Distal nerve degeneration refers to the process of disintegration of a neuron or neuronal circuit as a consequence of distal damage. The end result of multiple etiologies, this finding is becoming common due to the increasing number of imaging tests done. This paper aims to define the different types of distal nerve damage, review the anatomy and function of the most commonly affected tracts, and illustrate distal nerve damage through diagrams and representative cases from routine practice.
ConclusionKnowing the distant response that can be expected according to the topography of a neuronal lesion is crucial to avoid diagnostic errors. Axonal degeneration and transsynaptic degeneration can be both antegrade and retrograde. Studies of cerebral metabolism, perfusion sequences, and diffusion sequences are showing increasingly earlier changes related to the same process; radiologists need to be aware of these changes.
La degeneración neuronal distal (DND) define el proceso de desintegración de una neurona o un circuito neuronal como consecuencia de una noxa localizada a distancia. Es un hallazgo frecuente debido al creciente número de realización de pruebas de imagen y a que refleja un desenlace común a múltiples etiologías. El objetivo de este artículo es definir los distintos tipos de daño neuronal a distancia, revisar la anatomía y función de los tractos más frecuentemente afectados e ilustrar la DND con esquemas y casos representativos de la práctica habitual.
ConclusiónEl conocimiento de la respuesta a distancia esperable según la topografía de una lesión neuronal es crucial para evitar errores diagnósticos. La degeneración axonal y transináptica puede ser tanto anterógrada como retrógrada. Los estudios de metabolismo cerebral, las secuencias de perfusión y de difusión muestran cambios cada vez más precoces del mismo proceso con los que debemos estar familiarizados.
Secondary or distal neuronal degeneration (DND) is defined as the process of demyelination and disintegration of a neuron or neural circuit as a result of a remotely located harmful agent. It describes a shared outcome in multiple aetiologies. With numbers of imaging tests growing, radiologists must be familiar with the remote signs that can be expected in DND of the main neural tracts. The objective of this article is to define the different types of remote nerve damage and to review the anatomy and function of the most commonly affected tracts.
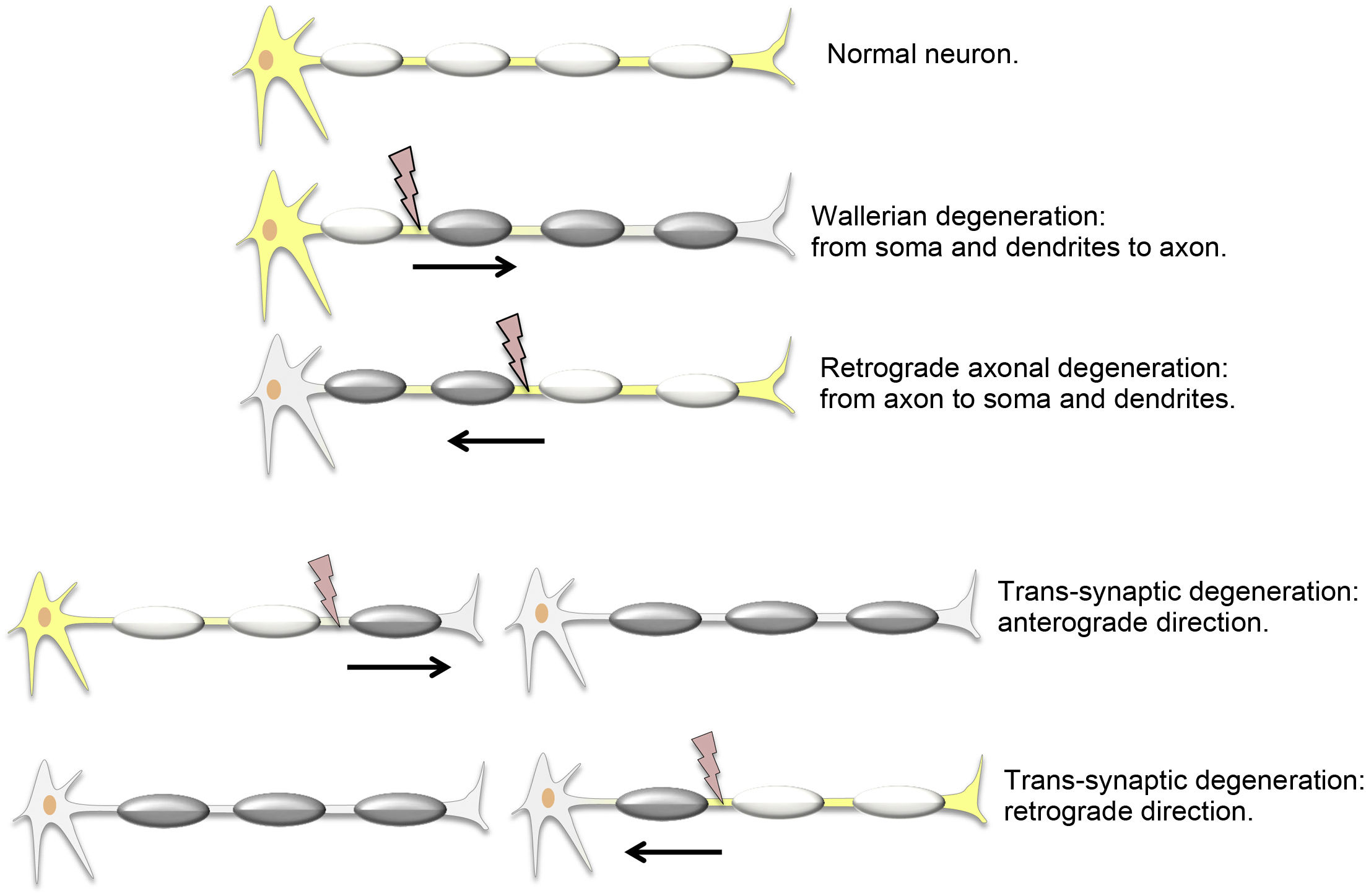
Types of distal neuronal degenerationAxonal degeneration (AD) is the disintegration of an axon due to damage to the soma of the same neuron. It was first reported by Waller,1 who applied the term to peripheral neurons, although it is used indiscriminately for the central nervous system. Anterograde AD, from the soma to the axon, is called Wallerian degeneration (WD). Retrograde AD may also occur when the soma of a neuron degenerates as a result of axonal damage.
Transneuronal or trans-synaptic degeneration (TD) is the disintegration of a neuron other than the one that sustained damage. It affects intact but synaptically linked neurons. Anterograde TD is the impairment of the distal, postsynaptic neuron, from the axon to the soma and dendrites. In retrograde TD, the proximal, presynaptic neuron degenerates, from the soma and dendrites to the axon2 (Fig. 1).
Remember: both AD and TD can be either anterograde or retrograde.
Following damage, the physical disintegration of the axon has no remote repercussions. After four weeks, fragments of myelin protein are destroyed, but lipids are preserved, increasing the lipid-to-protein ratio. The hydrophobic environment yields a hypointense signal on long repetition-time (TR) sequences. In this early phase, diffusion restriction that may resolve has been documented and is called acute AD or pre-Wallerian degeneration.3,4 After approximately three months, lipid reabsorption and astrocyte response create a more hydrophilic environment and increase the T2/FLAIR signal, with resolution in the diffusion sequence (Fig. 2). Loss of volume and atrophy of the axonal tracts occur over a long-term period (Fig. 3)5,6.
