Palpable tumors in children are a common reason for consulting a radiologist. The origin of these lesions varies widely, and although they are common, classic radiology books do not cover some of them.
This series of two articles aims to review the clinical and radiological characteristics of a selection of palpable tumors in children that radiologists need to be familiar with.
Las tumoraciones palpables en los niños son un motivo de consulta muy habitual para el radiólogo. El origen de las lesiones es muy variado, y algunas de ellas no reciben atención en los libros clásicos de Radiología, pese a encontrarse frecuentemente.
El objetivo de esta serie de 2 artículos es revisar las características clínico-radiológicas de una selección de estas entidades que consideramos importante conocer.
Palpable tumours in paediatric patients might manifest due to a variety of different diseases with diverse aetiologies and courses. Most of these tumours are benign. As discussed in part one of this article, in most cases the most useful imaging test is ultrasound. The aim of part two is to describe those lesions which are most frequently observed in clinical practice and which are yet to be mentioned. Familiarity with these lesions enables accurate diagnoses and the avoidance of unnecessary—and at times invasive—tests.
PilomatricomaPilomatricomas, also known as pilomatrixomas or ‘calcifying epitheliomas of Malherbe’, are benign skin neoplasms arising from the cells at the base of hair follicles.1 They can appear at any age but are most frequent in children.2 They are the most commonly resected benign neoplasms in paediatric patients after epithelial cysts and lymph nodes.3
They are most commonly located on the head and neck, most typically on the periauricular area and the cheeks.4 The next most common sites are the upper and lower extremities and the trunk. They do not appear on the palms of the hands, the soles of the feet, the genital area, or mucous membranes.
A solitary neoplasm may appear or there may be several, usually smaller than 2 cm. The presence of seven or more suggests that a syndromic condition is associated (namely Turner syndrome, Gardner syndrome or myotonic dystrophy).
They present as painless, slow-growing, subcutaneous nodules. They are hard, with well-defined and irregular borders, and are not adherent to underlying tissue. The overlying skin may be normal in colour or more often present red, blue or purple hues.5
Even when the clinical presentation is typical, an ultrasound is often requested to confirm the diagnosis and assess the extent to which the underlying tissue is affected. An ultrasound scan reveals well-defined round subcutaneous nodules of heterogeneous echogenicity with scattered hyperechoic foci within the nodules, corresponding to microcalcifications, resulting in poor sound transmission or posterior acoustic shadowing. Typical features are the presence of a hypoechoic halo around the lesion, increased echogenicity of the surrounding subcutaneous cellular tissue and a predominantly peripheral flow in the Doppler study (Fig. 1).6 They can become completely calcified.
(A) Clinical image of a 7-year-old girl with a preauricular pilomatricoma. (B) Grey-scale ultrasound image showing a lesion of heterogeneous echogenicity, with the presence of punctate hyperechoic foci compatible with microcalcifications. Note the hypoechoic halo at the periphery of the lesion. (C) Microvascular Doppler ultrasound image showing peripheral flow.
In the case of typical clinical and ultrasound findings, no further imaging tests are performed.
Despite being benign lesions, they are usually removed because of the slight possibility of malignant degeneration.7 Reoccurrence is very rare.
Myofibroblastic tumoursMyofibroblastic tumours belong to a large and heterogeneous subgroup of mesenchymal neoplasms which develop in a range of different ways from lesions that are self-limiting to those that are malignant.8 It would go beyond the scope of this article to describe each one individually. However, we will briefly analyse the ones that we are most likely to encounter.
Locally aggressive and malignant myofibroblastic neoplasms are masses with highly variable and often indeterminate radiological features. Histological examination is usually necessary for diagnosis. We are more interested in gaining a good understanding of the typical clinical and radiological features of benign myofibroblastic neoplasms, since they are more frequent and a good number of them are self-limiting, meaning that a conservative approach may be adopted if there is a strong radiological suspicion.
Fibromatosis colliFibromatosis colli tumours are benign, self-limiting masses that exclusively affect the sternocleidomastoid muscle (SCM).9 Their origin is uncertain, but they are associated with traumatic birth and fetal malposition.10
They present in new-born infants, usually as early as the second week of life, as a hard lateral neck mass. They are a frequent cause of congenital torticollis, as the shortening of the SCM caused by fibrosis can lead to the head tilting towards the affected side, and chin deviation towards the contralateral side.
Although the clinical presentation is distinctive, an ultrasound scan is usually requested to confirm the diagnosis and to rule out other causes of neck masses.11
Ultrasonography shows focal or diffuse thickening of the SCM, which usually takes on a spindle-shaped morphology, affecting the distal half of the SCM to a greater extent (Fig. 2A and B). In the thickened area, the muscle fibres lose their normal appearance and a poorly-defined area of heterogeneous echogenicity is identified, but no masses or enlarged lymph nodes are seen. A Doppler study may show hyperemia.12
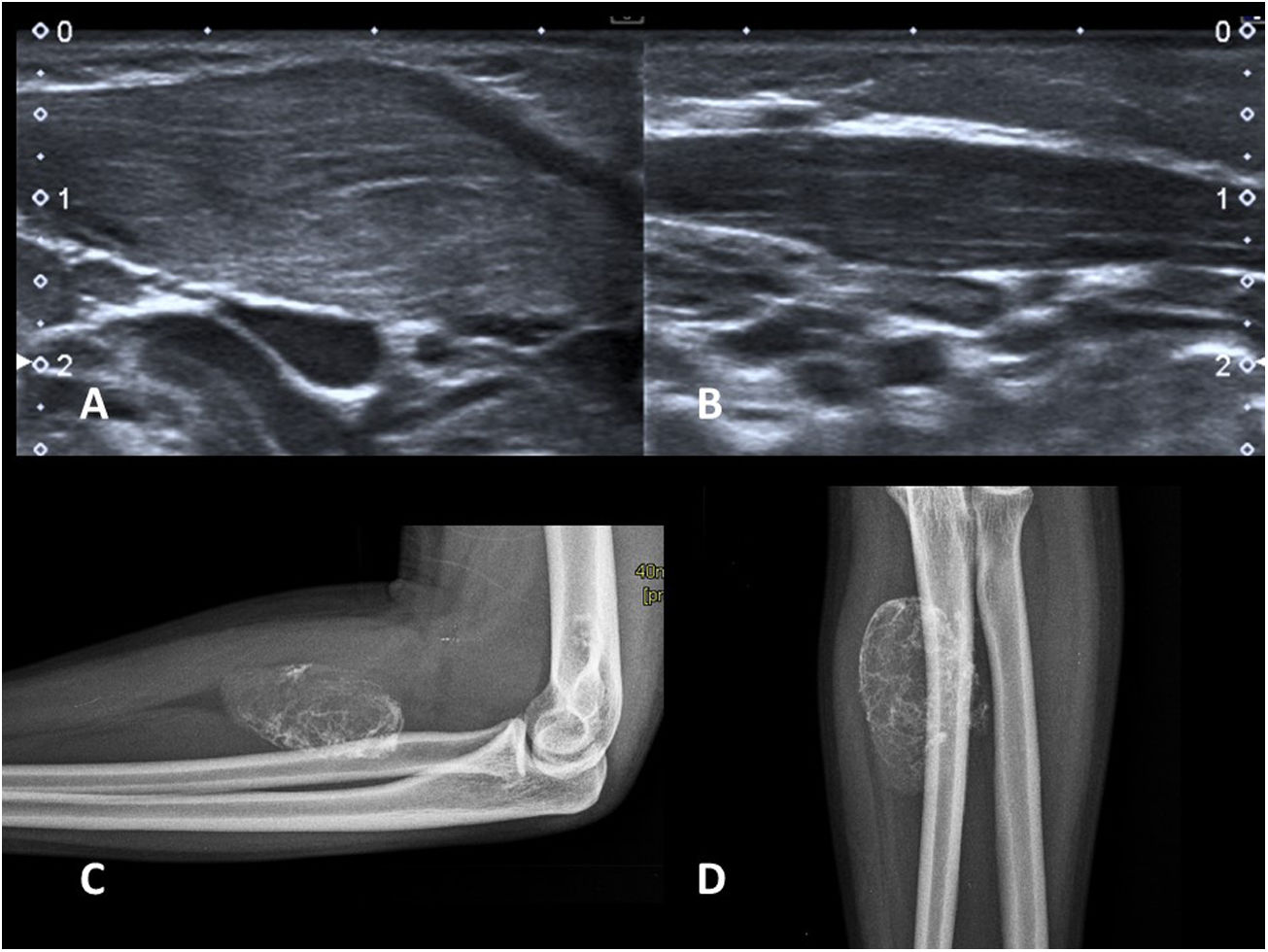
(A and B) Fibromatosis colli. Two-month-old infant with a palpable lateral neck nodule on the right side and torticollis. Longitudinal images of both sternocleidomastoid muscles (SCM) on grey scale ultrasound. Spindle-shaped thickening of the SCM on the affected side (A) versus normal (B). (C and D) Myositis ossificans. AP and lateral X-rays of the forearm of a 15-year-old adolescent with a palpable hard mass and a history of trauma. Typical appearance with peripheral calcification. No further imaging tests are necessary.
