Hearing loss is the most frequent complication of temporal bone trauma. The role of the radiologist is of great importance; the adequacy and selection of the imaging technique, as well as its correct interpretation, are crucial to establish the diagnosis, prognosis and enable the selection of appropriate treatment. With the aim of systematizing the most relevant concepts in the evaluation of image studies in this scenario, this review will be outlined according to the hearing loss type. The potential lesions of its components will be assessed; In each case the most appropriate imaging technique will be suggested and the findings will be described and depicted.
ConclusionIn postraumatic hearing loss, computed tomography is the initial technique of choice and will allow the detection of alterations that cause conductive hearing loss; magnetic resonance imaging will be useful in the evaluation of sensorineural hearing loss.
La hipoacusia es la complicación más frecuente del traumatismo del hueso temporal. El papel del radiólogo es de gran importancia; la adecuación y la selección de las pruebas radiológicas, así como su correcta interpretación, son cruciales para establecer el diagnóstico y el pronóstico, y para seleccionar el tratamiento idóneo. Con el objetivo de sistematizar los conceptos más relevantes en la valoración de los estudios de imagen en este contexto, se esquematizará el desarrollo del tema según el tipo de hipoacusia que condicione el traumatismo. De forma ordenada se valorarán las potenciales lesiones de sus componentes; en cada caso se sugerirá la técnica de imagen para su evaluación y se describirán e ilustrarán los hallazgos.
ConclusiónEn la hipoacusia postraumática, la tomografía computarizada es la técnica de elección inicial y permitirá la detección de alteraciones que condicionen hipoacusia conductiva; la resonancia magnética es útil en la valoración de la hipoacusia neurosensorial.
Hearing loss is an important consequence of traumatic brain injuries and a disability that should be systematically assessed by analyzing the different components of the auditory pathway. It is estimated that it affects around al 24–81% of patients with temporal bone traumas.1 Hearing losses can be categorized into conductive, or neurosensorial; the conductive hearing loss is the result of damage to the outer ear, and the neurosensorial hearing loss is the result of damage to the inner ear or the central auditory pathway.