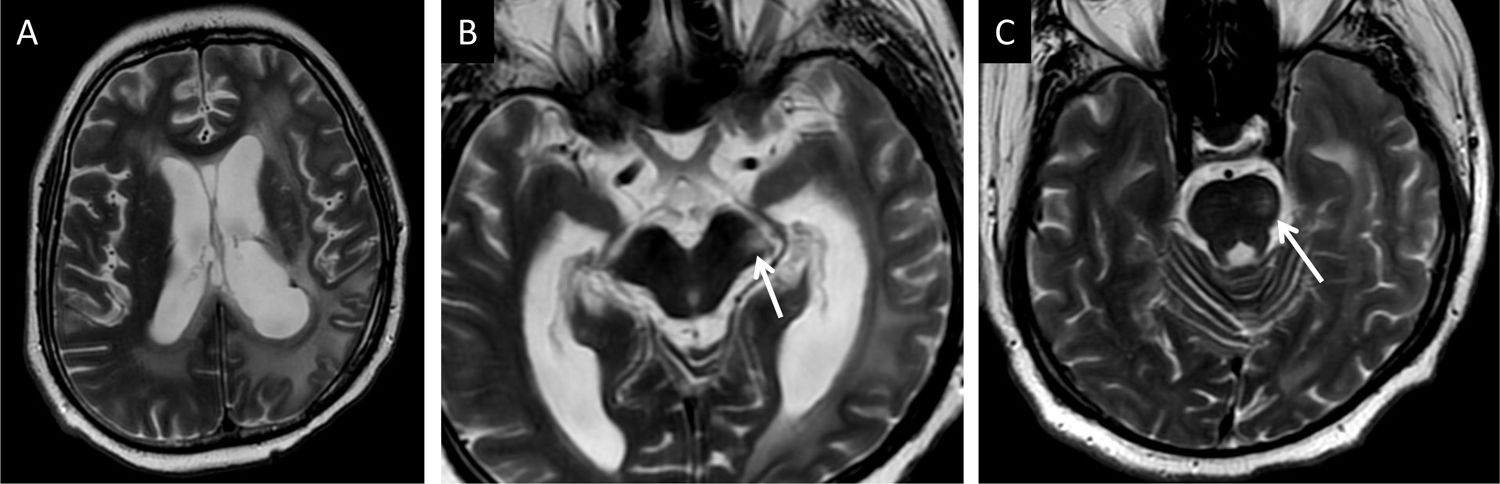
A 22-year-old man who underwent surgery and radiotherapy for a supratentorial glial tumour with associated Wallerian degeneration of the left corticospinal tract. He showed increased signal intensity on T2-weighted imaging in the cerebral peduncle (arrow in B) and the left half of the pons (arrow in C) with preservation of each structure's volume.
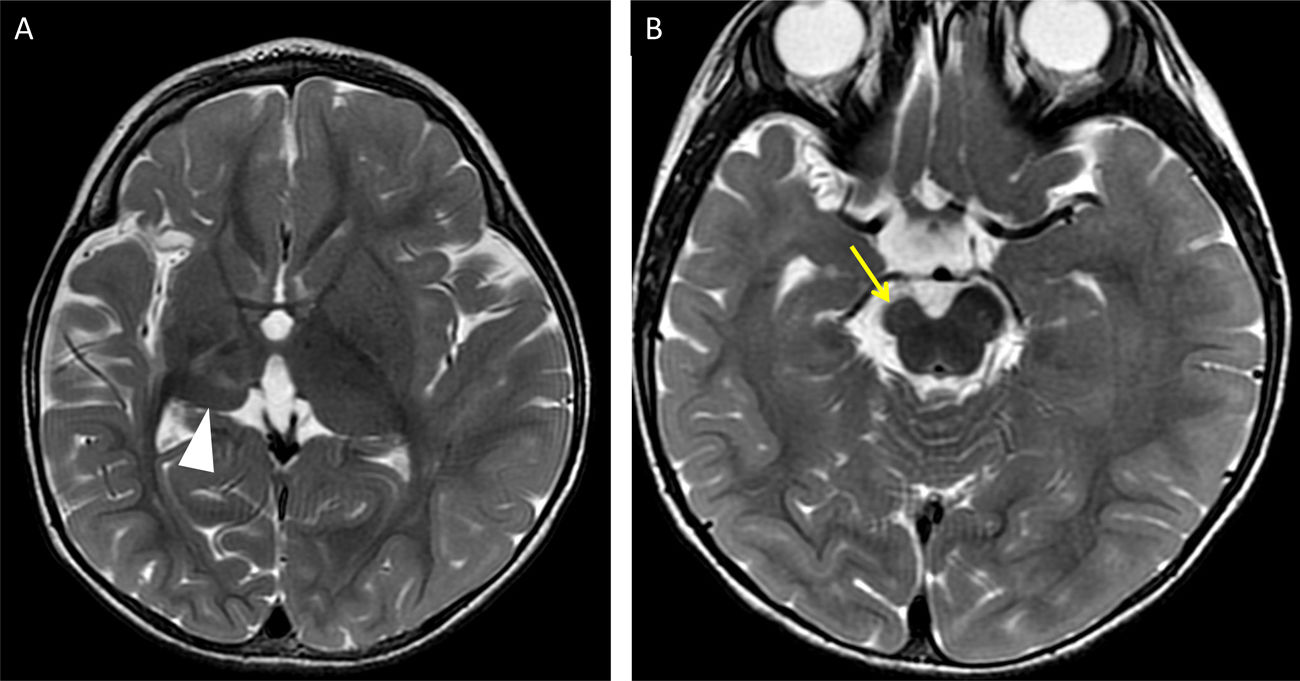
A three-year-old child with prior right middle cerebral artery infarction. T2-weighted imaging at different points in time showing loss of thalamic volume (arrow tip in A) resulting from retrograde trans-synaptic degeneration of the thalamocortical projections and of the right cerebral peduncle (yellow arrow in B) due to Wallerian degeneration of the corticospinal tract.
This originates from the somas of neurons of the primary motor cortex and supplementary area; descends through the corona radiata, genu and posterior third of the posterior limb of the internal capsule; and continues through the cerebral peduncles to the anterior aspect of the pons and medulla oblongata. Some 90% of the fibres cross over to the contralateral side at the pyramidal decussation in the inferior medulla oblongata and descend through the lateral corticospinal tract of the spinal cord.7 They transmit nerve impulses from the motor cortex to the alpha motor neurons of the ventral horns of the spinal cord.
This nerve tract is the one that most commonly presents DND due to ischaemic disease of the middle cerebral artery. WD is the most common response, but it is possible to visualise retrograde degeneration co-occurring with anterograde degeneration in initial impairment of the subcortical region and trans-synaptic abnormality in the lower motor neuron8,9 (Figs. 2 and 3).
