant transformation of bladder mucosa due to chronic irritation. Bladder exstrophy and glandular cystitis are also risk factors.57,7 Radiologically, they appear as solid cystic masses, in the dome, trigone or lateral wall of the bladder, and usually contain peripheral calcifications. In some cases, it is difficult to differentiate between a primary (Fig. 10A) or metastatic (much more common) lesion, since the bladder can be infiltrated by tumors originating in the colon, rectum or prostate (Fig. 10B).
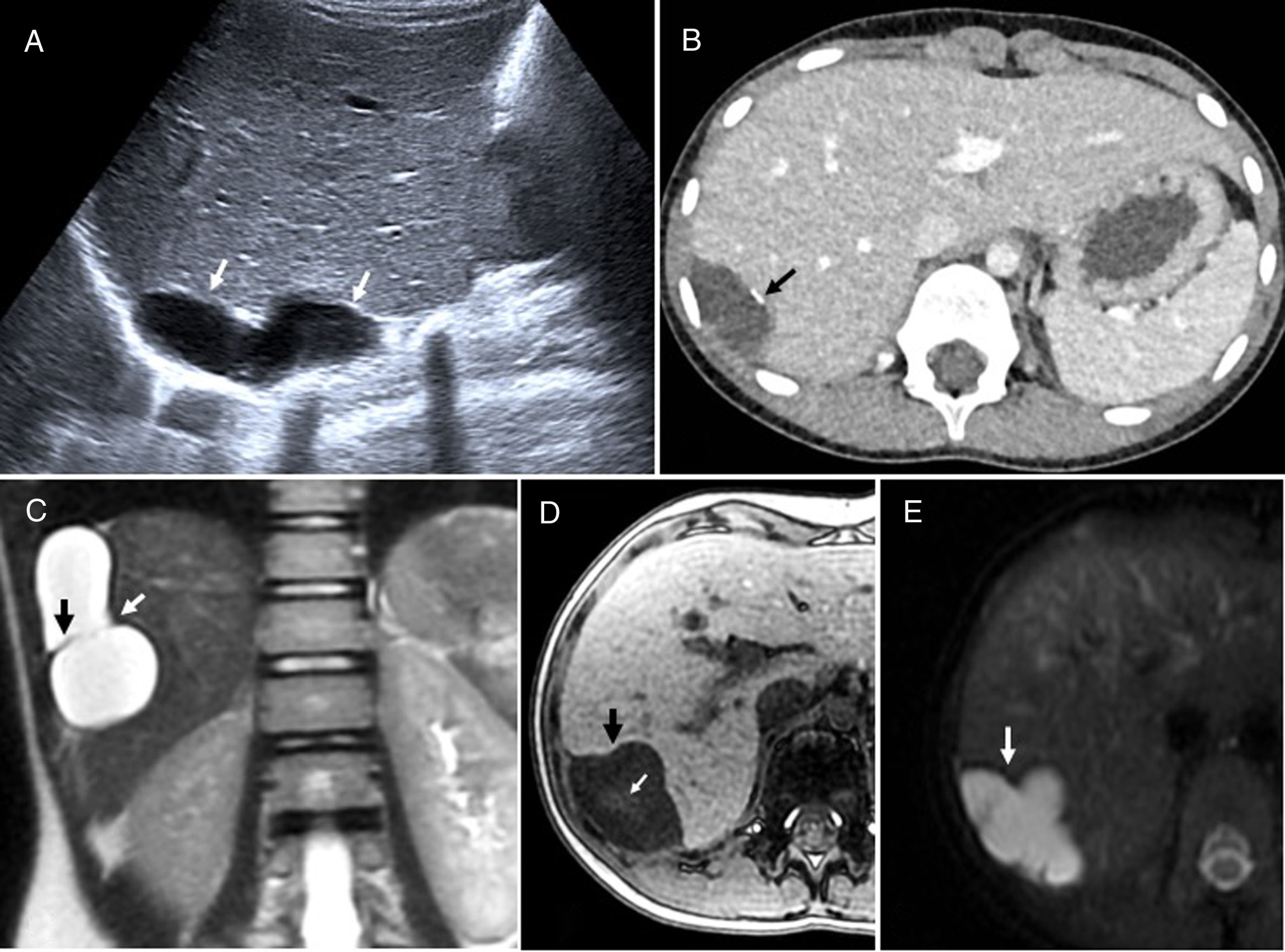
(A) Mucinous cystic neoplasm of the bladder in a 68-year-old woman. Contrast-enhanced computed tomography (CT) axial image showing a heterogeneous mass (white arrows), with hypodense zones, partially calcified (black arrow), and areas of contrast enhancement in the posterior wall of the bladder, with no cleavage plane with the vaginal vault (white dotted arrows). The arrowhead points to the tube of a bladder catheter. Cystectomy, hysterectomy, colpectomy and Bricker anastomosis were performed. Histopathology revealed perineural, vascular, lymph node extension and involvement of the surgical margin of the left ureter, so it was decided to start adjuvant chemotherapy. (B) Well-differentiated, low-grade mucosal adenocarcinoma of the rectum in a 77-year-old male patient. T2-weighted sagittal image showing full thickness infiltration of the posterior wall of the bladder (black arrow) by a hyperintense lesion, with hypointense irregular internal septa, compatible with mucinous neoplasm of the rectum (white arrows). In this region, it can be difficult to determine the origin of the lesion. Abdominoperineal resection and radical cystectomy were performed, followed by chemoradiotherapy. The patient has showed no signs of recurrence. (C and D) Mucinous adenocarcinoma of the urachus in a 47-year-old woman. Contrast-enhanced coronal CT scan (C) showing a heterogeneous cystic mass in the midline, hypodense, with irregular, predominantly peripheral enhancement (white arrows), which infiltrates the dome of the bladder (black arrow). The CT sagittal image (D) shows the lesion (white arrows) infiltrating the dome of the