The term contrast-induced nephropathy is used to describe acute deterioration of renal function after the intravenous administration of iodinated contrast material. We aimed to estimate the incidence of contrast-induced nephropathy and to analyze the evolution of different biomarkers of renal function in patients who underwent computed tomography with intravenous contrast administration after premedication with oral hydration and N-acetylcysteine.
Material and methodsThis prospective observational study included 112 patients with chronic renal failure (glomerular filtration rate (GFR) 30ml–60ml/min/1.73m2) scheduled for computed tomography with intravenous iodinated contrast material. We recorded demographic variables, dose of contrast material, diabetes mellitus, hypertension, and serum hemoglobin. We measured serum creatinine and GFR after premedication and after the CT examination. We summarized variables as means, standard deviations, and percentages. We used the Wilcoxon and Mann–Whitney tests to compare pre- and post-CT values and Pearson's r to analyze correlations.
ResultsIncidence acute kidney injury: 0.9%; 95%CI: 0.36–1.4. Mean difference between pre- and post-CT creatinine: 0.04; 95%CI: 0.002–0.09, p<0.004. Mean difference between pre- and post-CT GFR: −3.06; 95%CI: −4.66 to −1.47), p<0.001.
ConclusionsThe incidence of contrast-induced nephropathy in patients with chronic renal failure and GFR 30ml–60ml/min/1.73m2 is low. The biomarkers of renal function analyzed improve in patients who receive premedication and the minimum dose of contrast material.
Nefropatía inducida por contraste (NIC) es el deterioro agudo de la función renal tras la administración intravascular de contraste yodado. Nuestros objetivos son estimar la incidencia de NIC y analizar la evolucion de distintos biomarcadores de funcion renal, en pacientes tras una tomografía computarizada (TC) con contraste yodado intravenoso (CIV) y premedicación (hidratación oral y N-acetilcisteína).
Material y métodosEstudio observacional prospectivo. Fueron seleccionados 112 pacientes, con una TC con CIV programada y enfermedad renal crónica (ERC) con un filtrado glomerular (FG) entre 30 y 60ml/min/1,73 m2. Se obtuvieron datos demográficos, dosis del CIV, diabetes mellitus, hipertensión arterial y hemoglobina sérica. Se midió la creatinina sérica y el FG posterior a la premedicación y a la TC. Se utilizaron medias, desviaciones estándar y porcentajes, así como las pruebas de Wilcoxon y Mann-Whitney para determinar diferencias significativas, y la de Pearson para análisis de correlación.
ResultadosIncidencia de insuficiencia renal aguda: 0,9%; intervalo de confianza (IC) 95%: 0,36 a 1,4. Media de la diferencia de creatinina pre- y post-TC: 0,04 (IC: 0,002 a 0,09), p < 0,004. Media de la diferencia de FG pre- y post-TC: -3,06 (IC: -4,66 a -1,47), p < 0,001.
ConclusionesLa incidencia de NIC en pacientes con ERC y FG entre 30 y 60ml/min/1,73 m2 es baja. Los pacientes que recibieron premedicación y la mínima dosis de CIV presentaron mejoría en los biomarcadores de función renal analizados.
Contrast-induced nephropathy (CIN) is understood to refer to acute deterioration of kidney function occurring two to five days following administration of an iodinated intravascular contrast (IVC) medium.1,2 Some associations, such as the American College of Radiology (ACR), are more restrictive and consider CIN to be just a subgroup of types of kidney failure that occur following administration of IVC, in which the aetiological agent is the IVC itself, in the absence of another aetiological explanation.3
CIN may be diagnosed based on an absolute increase in creatinine levels by 0.3mg/dl or a relative increase in creatinine levels greater than 50% compared to baseline.4
Its onset has been linked to patient risk factors (chronic kidney disease, diabetes mellitus, advanced age, anaemia, hypotension, hypovolaemia, obesity and critical tissue ischaemia, among others) and to procedures (administration of large contrast volumes, high concentration, high osmolarity, route of administration, etc.).1,2,5–7
Although the existence of CIN has been doubted, some studies have affirmed that it is a real condition, if one that is less common than initially thought.1,2,8,9 Despite this, it seems reasonable to use prophylactic measures to minimise the risk of suffering from CIN, as well as to find groups of patients at higher risk of developing this complication. This takes on greater importance if the increased use of procedures involving radiological contrast is taken into consideration, especially in patients who require multiple monitoring studies due to their underlying disease and/or patients who present greater frailty and morbidity.1,2
The best preventive measures are proper patient assessment and good communication between requesting physicians and radiologists, for the purpose of selecting the most appropriate test for a particular patient.10,11 In addition, prophylactic measures have been proposed for risk patients; volume expansion is the most accepted, but there is no consensus regarding the agent with which it is to be performed or the use of supplementary N-acetylcysteine as a supplement.12–16
The objectives of this observational study were to determine the incidence of CIN at our centre, following a scheduled computed tomography (CT) scan with IVC, in a sample of patients with chronic diseases and moderate kidney damage, and to analyse overall changes in laboratory parameters of kidney function and their variation in relation to different risk factors.