Corpus callosumThe corpus callosum (CC) is the large commissure of interhemispheric connection with the main function of connecting the two cerebral hemispheres. Most neurons project from layer III to the contralateral hemisphere and form synapses in contralateral neurons of layers III and IV. Layer III in particular is the most vulnerable to ischaemia.2,3,5
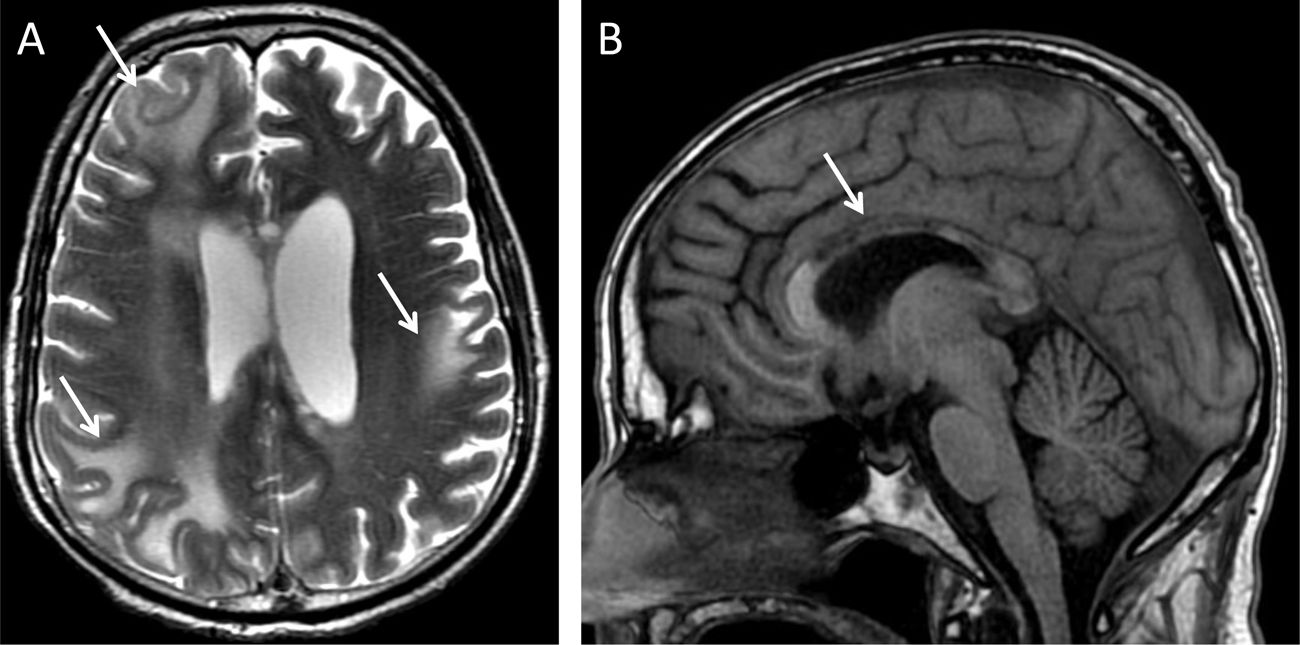
The same harmful agents that cause WD of the corticospinal tract in one hemisphere can cause WD of the axon that crosses over to the contralateral hemisphere. When the number of gliotic fibres is limited to a specific tract, focal atrophy of the CC can occur, with topographic correspondence between focal atrophy of the CC and the corresponding cerebral cortical atrophy.10 By contrast, degenerative diseases, such as vascular dementia and Alzheimer's disease, show diffuse atrophy of the CC.11 Normal ageing also leads to thinning of the genu and anterior part of the body of the CC, as an indirect sign of atrophy of the frontal lobes and interhemispheric fibres (Figs. 4 and 5).12
An 18-year-old man with acute disseminated encephalomyelitis following a poor course. (A) T2-weighted transverse imaging: multiple areas of signal abnormality and loss of subcortical white-matter volume were identified (arrows). Secondarily, abnormal signal intensity and thinning of the body of the CC and, to a lesser extent, the splenium were confirmed (arrow in B, T1-weighted imaging).
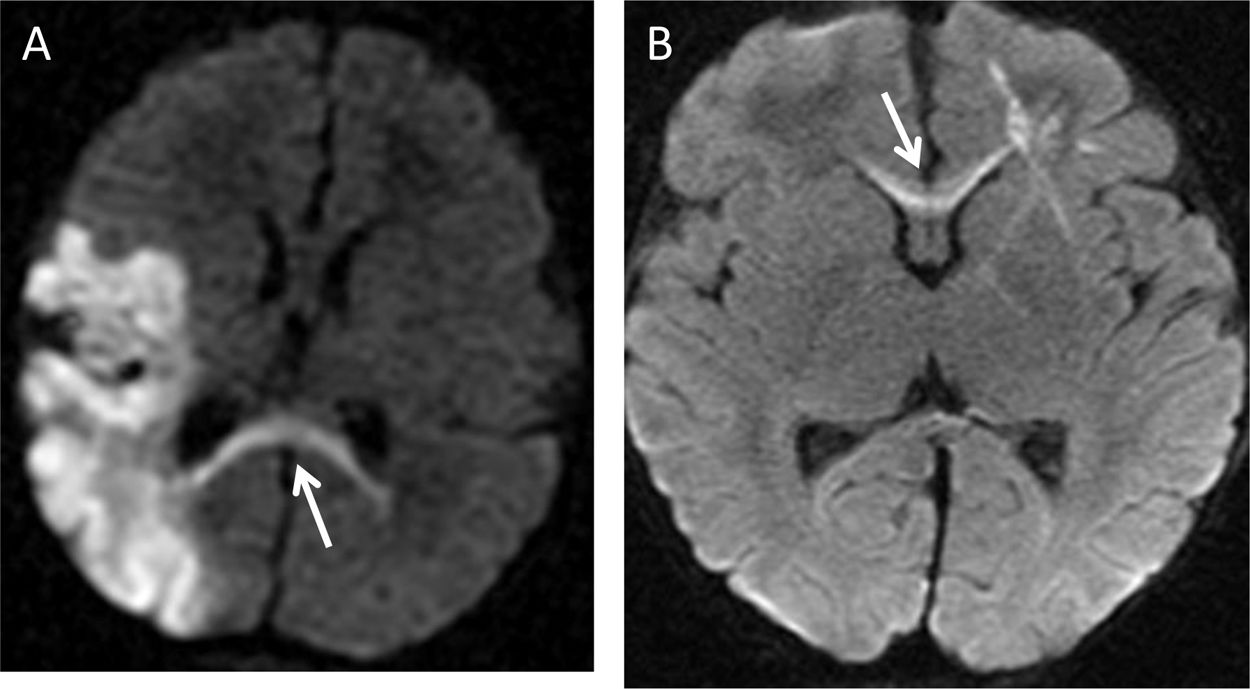
A 10-month-old male infant with left parieto-occipital ischaemic infarction. (A) Diffusion sequence identifying acute Wallerian degeneration in the splenium (arrow). (B) A 15-month-old female infant with right frontal venous infarction and associated axonal tract restriction in the genu of the corpus callosum (arrow).
The striatum includes the caudate nucleus and the putamen. It receives afferences almost exclusively from the frontal cortex, and the frontostriatal projections are organised according to a ventromedial-to-dorsolateral gradient. This tract carries out cognitive, limbic and motor functions. WD occurring exclusively in the striatum after surgery or ablation of the frontal lobes due to frontostriatal impairment is highly representative.13 Early remote damage or acute AD can be evaluated in the diffusion sequence. There have been reports of diffusion restriction in early-stage DND, both Wallerian and transneuronal. This restriction usually develops a few days after damage, showing a minimum apparent diffusion coefficient (ADC) value at seven days and then recovery of normal signal intensity at around 30 days3,4,13 (Figs. 5 and 6).