Resolution occurs spontaneously in approximately 4–8 months. Physiotherapy is usually used to accelerate healing and avoid cranial deformities associated with torticollis.
Myositis ossificiansMyositis ossificans (MO) is a benign heterotopic ossification in soft tissue. The condition often occurs in skeletal muscle tissue, but can also affect tendons, nerves and subcutaneous fat.13
The typical clinical profile is that of an adolescent presenting with a painful lump on a limb, and a history of trauma to the area. Sometimes the presentation is atypical, as there is no history of trauma or pain, or the site of onset or age may be less typical.
Imaging reveals a soft tissue mass with peripheral calcification (Fig. 2C and D).14 This peripheral calcification is the key to the diagnosis, as calcifications are usually central in soft tissue sarcomas. It is often easier to identify the calcium distribution with a computed tomography (CT) scan. Sometimes in the early stages, calcium is not yet visible, or it might not clearly present the characteristic circumferential pattern. If the imaging findings are inconclusive, but there is a suspicion of MO, a follow-up radiograph or CT scan after two weeks will reveal the progression of calcification and facilitate the diagnosis. It is not uncommon for periosteal reaction to present in adjacent bone.
Magnetic resonance imaging (MRI) and biopsies are best avoided, especially in the early stages. Calcium can be difficult to detect on MRIs and, as with biopsy, MO can mimic soft tissue sarcomas.15 It is the classic example of a “do not touch” soft tissue lesion.
MO is usually self-limiting and greatly diminishes or completely disappears within approximately two years.
Nodular fasciitisNodular fasciitis is a benign myofibroblastic tumour.16 Due to their rapid initial growth as well as high cellularity and mitotic activity in biopsy samples, they have also been referred to as “nodular pseudosarcomatous fasciitis”.
They typically manifest in adolescent patients as solitary, fast-growing, palpable and occasionally painful nodules.
Most lesions occur in the subcutaneous cellular tissue and there is usually extensive contact with muscle fascia. Although they can occur anywhere in the body, the upper extremities are most frequently affected (46%).17
While they may be self-limiting, these are often removed because of aggressive aspects that are observed in imaging and biopsy.
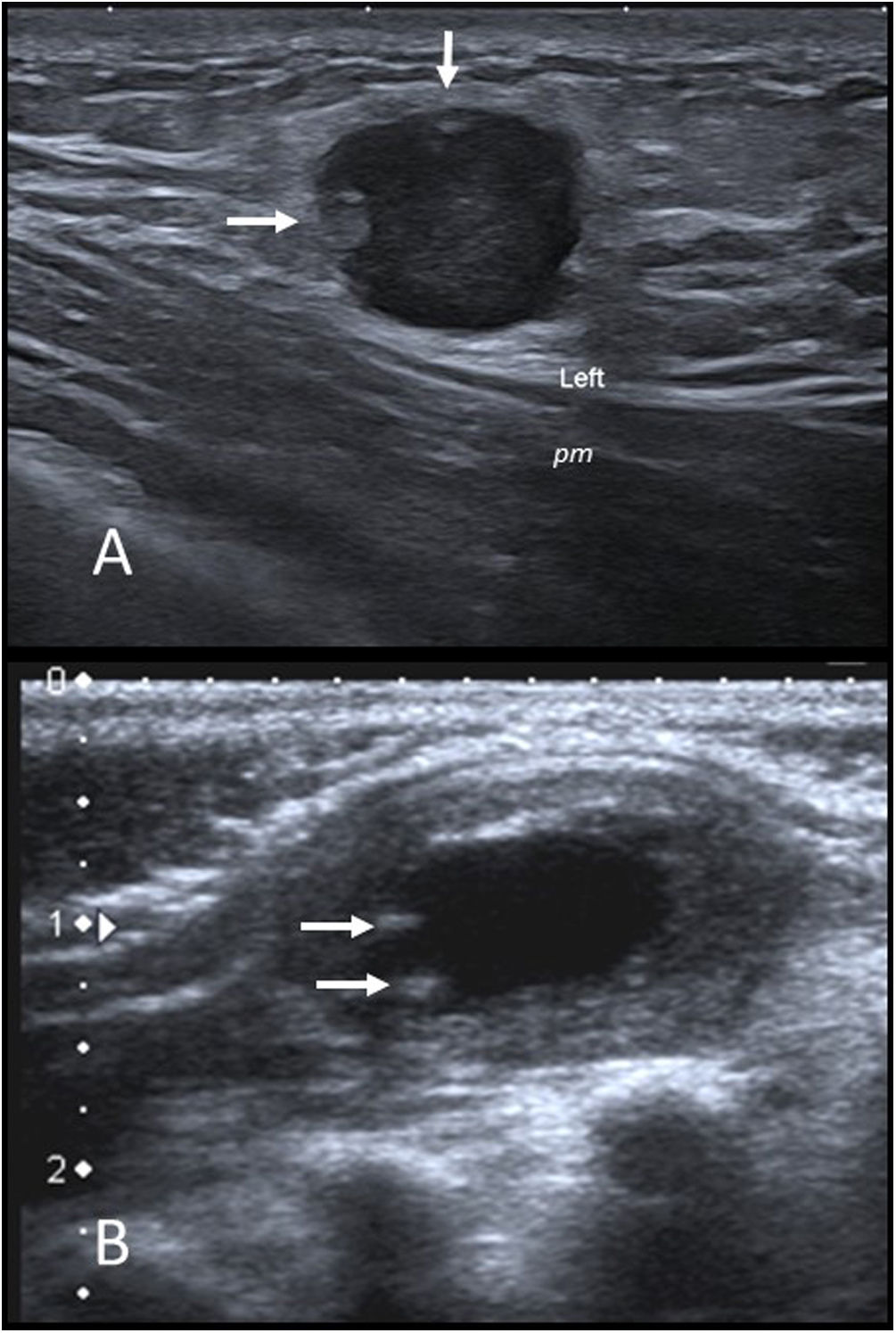
On ultrasound, they are predominantly visible as hypoechoic nodular lesions with lobulated borders that are in contact with, or even impinging on, muscle fascia. A perilesional hyperechoic halo and peripheral intralesional hyperechoic nodular images are common (Fig. 3A). They may show a moderate flow on a colour Doppler ultrasound.
(A) Nodular fasciitis. Grey-scale ultrasound image of a left infraclavicular nodule in a 14-year-old girl. There is a hypoechoic nodule with a well-defined lobulated border that impinges upon the pectoralis major muscle (pm). It shows some peripheral hyperechoic nodular images (arrows) and increased echogenicity of the surrounding subcutaneous fat. (B) Infantile myofibroma. Grey-scale ultrasound of a palpable nodule in the left scapular region of a 2-month-old infant. There is an oval nodule with well-defined borders in the trapezius muscle. The periphery is isoechoic and the muscle and the centre are anechoic with small calcifications in the periphery (arrows).
MRI is not often very useful as these lesions do not have a specific appearance, appearing as isointense nodules with muscle on T1, hyperintense on T2 and with variable gadolinium enhancement.
Infantile myofibromaInfantile myofibromas are the most common fibrous tumours in infants. Most cases involve a solitary tumour, but the term “myofibromatosis” is used when multiple tumours are present.18 They present as palpable nodules with normal or slightly purple skin discolouration.19 They usually form on the skin or in muscle tissue in the head, neck or trunk; bone and visceral involvement is also possible. Most lesions disappear spontaneously within two years, so the prognosis is excellent with the exception of cases with multiple visceral involvement.