bladder (black arrow) with the urachal remnants (arrowhead). Given the suspicion of superinfected urachal cyst or endometriosis, fine-needle aspiration of the lesion was performed using the supravesical approach. The analysis showed the presence of mucoid material associated with a few three-dimensional clusters of atypical epithelial cells. The patient underwent partial cystectomy with mass resection and radiotherapy, and is currently in follow-up.
Urachal cancer presents with haematuria, mucosuria or palpable suprapubic mass. It may be clinically silent due to its extravesical location, resulting in late and advanced presentations, and, therefore, a worse prognosis.57,58 Unlike most bladder tumors, which are urothelial, most urachal tumors are adenocarcinomas, possibly due to their origin in the remains of embryonic enteric cloaca cells. They appear as a mixed, solid or cystic mass anterior and superior to the dome of the bladder, in the middle line, with extension to the retropubic space3,57,7,58 (Fig. 10C and D). On CT, most urachal tumors show hypodense mucinous components. Like other mucinous adenocarcinomas, they may show peripheral calcifications, which in this case are considered almost pathognomonic. Areas of high signal intensity from mucin are observed on T2-weighted images. The solid component of the tumor is isointense on T1 and, unlike collections or necrosis, enhances with intravenous contrast.3,57,7 Bladder and urachal adenocarcinomas are resistant to chemoradiotherapy, so the treatment of choice is surgery59,60: radical cystectomy for bladder tumors, with en bloc resection of the urachal mass, posterior rectal fascia, peritoneum and abdominal wall in the case of the urachal tumors.
ProstateMucinous prostate adenocarcinomas account for 0.4% of prostate adenocarcinomas, and have a similar or, in some cases, better prognosis than conventional prostate cancers.61 They are hyperintense on T2-weighted images, and PI-RADS standards are not useful for detection due to their tendency to show low apparent diffusion coefficient (ADC).62
The differential diagnosis is established with cystic hyperplasias, abscesses and cystadenomas.