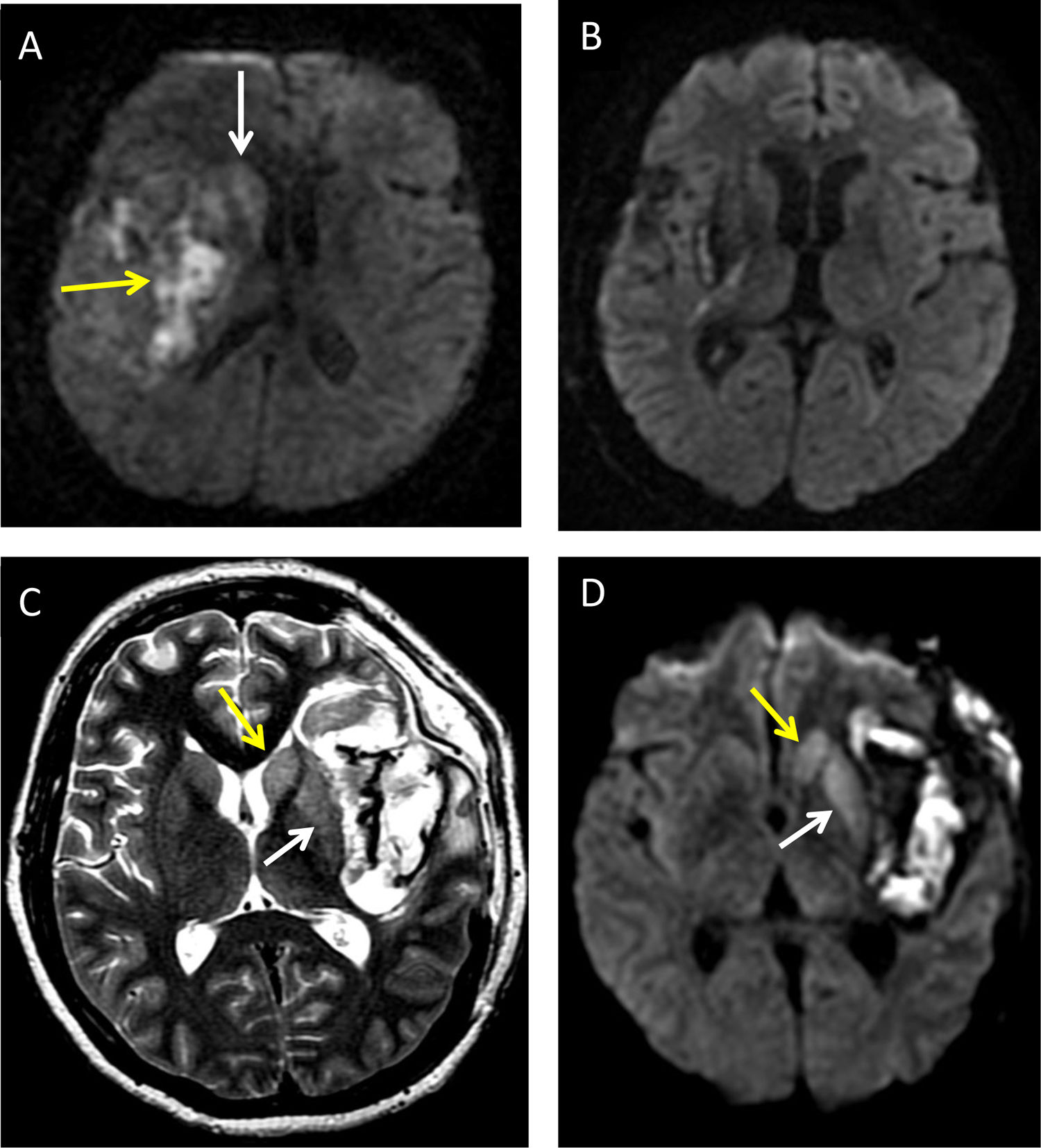
An eight-year-old girl with right insular haemorrhage (A and B). Diffusion imaging a few days after the event identifying heterogeneous restriction of bleeding (yellow arrow) and acute caudate restriction (white arrow) which resolved within weeks (B). A 35-year-old woman with spontaneous subarachnoid haemorrhage and an associated left insular intraparenchymal component (C and D) indicating associated acute axonal degeneration of the caudate nucleus (yellow arrow in C and D) and putamen (white arrow in C and D) on T2-weighted imaging and a diffusion sequence.
The thalamus groups nuclei and connections at which multiple sensory stimuli arrive before projecting to the cortex. Cortical damage may cause retrograde degeneration through thalamocortical projections. The damaged cortical areas cause secondary degeneration that follows the anatomical distribution of the connections with their nuclei; thus, occipital lobe injury will cause retrograde abnormality of the pulvinar, while a harmful agent in the anterior cingulum leads to degeneration of the anterior nucleus.14 It is important to be aware of this correspondence and to consider possible acute retrograde AD in the event of an increase in diffusion as an alternative to an ischaemic event (Fig. 6).
Remember: acute AD is a common finding in a diffusion sequence, but it is likely to resolve.
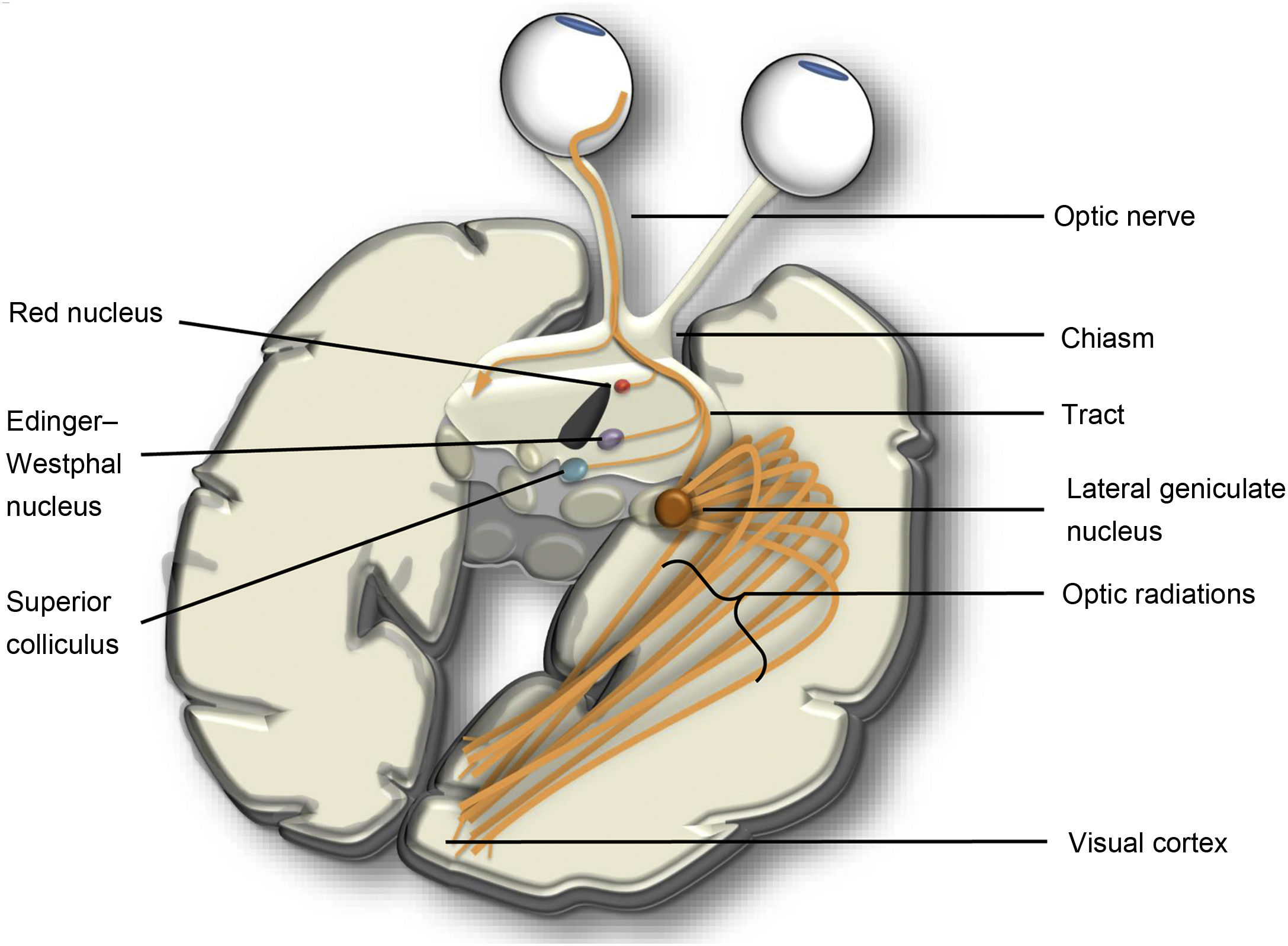
Optic tractThe visual or optic tract consists of multiple neurons vulnerable to both anterograde and retrograde TD. The first two synapses of the optic tract are in the retina and connect photoreceptors to bipolar cells and ganglion cells. Posteriorly, the optic nerve joins the chiasm with the retina. At the chiasm, the nasal fibres decussate and join with the contralateral temporal fibres to form the optic tract which synapses in the lateral geniculate nucleus (LGN). The LGN connects to the cortex of the calcarine sulcus through optic radiations. In addition to the main connection, there are also connections with the pretectal nuclei, hypothalamus and superior colliculus (Fig. 7).