Ultrasonography is the most commonly used diagnostic technique, although due to the variable and non-specific appearance of this tumour, surgical removal is usually necessary for final diagnosis. They most typically present as nodules with anechoic centres, with thin peripheral calcifications and solid hypoechoic surrounding areas (Fig. 3B).20
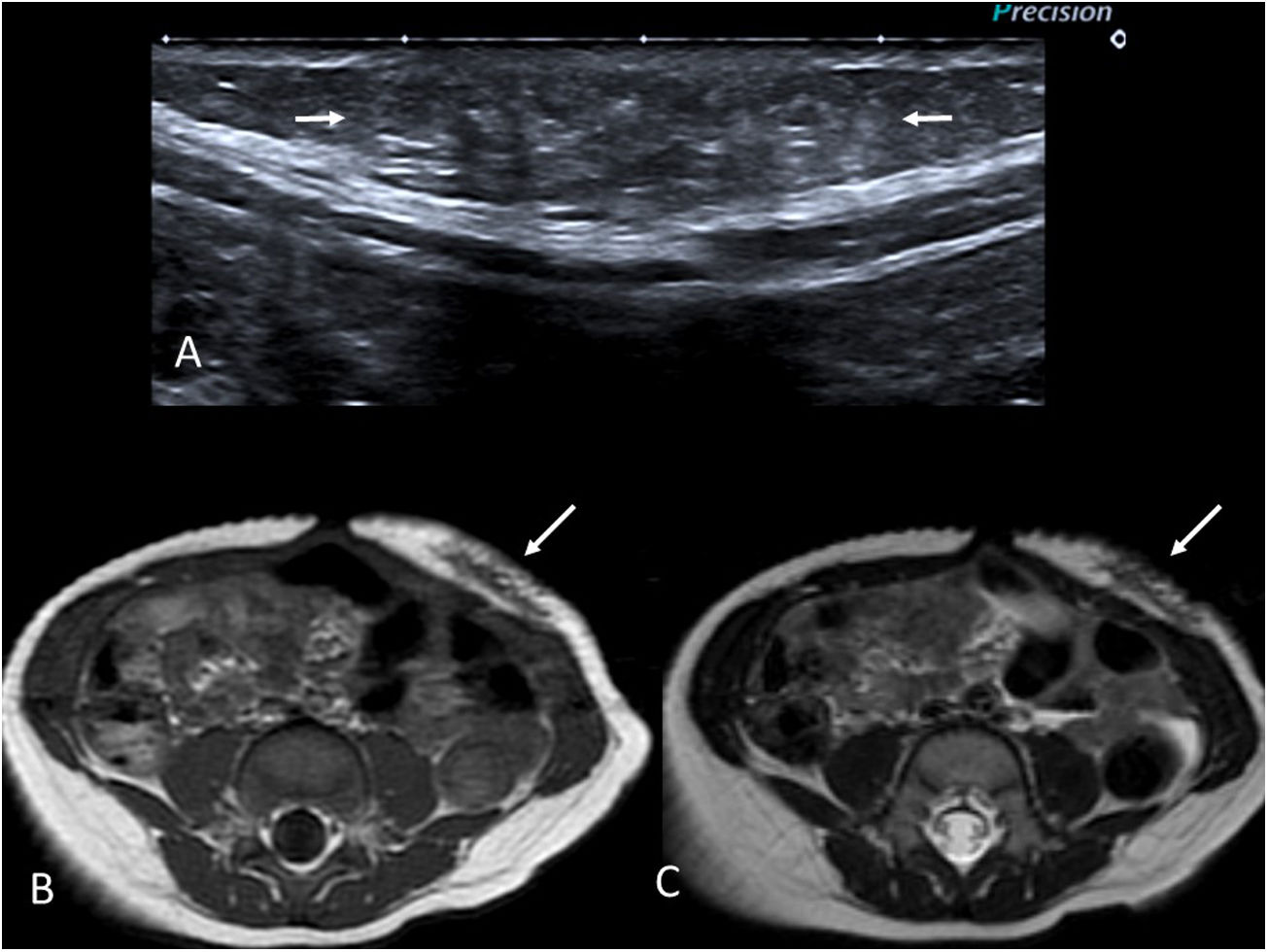
Desmoid-type fibromatosisDesmoid-type fibromatosis tumours, also known as desmoid tumours, are a group of locally aggressive fibrous mesenchymal neoplasms that do not metastasise to distant sites.
They usually present as firm palpable masses, most commonly appearing in the muscle tissue of the extremities, followed by the head-neck region and the trunk.21 The presence of an abdominal desmoid tumour is strongly associated with the presence of Familial adenomatous polyposis.22
Ultrasounds show lesions of variable echogenicity, often with imprecise borders (Fig. 4A). In MRIs, this kind of tumour should be suspected if low signal areas are detected in T2, as this is typical of fibrous lesions (Fig. 4B and C). Gadolinium enhancement is variable. While the scans may raise suspicions, biopsy is recommended before making any therapeutic decisions. Stable asymptomatic lesions are usually monitored, while symptomatic lesions should be treated, with surgery being the treatment of choice wherever technically possible.
9-month-old boy with a desmoid tumour on the anterior abdominal wall. First-degree family history of familial adenomatous polyposis. (A) Grey-scale ultrasound image showing a slightly hyperechoic lesion with poorly defined borders in the subcutaneous fat (arrows). (B and C) T1- and T2-weighted MRI images, respectively, in the axial plane. A lesion with poorly defined borders is identified in the subcutaneous cellular tissue of the anterior abdominal wall (arrows). Its low signal intensity on T2 is notable due to its fibrous nature.
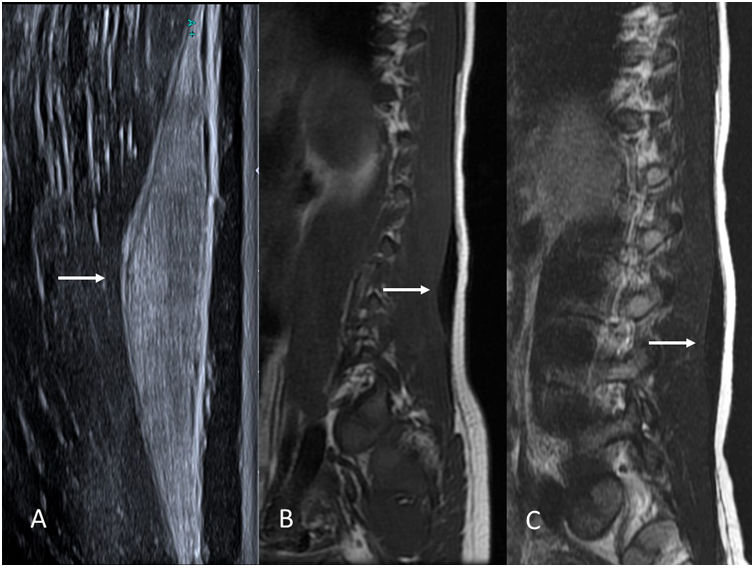
Gardner fibromas are benign fibrous soft-tissue lesions. They present as painless, often paraspinal, tumours in children under 10 years of age.
Their radiological appearance is very distinctive. On ultrasound, they are homogeneously hyperechoic spindle-shaped lesions. On MRI, they have a low signal on T1 and T2, due to their fibrous nature (Fig. 5). They resemble significantly thickened muscle fascia.
9-year-old boy with a Gardner fibroma in the erector spinae muscles. (A) Grey-scale ultrasound image in the sagittal plane showing a markedly hyperechoic spindle-shaped lesion in the muscle fascia (arrow). (B and C) T1- and T2-weighted MRI images of the same patient, respectively. Note the low signal intensity of the lesion on T1 and T2 due to its fibrous nature (arrows).
Even though these lesions are benign and it is not clear that the patient will benefit from their removal, the importance of recognising these masses lies in the fact that, like abdominal desmoid fibromatosis, they are a classic sentinel sign of several familial adenomatous polyposis syndromes.23 For this reason, the patient and immediate family members should be screened for these disorders early and treated accordingly. A Gardner fibroma may precede the development of colon polyps.