Materials and methodsPatientsA total of 112 consecutive patients scheduled for a CT scan at our centre, between 1 July and 30 November 2018, for follow-up of a chronic disease, with laboratory data in relation to chronic kidney disease in the past 3 months and an estimated glomerular filtration rate (GFR) of <60ml/min/1.73m2 according to the Modification of Diet in Renal Disease (MDRD-4) formula,17 who agreed to take part in the study and signed the informed consent form, were selected. Patients under 18 years of age, patients who were admitted and patients with an estimated GFR of <30ml/min/1.73m2 were excluded.
MethodsThis was a prospective cohort study that consisted of several phases:
- 1.
Review of patients and generation of laboratory orders. Three days before the test, the list of patients scheduled for a CT scan with IVC at our centre was obtained and the requests were selected. These patients were confirmed to have had a laboratory test performed at our centre in the past 3 months, with a GFR of >30 and <60ml/min/1.73m2. In these cases, the patient was contacted to inform him or her of the study and, once the patient granted consent to take part in the study, he or she was advised to take the prophylactic measures established according to the nephrology department at our hospital (hydration with 2l of water per day and 600mg every 12h of N-acetylcysteine in the 2 days prior to the test).
- 2.
On the day of the test, patient data concerning established risk factors were filled in:
- a.
Diagnosis of hypertension.
- b.
Contrast dose and type.
- c.
Diagnosis of diabetes mellitus.
- d.
Serum haemoglobin (Hb).
- e.
Age, sex and race.
- a.
- 3.
Following the test, the patient was provided with a request for laboratory testing and instructions to go to the laboratory 2–5 days after the test.
- 4.
On the day of the follow-up laboratory testing, the patients went to the laboratory, the testing was done, the results were reviewed at the laboratory and variations in creatinine and GFR values compared to baseline were assessed for a diagnosis of CIN. A diagnosis of CIN could be made based on an absolute increase in creatinine levels by 0.3mg/dl or by a relative increase in creatinine levels greater than 50% compared to baseline. In the patients reviewed in whom the presence of CIN was presumed, their primary physician was notified for proper treatment and follow-up.
The data were electronically processed using a database in the form of a Microsoft Excel workbook, which was later imported for statistical processing into the Statistical Package for the Social Sciences (SPSS) software programme, version 23.
Mean and standard deviation were used to describe continuous quantitative variables. Absolute frequencies and relative frequencies expressed as percentages were used to describe qualitative variables.
Statistical tests for samples with a non-parametric distribution were chosen, due to the small sample size and the difficulty of being certain of a normal distribution. Differences with a probability of error of less than 5% (p<0.05) were considered statistically significant.
Pairwise comparisons between continuous quantitative variables (mean serum creatinine and mean GFR before and after CT with IVC, as well as mean differences) were performed using the Wilcoxon test for paired data. This enabled estimation of the magnitude of overall variation in laboratory parameters of kidney function following CT with IVC.
Comparisons between continuous quantitative variables, between independent groups (internal stratification by sex, diabetes mellitus and hypertension), were primarily performed using the Mann–Whitney U test. This enabled estimation of the magnitude of variation in the mean differences between serum creatinine and GFR values, before and after CT with IVC, in relation to the internal stratification.
Pearson's correlation coefficient was used for the analysis of correlation between continuous quantitative variables (age, dose and serum haemoglobin).