Severe retinal abnormalities such as pigmentary degeneration lead to changes in the cortex; an increase in the width of the calcarine sulcus is a marker of anterograde TD of the entire tract.15 A relationship between loss in LGN height and clinical staging of glaucoma has also been demonstrated; this acts as a noninvasive imaging marker of the state of integrity of the tract that can be visualised on high-resolution T2-weighted images16,17 (Fig. 8). One example of retrograde TD is retinal thinning, seen on optical coherence tomography following occipital infarctions of the posterior cerebral artery.18,19
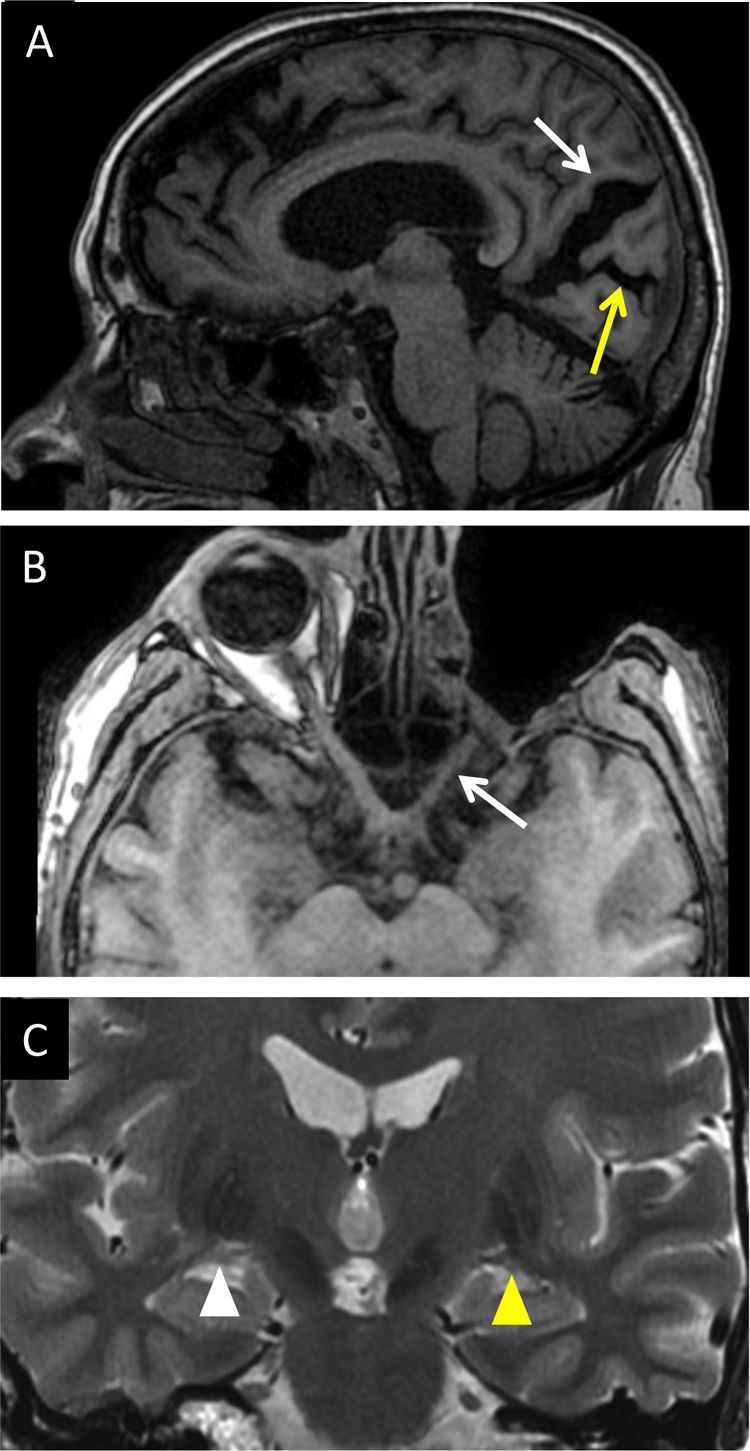
(A) A 65-year-old man with long-standing diabetes having undergone vitrectomy. T1-weighted sagittal imaging: trans-synaptic degeneration was evident with visible enlargement of the parieto-occipital sulcus (white arrow) and the calcarine sulcus (yellow arrow) due to visual cortex atrophy. (B) A 30-year-old man with exenteration due to orbital rhabdomyosarcoma. T1-weighted transverse imaging: secondary axonal degeneration of the left optic nerve (white arrow) and chiasm was visualised. Coronal T2-weighted imaging in the same patient (C) revealed the left lateral geniculate nucleus to have a lower volume (yellow arrow tip) than its contralateral counterpart (white arrow tip).
Remember: TD of the optic tract involves LGN abnormality, and therefore LGN preservation is an indicator of the integrity of the tract.
Cerebellum and pedunclesThe cerebellum is a complex centre of neuronal interconnection with multiple tracts and nuclei. Notable among the afferent tracts is the corticopontocerebellar tract, whose fibres descend through the corona radiata and the posterior limb of the internal capsule to synapse with the pontine nuclei. The transverse fibres then originate from the pons, cross the midline and enter the cerebellar hemisphere through the middle cerebellar peduncle. It is the most important tract in the preparation, initiation and execution of movement. The tract can sustain not only acute damage, but also chronic damage in multisystem atrophy, with visualisation of the typical cross sign.20 With regard to efferences, most axons synapse in deep nuclei before departing the cerebellum through the peduncles. One of the main tracts comprising the superior cerebellar peduncle (SCP) is the dentatorubrothalamic tract. In addition to acute causes, there are also degenerative diseases, such as progressive supranuclear palsy and Friedrich's ataxia, leading to atrophy of the SCP as a sequela of secondary axonal degeneration (SAD).21 As a general principle, the superior cerebellar peduncles connect the cerebellum to the midbrain, the middle cerebellar peduncles connect the cerebellum to the pons and the inferior cerebellar peduncles connect the cerebellum to the medulla oblongata (Fig. 9).22,23
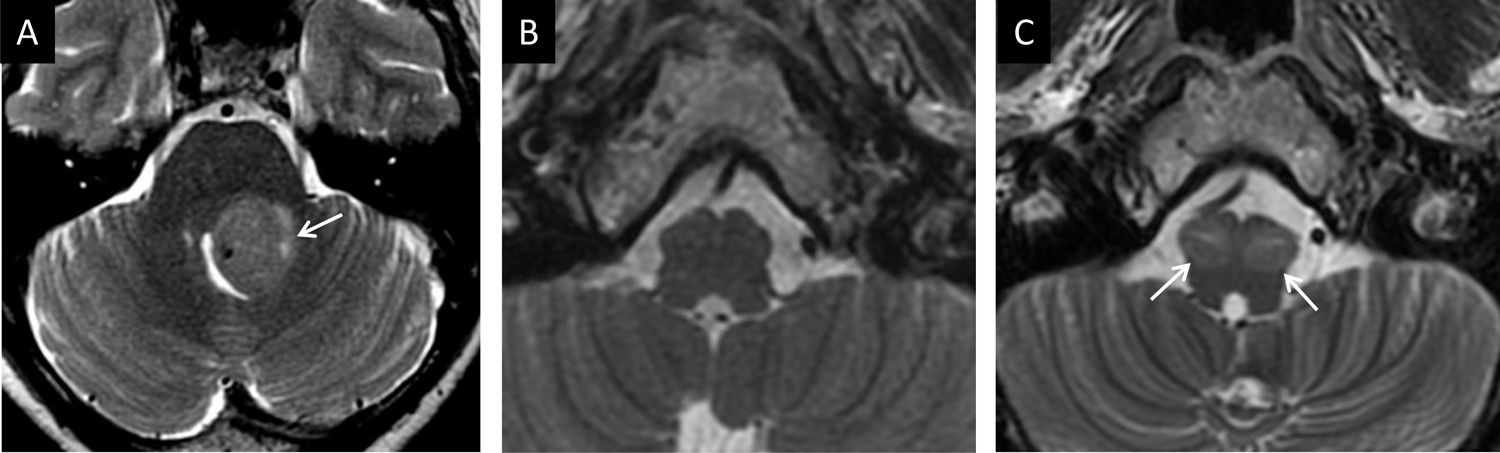
A 48-year-old woman with cerebellar atrophy due to haemorrhage. (A) Transverse T2-weighted imaging: thinning of the superior cerebellar peduncle was identified (white arrow); this was confirmed on T1-weighted coronal imaging (white arrow in (B) upon comparison to the contralateral side (yellow arrow).