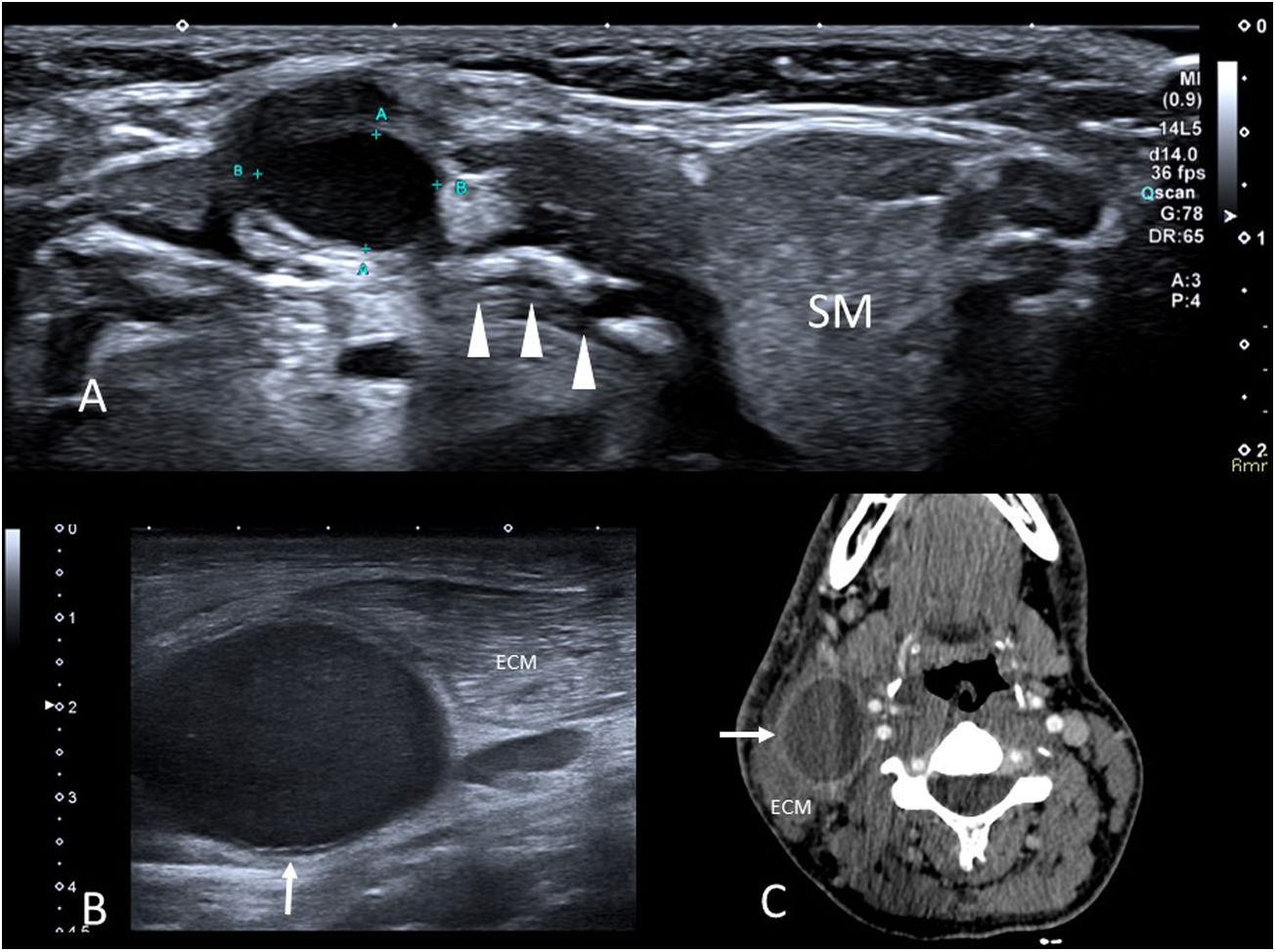
Thyroglossal cystsThyroglossal cysts are the most common congenital cystic lesions of the neck affecting children. The thyroid gland develops at the base of the tongue in the first weeks of gestation and migrates to its normal position via the thyroglossal duct, which is closely related to the forming hyoid bone. If, following thyroid descent, the thyroglossal duct does not completely involute, epithelial remnants of the duct persist and a cyst is formed.24,25
Cysts can occur anywhere in the thyroglossal duct, although 85% are infrahyoid or closely related to the hyoid.26
They usually present as a painless, slow-growing nodule in the anterior region of the neck, at or near the midline. They usually move when the patient moves their tongue or swallows. They may be complicated by infection or bleeding. Malignancy is very rare.
Ultrasound is the test of choice to confirm the diagnosis and rule out complications. In the absence of complications, these cysts present as well-defined nodules that are anechoic or hypoechoic depending on their content, and which reveal posterior acoustic enhancement and an absence of flow in Doppler ultrasound studies (Fig. 6A). When complications are present, their echogenicity is variable and heterogeneous, and there are usually adjacent inflammatory changes. MRIs are generally unnecessary and show signals that are hypointense in T1 and hyperintense in T2, although hyperintensity may be present in T1 due to the presence of proteinaceous content.27 When complications are present, their content is more heterogeneous and surrounding inflammatory changes are observed, such as in the ultrasound scan.
(A) Thyroglossal cyst. Axial grey-scale ultrasound image of a nodule in the anterior midline of the neck in a 4-year-old boy. A hypoechoic lesion with well-defined borders is seen in intimate contact with the body of the hyoid bone (arrowheads) (SM: submandibular gland). (B) Cyst of the second branchial arch. Axial grey scale ultrasound image of the right lateral neck region. A cystic lesion (arrow) is seen adjacent to the sternocleidomastoid muscle (SCM). (C) Axial image of the pre-surgical contrast-enhanced CT scan of the previous patient. The cyst exhibits a thin wall with enhancement (arrow) and is located between the SCM and the carotid vessels.
The differential diagnosis typically involves submental lymph nodes and dermoid cysts in the anterior midline of the neck. If the lymph nodes do not present complications, they have a central fatty hilum and are easy to differentiate. When purulent or necrotic contents are found (for example, in non-tuberculous mycobacterial adenitis) they may have a similar sonographic appearance, and the relationship between the lesion and the hyoid is helpful in deciding between different diagnoses. Dermoid cysts exhibit posterior enhancement, but have a uniform, medium echogenicity and do not come into contact with the hyoid.
Ultrasound should also be used to confirm that the thyroid gland is correctly positioned and to rule out the presence of ectopic thyroid tissue.28
Treatment involves surgical resection, usually using the Sistrunk procedure, which includes the cyst, its tract and the middle third of the hyoid to prevent recurrence.
Branchial cleft cystsThese are benign lesions caused by anomalies in the closure and reabsorption of the branchial structure. They usually present clinically as slow-growing and movable neck masses that are typically painless except if they bleed or become infected. They are classified according to the relevant branchial arch.29
Second branchial cleft cysts are the most common (accounting for more than 90%). They can be located anywhere in the space between the anterior border of the SCM muscle and the parapharyngeal region, although their most frequent location is lateral to the carotid vessels, between the SCM muscle and the submandibular gland. They most frequently present when the patient is in the second or third decade of life.
First branchial cleft cysts (5%–8%) are closely associated with the auricle, the external auditory canal and the parotid gland. They occur most frequently in children under 10 years of age.
Third and fourth branchial cleft cysts are very rare.
These cysts are initially assessed by ultrasound (Fig. 6B), where they appear as well-defined, thin-walled lesions with contents that are usually anechoic, although sometimes they may be echogenic or even solid in appearance (but without flow on Doppler). CT scans and MRI are performed if there are diagnostic uncertainties or for surgical planning (Fig. 6C). In both techniques, these lesions exhibit thin walls and liquid content. If superinfection or bleeding is present, the wall is thickened and the appearance of the contents changes.
Treatment is surgical resection.