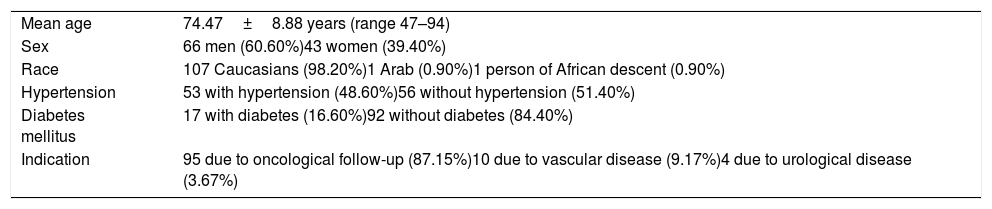
ResultsIn total, 112 patients agreed to take part in the study. Subsequently, 3 were excluded as they did not undergo laboratory testing following the CT scan. Table 1 summarises the demographic and clinical variables corresponding to this sample.
Demographic and clinical variables of patients.
| Mean age | 74.47±8.88 years (range 47–94) |
| Sex | 66 men (60.60%)43 women (39.40%) |
| Race | 107 Caucasians (98.20%)1 Arab (0.90%)1 person of African descent (0.90%) |
| Hypertension | 53 with hypertension (48.60%)56 without hypertension (51.40%) |
| Diabetes mellitus | 17 with diabetes (16.60%)92 without diabetes (84.40%) |
| Indication | 95 due to oncological follow-up (87.15%)10 due to vascular disease (9.17%)4 due to urological disease (3.67%) |
Ioversol (Optiray®) 300mg was the most common iodinated contrast medium, used in 97.26% of cases. Iomeprol (Iomeron®) was used at a dose of 300mg in only 1.82% of cases, and at a dose of 400mg in only 0.92% of cases. Volumes of 120–125ml were used, with a mean of 121.90±4.70ml (range 80–125) and a flow rate of 2ml/s.
Among the patients enrolled, there was only 1 case of acute kidney failure (AKF), amounting to an incidence of 0.91% with a 95% confidence interval (CI) of 0.36 to 1.40. This patient showed an increase in serum creatinine from a baseline level of 1.84 to 3.07mg/dl and a decrease in GFR from 35 to 18ml/min/1.73m2, 3 days after the CT scan. He was not found to have any risk factors such as diabetes mellitus or hypertension. However, given that his AKF occurred concomitantly with an acute infection, hospital admission was indicated. His kidney function normalised after a week (serum creatinine 1.57mg/dl and GFR 43.30ml/min/1.73m2). All other patients had neither intercurrent infectious signs or symptoms nor hospital admissions due to another cause.
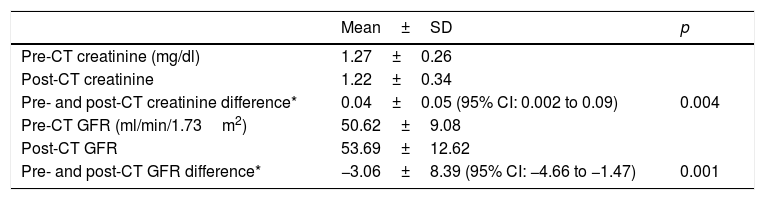
Table 2 shows the magnitude of the changes in the laboratory parameters of kidney function evaluated.
Overall changes in laboratory parameters of kidney function.
| Mean±SD | p | |
|---|---|---|
| Pre-CT creatinine (mg/dl) | 1.27±0.26 | |
| Post-CT creatinine | 1.22±0.34 | |
| Pre- and post-CT creatinine difference* | 0.04±0.05 (95% CI: 0.002 to 0.09) | 0.004 |
| Pre-CT GFR (ml/min/1.73m2) | 50.62±9.08 | |
| Post-CT GFR | 53.69±12.62 | |
| Pre- and post-CT GFR difference* | −3.06±8.39 (95% CI: −4.66 to −1.47) | 0.001 |
GFR: glomerular filtration rate; CI: confidence interval; p: statistical significance (Wilcoxon test for paired data); CT: computed tomography.