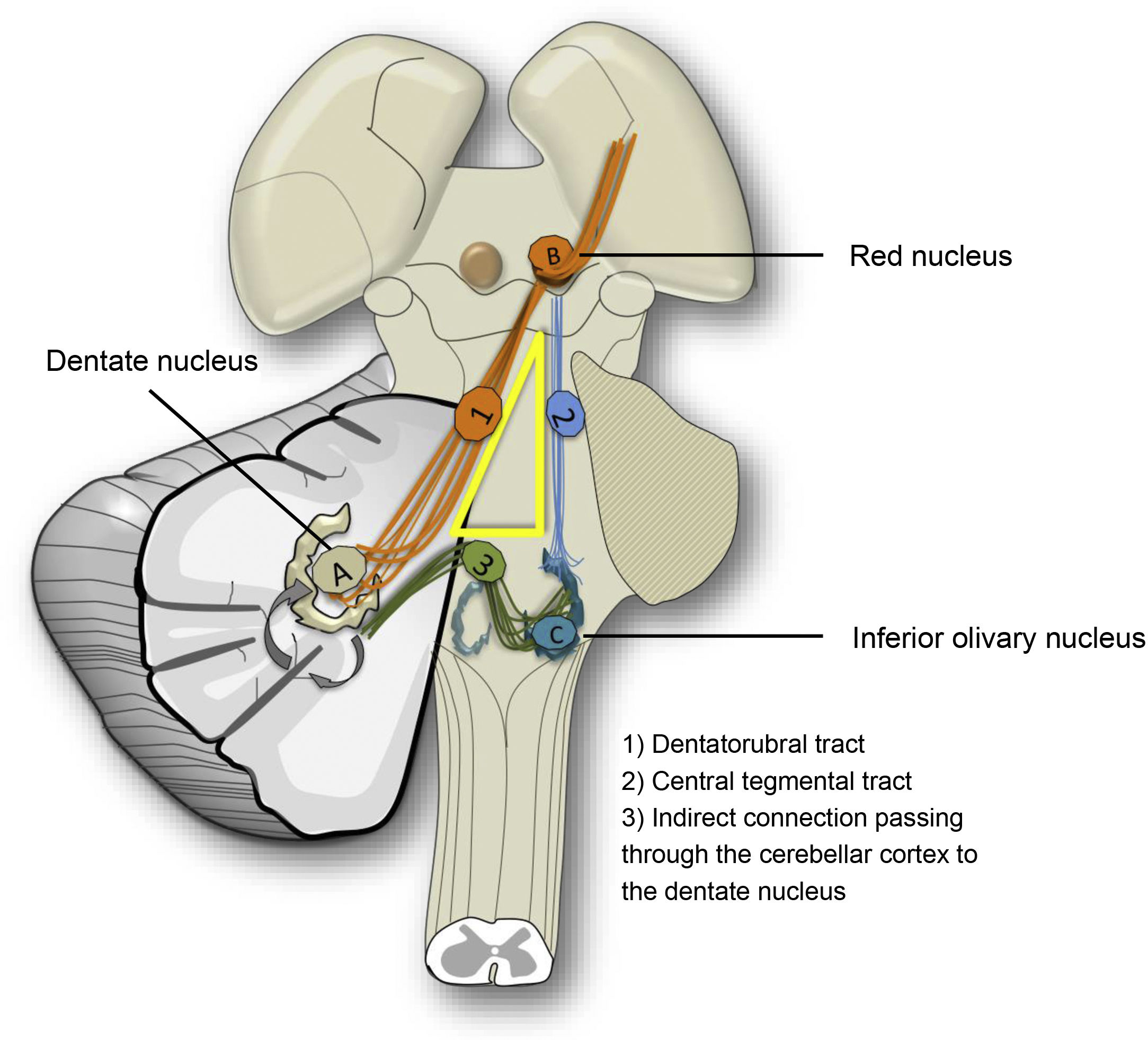
Hypertrophic olivary degeneration (HOD) is a type of TD known for its paradoxical morphological response. It occurs due to lesions in the dentatorubro-olivary tract. The connection of the dentate nucleus to the contralateral red nucleus and inferior olivary nucleus is known as the myoclonic or Guillain–Mollaret triangle, as patients may develop palatal myoclonus (Fig. 10).
The hypertrophic response of the olivary nucleus is due to disconnection following injuries that affect the central tegmental or dentatorubral tract. Synapse loss leads to hypertrophy due to vacuolar degeneration of the cytoplasm.24 Unilateral, contralateral or bilateral olivary hypertrophy may occur, depending on the structures affected. Initially, following damage, signalling increases on T2-weighted imaging without hypertrophy. At six months, the characteristic hypertrophy is added, and after a year, the hypertrophy resolves but the hyperintensity persists. An absence of enhancement following administration of contrast in any phase is important in distinguishing it from other entities (Fig. 11).25
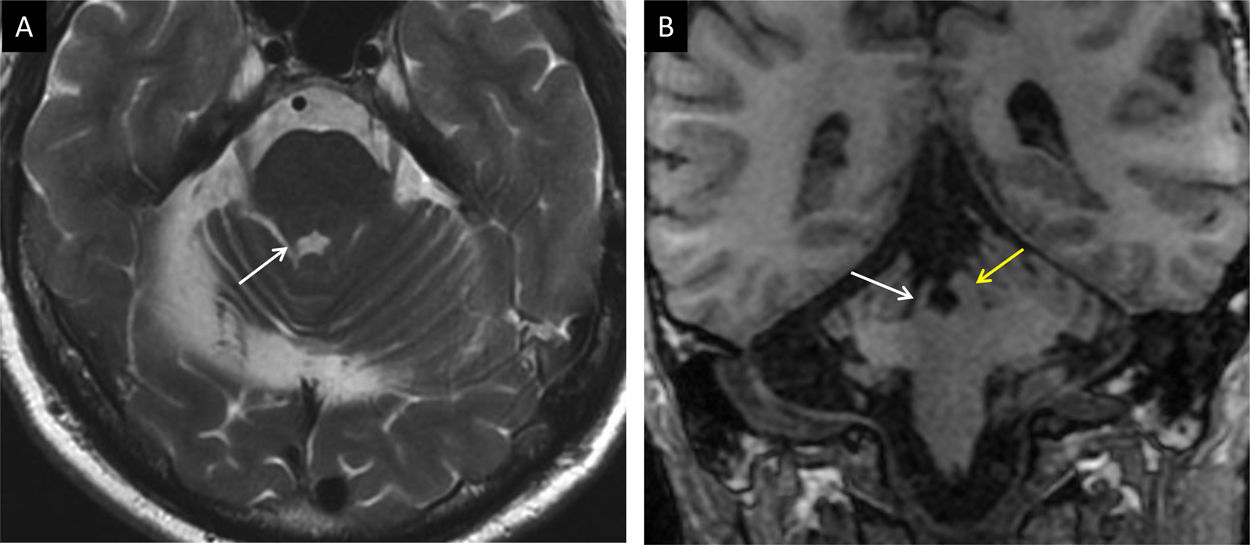
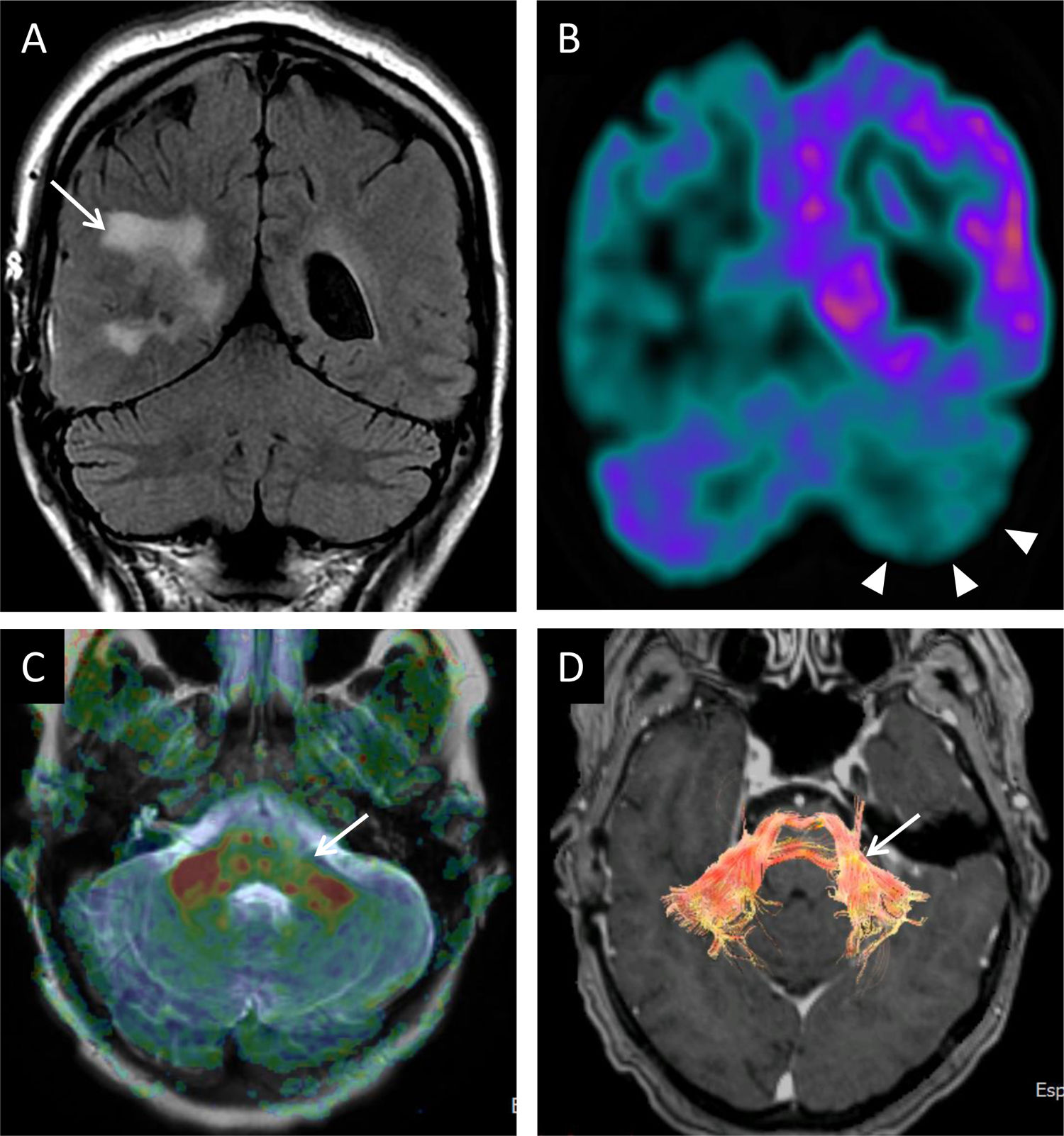
A 35-year-old man with pedunculopontine exophytic ganglioglioma (arrow in A). No volume or signal abnormality was identified in the olivary nuclei at diagnosis (B). Four months after biopsy, the volume and intensity of the olivary nuclei increased due to bilateral hypertrophic degeneration (arrows in C).