OvaryOMNs can either be primary tumors, or a metastasis of a mucinous adenocarcinoma. They are usually located in the gastrointestinal tract.63 OMNs, which originate in the secretory epithelium, are slightly less common than ovarian serous neoplasms (OSN); they are benign (cystadenomas) in 80% of cases, and occur between the ages of 30 and 50. The rest are malignant (cystadenocarcinomas) or borderline, and present at a more advanced age. As in other locations, a progressive transformation from adenoma to adenocarcinoma is believed to occur.64
They generally appear as large, unilateral, multiloculated masses (greater than 10cm) that can rupture, causing PMP. Loculi may present different signals in MRI according to their haemorrhagic, protein or mucinous content, which gives them a “stained glass” appearance.61,62,64 Clusters of very small OMNs can give a “honeycomb” appearance63,64 (Fig. 11).
Ovarian mucinous neoplasms. (A) Abdominal-pelvic ultrasound image in a 61-year-old male patient with an intestinal-type mucinous cystadenocarcinoma of the ovary, which was resected. Complex cystic lesion in the pelvis (arrows), with solid nodules (dotted arrow) and irregular septa (arrowheads) (“honeycomb” pattern). (B) Computed tomography axial image with oral and intravenous contrast in a 67-year-old female patient, showing a voluminous hypodense mass (arrows) with contrast-enhancing septa (arrowhead) associated with borderline mucinous ovarian neoplasia of low malignant potential concomitant with a low-grade mucinous tumor of the appendix (dotted arrow). Surgery was uneventful, and the patient made satisfactory progress. (C–F) A 53-year-old woman with a histopathological diagnosis of mixed, endocervical and intestinal type, moderately differentiated mucinous ovarian cystadenocarcinoma. Oblique CT image (C) with oral and intravenous contrast showing a large multiloculated lesion with calcifications (black arrow), cystic areas (asterisks), and areas of heterogeneous enhancement (white arrows). Intravenous contrast-enhanced T2 (D), T1 (E), and T1 with fat suppression axial images (F) showing a mixed, multiloculated lesion with different signal intensity (“stained glass” appearance), cystic (asterisks) and solid components, with intense (arrows) and faint (+ sign) contrast enhancement. The patient remains disease-free after radical surgery and chemotherapy.
Benign OMNs are characterised by the absence of septa or solid portions. Borderline OMNs may present small papillary projections, with type 2 enhancement curves (plateau) in dynamic studies, and with the solid component showing hypointensity and high ADC values in diffusion. Malignancy should be suspected when the lesion shows thick septa measuring more than 3mm, solid enhancing components, type 3 (washout) curves,63–66 and when the solid component shows hyperintensity in diffusion and low ADC values. The optimal ADC threshold value to differentiate malignant from borderline OMNs is 1.039×10−3mm2/s.67
OMNs are usually associated with Brenner's tumor and vice versa. In addition, OMN surgery guidelines recommend evaluating the appendix and removing it if it appears pathological, due to an association between OMN and AMN68 (Fig. 11B).
Differential diagnosis is established with serous neoplasms, which are usually smaller, unilocular, bilateral, and with a homogeneous signal; also with metastasis, usually bilateral and smaller. If the ovarian lesion is believed to be secondary, the primary tumor should be located, and the first-line treatment is chemotherapy. In the case of OMN, however, CT must be performed to rule out carcinomatosis, and treatment should start with pelvic surgery.63,68
UterusA small percentage of endometrial adenocarcinomas show predominantly mucinous differentiation and, in these cases, mucin can be macroscopically visible.
