This update covers the management of solitary or multiple pulmonary nodules detected incidentally in imaging studies done for other reasons. It describes the most appropriate computed tomography technique for the evaluation of these nodules, how they are classified, and how the different types of nodules are measured. It also reviews the patient-related and nodule-related criteria for determining the risk of malignancy. It discusses the recommendations in the guidelines recently published by the Fleischner Society for the management and follow-up of each type of nodules according to its size and risk of malignancy.
En esta actualización se aborda el manejo de los nódulos pulmonares, solitarios o múltiples, detectados incidentalmente en estudios radiológicos que se realizan por otros motivos. Se describe la técnica de tomografía computarizada más adecuada para su evaluación, y cómo se clasifican y se miden los diferentes tipos de nódulos. También se revisan los criterios que permiten establecer el riesgo de malignidad, tanto asociados al paciente como a las características del nódulo, y, por último, las recomendaciones de manejo y seguimiento para cada tipo de nódulo en función del tamaño y el riesgo de malignidad, siguiendo fundamentalmente las guías recientemente publicadas por la Sociedad Fleischner.
A large number of pulmonary nodules are detected incidentally during studies performed for other reasons. The prevalence is estimated at 25–51% in healthy volunteers and in patients undergoing lung cancer screening.1,2 Most of these nodules have a low probability of malignancy,3 but they are an important source of anxiety for the patient and a challenge for clinicians and radiologists who, while endeavouring to avoid unnecessary invasive examinations and procedures, must not overlook the diagnosis of a malignant nodule.
A meticulous analysis of the nodule in terms of size, morphology and density, using an appropriate acquisition technique, will aid in decision-making.
Various guidelines aimed at standardising the management of incidental pulmonary nodules have been published, and are continually updated. Although the starting point for diagnosis is size, guidelines give greater importance to the probability of malignancy associated with the nodule itself and other patient risk factors.4–9
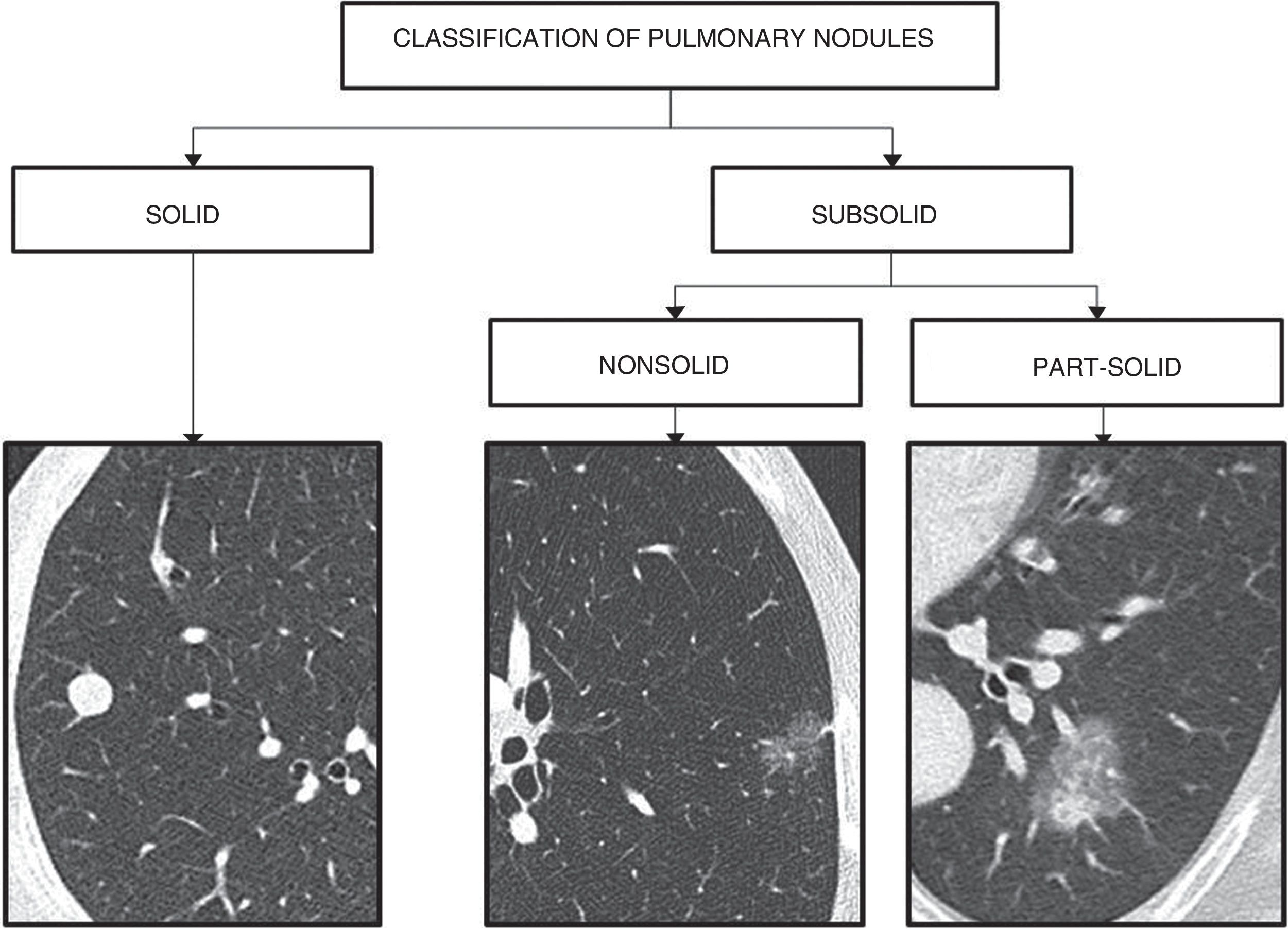
Classification of pulmonary nodulesA solitary pulmonary nodule is defined as a discrete, well-marginated, rounded opacity, less than or equal to 3cm in diameter, which is completely surrounded by lung parenchyma and is not associated with adenopathy, atelectasis, or pleural effusion. Lesions greater than 3cm are called masses, and are considered malignant until proven otherwise.