Diaschisis is the inhibition of function of an area as a result of remote damage. Crossed cerebellar diaschisis is, therefore, remote impairment of the cerebellum by a contralateral supratentorial injury. Functional abnormality, due to deafferentation, may persist and cause morphological changes. Reduction of cross-metabolism in positron emission tomography (PET) studies, peak transit time and relative cerebral blood flow in perfusion studies have been reported. Diffusion tensor magnetic resonance imaging (DTI) represents an intermediate step between functional and early morphological changes. The lesser degree of restricted diffusion through white-matter tracts on the affected side can be measured by fractional anisotropy26–28 (Fig. 12).
A 56-year-old woman having undergone surgery for right choroid plexus carcinoma (arrow in A). (A) Coronal FLAIR imaging: there were no cerebellar or signal-intensity abnormalities. Coronal imaging from PET with fluorodeoxyglucose (FDG) showed right temporoparietal and left cerebellar hemispheric hypometabolism (arrow tips in B) that could be attributed to crossed cerebellar diaschisis. C) Anisotropy map and DTI reconstruction (D) revealing thinning of the fibres in the left middle peduncle (arrows in C and D).
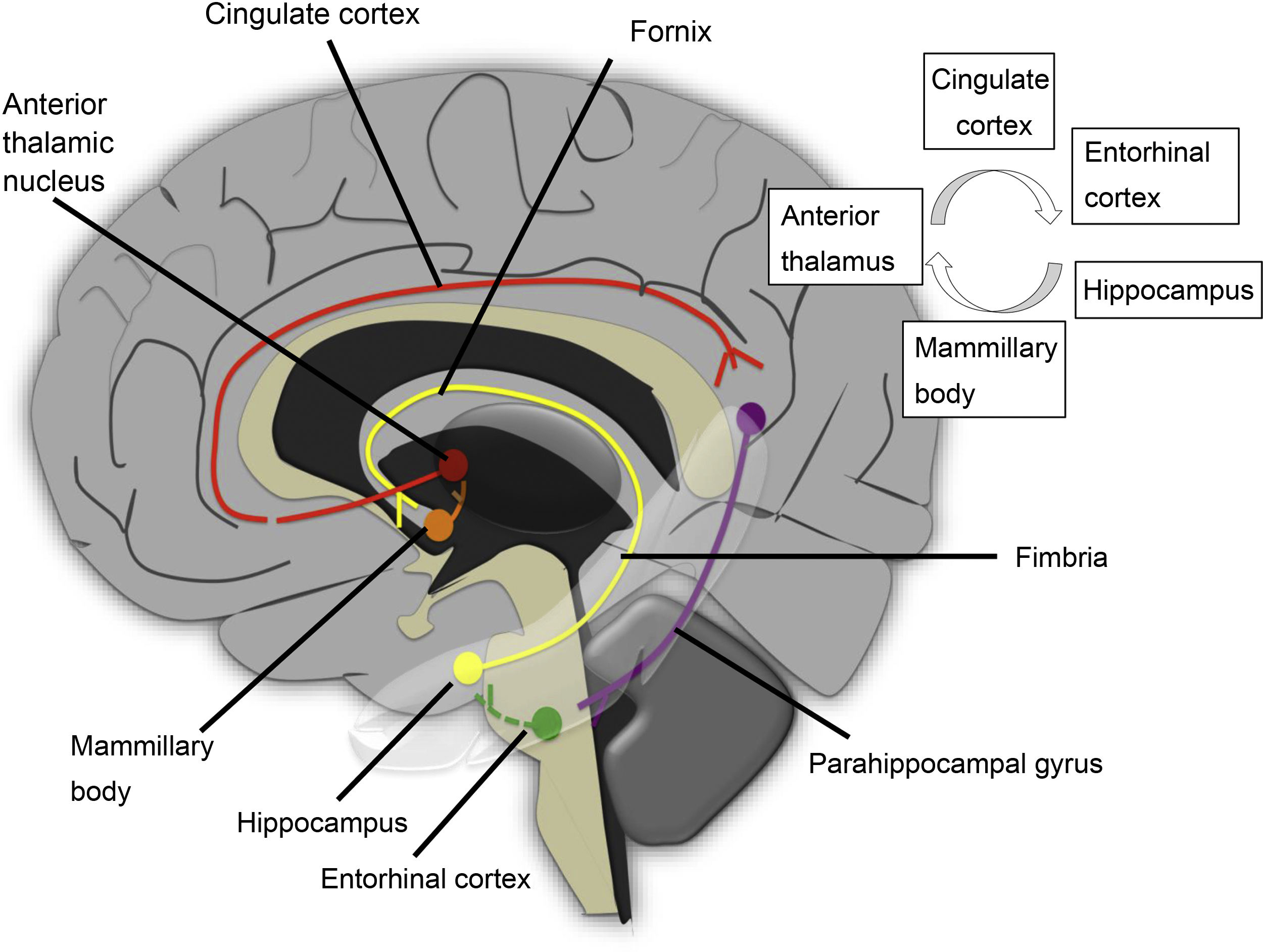
This is a complex neural network consisting of a closed main circuit, the Papez circuit, and multiple afferences. The circuit starts in the hippocampus, where the subiculum and the cornu ammonis connect through the first neuron to the mammillary body, through the tract located in the fornix. The first synapse occurs in the mammillary body, and the second neuron runs through the mammillothalamic tract to the anterior thalamic nucleus. In this location, another synapse is made, and a third neuron projects to the cingulate gyrus. The circuit is completed by impulses sent to the hippocampus through the parahippocampal gyrus (Fig. 13).