Traditionally, the role of the radiologist in the assessment of the temporal bone trauma was limited to detecting the fractures and classifying them as transverse or longitudinal based on the direction of the main trace with respect to the petrous part of the temporal bone.2 However, today, on top of using other classification systems such as those based on the involvement of the otic capsule with greater therapeutic and prognostic implication,3,4 we are able to assess other components of the auditory pathway that can be damaged without the existence of a temporal bone fracture, such as the ossicular chain, the inner ear, or the central auditory pathway. We should remember that when dealing with temporal bone fractures, the direction of the trace, the portion affected, and the involvement of the otic capsule should be described.
We need to have a complete anatomical knowledge of the auditory system and the possible lesion mechanisms. We should also know what radiologic imaging modalities are available and what their indications are. In most cases, choosing the right imaging modality and making correct interpretations of the findings will allow us to establish functional diagnoses and prognoses, and eventually choose the optimal therapy. In general, the computed tomography (CT) scan is the initial modality of choice for the management of temporal bone traumas, since it allows fast, minimal manipulations of the patient who may have severe lesions, with sub-millimeter resolution. The deferred magnetic resonance imaging (MRI) allows us to assess the inner ear, the central auditory pathway, and other possible complications.
In an attempt to systematize the most relevant concepts in the assessment of imaging studies in this scenario, we will be reviewing this topic based on to the type of hearing loss conditioned by the trauma. The potential lesions of its components will be assessed in an orderly fashion; in each case the most appropriate imaging modality will be suggested, and the findings will be described and depicted.
Conductive hearing lossIt is due to alterations in both the outer and inner ears. Among these lesions we find the accumulations of blood deposits or debris, fractures located in the external auditory canal, and damage to the ossicular chain. During the first days following the trauma, the hearing loss is not easy to assess, especially in the presence of hemotympanum causing the reduced, temporary sound transmission. Should the hearing loss persist after the hemotympanum has been resolved and the tympanic membrane has been re-established, then we should suspect structural damage to the ossicular chain.5
The CT scan allows us to make accurate assessments of the temporal bone and is considered the imaging modality of choice.6,7 The hemotympanum can make the assessment of the ossicular chain a difficult task. Making comparisons with the healthy side together with the use of multiplanar and volumetric reconstructions is useful for visualization purposes.