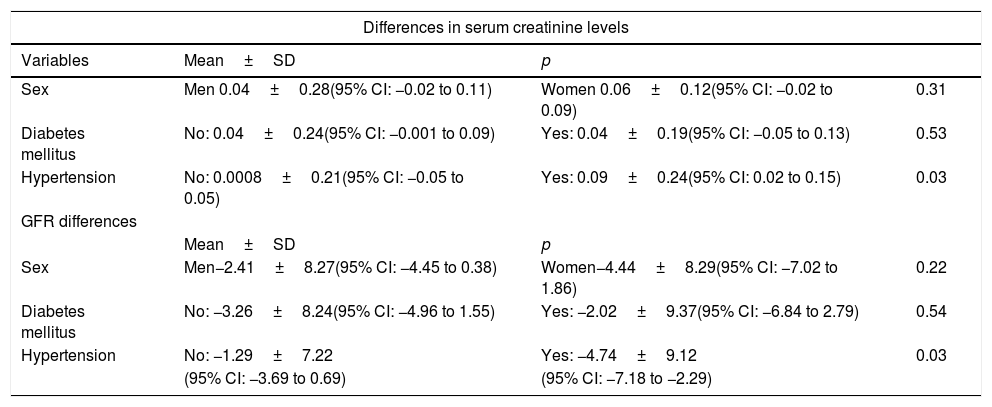
In addition, variation in mean differences between serum creatinine and GFR values, before and after CT with IVC, in relation to the internal stratification by risk factors (sex, diabetes mellitus and hypertension) was estimated (Table 3). In the race and contrast type variables, no statistical power was found to demonstrate significant differences, due to the small sample size of the groups.
Variation in mean differences of serum creatinine and glomerular filtration rate, before and after the CT scan with intravenous contrast, in relation to risk factors.
| Differences in serum creatinine levels | |||
|---|---|---|---|
| Variables | Mean±SD | p | |
| Sex | Men 0.04±0.28(95% CI: −0.02 to 0.11) | Women 0.06±0.12(95% CI: −0.02 to 0.09) | 0.31 |
| Diabetes mellitus | No: 0.04±0.24(95% CI: −0.001 to 0.09) | Yes: 0.04±0.19(95% CI: −0.05 to 0.13) | 0.53 |
| Hypertension | No: 0.0008±0.21(95% CI: −0.05 to 0.05) | Yes: 0.09±0.24(95% CI: 0.02 to 0.15) | 0.03 |
| GFR differences | |||
| Mean±SD | p | ||
| Sex | Men−2.41±8.27(95% CI: −4.45 to 0.38) | Women−4.44±8.29(95% CI: −7.02 to 1.86) | 0.22 |
| Diabetes mellitus | No: −3.26±8.24(95% CI: −4.96 to 1.55) | Yes: −2.02±9.37(95% CI: −6.84 to 2.79) | 0.54 |
| Hypertension | No: −1.29±7.22 | Yes: −4.74±9.12 | 0.03 |
| (95% CI: −3.69 to 0.69) | (95% CI: −7.18 to −2.29) | ||
IVC: intravenous contrast; GFR: glomerular filtration rate; CI: confidence interval; p: statistical significance (Mann–Whitney U test); CT: computed tomography.
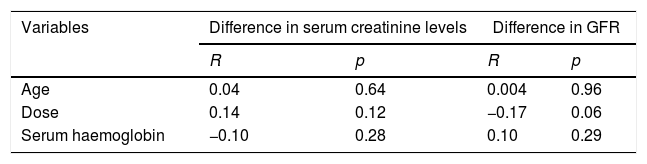
Finally, regarding the correlation between differences in serum creatinine and GFR, before and after CT with IVC and the quantitative variables included (age, contrast dose and serum haemoglobin), a correlation was found with a negligible, statistically insignificant magnitude (Table 4).
Correlation between differences in serum creatinine and glomerular filtration rate, before and after the CT scan with intravenous contrast and the quantitative variables included.
| Variables | Difference in serum creatinine levels | Difference in GFR | ||
|---|---|---|---|---|
| R | p | R | p | |
| Age | 0.04 | 0.64 | 0.004 | 0.96 |
| Dose | 0.14 | 0.12 | −0.17 | 0.06 |
| Serum haemoglobin | −0.10 | 0.28 | 0.10 | 0.29 |
IVC: intravenous contrast; GFR: glomerular filtration rate; p: statistical significance (Pearson's correlation coefficient), R: correlation coefficient; CT: computed tomography.