Malignant adenoma is a rare and aggressive variant of cervical adenocarcinoma associated with Peutz–Jeghers syndrome. It is typically a multicystic lesion that is isointense or hyperintense on T1-weighted images and hyperintense on T2-weighted images. It has a solid component of varying size that enhances with contrast, a feature that differentiates it from Nabothian cysts.3
Pseudomyxoma peritoneiPMP is a rare clinical entity characterised by the accumulation of mucinous material in the peritoneal cavity due to the rupture of benign or malignant, generally appendiceal mucin-producing tumors.69 Even when an ovarian tumor is found in the context of PMP, the most common origin of PMP will be appendiceal. Histologically, it can be acellular, low- or high-grade, and involve signet ring cells.70
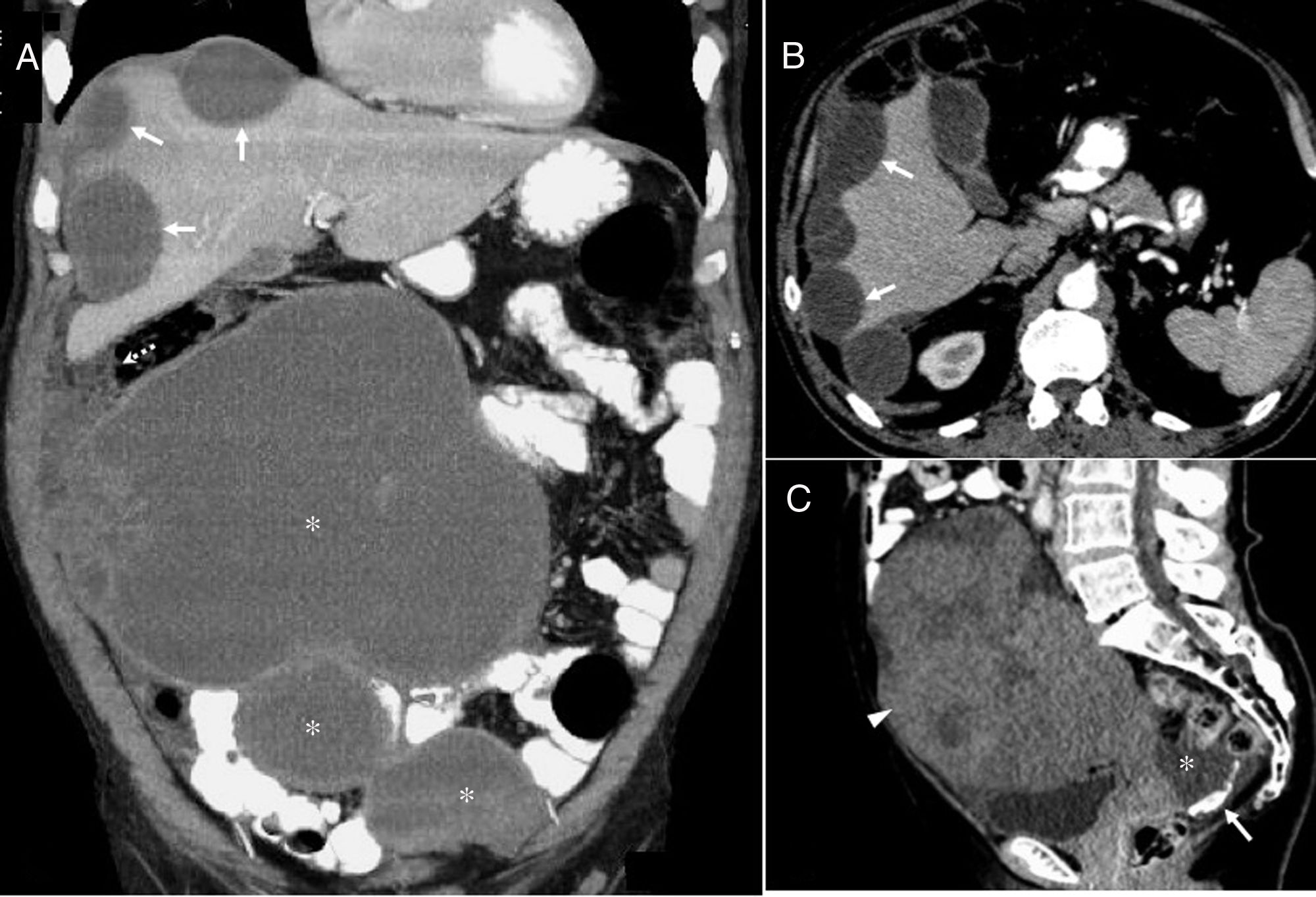
The tumor progresses slowly, and the most common clinical presentation is abdominal pain, distension or weight loss. Due to the increase in intra-abdominal pressure, hernias and intestinal obstruction are common presenting symptoms. On ultrasound, it appears as avascular echogenic masses or ascites with immobile echogenic foci (unlike purulent ascites or haemoperitoneum) (Fig. 1B). On CT, mucin deposits appear as hypodense fluid, often loculated, mainly in gravity-dependent areas such as the peritoneal recesses and the recto-uterine pouch, and in areas of peritoneal fluid absorption, such as the omentum and the right diaphragm. The peritoneal surfaces of the intestine and the mesentery, however, are spared, possibly due to their peristaltic action. Curvilinear calcifications in peritoneum are a frequent finding.70,71 In MRI, the signal varies, and is generally low on T1-weighted images and high on T2-weighted images, although it can show hyperintensity on T1-weighted images and hypointensity on T2-weighted images if thick mucin is present. Unlike serous fluids, mucinous ascites has a mass effect on the liver and hollow viscera that causes a characteristic scalloping pattern, mainly of the liver. This sign can differentiate it from other ascites, such as peritoneal carcinomatosis72 (Fig. 12).