Pulmonary nodules are classified as solid and subsolid, according to their density. Subsolid nodules are in turn divided into non-solid (ground-glass opacity that does not obscure the bronchovascular structures) and part solid (ground-glass opacity with a solid component)8,10–12 (Fig. 1).
Solid nodules are granulomas, scars, lymph nodes, primary malignancies or metastases.
Most subsolid nodules are transient and secondary to infection or haemorrhage. When they persist, they often represent pathology in the spectrum of adenocarcinoma; nonsolid nodules correspond to non-invasive, minimally invasive or lepidic-predominant adenocarcinoma, and most part-solid nodules correspond to invasive adenocarcinomas.13–15
Differentiating between these types of nodule is no easy task, and is subject to considerable interobserver variability. Even experienced radiologists only achieve a “good” agreement rate of 0.62 and 0.67 for solid and nonsolid nodules, respectively.16,17 According to one published study, only 58% of nodules were correctly classified,16 while in another disagreement occurred in 36.4% of cases.17
Computed tomography techniqueStrategies involving low doses of radiation that do not exceed 3 mGy on the Computed Tomography Dose Index (CTDI), but that are able to correctly characterise the nodule, should be prioritised.
The most important technical factors to be taken into account are slice thickness and the reconstruction filter. Slices thickness should be equal to or greater than 1.5mm to avoid the partial volume effect, and images should be reconstructed using edge enhancement and soft tissue filters in order to correctly measure and classify the nodules as solid, nonsolid or part-solid, and to identify the presence of fat or calcium deposits that can characterise the nodule and avoid subsequent follow-up8,9,18,19 (Fig. 2).
The administration of intravenous contrast is not necessary, especially in follow-up studies.
Lung (level −700 to −500 Hounsfield units [HU], width 1500–2000HU) and mediastinum (level 30–70HU, width 350–400HU) windows should be analysed, both in the axial plane and in the sagittal and coronal planes, which will help differentiate nodules from scars.
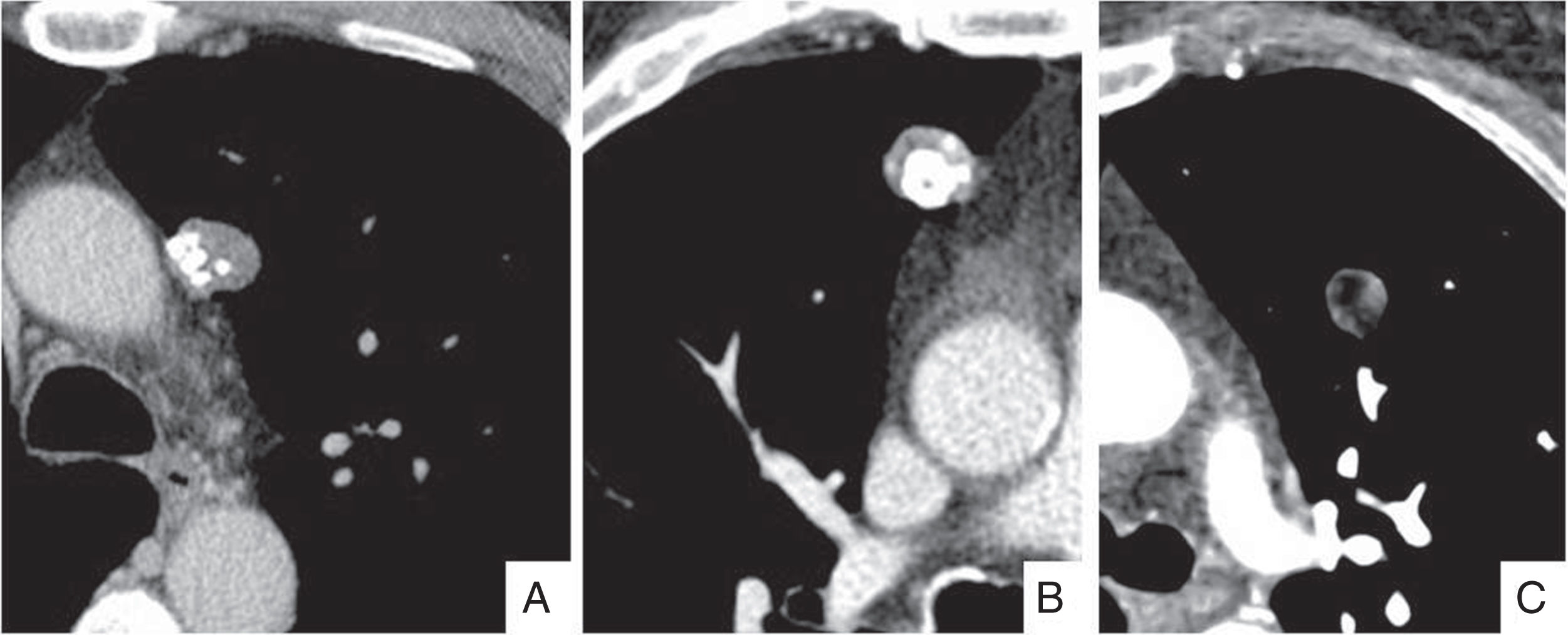
When a nodule is detected incidentally on a computed tomography (CT) scan with thicker slices, a short-term study with slices equal to or less than 1.5mm should be performed and used as a reference study for follow-up. To ensure homogeneity, follow-up studies should be performed using the same technique (Fig. 3).
A 70-year-old man in whom a part-solid nodule was incidentally detected in the left upper lobe. (A) Image of 2mm slice reconstructed using a soft-tissue filter. (B) Computed tomography follow-up at 3 months with a slice thickness of 1.5mm and edge enhancement reconstruction filter. The image confirms the persistence of the nodule and identifies the different components more clearly, and also shows the presence of pseudocavitation (white arrow). Biopsy confirmed the suspicion of lepidic-predominant adenocarcinoma.
Measurements should be taken using lung window images and edge enhancement filters, in the plane showing the largest diameter of the nodule (preferably the axial plane). Measurements in oblique planes are not recommended because they are not reproducible.8,18
The standard practice is manual linear measurement using electronic calibrators. Automated or semi-automated measurement of nodule volume appears to be more sensitive to changes in size, and is recommended by different lung cancer screening groups:
Manual linear measurementNodules measuring less than 3mm do not need to be measured, and we will refer to them as micronodules.