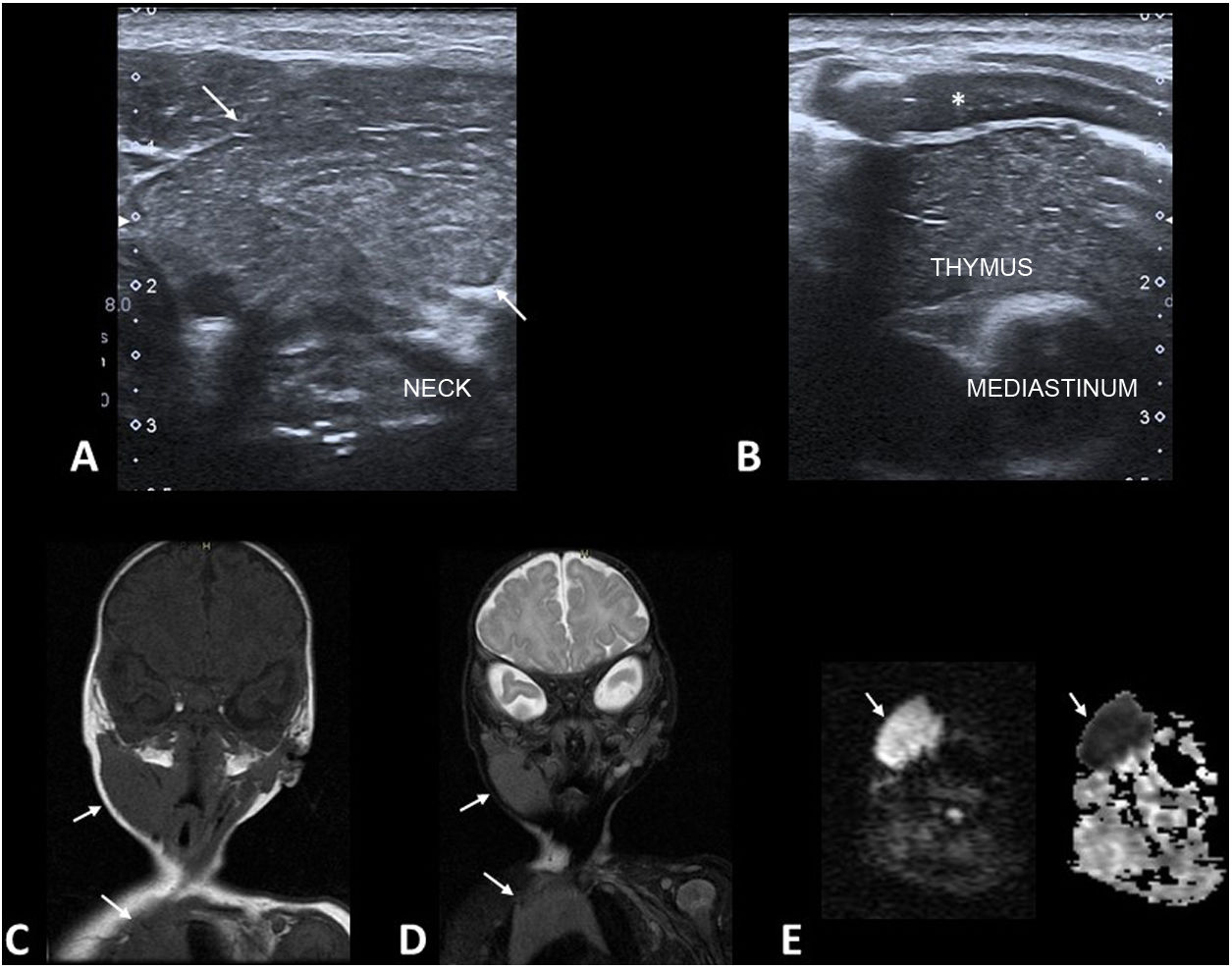
Ectopic cervical thymusIf the thymus fails in any way to descend to the mediastinum during embryonic development, ectopic thymic remnants may develop.30 These are rare lesions, probably underdiagnosed because they are asymptomatic.
They present in infants as firm neck masses. They can occur anywhere along the pathway of the thymopharyngeal duct, from the angle of the mandible to the mediastinum, with the most typical locations being the lateral cervical region (closely related to the neurovascular bundle of the neck) and the thyroid gland. In 85% of cases, they are unilateral.
The main diagnostic test used is ultrasound (Fig. 7A and B). The ultrasound appearance of the thymus is very distinctive and the ectopic thymus exhibits an identical echotexture to that of the orthotopic thymus.31 MRI is only necessary in cases of diagnostic uncertainty or to assess large lesions. On MRI, the signal intensity is identical to that of the mediastinal thymus in all sequences (Fig. 7C and D). Severely restricted diffusion is normal in the thymus of infants (Fig. 7E).
Ectopic cervical thymus. (A and B) Grey-scale ultrasound images of a 2-month-old infant with a palpable soft right lateral neck mass. The lesion (A, arrows) has an identical echotexture to that of the orthotopic thymus (B) (*: costal cartilage). (C and D) Coronal plane T1-weighted (C) and T2-weighted fat-saturated (D) MRI images with identical signal intensity in the cervical lesion and mediastinal thymus (arrows). (E) Diffusion and ADC-weighted MRI images show the severely restricted diffusion of the lesion (arrows), a normal finding in the thymus of an infant.
If the findings are typical, biopsy is not recommended and is moreover, often inconclusive. Conservative management is recommended. Ectopic thymic tissue involutes in parallel to orthotopic thymic tissue, so only ultrasound monitoring is recommended. Malignant transformation to a thymoma is exceptional, the risk being similar to that of the mediastinal thymus. Surgery is only considered in the case of large, symptomatic lesions.
Epigastric herniaThese hernias are common and present as palpable nodules in the midline of the anterior abdominal wall, somewhere between the xiphoid process and the umbilicus.
They correspond to small accumulations of properitoneal fat herniated through millimetric defects in the linea alba. The defect is so small that there is usually no space for other abdominal contents to herniate. It is not uncommon for the same patient to have two or more hernias.32
The defect in the linea alba and herniation of the properitoneal fat can be seen on ultrasound.
They are usually surgically repaired.
Prominent costal cartilageA common complaint, usually in adolescent boys, involving a hard, painless lump on the anterior chest wall.33 They are usually caused by an asymmetry in the costal cartilages, with one or more being more convex towards the front on the prominent side. They may be associated with sternal tilt. It is very prevalent, affecting one third of the population,34 and is regarded as a normal variant. In our experience, it is more common for the prominence to be on the left side of the sternum.