Pseudomyxoma peritonei. (A and B) A 66-year-old male patient with mucinous adenocarcinoma of the appendix and pseudomyxoma peritonei. Computed tomography (CT) coronal image (A) with oral and intravenous contrast showing hypodense, loculated, cystic lesions compatible with large hepatic (arrows) and peritoneal capsular implants (asterisks). Also note the involvement of the omentum (dotted arrow). CT axial image (B) with oral and intravenous contrast showing the typical hepatic scalloping (arrows) produced by pseudomyxoma peritonei. Given the massive involvement, any type of surgical intervention was ruled out and chemotherapy was initiated. The patient died 25 months after diagnosis. (C) An 81-year-old woman. Contrast-enhanced CT sagittal image showing a heterogeneous mass with faint diffuse enhancement, consistent with an ovarian mucinous adenocarcinoma (arrowhead). Free fluid (asterisk) is observed at the bottom of the recto-uterine pouch, with the curvilinear calcifications typical of pseudomyxoma peritonei (arrow). Histopathology revealed WHO grade 1 ovarian mucinous carcinoma with mucinous tumor deposits in the peritoneum, mesentery, omentum, and atypical mucinous cells in ascitic fluid. Hysterectomy, double adnexectomy, appendectomy, omentectomy and peritonectomy were performed. The patient died after one year of chemotherapy.
The curative treatment for PMP is cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. However, this is associated with a high rate of morbidity and mortality,73 and the real diagnostic challenge is correct surgical candidate selection. Bouquot et al. published a preoperative radiological scoring system, based on CT findings, which evaluates tumor burden and predicts resectability.74 They suggest that perihepatic involvement is associated with incomplete cytoreductive surgery and, therefore, with a worse prognosis. Postoperative follow-up is recommended.70 In the event of recurrence, surgery can be repeated.70,71
ConclusionsMucinous tumors of the abdomen and pelvis share radiological characteristics that facilitate identification, but they differ in prognosis and clinical evolution. While in some locations such as the liver, pancreas or kidney, their course varies and is sometimes indolent, in others such as the colon, rectum, stomach, bladder or urachus, they are usually aggressive. Radiology is useful for follow-up and to suggest a diagnosis, since diagnostic yield from biopsies and cytologies is usually low in mucinous lesions. Nevertheless, histological findings will ultimately determine the therapeutic management in most cases.
Authorship- 1.
Responsible for the integrity of the study: LRR.
- 2.
Study conception: LRR.
- 3.
Study design: LRR.
- 4.
Data collection: LRR, NFG, DJTS, RGI, TBDS.
- 5.
Data analysis and interpretation: LRR, NFG, DJTS, RGI, TBDS.
- 6.
Statistical processing: NA.
- 7.
Literature search: LRR, NFG, DJTS.
- 8.
Drafting of the article: LRR, NFG.
- 9.
Critical review of the manuscript with intellectually significant contributions: LRR, NFG, DJTS, RGI, TBDS.
- 10.
Approval of final version: LRR, NFG, DJTS, RGI, TBDS.
The authors declare that they have no conflicts of interest.
Please cite this article as: Raposo Rodríguez L, Fernández García N, Tovar Salazar DJ, Gómez Illán R, Díaz Sánchez TB. Hallazgos en imagen en los tumores mucinosos de abdomen y pelvis. Radiología. 2019. https://doi.org/10.1016/j.rx.2019.03.003