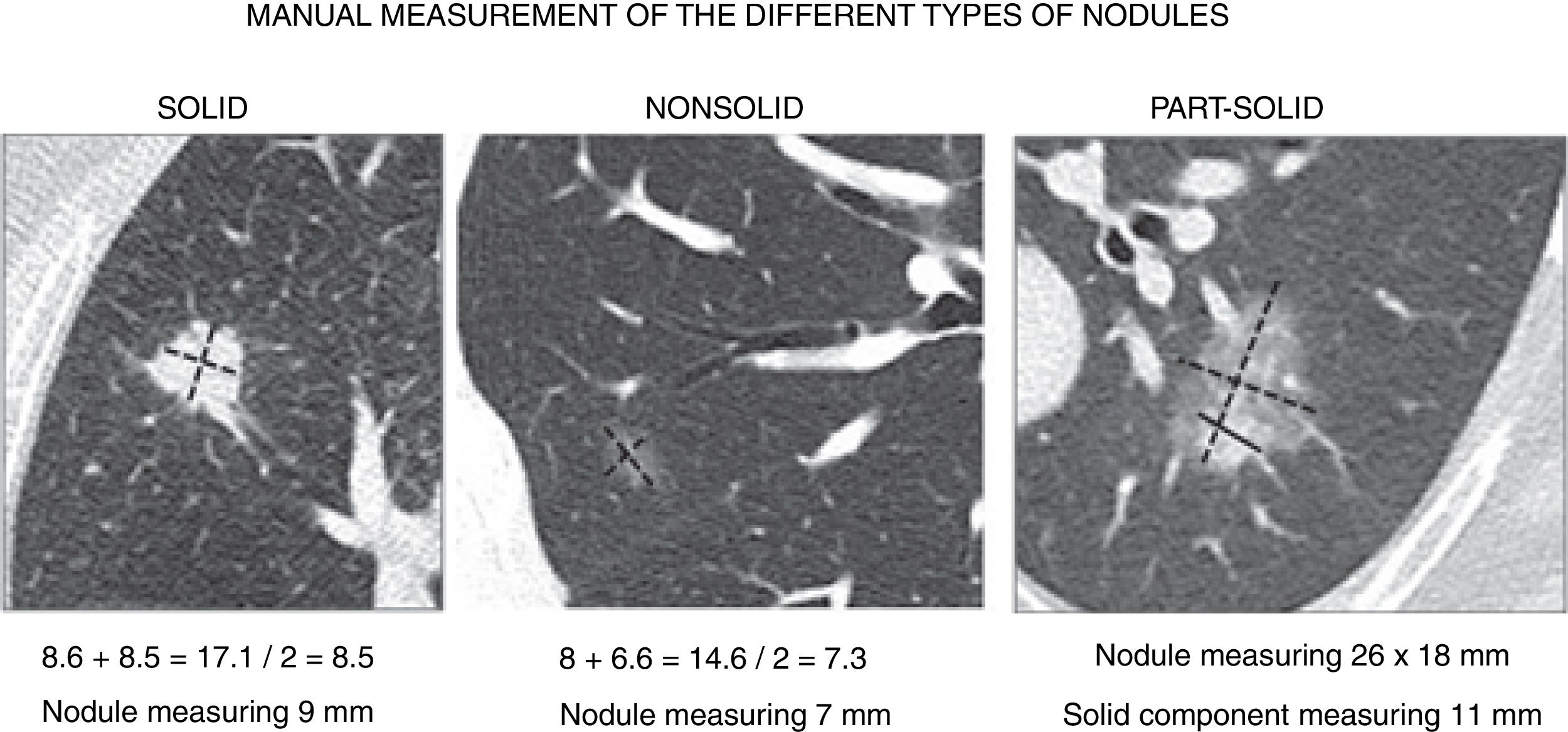
In nodules of less than 10mm, the Fleischner society4,8,18 recommends measuring the maximal long-axis and perpendicular maximal short-axis in the same plane (axial, coronal or sagittal). The final size is the average of these two diameters. Decimal numbers are rounded to the nearest whole number, in other words. 5.1–5mm, 5.5–6mm, 5.6–6mm, etc. (Fig. 4).
Manual measurement of the different types of nodules. In nodules measuring less than 10mm, the final size will be the average of the diameter of both the long and short axis, and in nodules larger than 10mm both diameters, instead of the average, should be reported. In part-solid nodules, the total measurement and the largest diameter of the solid component should be reported.
In nodules of less than 10mm, the mean of the two diameters correlates more closely with the volume and with the probability of malignancy than the measurement of the greatest diameter.18 In addition, the roundness of the nodule appears to influence prognosis, insofar as more irregular nodules have a poorer prognosis.20
In part-solid nodules, the total measurement must be obtained. This includes the area of ground glass, as in solid nodules, and also the largest diameter of the solid component when it is greater than 3mm. If there are several solid components, the largest one is measured and the others are described without measuring them.
Part-solid nodules can also be analysed by comparing lung and mediastinal window images. Any component of the nodule visible in the mediastinal window is considered solid, and any component that is not visible is ground glass. This analysis technique improves interobserver agreement and increases specificity for the diagnosis of invasive adenocarcinoma.10
In spiculated nodules, only the solid centre should be measured, excluding the spiculations.
In nodules measuring over 10mm, the diameter of the long and short axis should be given, instead of the average. In lung cancer staging, the greatest diameter will be used in order to evaluate the T descriptor and response to treatment.21,22
When multiple nodules are found, only the largest, or the most suspicious, should be measured.
Automated or semi-automated volumetric measurementThis is less sensitive to variability and, therefore, more reproducible, but the measurements obtained are highly influenced by the acquisition technique, the type of software used, and the characteristics of the lesion.23–25 Therefore, this method cannot be generally recommended until the factors causing the variability between CT studies and different software are better understood. In any event, measurements must always be obtained using the same method to maximise accuracy when comparing measurements. As a general rule, 100mm3=6mm, 100–250mm3=6–8mm, and over 250mm3=8mm or greater.