If there is clinical doubt, ultrasound is sufficient to confirm costal cartilage asymmetry35 and rule out masses of other origins, without the need to resort to other tests.36
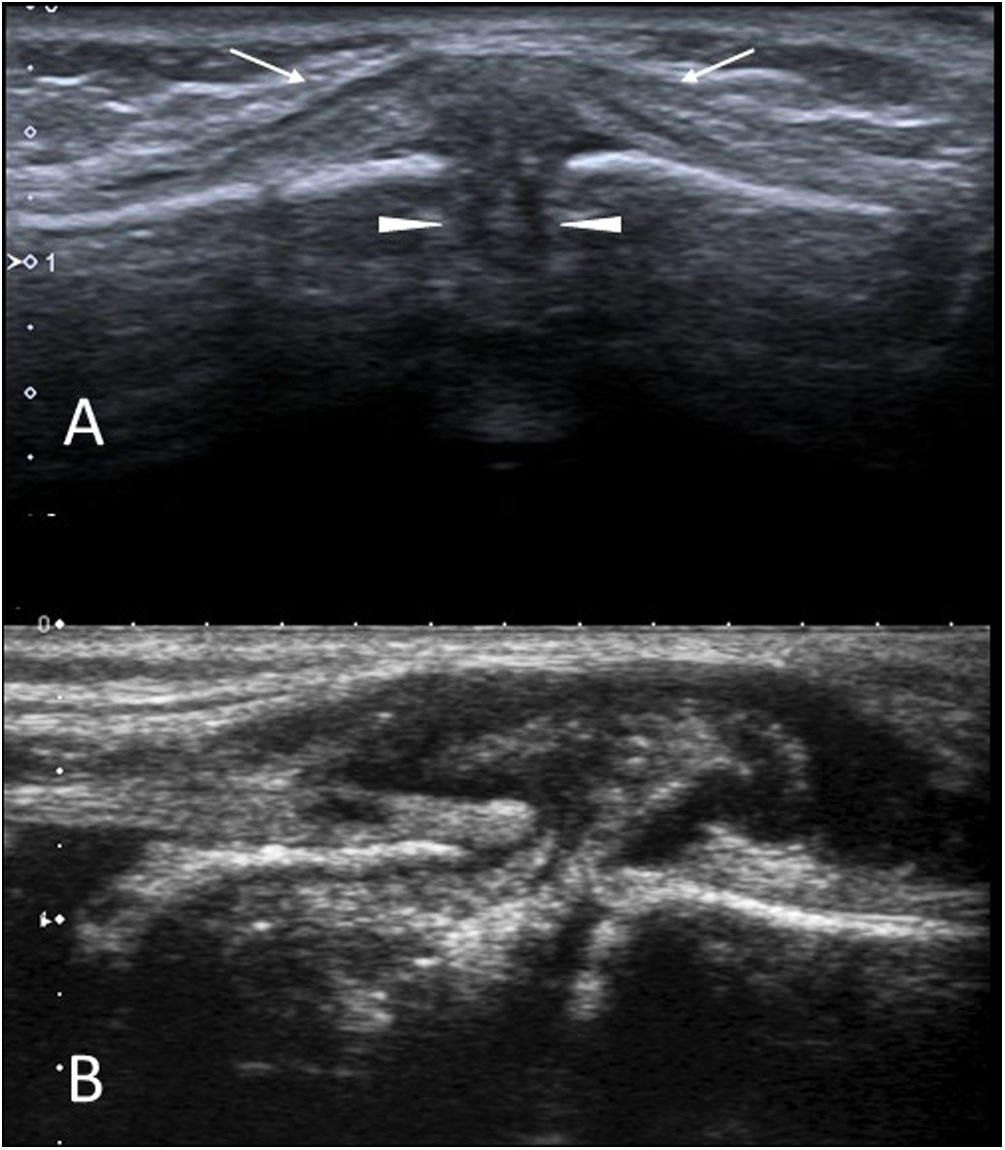
Self-Limiting Sternal Tumours of Childhood (SELSTOC)Self-Limiting Sternal Tumours of Childhood (SELSTOC) are a relatively little-known condition involving self-limiting aseptic inflammation of unknown aetiology in the sternums of young children (the condition has not been reported in children over five years old).37–39 Awareness of their existence helps prevent unnecessary biopsies.
These lesions are asymptomatic or not very painful, appearing as a 2–3 cm presternal or parasternal tumour. There may be reddening of the skin. The child is sometimes brought in for another reason and the comorbidities (fever, respiratory infection, etc.) generate diagnostic confusion. There are no abnormalities in the blood tests.
The initial study should be performed with ultrasound. The tumour exhibits an hourglass-shaped hypoechoic lesion between the ossification nuclei of the sternum, separating them and giving the appearance of bone destruction (Fig. 8). When clinical and ultrasound findings are typical, a wait-and-see approach is usually maintained, with complete resolution typically occurring within one to six months. No further imaging tests are usually necessary.
SELSTOC. (A) Sagittal plane grey-scale ultrasound image of a 2-year-old boy with a hard, painless presternal nodule. The lesion (arrows) is predominantly hypoechoic and is located between the ossification nuclei of the sternum, separating them (arrowheads). (B) Sagittal plane grey-scale ultrasound image of a 4-year-old girl with clinical and imaging findings similar to those of the previous patient.
If there is any uncertainty about the diagnosis or it does not follow the expected course, a biopsy is performed to rule out a neoplastic process or osteomyelitis. A non-specific inflammatory infiltrate is typically found with no evidence of neoplastic cells. Microbiological studies come back negative.
Lipoma and lipoblastomaLipomas are benign lesions comprised of encapsulated mature adipocytes. While they are the most common adipocytic tumour in children, they are seen much more frequently in adults.40 On ultrasound, lipomas are identified as lesions with well-defined borders and homogeneous echogenicity, which may be higher or lower depending on the proportion of water and fat present. Their adipose nature can be confirmed by MRI.
The next most common adipocytic tumours in children are lipoblastomas. These are benign tumours comprised of mature and immature adipocytes. They are more frequent in children under three years of age and very rare after the age of eight. On ultrasound, these lesions are usually hyperechoic, but it is not uncommon for them to exhibit hypoechoic or cystic areas due to their fibromyxoid content. On MRI it is usual to see an encapsulated lesion with contents that are not completely fatty, especially at the periphery of the lesion. Treatment is surgical, with less than 20% risk of local recurrence.
Soft tissue sarcomasSarcomas are malignant solid tumours of mesenchymal cell origin.41 When palpable, they are usually referred for assessment with ultrasound, where their appearance is variable. On MRI they have a heterogeneous signal that is isointense or hypointense on T1 and hyperintense on T2, with areas of enhancement after the administration of contrast; they frequently exhibit restricted diffusion.
In some instances, imaging tests do not provide a conclusive diagnosis, but they are of great value in obtaining topographical information for planning a biopsy or surgery, as well as for assessing responses to treatment. It is worth remembering that, in general, when a soft tissue lesion is not clearly a self-limiting or benign entity, it should at least be closely monitored and, in most cases, be removed or at least biopsied.
The most frequently encountered soft tissue sarcomas in children are rhabdomyosarcoma, synovial sarcoma and Ewing’s sarcoma.
Rhabdomyosarcomas are the most common childhood sarcomas. The average age at diagnosis is five years. Although they are most commonly found in the head-neck region and the genitourinary tract, 20% occur in the extremities.
Synovial sarcomas are the next most common sarcomas in children. Their name is misleading as, although they usually occur near joints, they do not originate from the synovium and most are extra-articular. They are usually well-demarcated lesions with variable radiological features, ranging from solid tumours with cystic and bleeding areas to completely cystic lesions with an “innocent” appearance that can be mistaken for benign lesions such as ganglions. On MRI, the presence of a soft tissue mass with three different signal intensities on T2 is considered highly indicative of synovial sarcoma.42
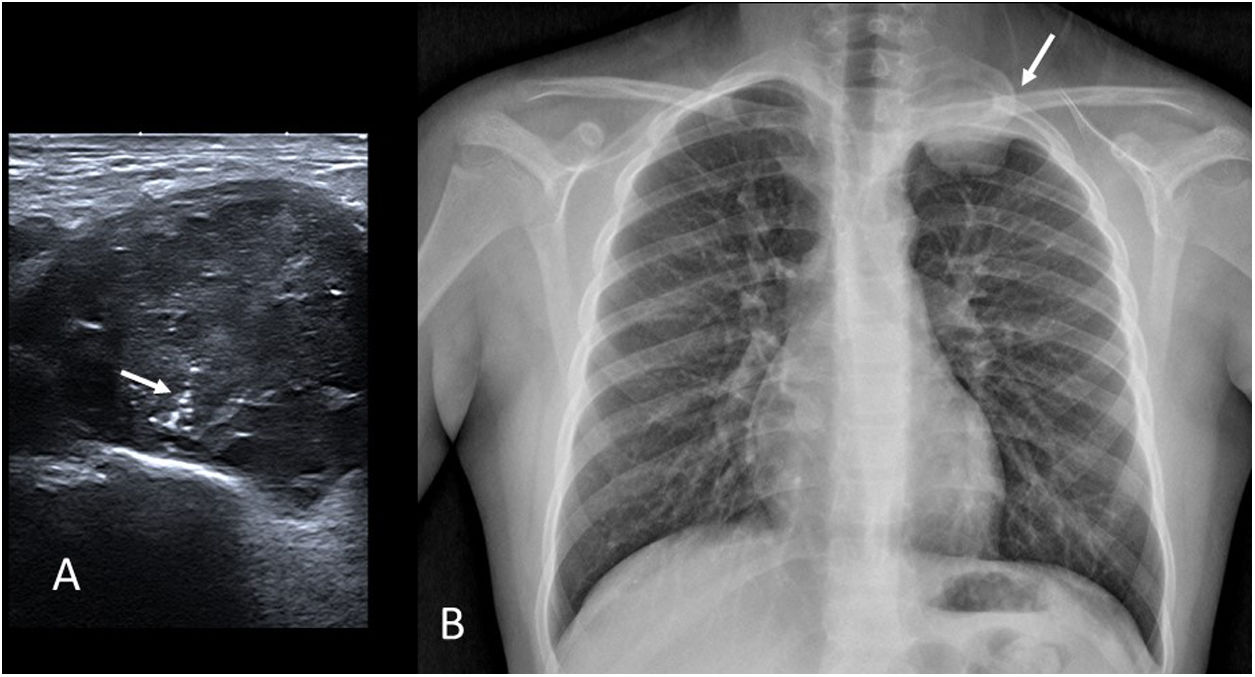
Ewing’s sarcomas are primitive small round cell tumours. They are typically found in patients under 20 years of age and, although they are most frequently found in bone, 20% are extra-skeletal. They often present as a palpable mass, often with local pain (Fig. 9).