A low incidence of CIN as a cause of iatrogenic AKF is to be expected according to the scientific literature. It ranges from 0.6% to 2.3%, usually in patients with concomitant heart disease. However, some studies have not found significant differences in CIN incidence or serum creatinine levels among patients who received or did not receive IVC, even in those at high risk.1,18,19
In fact, according to our results, following a CT scan with IVC, there is overall improvement in laboratory parameters of kidney function. The mean difference between serum creatinine before and after CT with IVC was 0.04 (CI: 0.002 to 0.09) and the mean difference between GFR before and after CT with IVC was −3.06 (CI: −4.66 to −1.47); both were statistically significant. Other authors have found the same in their own studies.20,21 Ferrer et al. found that a CT scan with IVC not only is not associated with AKF development, but also is a protective factor against AKF development. A possible explanation for this would be the hydration involved in administration of contrast volume, its osmotic diuretic effect and the subsequent resulting hydration.21
Our study detected only one case of AKF. This episode coincided with intercurrent infectious signs and symptoms requiring hospital admission, with normalisation of kidney function after a week of treatment of the infectious focus. Given that not all variation in serum creatinine levels following IVC administration is dependent thereon and that sepsis may significantly affect kidney function, it could even be concluded that these circumstances suggested that CIN was actually absent from our study population. Other conditions that may affect the kidney function of hospitalised patients include administration of nephrotoxic drugs, glomerulonephritis, haemolysis, rhabdomyolysis, low cardiac output, acute obstructive uropathy, surgery, burns and bowel obstruction.8
Such is how Hong et al. retrospectively analysed kidney function in 820 patients with active oncology disease, before and after they underwent a CT scan with IVC. Based on their results they concluded that the incidence of CIN was significant — 8% — and that it was explained in part by the greater frailty of these patients and the adverse effects of their medication.22,23 Fukushima et al. conducted a retrospective study of a cohort of 216 patients with a GFR of less than 60ml/min/1.73m2 who underwent a CT scan with IVC. Among the variables analysed, chronic kidney disease, diabetes mellitus, IVC volume administered, prior hydration, a left ventricular ejection fraction (LVEF) of less than 60% and need for admission to an intensive care unit (ICU) were found. A significant association was only found between development of CIN and admission to an ICU or a LVEF of less than 60%.24
Moreover, notable among the chronic conditions that affected the patients studied on an outpatient basis was diabetes mellitus, which has been classically considered a risk factor for developing CIN.25 In our study, patients with a history of diabetes mellitus showed no significant variations in serum creatinine or GFR values following a CT scan with IVC. This was consistent with other recent studies which found no significant association.22,24
Hypertension has been considered to be a risk factor for the development of CIN independent of diabetes mellitus. Significant differences have been found with respect to CIN incidence in hypertensive versus non-hypertensive patients (41.6% versus 14.7%, p=0.044).23 In our study, even though there were significant differences between mean differences in creatinine and GFR, in hypertensive versus non-hypertensive patients, the former showed an improvement in the indicators of kidney function analysed following a CT scan with administration of IVC.
Administration of high-osmolarity agents, a contrast volume greater than 350ml or more than 4ml/kg and further administration of IVC before 72h have elapsed following initial administration of IVC were associated with an increased risk of CIN.26 The use of non-ionic compounds with a low osmolarity and a better nephrotoxicity profile such as Ioversol (Optiray®), and the use of a contrast volume of less than 140ml in all cases, also accounted for the low incidence of CIN in our sample.26,27
Volume expansion (oral or intravenous) prior to administration of radiological contrast and/or use of N-acetylcysteine have been traditionally recommended to decrease the risk of developing CIN.28,29 The main preventive measure consists of volume expansion with isotonic solutions prior to administration of IVC, essentially in interventional procedures. This effect is believed to be due to an increase in renal blood flow which induces an increase in diuresis, dilution of the IVC in the kidney tubules, suppression of the renin–angiotensin–aldosterone axis and lesser inhibition of production of endogenous vasodilators.28–30,13 The ideal infusion rate and volume are unknown. However, the current ACR guidelines recommend the use of isotonic saline solution at an infusion rate of 100ml per hour for 6 to 12h before and 4 to 12h after an angiography.3 Oral hydration has also been used, but