It is important to measure attenuation in order to show the presence of calcium or fat, which will affect diagnosis. A region of interest and not a single point should be used, choosing for this purpose an image with the minimum available slice thickness reconstructed with a soft tissue filter and mediastinal window.
The considerable inter- and intra-observer variability in measurements must always be taken into account, particularly in nodules with complex morphology and in part-solid nodules. This variability is due to the observer, the CT technique, the patient's depth of inspiration, other lesions in the adjacent parenchyma, etc., and can give rise to false positives and negatives when evaluating the growth of the nodule in follow-up studies. To avoid this problem, a nodule is considered to have changed in size when the mean diameter has increased or decreased by at least 2mm, rounded to the nearest millimetre.6,7,18
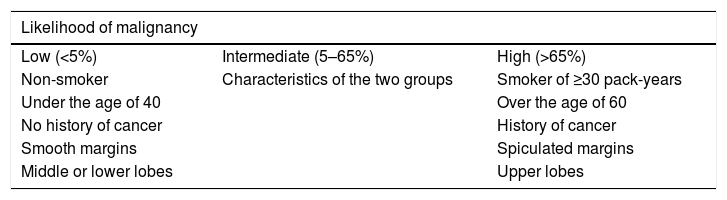
Risk factors for malignancyThe first step in the management of an incidentally-detected pulmonary nodule is to assess the risk of malignancy, based on the characteristics of both the nodule and the patient him/herself. Patients with low pre-test risk (0–5%) can be managed conservatively. Patients with intermediate risk (5–65%) (almost half of all patients) require additional studies, and high-risk patients (more than 65% of all patients) should be managed more aggressively. Risk estimation models have been developed for this purpose (see http://www.brocku.ca/cancerpredictionresearch)3,6,7 (Table 1 and Fig. 5).
Risk factors for malignancy.
| Likelihood of malignancy | ||
|---|---|---|
| Low (<5%) | Intermediate (5–65%) | High (>65%) |
| Non-smoker | Characteristics of the two groups | Smoker of ≥30 pack-years |
| Under the age of 40 | Over the age of 60 | |
| No history of cancer | History of cancer | |
| Smooth margins | Spiculated margins | |
| Middle or lower lobes | Upper lobes |
Nodules with a high risk for malignancy. (A) A 70-year-old man with a solid nodule with spiculated margins in the right upper lobe. (B) Part-solid nodule with signs of pseudocavitation (arrow). (C) An 85-year-old man with extensive emphysema and solid nodule with lobulated margins in the left upper lobe (arrow). In all three cases, biopsy was indicated. In (A) and (C), the diagnosis was squamous cell carcinoma, and in (B) invasive adenocarcinoma was diagnosed.
The characteristics to be considered, with respect to the pulmonary nodule and the patient, in order to determine the risk of malignancy are as follows:
SizeSize is clearly associated with the risk of malignancy, and is one of the main factors in nodule management. Risk of malignancy is estimated at less than 1% for solid nodules smaller than 6mm, and between 0.5% and 2% for nodules measuring 6–8mm in high-risk patients.3 In the screening population included in the NELSON study, the incidence of malignancy in nodules greater than 10mm was 15.2%.26
Density and morphologyNodules are classified according to their density as solid and subsolid (part-solid and nonsolid).27–29
Many subsolid nodules are transient and secondary to infection or haemorrhage. When they persist, the size and presence of a solid component is associated with invasive behaviour. Persistent part-solid nodules with a solid component larger than 6mm are considered malignant until proven otherwise. They present slower growth and a higher risk of malignancy than solid nodules.30,31 Henschke et al. found 63% of part-solid nodules to be malignant, while 18% of nonsolid nodules and 7% of solid nodules were also malignant.28 The appearance of a solid component during the evolution of these nodules was associated with invasive adenocarcinoma in 100% of cases, while size increase alone was associated with malignancy in only 44.4% of cases.15
Lung nodules with spiculated margins are associated with a high likelihood of malignancy. Likelihood of malignancy is intermediate in nodules with lobulated margins and low in those with smooth margins. The presence of an air bronchogram or air bubbles (pseudocavitation) in a nodule is a sign of malignancy that is seen more frequently in adenocarcinomas.32
Diffuse, central, laminar or popcorn calcifications are considered benign patterns, unless the patient has a history of cancer. Diffuse calcifications can be seen in chondrosarcoma and osteosarcoma metastases, and central and popcorn calcification is seen in tumours of the gastrointestinal tract or those who have undergone chemotherapy.19
The presence of fat is a sign of benignity that can be seen in hamartomas, lipomas or lipoid granulomas.33
LocationMost cancers are located in the upper lobes, particularly in the right upper lobe.