Ewing’s sarcoma. (A) Grey-scale ultrasound image of a 13-year-old male referred for a palpable left supraclavicular mass. We observe a lesion of heterogeneous echogenicity with fine linear calcifications (“the rising sun”, arrow). (B) Chest X-ray of the same patient showing a left extrapleural mass originating from the second rib (arrow).
The vast majority are benign peripheral nerve sheath tumours (PNSTs). Other entities, such as neural fibrolipoma and malignant lesions, are much less common. There are two types of PNST: schwannomas (also called neurilemomas or neurinomas) and neurofibromas. They usually appear between the age of twenty and thirty.43
They generally present as solitary lesions. Multiple lesions are more common in the case of neurofibromas in patients with neurofibromatosis type 1 (NF-1).
They are most frequently located in the head and neck area, the flexor surface of the extremities, mediastinum and retroperitoneum, usually as painless, slow-growing masses. Sometimes, especially in the case of large lesions, they may be associated with pain or other neurological symptoms.
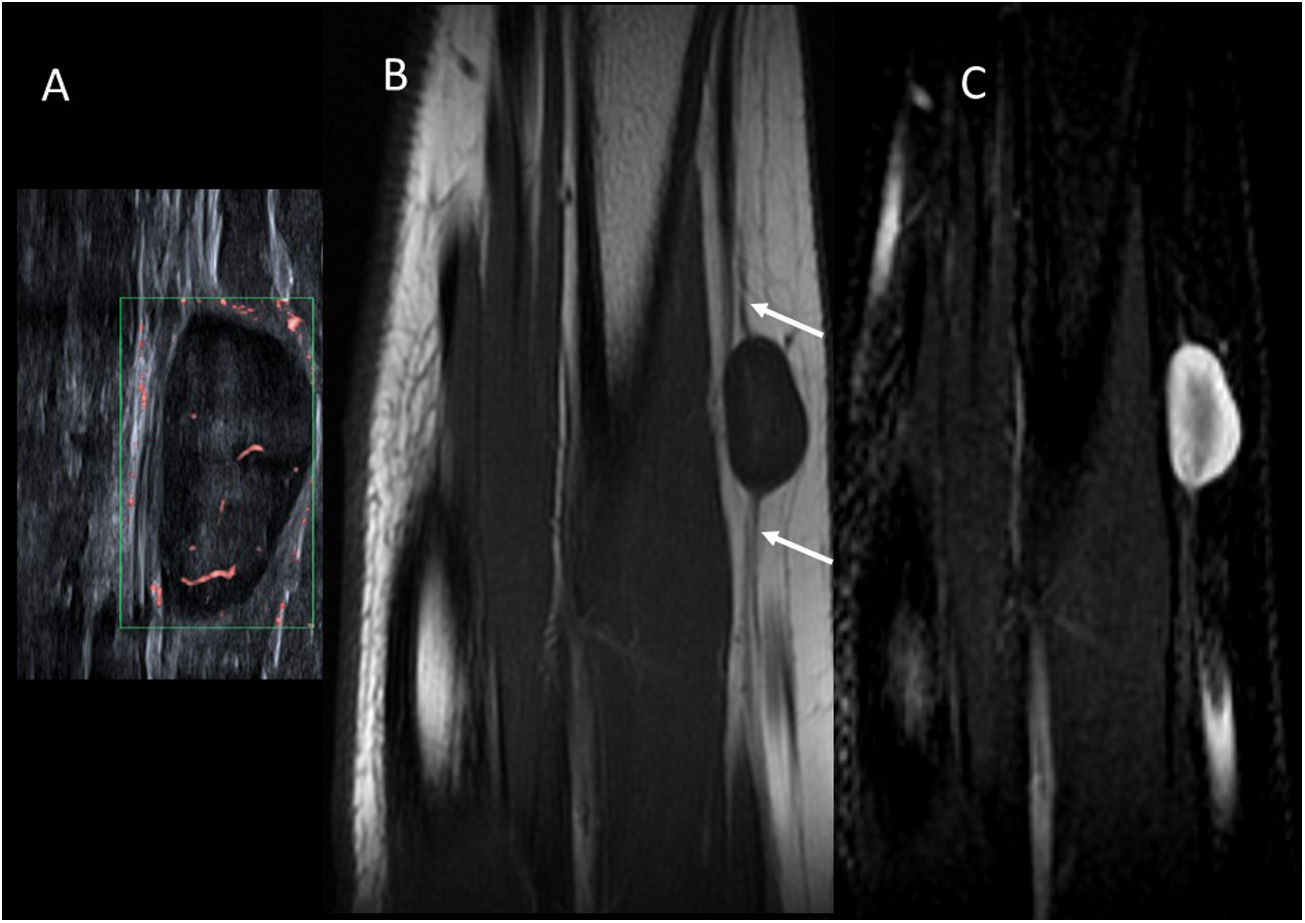
In imaging tests (Fig. 10) they exhibit well-defined spherical or spindle-shaped masses. They are found along a peripheral nerve, whether proximal or distal, and this is one of the most specific signs to indicate their neurogenic origin. On ultrasound, they are usually homogeneous hypoechoic lesions with low flow in the Doppler study. On MRI, they are usually hypointense on T1 and hyperintense on T2. The presence of a central hypointense area in T2 (target sign) is the most distinctive feature and upon administration of intravenous contrast, central enhancement may be present. Sometimes there is muscle atrophy in the muscles innervated by the affected nerve.44,45
Schwannoma of the radial nerve. (A) Microvascular Doppler ultrasound image of an 11-year-old girl with a palpable nodule on the outer surface of the forearm. There is a hypoechoic oval lesion with well-defined borders and a slightly hyperechoic centre, with internal flow. (B) T1-weighted MRI image in the coronal plane. There is eccentric growth of the lesion from the radial nerve (arrows). (C) T2-weighted MRI image with fat suppression in the coronal plane. The periphery of the lesion is hyperintense and the central region is hypointense (“target” sign).
It may be impossible to differentiate between schwannomas and neurofibromas through imaging, although the former more frequently present cystic, haemorrhagic or necrotic areas, so their signal may be more heterogeneous. Neurofibromas exhibit an intrinsic relationship with the nerve, whereas schwannomas are eccentric, so it is easier to distinguish between them in large lesions by assessing their relationship with the nerve of origin.
They are treated surgically. The neurofibroma is inseparable from the nerve, which cannot therefore be preserved in surgery, whereas schwannomas are eccentric and so the nerve can usually be conserved.
Malignant PNSTs usually correspond to the malignant transformation of a plexiform neurofibroma (a variant of neurofibroma that affects a longer segment of the nerve and its branches, and it is indicative of NF-1). On MRI, the heterogeneity of the lesion, peripheral enhancement and perilesional oedema are reasons to suspect malignancy, although sensitivity and specificity for differentiating between benign and malignant lesions is relatively low.