Accumulations in the outer and middle earsThese accumulations are mainly hemorrhages and are relatively common in temporal bone fractures, and can be the early sign of one subtle fracture easily misdiagnosed (Fig. 1). The hemotympanum can be accompanied by otorrhagia when in the presence of tympanic perforation. The conductive hearing loss is transient and usually resolves within 5–6 weeks.5,6
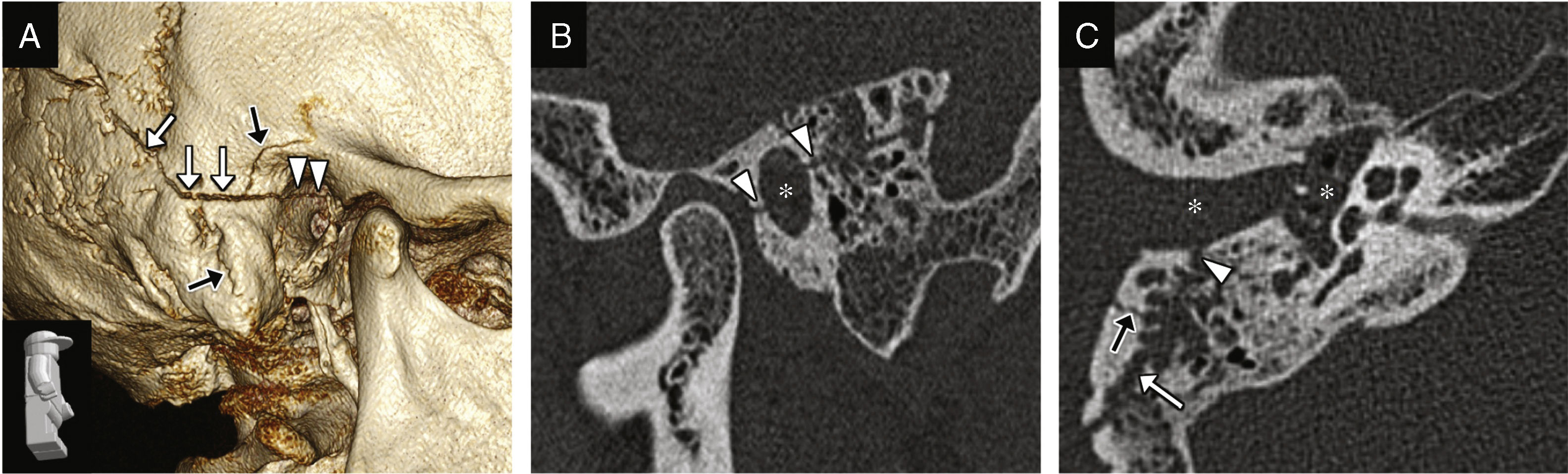
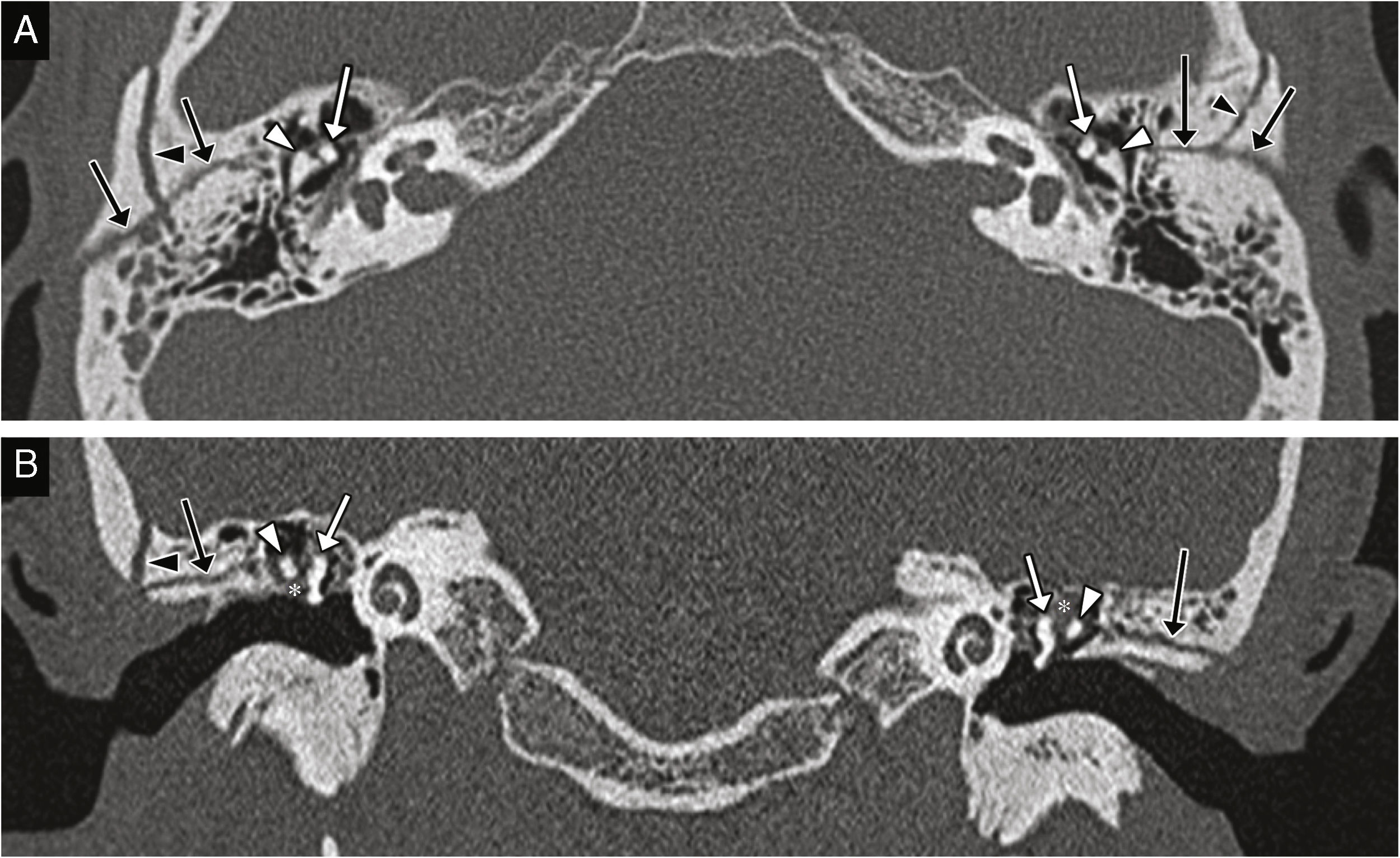
Fracture of the external auditory canal, hemorrhage in the external auditory canal and the middle ear. (A) CT-volumentric reconstructions showing the fracture of the temporal bone with damage to its mastoid and tympanic portions (white arrows) spreading toward the external auditory canal (arrow heads); other fracture traces can be seen (black arrows). (B) CT-sagittal reconstructions showing damage to the anterior and posterior walls of the external auditory canal due to the fracture trace (arrow heads), and lumen occupation (asterisk) due to blood deposits. (C) Transverse CT image showing the fracture trace (white arrow), damage to the external auditory canal (arrow head), another fracture trace (black arrow), and the external auditory canal and middle ear occupation by a material consistent with a hemorrhage in the clinical exploration (asterisks).
There can be temporal bone fractures running across the external auditory canal, or isolated fractures in the anterior wall due to mandibular condyle impaction (Fig. 1).8 They should not be interpreted as tympanosquamous and petrotympanic fissures, which is why knowing their trajectory and appearance is a must.
Ossicular chain dislocationsThey are much more common than ossicular fractures. The CT scan allows us to perform accurate assessments of the ossicular chain, which in turn allows us to perform assessments of the actual position and movement of the small bones.
- •
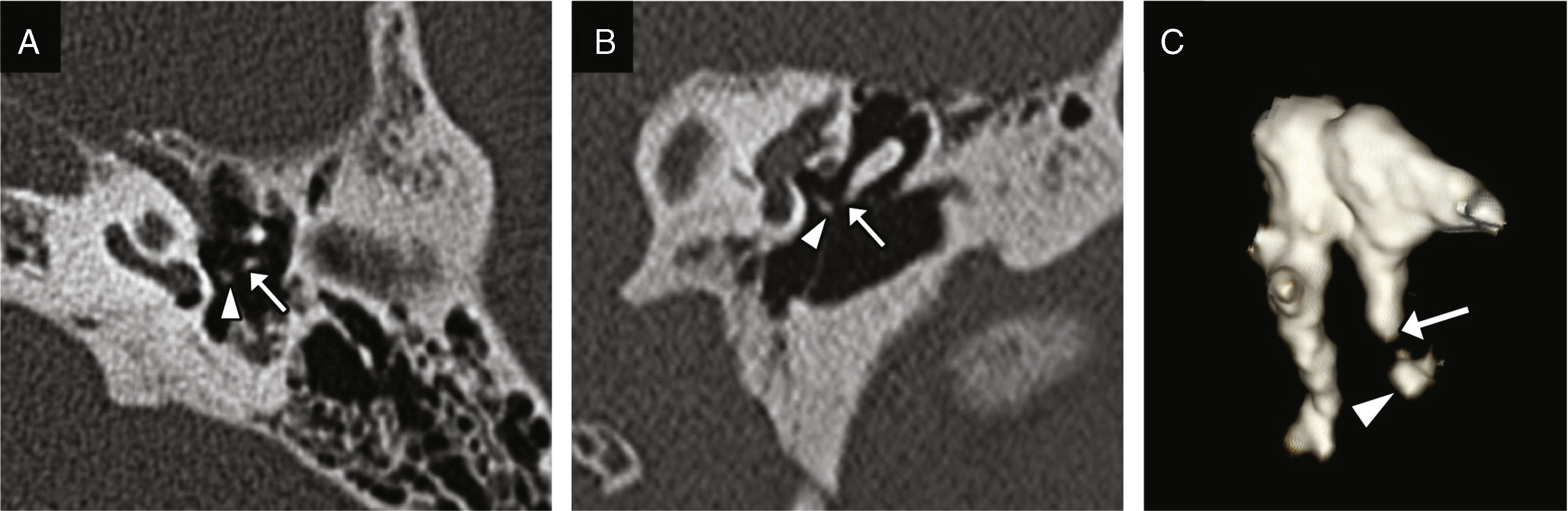
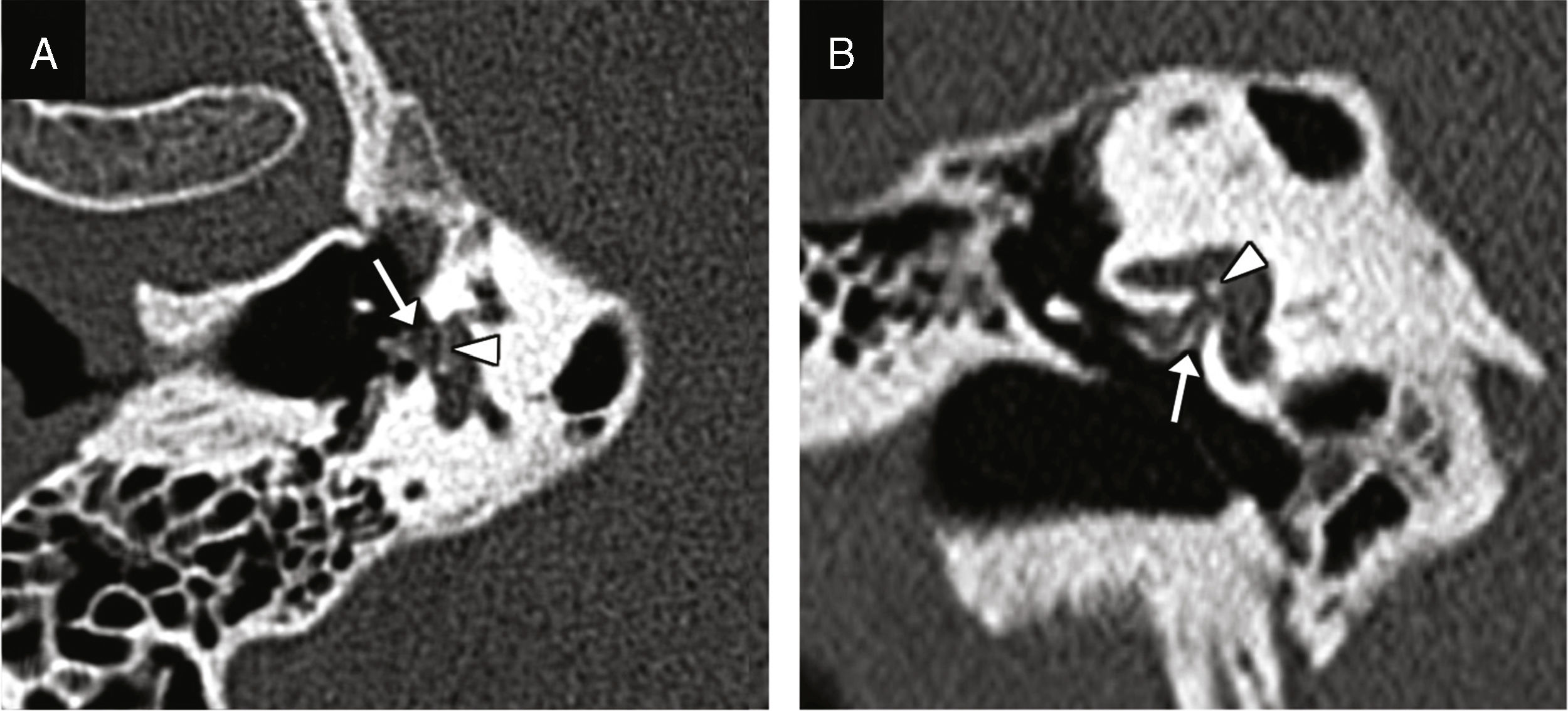
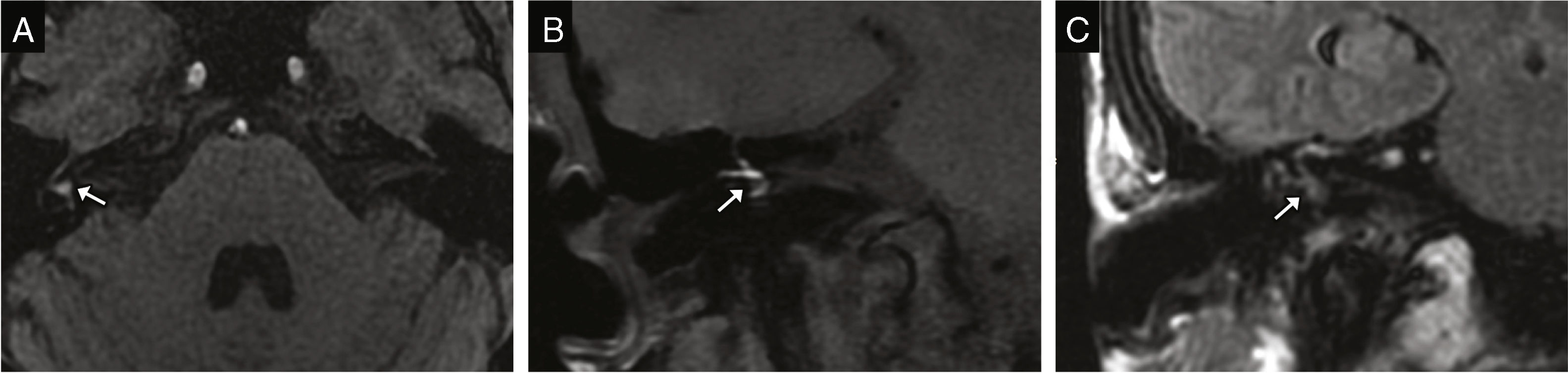
Incudostapedial dislocation: it is the most common dislocation found during surgical exploration, although its incidence is not consistent with data from the radiological studies probably due to how difficult it is to find.6 It is especially sensitive to trauma because it is one diarticular joint located between two axes of rotation; a powerful enough trauma can displace the incus and the stapes causing its dislocation.9 The oblique plane perpendicular to the oval window allows us to see the lack of contact between the incus lenticular apophysis and the head of the stapes (Fig. 2).9
Figure 2.Incudostapedial dislocation. Transverse CT image (A) and coronal reconstruction (B) showing lack of contact between the incus lenticular apophysis (white arrow) and the head of the stapes (arrow head). (C) This volumetric reconstruction shows the dislocation and may be useful for its detection.