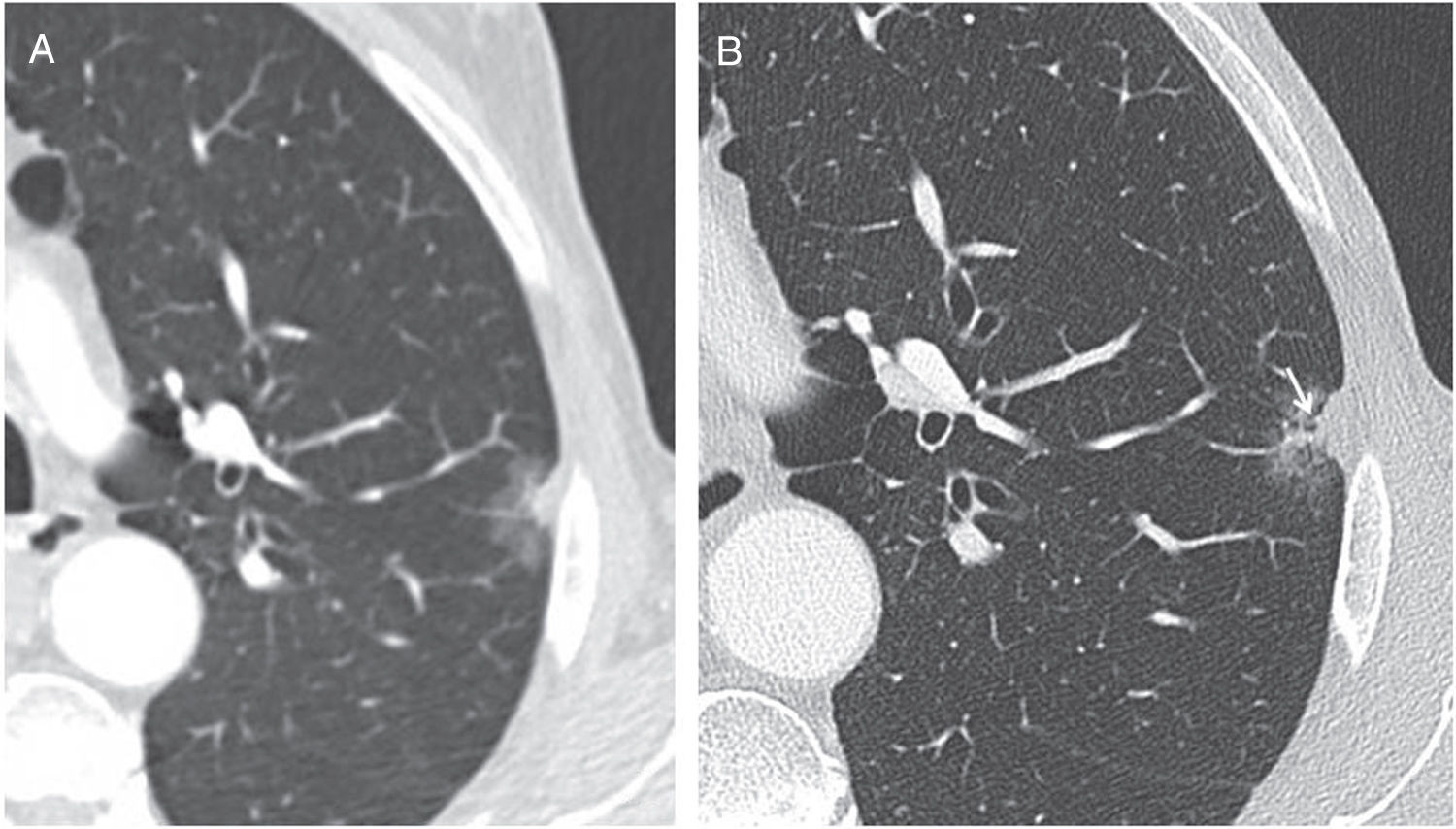
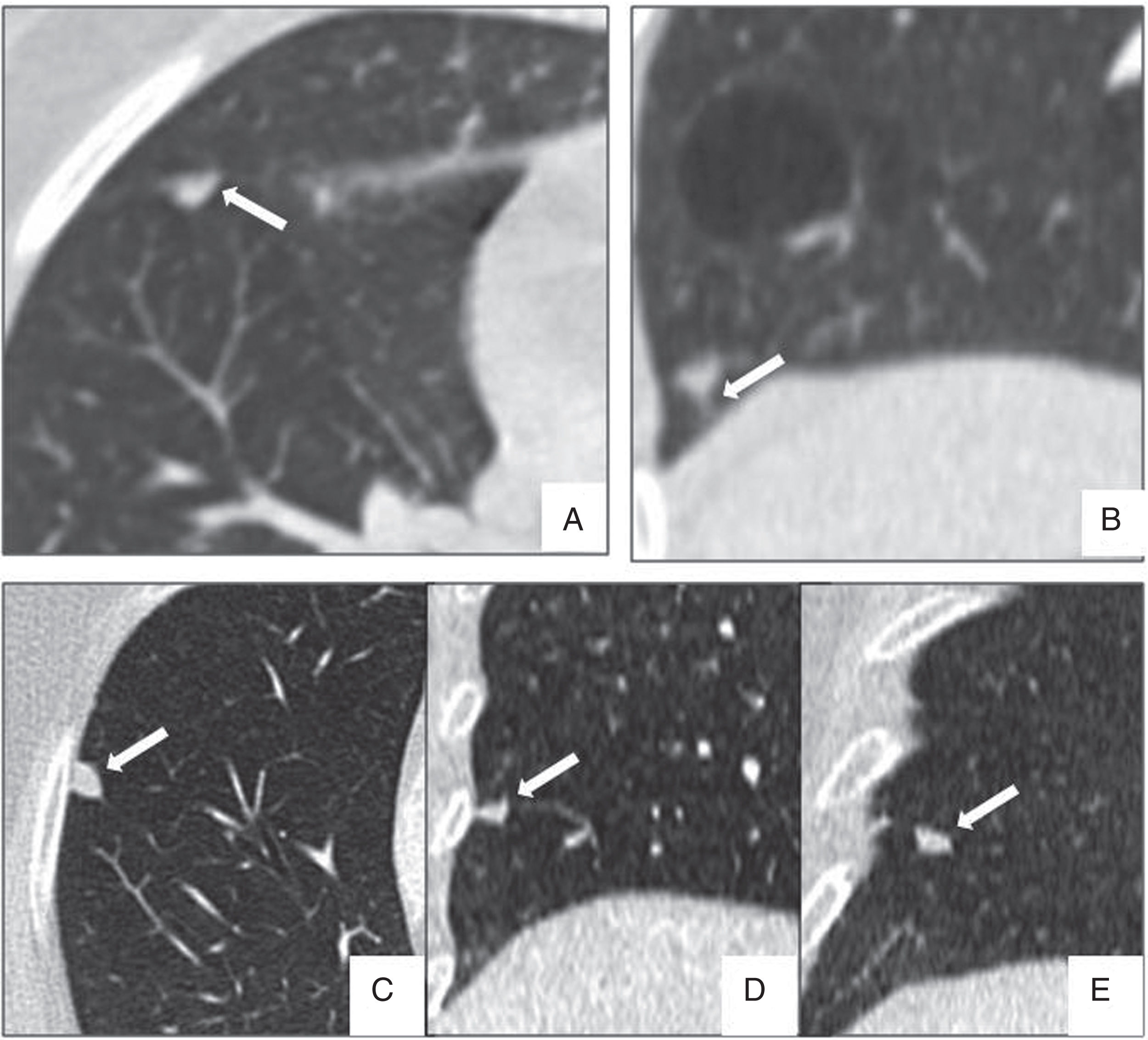
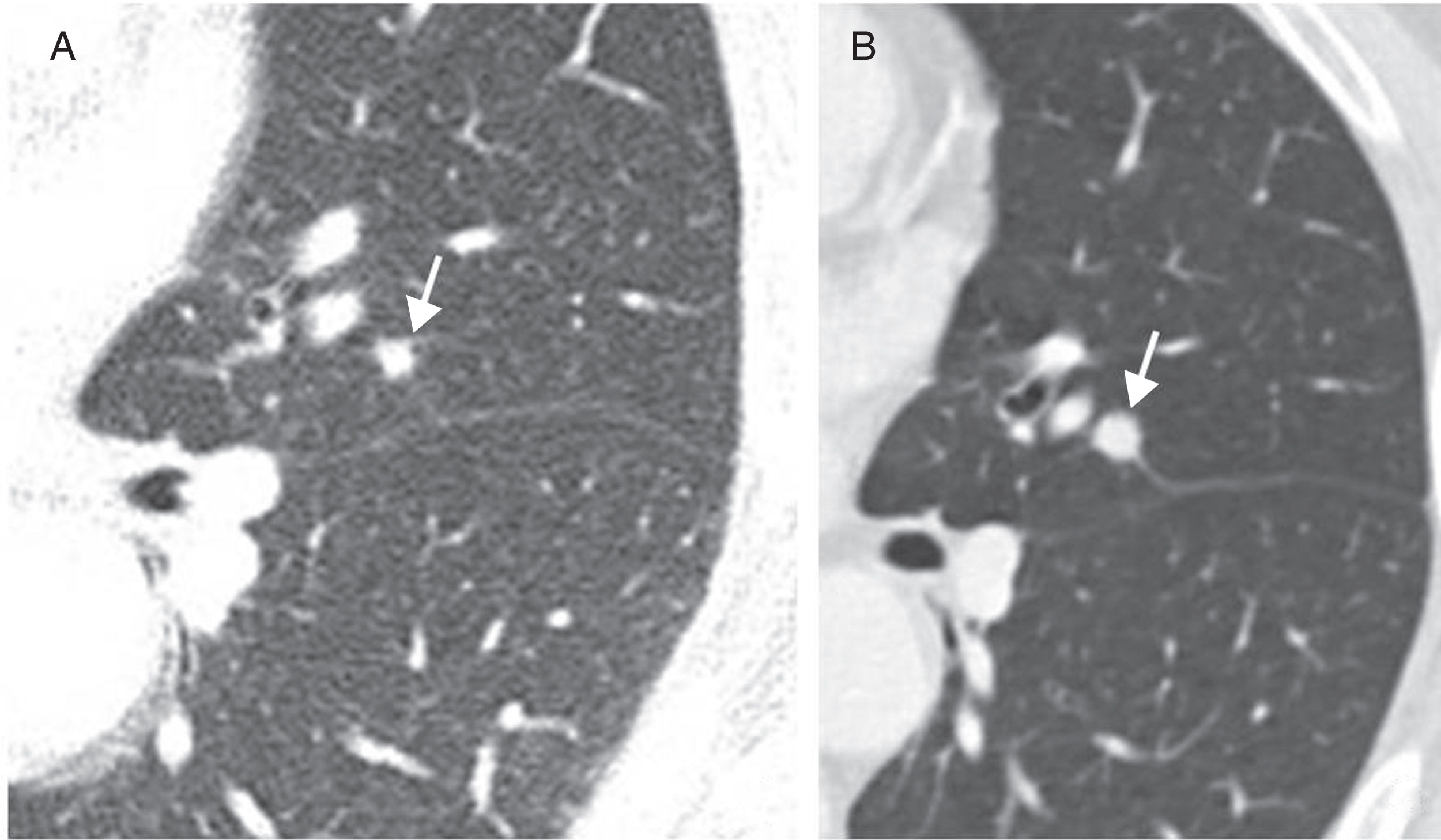
Perifissural and intrapulmonary ganglia are solid, homogeneous nodules with well-defined, smooth margins. They can be rounded, trapezoid, triangular or lentiform in shape, are frequently located below the carina, in contact with a fissure or less than 15mm from the pleural surface, and often attached to the pleura via a septum.34–36 In a screening population, none of the malignant tumours diagnosed originated from a perifissural nodule after a follow-up of 7.5 years.34 Multiplanar reconstructions are very useful for identifying the typical morphology of these intrapulmonary ganglia, which can grow and do not need follow-up even when they measure over 6mm (Fig. 6).
Typical morphological characteristics of benign perifissural and intrapulmonary ganglia. (A) Axial image of incidental nodule in a patient with heart failure, presenting triangular morphology based on the minor fissure (arrow). (B) Highly characteristic linear septum extending to the diaphragmatic pleura (arrow). (C–E) Multiplanar reconstructions showing an intrapulmonary ganglion in contact with the pleura, with lenticular morphology in the axial plane (C), triangular in the coronal plane (D) and trapezoid in the sagittal plane (E).
In the NELSON lung cancer screening study, the risk of malignancy was observed to increase when the number of nodules increased from one to four, but decreased when there are more than five nodules, in which case they are most likely to be due to granulomatous disease. The probability of cancer was 3.6% in patients with only one nodule, 4.1% when there were two nodules, 4.8% for three nodules, 6.3% for four nodules and 3.3% for more than four nodules, although these differences were not significant.37
Emphysema and fibrosisEmphysema and idiopathic pulmonary fibrosis, which may be associated with incidental nodules, are independent risk factors for malignancy.
Tobacco and other inhaled carcinogensCigarette smoking is the most important risk factor for the development of lung cancer, with an increased risk of 10–35% compared to non-smokers. This association is stronger in small squamous cell carcinoma, and weaker in adenocarcinoma. A history of 30 pack-years or more, or having quit smoking in the last 15 years, is considered a high risk factor in solid lung nodules. Passive smokers also present an increased risk, albeit to a lesser extent.
The way in which tobacco affects the development of adenocarcinoma is unclear, and, for this reason, recommendations for the management of subsolid nodules are independent of this risk category.
There is no evidence that any components of electronic cigarettes are risk factors.
Other inhaled carcinogens to consider are asbestos, uranium, radon and silica.