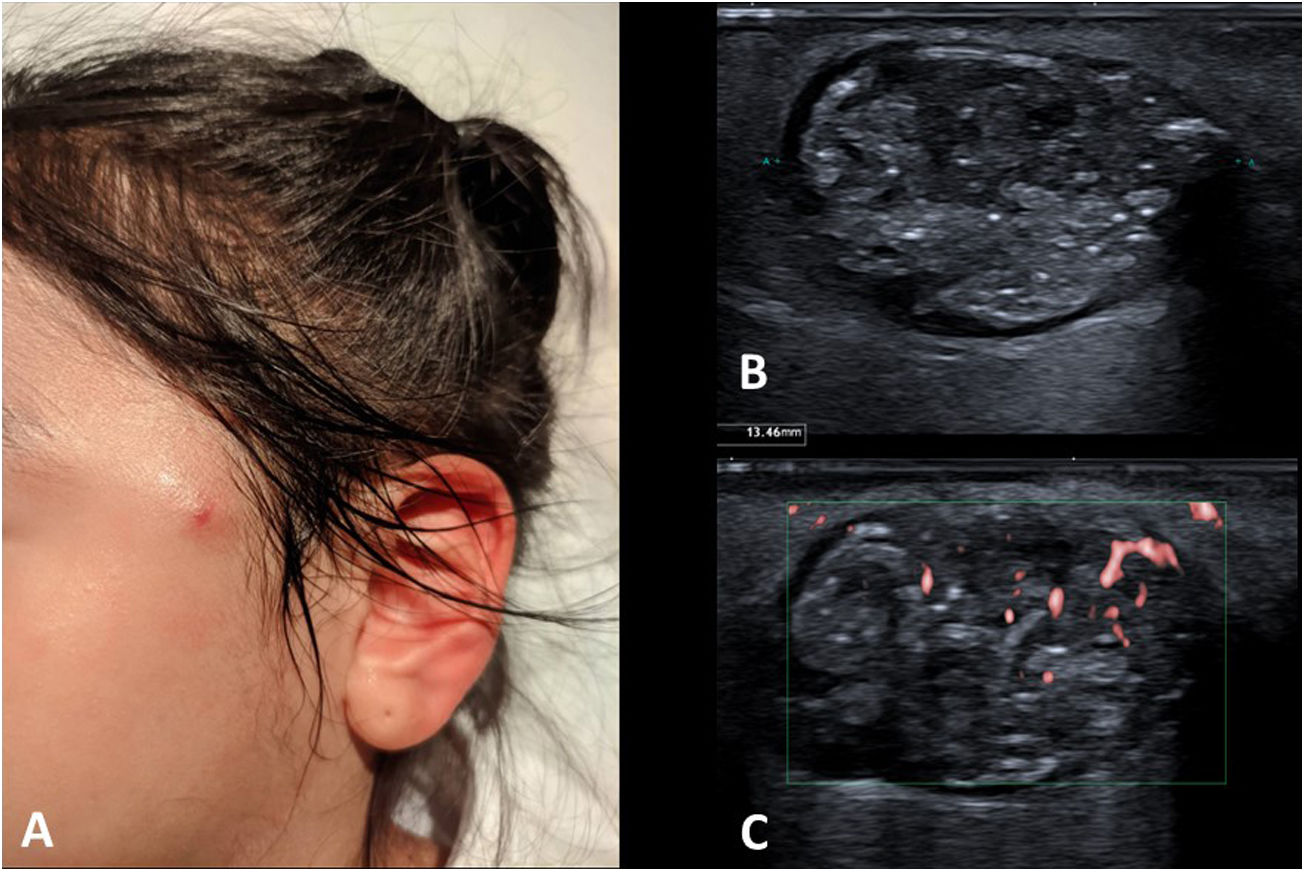
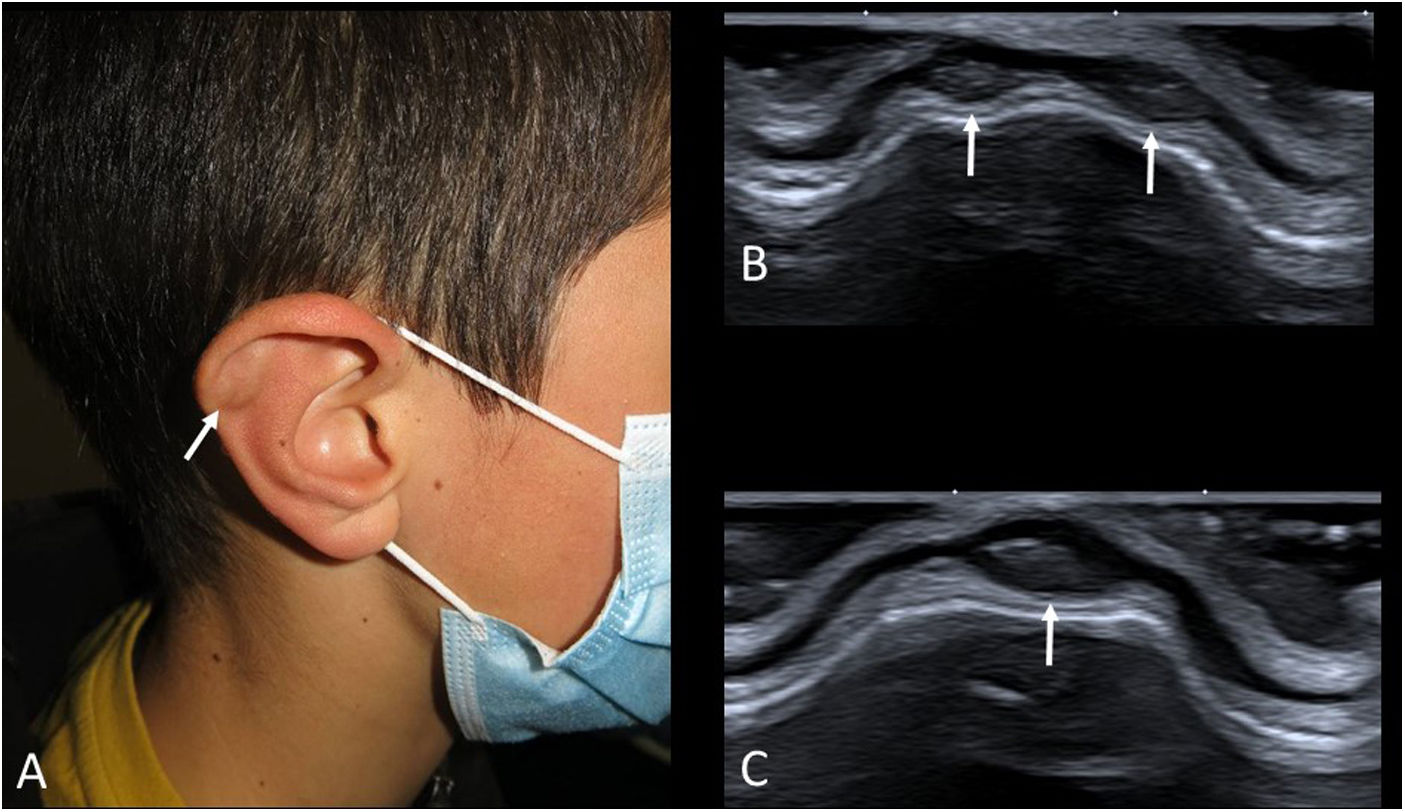
Auricular pseudocystsAuricular pseudocysts consist of small fluid collections in the cartilage of the auricle. Their origin is attributed to repeated trauma. Until recently they were rare in paediatric patients (sometimes seen in children with the habit of repeatedly bending their ears), but with the prolonged use of facemasks and earphones, their frequency has increased.46 On examination, they are small painless nodules in the helix, with normal colour or slight redness (Fig. 11A).
As these are relatively little-known lesions, an ultrasound scan is often requested. Small “cysts” are seen in the thick cartilage of the auricle (Fig. 11B and C). Their echogenicity varies from anechoic to hyperechoic, depending on their haematic content. If the trauma ceases, they usually resolve on their own, but if they are very large or persistent, they may be aspirated.
ConclusionPalpable tumours in children are one of the most common reasons for consultations in paediatric radiology. In this two-part article series, we have examined the clinical and radiological features of the most common kinds. They are usually benign lesions, although it is important always to be alert to the possibility of finding a more serious disease.
Ultrasound is the most widely used imaging technique due to its availability, safety and high-quality results. An accurate radiological diagnosis is extremely important, because it usually determines the course of care to be followed and can prevent unnecessary tests, iatrogenesis and delays.
Authors- 1.
Research coordinators: DL and IP.
- 2.
Development of study concept: DL and IP.
- 3.
Study design: DL and IP.
- 4.
Data collection: DL, IP and LC.
- 5.
Data analysis and interpretation: DL and IP.
- 6.
Statistical processing: not applicable.
- 7.
Literature search: DL and IP.
- 8.
Article authors: DL, IP, LC and LA.
- 9.
Critical review of the manuscript with intellectually relevant contributions: DL, IP, LC, JA and LA.
- 10.
Approval of the final version: DL, IP, LC, JA and LA.
The authors declare that they have no conflicts of interest.