(0.25MB). - •
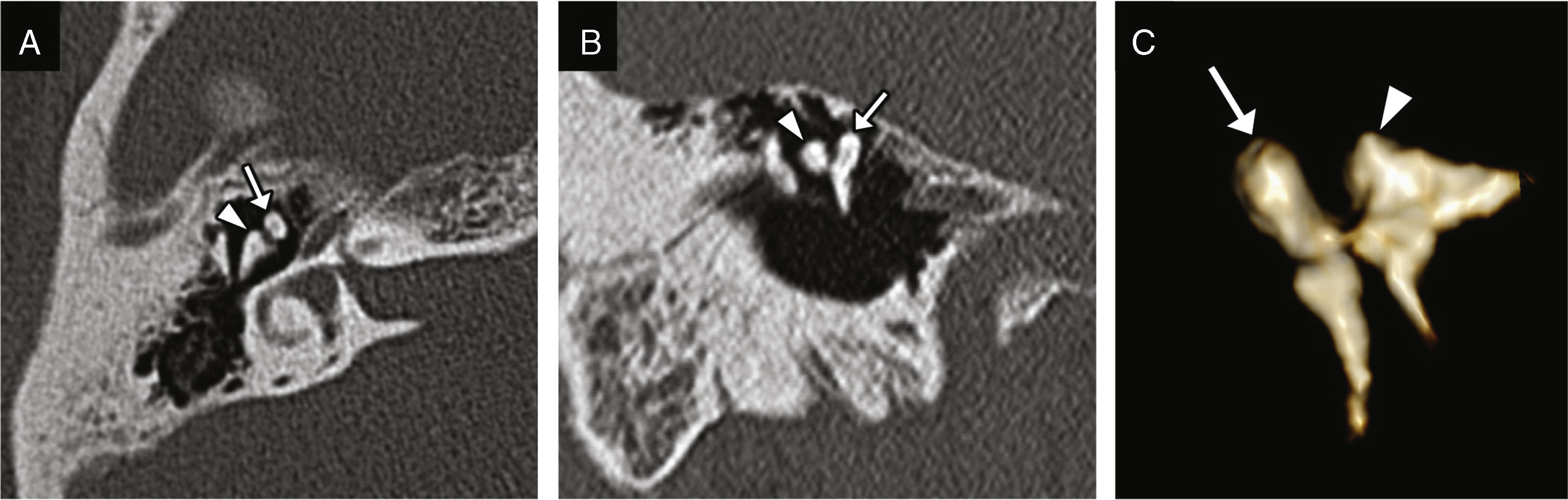
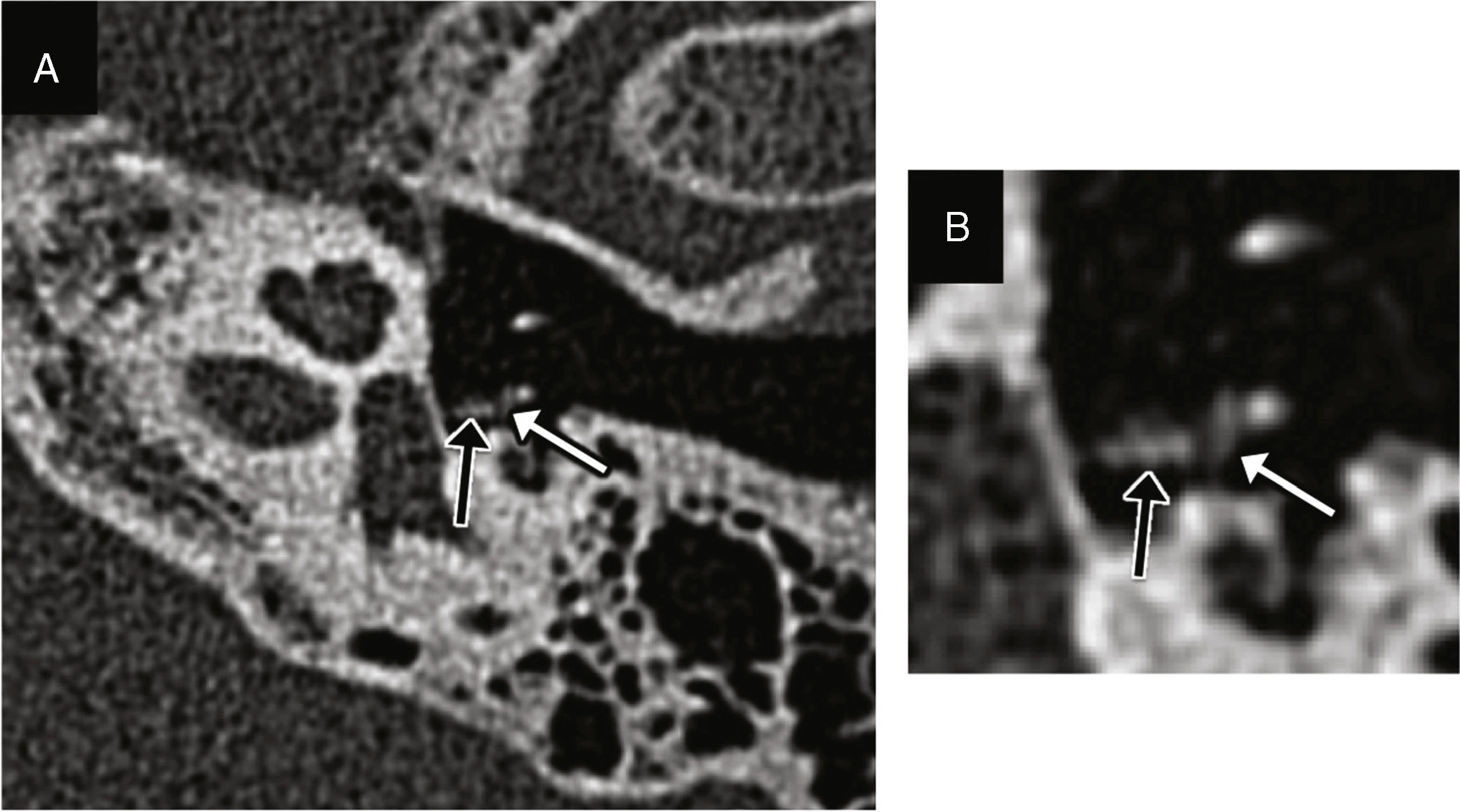
Incudomalleolar dislocation: it can be easily identified and together with the previous one is the most common of all.10 The transverse plane shows a separation between the malleus head and the incus (the “ice-cream ball” corresponding to the malleus is separated from the “cone” corresponding to the body and short apophysis of the incus), while the coronal plane shows an additional perspective of the lack of alignment (Fig. 3).1,9 It is commonly associated with longitudinal fractures of the temporal bone (Fig. 4).
Figure 3.Incudomalleolar dislocation. Transverse CT image (A) and oblique coronal reconstruction (B) showing the lack of contact between the malleus head (white arrow) and the body of the incus (arrow head). (C) The volumetric reconstruction allows its representation and may be useful for its detection.
(0.26MB).Figure 4.Incudomalleolar dislocation and bilateral longitudinal fracture. Transverse TC image (A) and coronal reconstruction (B) showing one longitudinal fracture (black arrows), one incudomalleolar dislocation on both sides, and the lack of contact between the malleus head (white arrow) and the body of the incus (arrow head). Another fracture trace with transverse direction is identified (black arrow head) together with the partial occupation of both epitympana (asterisks) probably due to blood deposits.
(0.44MB). - •
Incudomalleolar complex dislocation: it is often due to high energy traumas. It is the en bloc displacement of the malleus head and the body of the incus toward the mesotympanum or hypotympanum with respect to the incudomalleolar joint. These cases can coexist with an incudostapedial dislocation.