AgeAdvanced age is a risk factor. Lung cancer is very rare before the age of 35, and rare before the age of 40, but from this age the frequency increases with each decade.
GenderFemale gender has been associated with an increased risk in subsolid nodules.
Family historyA family history of lung cancer increases the risk of cancer in both smokers and non-smokers. It is associated with a relative risk of 1.5 when there is an affected sibling, and 1.8 when the sibling is a twin.
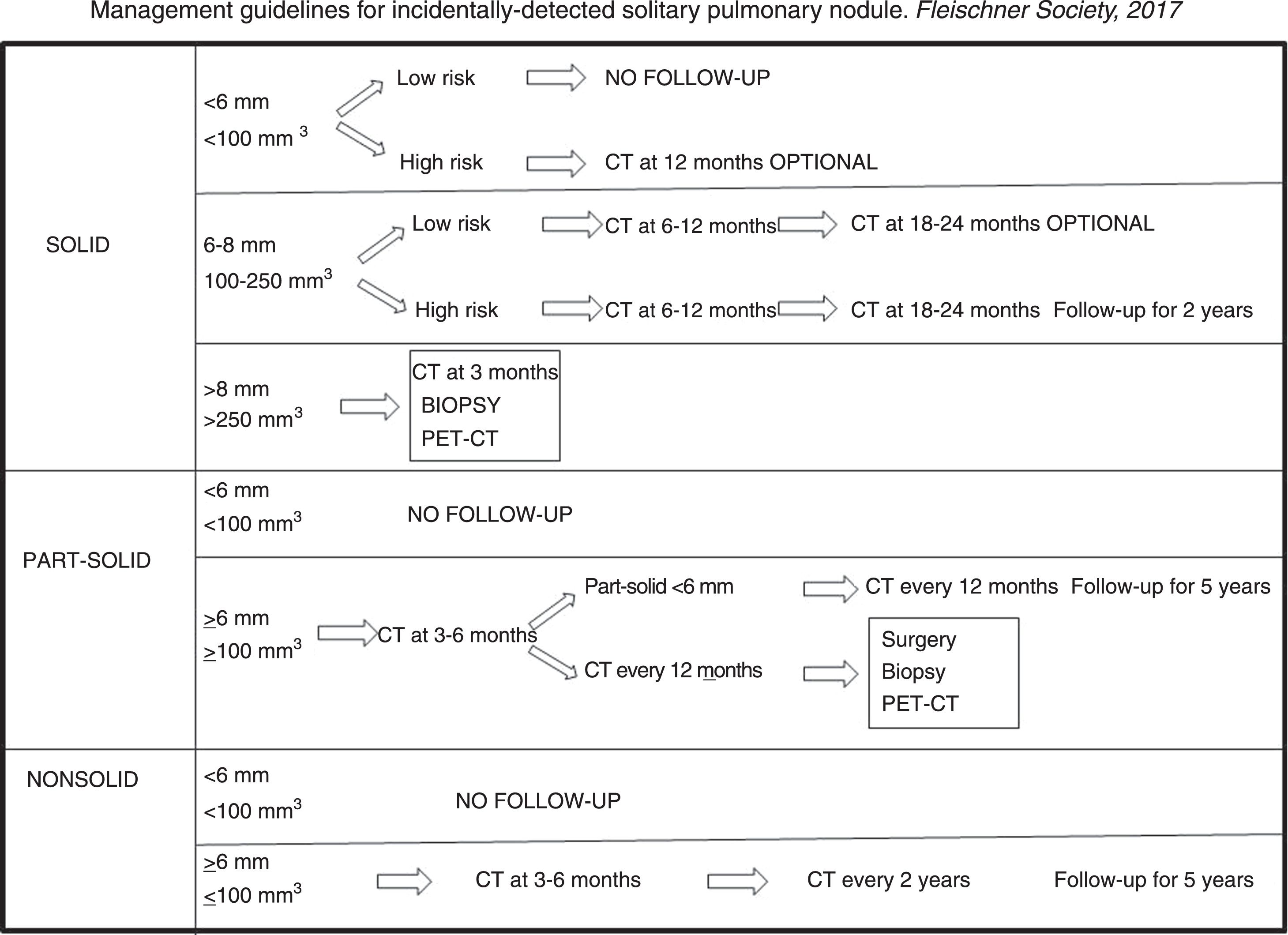
Guidelines for the management of incidental solitary pulmonary noduleThe growth rate of malignant tumours varies widely, depending on morphology, density and histology. The aim of recommended follow-up intervals is, on the one hand, to minimise the number of examinations, and on the other, to prevent a growing cancer from advancing during the follow-up interval.
The volume doubling time of solid nodules is 100–400 days,38 and three to five years for subsolid nodules, which is why longer follow-up intervals are recommended in these cases.39,40
In 2005, the Fleischner Society published the first management guidelines for solid solitary pulmonary nodules4; in 2013 the Society published its guidelines for subsolid nodules,5 and, in 2017, it published the latest guidelines for the management of incidentally detected solitary, solid and subsolid pulmonary nodules.8 The Fleischner Society guidelines, together with those of the British Thoracic Society published in 2015, are the most widely followed.6,7
The aim of these new guidelines is to reduce the number of unnecessary studies, and give clinicians, radiologists and patients more autonomy in the management of pulmonary nodules. This means that risk factors and patient preferences will determine whether a more aggressive or more conservative management strategy is implemented, and follow-up periods will be expressed in time intervals.
The Fleischner Society guidelines cover incidental nodules found in patients over 35 years of age. They are not applicable in the following clinical scenarios:
- •
Nodules detected in patients under 35 years of age. These must be managed individually because the risk of cancer is low, an infectious aetiology is the most likely, and follow-up studies should be avoided.
- •
Nodules in patients with known primary tumours with a high risk of metastasis.
- •
Nodules in immunosuppressed patients with high risk of infection.
- •
Nodules found during lung cancer screening, for which specific guidelines already exist.
No routine follow-up is expected when the risk of cancer is less than 1% (solid nodules measuring less than 6mm).
Exceptionally, nodules that are clearly identified on a chest X-ray can be followed up with radiography, which is less costly and involves lower doses of radiation.