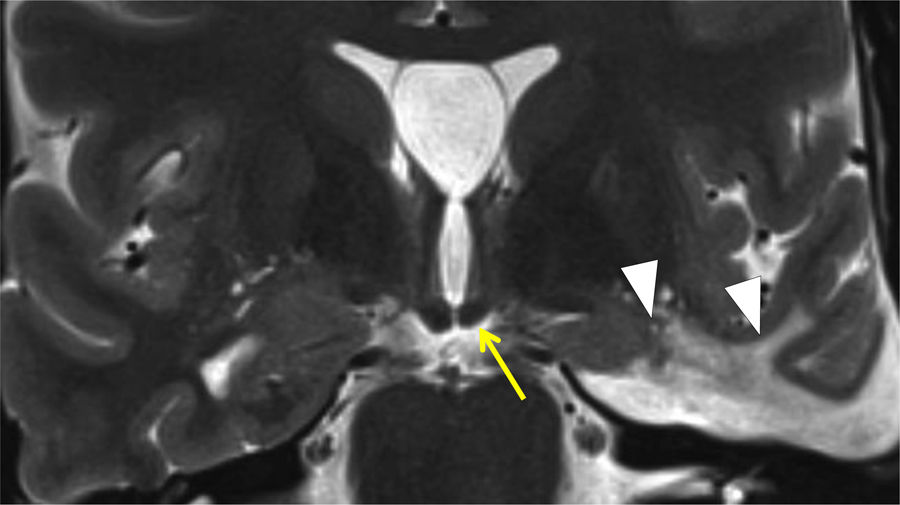
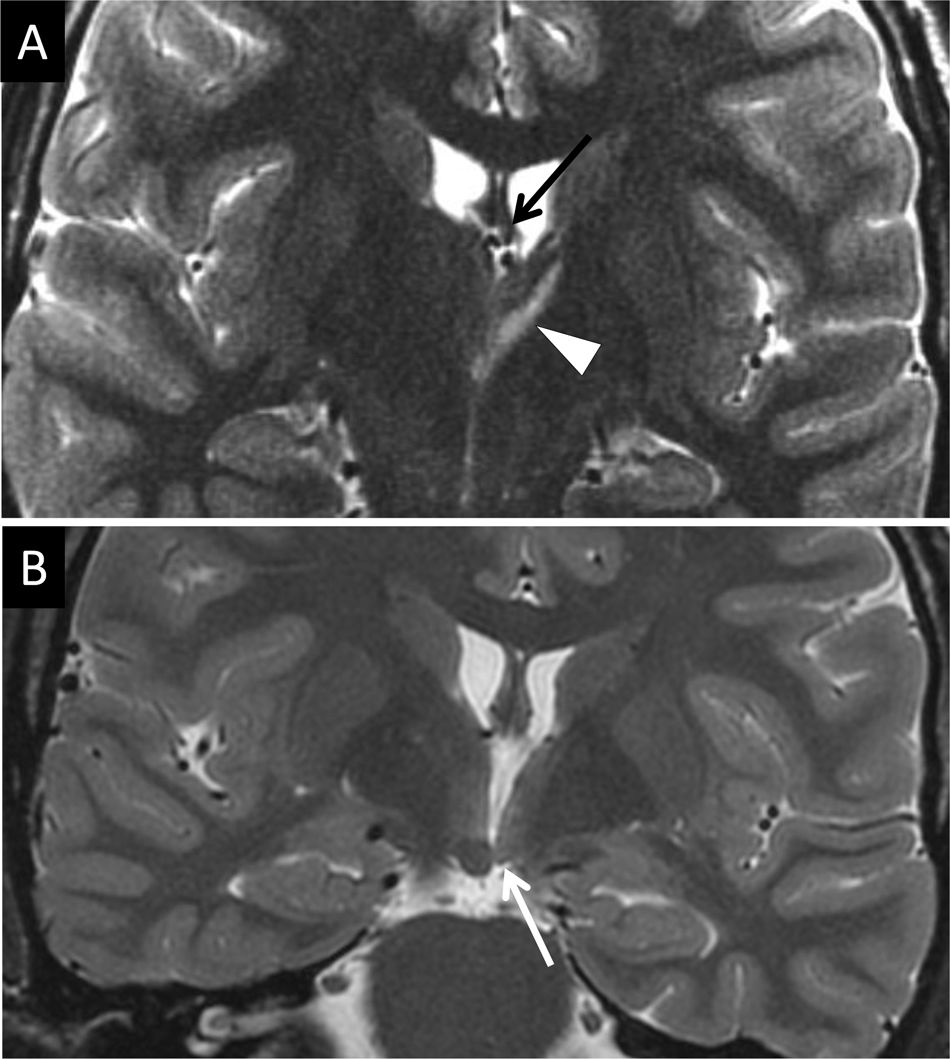
Hippocampal sclerosis is a common cause of temporal lobe epilepsy. This damage can cause atrophy of the ipsilateral fornix as a result of WD of the first neuron and cause mammillary body atrophy due to TD. Mammillary body atrophy can also be due to impairment of interconnected structures outside the limbic system (Fig. 14). Impairment of the second neuron leads to a reduction in the size of the mammillary body and the anterior thalamic nucleus due to injury to the mammillothalamic tract. This is generally a result of retrograde TD, with variable fornix abnormality29 (Fig. 15). In these cases, DTI is very useful in visualising cingulate gyrus fibres on the affected side. Infarction due to occlusion of the hippocampal artery, a branch of the posterior cerebral artery, is another cause of middle temporal impairment and secondary damage to the fornix and the mammillary body.30
DND is a common finding in routine radiology practice and a shared outcome in many disease processes. Knowledge of the expected remote response based on lesion topography is crucial for preventing diagnostic errors. Moreover, studies of cerebral metabolism and perfusion and diffusion sequences are showing increasingly early changes in the same process; hence, radiologists must be familiar with them.
FundingThere is no funding to declare.
Authorship- 1
Responsible for study integrity: AM-F.
- 2
Article concept: AM-F, JCP.
- 3
Article design: AM-F, JCP.
- 4
Data collection: AM-F, JCP.
- 5
Data analysis and interpretation: not applicable.
- 6
Statistical processing: not applicable.
- 7
Literature search: AM-F, JCP, MG-JA, POF.
- 8
Drafting of the article: AM-F, JCP, MG-JA.
- 9
Critical review of the manuscript with intellectually significant contributions: JCP, POF, MG-JA.
- 10
Approval of the final version: AM-F, JCP, POF, MG-JA.
The authors declare that they have no conflicts of interest.
Please cite this article as: Montoya-Filardi A, García-Junco Albacete M, Ortolá Fortes P, Carreres Polo J. Carretera perdida: imagen de la degeneración neuronal secundaria. Radiología. 2022;64:145–155.