- •
Incus dislocation: it is the concomitant result of one incudostapedial and incudomalleolar dislocation. The incus may remain in the epitympanic recess, prolapse into the hypotympanum, or cause the extrusion of the external auditory canal.3
- •
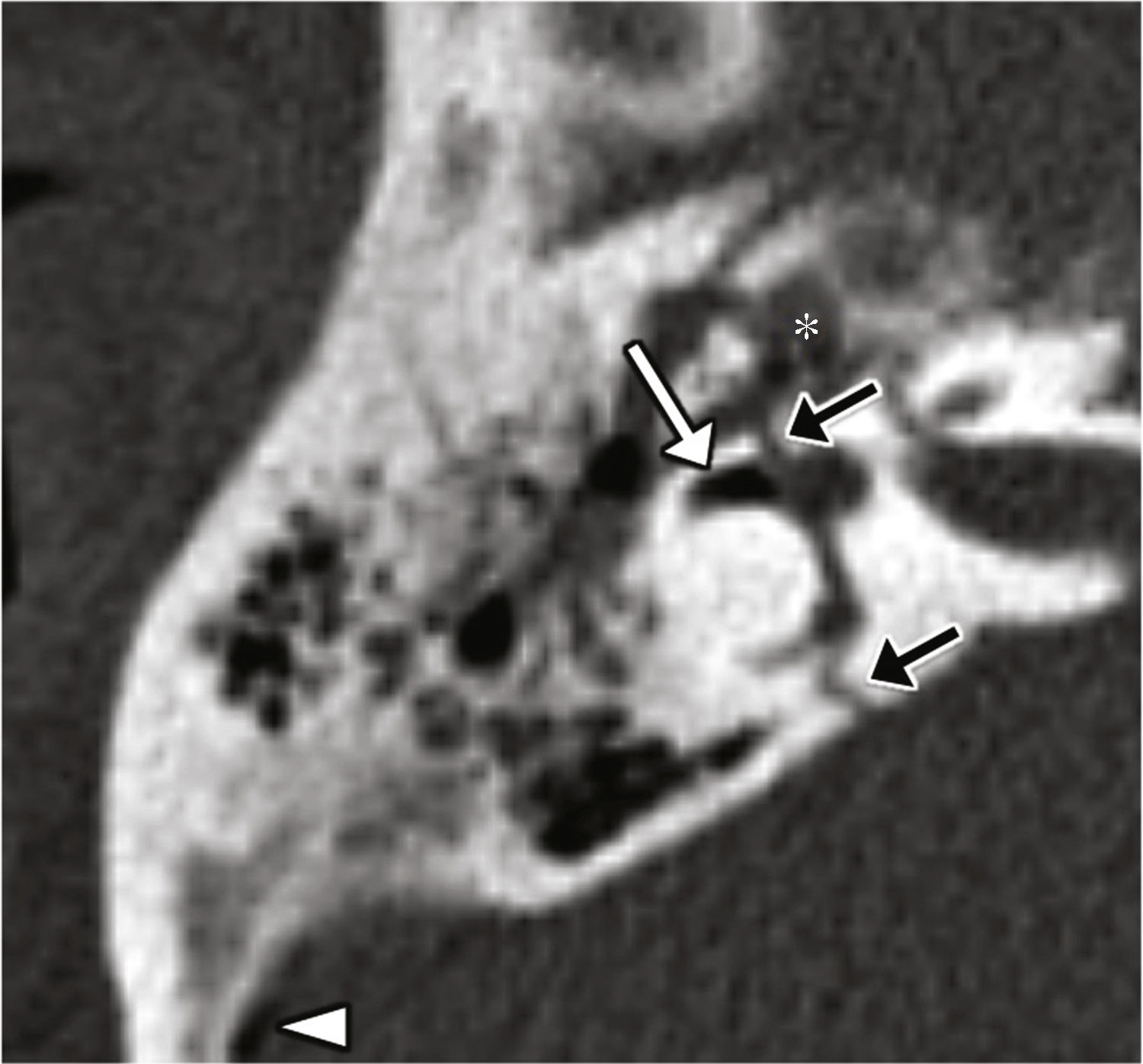
Vestibule-stapedial dislocation: it is rare and usually the consequence of a direct, penetrating trauma in the outer ear.6 Damage to the annular ligament or footplate can cause one perilymphatic fistula (Fig. 5).3,6
Figure 5.Vestibule-stapedial dislocation and perilymphatic fistula. Transverse CT-reconstruction on the oval window plane (A) and coronal reconstruction (B) showing the medial displacement of the footplate (arrow head) and the distal portion of both cruces of the stapes toward the inside of the vestibule due to damage to the annular ligament. Occupation of the oval window niche (arrow) probably due to fluid from the perilymphatic fistula.
(0.26MB).
We should remember that if the transmission hearing loss persists for another 5–6 weeks after sustaining the trauma, and the tympanic membrane is intact, then we will need to consider structural damage to the ossicular chain.
Ossicular chain fractures- •
Fracture of the incus: it is the most common of all since it is the heaviest bone of all the smallest bones, without muscle insertions, and with less ligament support.9 Due to its fragility it is the portion that fractures the most, but damage to the body and the short apophysis is rare. The transverse and coronal planes and the reconstructions acquired through the long apophysis axis are the most useful ones for visualization purposes.6
- •
Fracture of the malleus: the isolated fracture of its handle is rare, and it is usually associated with the digital manipulation of the external auditory canal with a suction mechanism.11,12 It can be adequately identified through handle reconstructions.
- •