with less demonstrated effectiveness.30,13,31 Our study found a negative mean difference between GFR before and after administration of IVC of −3.06 (CI: −4.66 to −1.47), denoting an improvement in kidney function. This improvement could be explained not only by the hydration involved in the administration of the contrast volume itself and its diuretic effect, but also by the oral hydration received as a preventive measure. Recently, a prospective, randomised, non-inferiority study compared the incidence of CIN following administration of IVC between patients with a GFR of 30–60ml/min/1.73m2 who did not receive intravenous hydration and patients who did. The difference in the incidence of CIN between these two groups was −0.10% (95% CI −2.25 to 2.06; p=0.47), which did not exceed the non-inferiority margin of 2.1%. Therefore, non-administration of intravenous hydration was not inferior in preventing CIN. In addition, non-use of this prophylaxis reduced costs and prevented associated complications such as congestive heart failure, hyponatraemia and cardiac arrhythmias, present in up to 5.5% of the patients in this study.32
Moreover, the use of N-acetylcysteine to reduce the incidence of CIN has been debated. Some studies and meta-analyses have yielded contradictory results as to whether or not this agent reduces the incidence of AKF.33–35 There is evidence indicating that N-acetylcysteine reduces serum creatinine in healthy subjects without altering levels of cystatin C, which is the best marker of GFR. This raises the suspicion that N-acetylcysteine simply reduces creatinine levels without really preventing AKF. At present, there is insufficient evidence of its efficacy and it is not recommended as a substitute for proper patient selection and volume expansion.33 A recent randomised study compared the use of intravenous sodium bicarbonate 1.26%, intravenous sodium chloride 0.9% and oral N-acetylcysteine 1200mg twice daily for 5 days or placebo in patients who were to undergo an angiography and were at high risk of renal complications (patients with diabetes mellitus or stage 3 or 4 chronic kidney disease). In the N-acetylcysteine group, 114 of 2495 (4.6%) patients presented a need for dialysis, died or developed CIN subsequent to the procedure, with a relative risk of 1.02 (95% CI: 0.78 to 1.33; p=0.88). No significant differences were found between the different groups with respect to decreased development of CIN.36
To our knowledge, few prospective studies have used volume expansion with oral hydration and N-acetylcysteine as a preventive measure in patients at high risk of developing CIN, the population for which the guidelines have been written. In addition, most studies have been conducted with a population with specific conditions, whereas our study was conducted with a population studied on an outpatient basis which required frequent monitoring and therefore would need multiple CT scans with administration of IVC in the follow-up of their underlying disease. However, as an observational study was conducted according to our institution's protocols, with the limitation of not having a control group, it was not possible to determine the incidence of CIN in the patients studied on an outpatient basis at high risk of developing it without administration of premedication.
In conclusion, our study supported the notion that the incidence of CIN in patients studied on an outpatient basis, at high risk, with a GFR between 30ml/min/1.73m2 and 60ml/min/1.73m2, is low. In our sample, an overall improvement in laboratory parameters of kidney function was observed in patients who were administered the minimum dose of low-osmolarity non-ionic iodinated IVC, oral hydration and N-acetylcysteine. However, we intend to conduct a prospective, randomised, controlled study with the same characteristics and a larger sample size in order to compare the incidence in non-premedicated patients.
Authors’ contributions- 1.
Study integrity: ABL, JIR, FGM, BM, RGR and CMH.
- 2.
Study concept: ABL, JIR, RGR and CMH.
- 3.
Study design: ABL, JIR, RGR, JDS and CMH.
- 4.
Data acquisition: ABL, JIR, FGM and BM.
- 5.
Data analysis and interpretation: ABL, JIR, FGM, JDS and RGR.
- 6.
Statistical processing: RGR.
- 7.
Literature search: ABL, JIR, FGM, BM and RGR.
- 8.
Drafting of the study: ABL, JIR and FGM.
- 9.
Critical review of the manuscript with intellectually significant contributions: RGR, JDS and CMH.
- 10.
Approval of the final version: ABL, JIR, FGM, BM, RGR, JDS and CMH.
The authors declare that they have no conflicts of interest. The study was approved by the Segovia Independent Ethics Committee.
We would like to thank the nursing staff for their dedication in taking patients’ vital signs and personal histories and the laboratory staff for analysing samples and promptly contacting patients’ primary physicians in cases in which patients had abnormal laboratory test values.
Please cite this article as: Barrios López A, García Martínez F, Rodríguez JI, Montero-San-Martín B, Gómez Rioja R, Diez J, et al. Incidencia de nefropatía inducida por contraste tras una tomografía computarizada. Radiología. 2021;63:307–313.