Fracture of the stapes: it is the consequence of a torsion mechanism that usually affects the superstructure, which is its most fragile portion; damage to the posterior crus of the stapes is more common than damage to its anterior crus (Fig. 6).3 The fractures of the footplate are usually secondary to transverse fractures across the oval window, and in these cases, we can have one perilymphatic fistula with pneumolabyrinth.6
We should remember that ossicular chain dislocations are more common than ossicular chain fractures, and the two most commonly affected joints are the incudomalleolar joint and the incudostapedial joint.
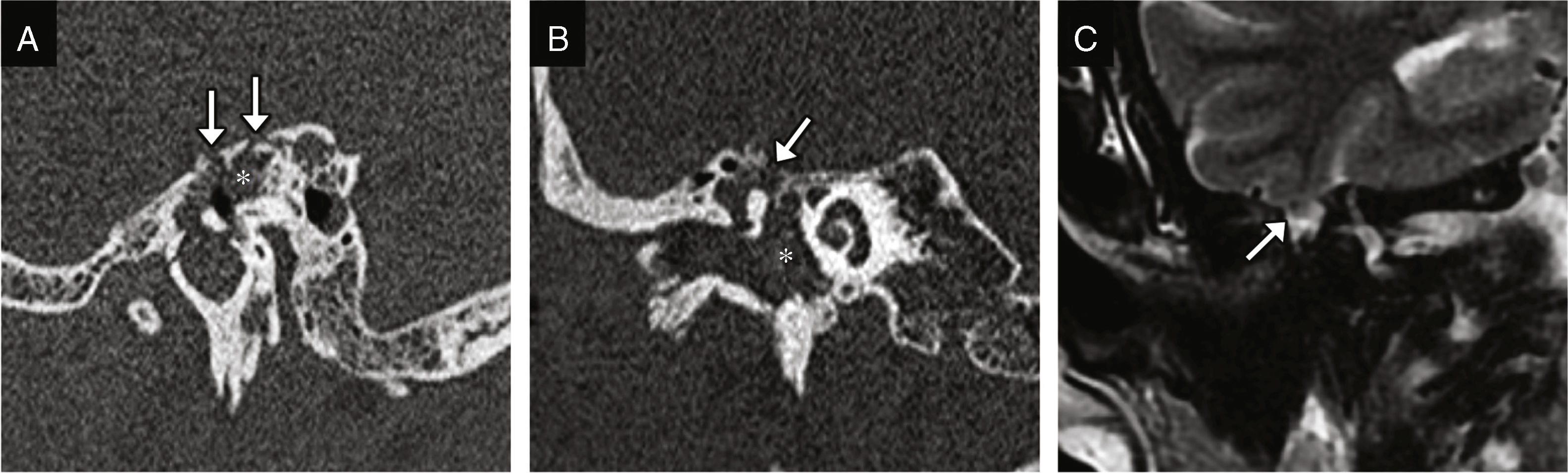
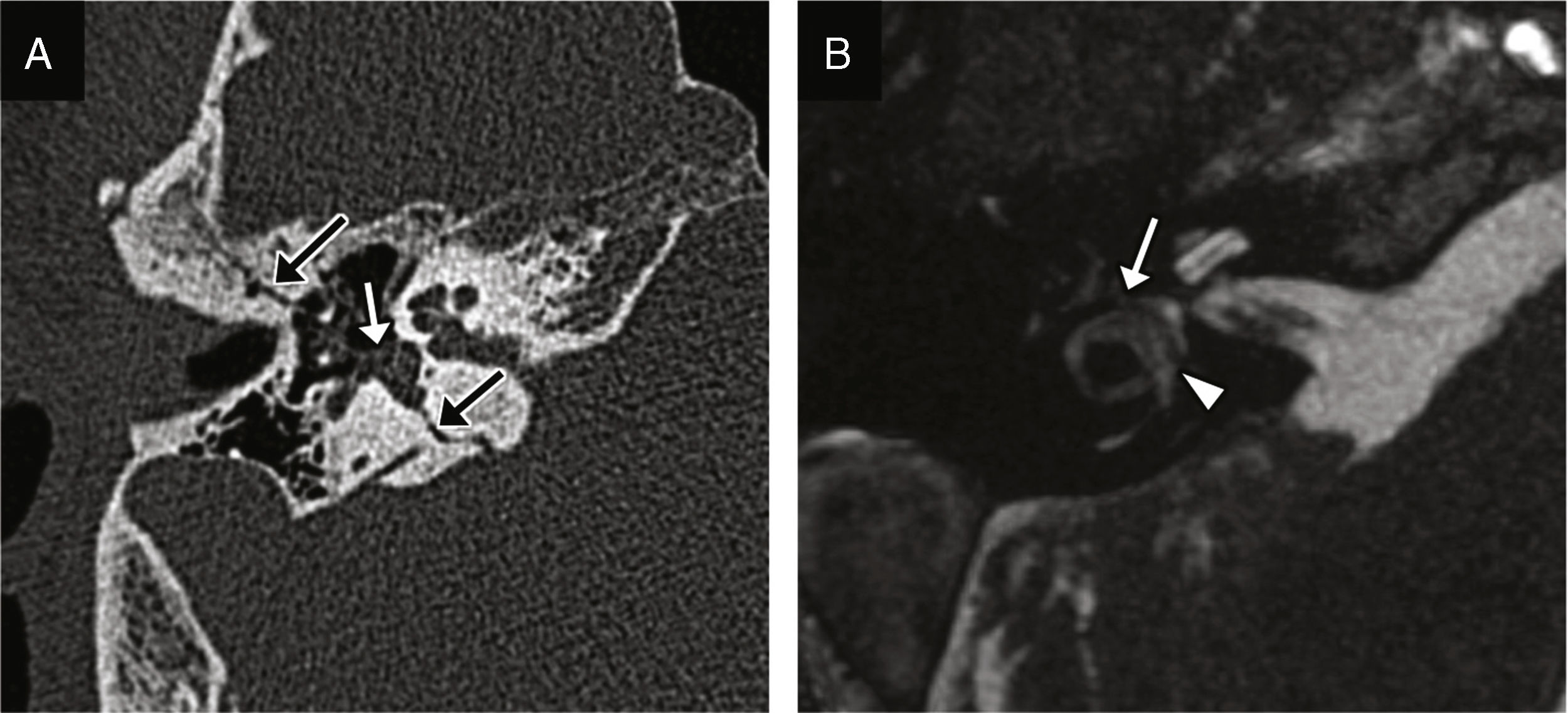
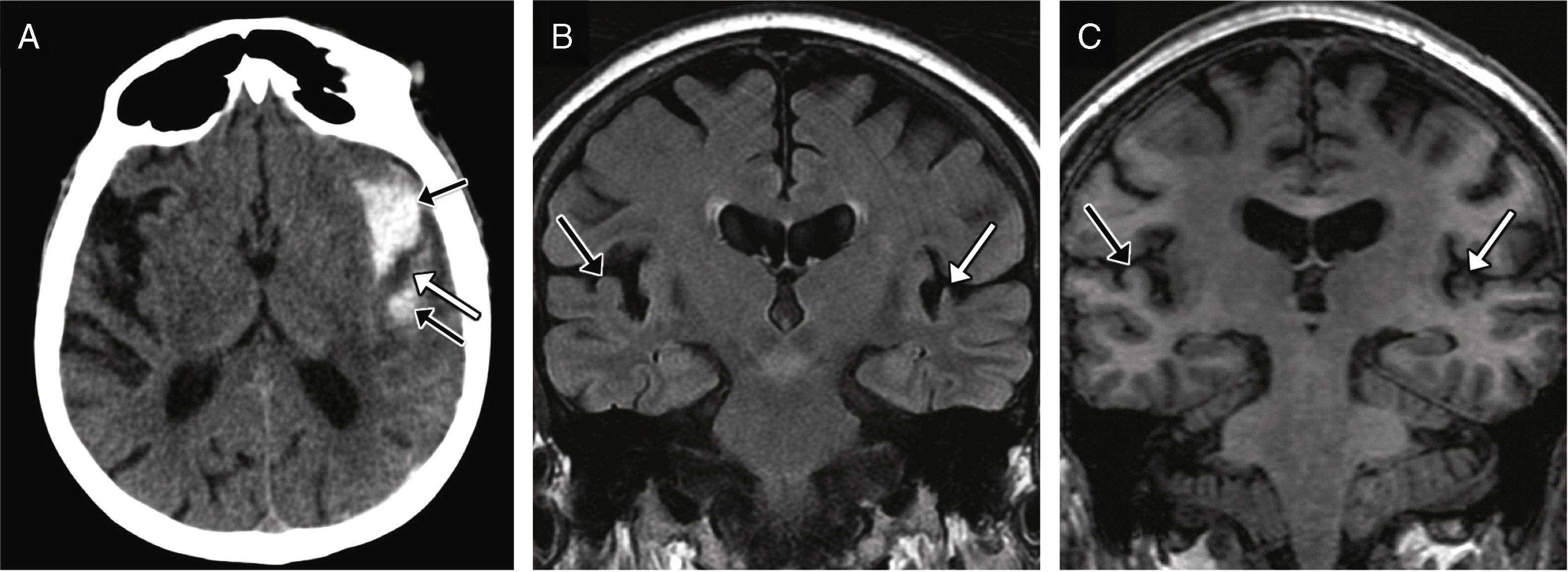
Cerebrospinal fluid-middle ear effusionThe fracture of the tegmen tympani or mastoideum is the common cause; it occurs in 11–45% of all temporal bone fractures.5 The middle ear accumulations of cerebrospinal fluid, or meningeal or brain hernias restricts the motility of the ossicular chain and leads to hearing losses. It associates a high risk of meningitis.1 The CT scan allows us to identify the fracture trace, while the MRI allows us to assess the possibility of protrusion of the meninges or brain to the middle ear (Fig. 7).
CSF effusion to the middle ear and the meningoencephalocele. CT-sagittal reconstruction (A) and CT-coronal reconstruction (B) showing several fracture traces affecting the tegmen tympani showing solutions of bone continuity (arrows). The occupation of the middle ear is shown here (asterisks) and may be due to blood or CSF deposits–or both. (C) Coronal MRI with T2-weighted image three months after sustaining the trauma acquired due to resistant hearing loss and showing the protrusion of the meninges and the lower portion of the temporal gyrus lower to the epitympanum (arrow); this finding is consistent with meningoencephalocele.
It can be the consequence of a fracture across the inner ear, or damage to the inner ear and the central auditory pathway.
They are the result of hits sustained in the occipital area and associate a higher incidence of neurosensorial hearing loss, facial paralysis, and cerebrospinal fluid fistula.3 In these cases, the CT scan allows us to identify the fracture trace and possible pneumolabyrinth, and the MRI allows us to make an assessment of the inner ear.
The neurosensorial hearing loss in the absence of a fracture may occur in cases of labyrinthine concussion, endolymphatic hydrops, damage to the central auditory pathway, and perilymphatic fistula without fracture. In this scenario, the MRI is especially important since it is the imaging modality that will allow us to make assessments of the inner ear and the central auditory pathway.
Labyrinthine concussionIt is defined as damage to the membranous labyrinth without a bone fracture. Through the MRI we can see the hemolabyrinth; the T1-weighted images show it with intrinsic hyperintensity due to its concentration of methemoglobin, yet the FLAIR sequence is more useful because it is more sensitive for the detection of subtle changes in the signal of the perilymph (Fig. 8).6,13
Labyrinthine concussion. T1-weighted MRIs on the transverse plane (A) and coronal plane (B) showing signal hyperintensity in the vestibule and the semicircular canals (arrows) due to hemorrhage. (C) Coronal MRI FLAIR sequences showing the signal hyperintensity of the vestibule and the semicircular canals (arrow) due to blood altering the composition of the perilymph.
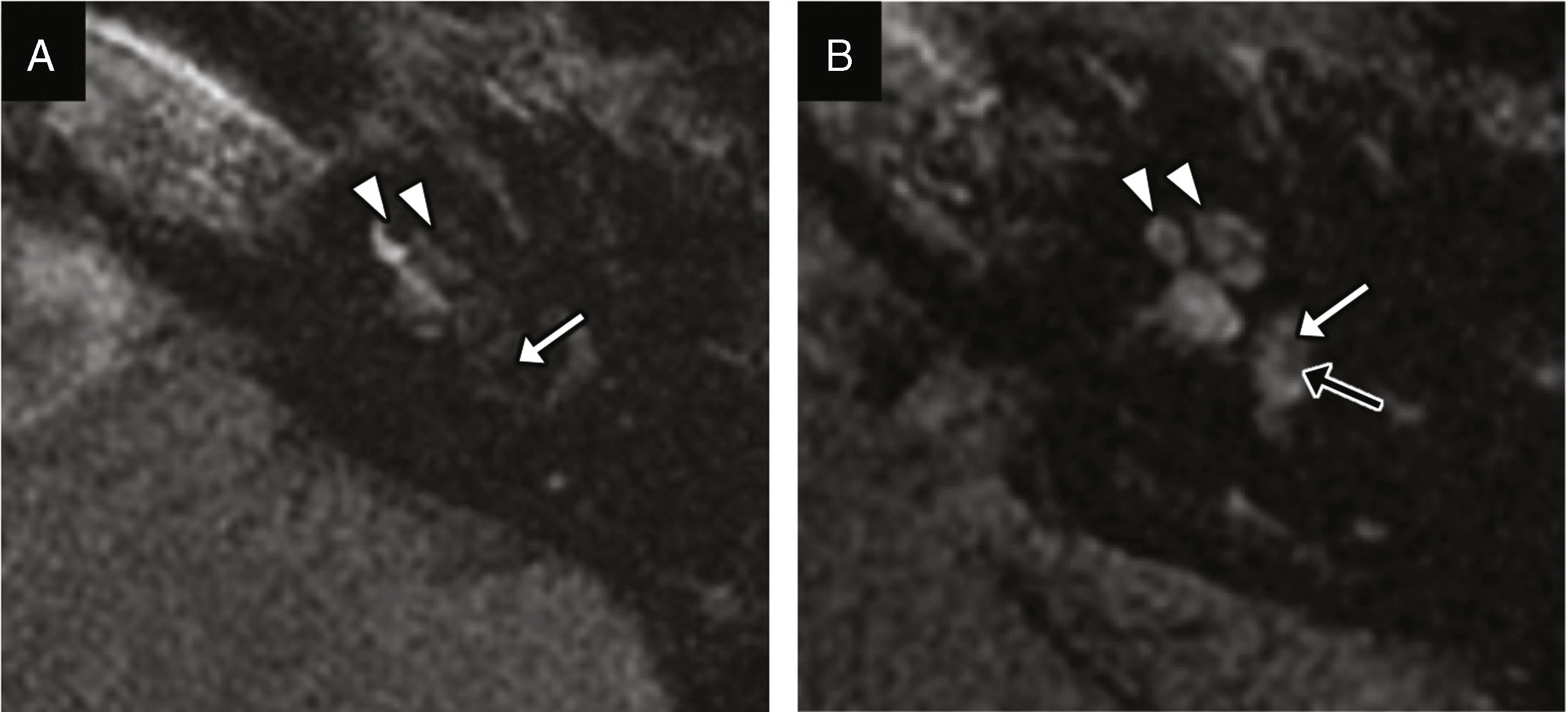
It is defined as the abnormal communication between the perilymphatic space and the middle ear. It may be secondary to fractures with damage to the windows; fractures of the footplate; or damage to the annular ligament. It may also occur in isolation without a fracture.14 The CT scan allows us to identify the possible fracture trace and the pneumolabytinth–a highly specific sign (Fig. 9).5 Other possible signs are the intra-vestibular displacement of the footplate and the occupation of the oval window niche (Fig. 5).15 The MRI can detect the perilymph of the middle ear, although it is hard to distinguish it from the hemotympanum (Fig. 10).6
Perilymphatic fistula, pneumolabyrinth and transverse fracture. Transverse CT image showing one transverse fracture with involvement of the otic capsule (black arrow) and pneumolabyrinth in the anterior portion of the lateral semicircular canal (white arrow)–a finding that is highly specific of perilymphatic fistula. We can also see the occupation of the middle ear due to blood deposits or perilymph (asterisk), and one isolated pneumoencephalus bubble (arrow head).
Perilymphatic fistula and transverse facture. (A) Transverse CT image showing one transverse fracture with involvement of the otic capsule running through the oval window (black arrows); occupation of oval window niche probably due to perilymph (white arrow). (B) Transverse reconstruction of steady-state, echo-gradient MRIs showing the fluid signal intensity alteration of the vestibule and semicircular canals probably due to hemorrhage (arrow head), and apparent content spread toward the oval window niche (arrow); although this finding is suggestive of perilymphatic fistula, it is difficult to interpret and may be indistinguishable from the hemotympanum with this location.
It is a known clinical entity; trauma is one of its causes although it is usually of unknown etiology. It consists of the post-trauma dilation of the endolymphatic space. In order to detect it, we use one delayed FLAIR sequence four hours after the administration of contrast that eventually shows the dilation of the vestibular saccule and utricle and cochlear duct toward the vestibular ramp (Fig. 11).
Post-trauma endolymphatic hydrops. (A) Transverse MRI FLAIR sequence four hours after the administration of contrast showing dilation of the endolymphatic duct that completely obliterates the vestibular ramp (arrow heads), and dilation of the saccule and utricle (arrow) that totally occupies the vestibule. (B) Comparative normal image of a patient without endolymphatic dilation; the endolymphatic duct appears as one hypointense laminar structure due to the lack of contrast uptake (arrow heads), while the perilymphatic space shows contrast uptake and looks hyperintense. The saccule (white arrow) and the utricle (black arrow) can be seen individually and have a round appearance.
The central auditory pathway should be assessed in its entire trajectory: cochlea, cochlear nerve, cochlear core, inferior colliculus, medial geniculate body, and auditory cortex. Damage can be secondary to axonal injuries, bruises, or hemorrhages.
The lesions prior to the decussation of the superior olivary nucleus lead to neurosensorial hearing loss, which should be distinguished from auditory agnosia.16 Isolated lesions to the thalamus, radiations, and auditory cortex may also cause hearing disorders.6
Initially, the CT scan is the modality of choice, but the MRI allows more accurate assessments (Fig. 12).
Damage to the central auditory pathway. Sixty-seven year-old-male reporting difficulties hearing sounds six months after sustaining a traumatic brain injury. (A) The urgent transverse CT image acquired after the accident shows one subarachnoid hemorrhage located in the Sylvian fissure and the lateral fossa of the left side (black arrows), and reduced attenuation of the transverse temporal gyrus with loss of cortical-subcortical differentiation (white arrow) probably due to the bruise. The coronal MRI FLAIR sequence (B) and coronal T1-weighted reconstruction (C) acquired 6 months after trauma show loss of volume due to encephalomalacia of the left transverse temporal gyrus (white arrow); for comparison purposes, the right transverse temporal gyrus is highlighted here (black arrow).
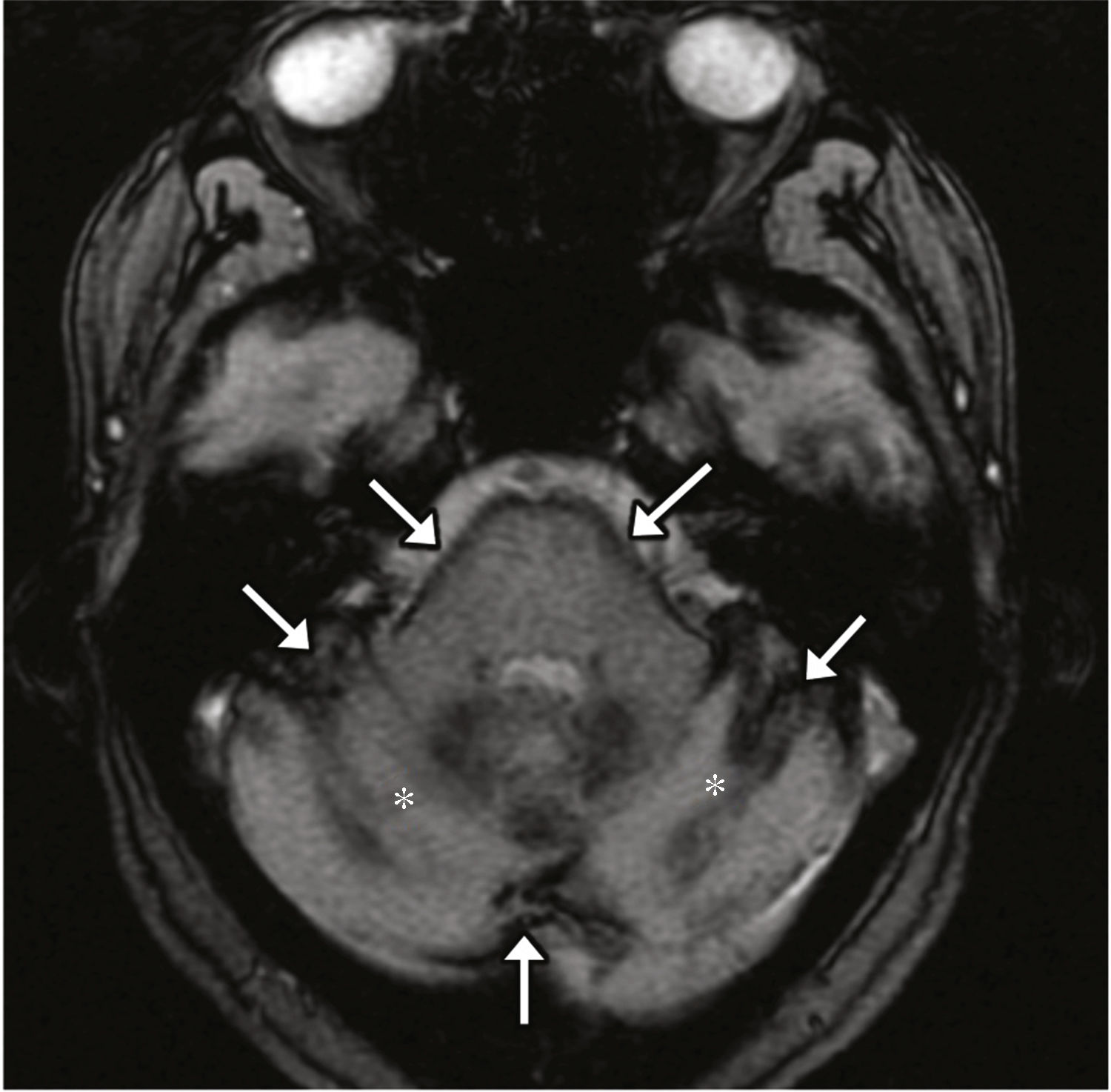
In the scenario of superficial siderosis, usually secondary to subarachnoid hemorrhages due to repeated trauma, there is hemosiderin deposition within the subpial brain layers.17 The 8th cranial nerve is the most frequently damaged one and the neurosensorial hearing loss is the most characteristic symptom; it is hypothesized that it is due to its long cistern trajectory and to the special susceptibility of its microglia.18 On the MRI it looks as a low intensity edge on the brain surface–more evident on echo-gradient and susceptibility sequences (Fig. 13).
Superficial siderosis. Forty-seven-year-old alcoholic-male with multiple traumatic brain injuries. The transverse echo-gradient T2-weighted MRI acquired shows one hypointense edge on the surface of the brain (arrows) consistent with siderosis. Cerebellar atrophy is identified here too (asterisks) probably due to the alcohol consumption.
For the correct assessment of auditory radiations, diffusion tensor images (DTI) can be used, and for the correct assessment of the auditory cortex, functional sequences can be used.19
ConclusionHearing loss is a common complication of traumatic brain injuries. The role of the radiologist is very important in this scenario and includes the assessment of fractures using classification systems with therapeutic and prognostic implications, and the assessment of the ossicular chain and the central auditory pathway.
The CT scan is the initial modality of choice since it detects the alterations that lead to transmission hearing loss; the MRI is useful for the assessment of neurosensorial hearing loss, since it allows us to assess the middle ear, the central auditory pathway, and other complications.
In this paper we tried to describe the trauma lesions of the auditory pathway by accurately depicting and describing the main radiologic findings.
Ethical responsibilitiesProtection of people and animalsThe authors declare that no experiments with human beings or animals have been performed while conducting this investigation.
Data confidentialityThe authors confirm that in this article there are no data from patients.
Right to privacy and informed consentThe authors confirm that in this article there are no data from patients.
Authors’ contributionMM and EP initiated the study design, followed by writing work, and took control to enforce integrity of the study; however, they together with NA conceived the study idea. Then, MM, EP, NA, JCP, and FME went for data mining, collected and collated all the References, made a comprehensive and critical review of the manuscript with intellectually relevant remarks, checked the written content, and finally approved it for submission. None in particular, but everyone sparingly contributed towards data analysis and interpretation as well as statistical analysis.
Conflict of interestsThe authors declare no conflict of interests associated with this article whatsoever.
Please cite this article as: Mazón M, Pont E, Albertz N, Carreres-Polo J, Más-Estellés F. Imagen de la hipoacusia postraumática. Radiología. 2018;60:119–127.