It is essential to review the patient's previous imaging studies. If these confirm that a solid nodule has remained unchanged for two years, or a subsolid nodule for five years, no further follow-up studies will be necessary.
Follow-up CT scans should be performed using the same technique as the baseline study in order to reduce the variability associated with the technique. The results of the current study should be compared with those of the previous study to estimate the growth interval, and also with the first study in which the nodule was detected. This will improve safety in estimating growth or long-term stability of the nodule.
These guidelines contain general suggestions for nodule management, and should never substitute individualised clinical management of each patient (Fig. 7).
Nodule measuring less than 6mm. Nodules measuring less than 6mm in low-risk patients do not need follow-up. The risk of cancer is less than 1% in smokers and even lower in low-risk patients. In the NELSON lung cancer screening programme, the risk of developing cancer was not greater in patients with a nodule volume of less than 100mm3 or measuring less than 5mm in diameter than in those with no nodules.26
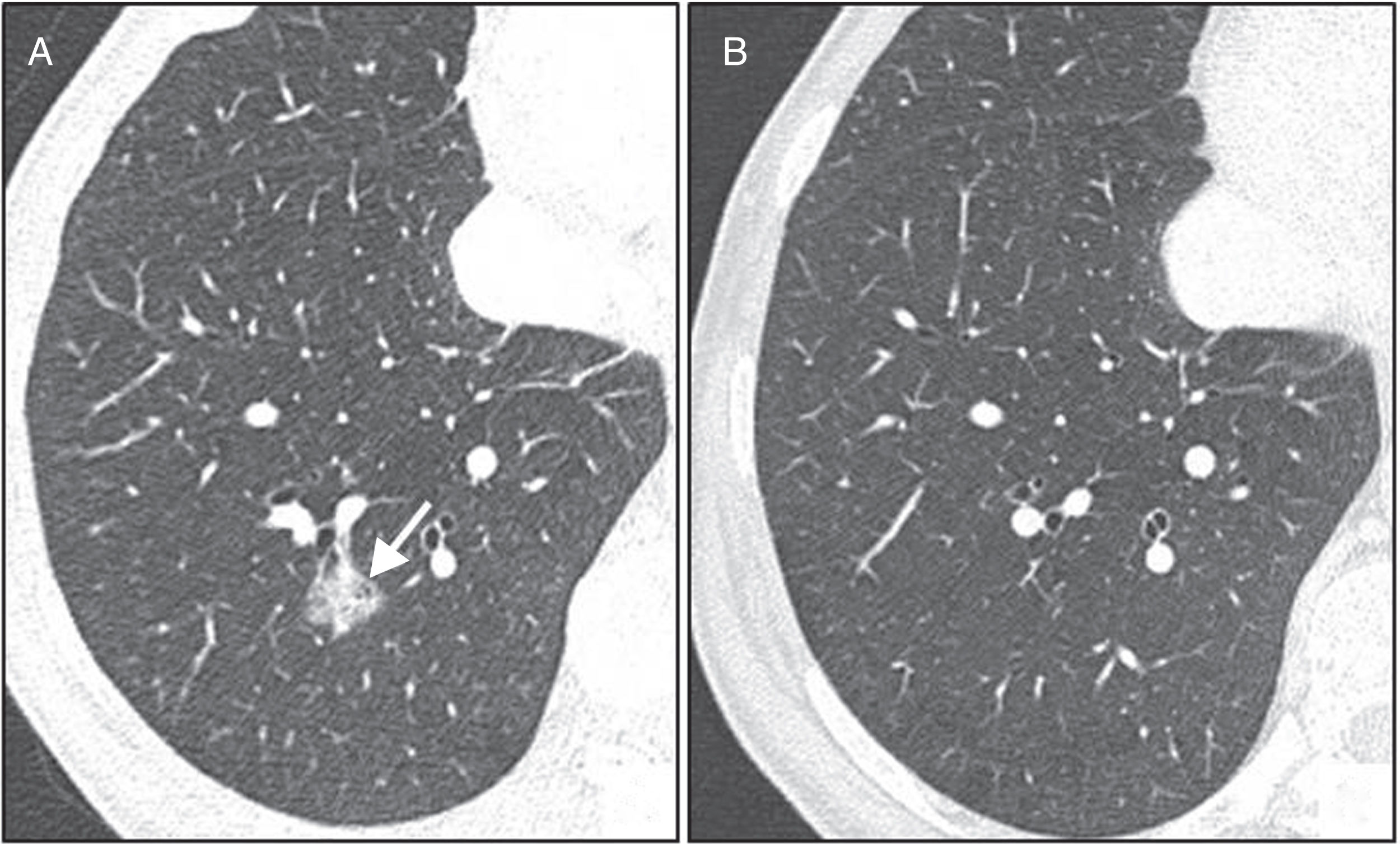
Twelve-month follow-up is only recommended in nodules with a suspicious morphology and/or nodules located in the upper lobes, which carry a 1% and 5% risk of cancer25 (Fig. 8). A shorter follow-up interval is not necessary because these nodes rarely change stage in a period of less than 12 months, and would only be necessary when the technical quality of the CT image is poor, or in patients in whom a long-term follow-up causes anxiety.
A 50-year-old woman with no risk factors. (A) Solid nodule measuring 4mm with slightly spiculated margins in left upper lobe (arrow). Given that the nodule is suspicious for malignancy, follow-up at 12 months is recommended. (B) On computed tomography at 12 months, the nodule measures 8mm (arrow). On this basis, biopsy is indicated, with the result of invasive adenocarcinoma.
Nodule measuring 6–8mm. In low-risk patients, follow-up at 6–12 months is recommended, depending on the morphology and size of the nodule, and the preferences of the patient. If the nodule has a benign morphology, is easy to measure, and unequivocally stable, there will be no need to complete a two-year follow-up schedule, a 12–18 month schedule will be adequate. If growth is unclear, or the morphology is suspicious of malignancy, a second follow-up should be performed at 18–24 months.
In high-risk patients, where the probability of malignancy is 0.5%-2%, an initial follow-up at 6–12 months is recommended, with a second control scan at 18–24 months. Two scans are sufficient in most patients; but, when growth is uncertain, additional scans can be performed.
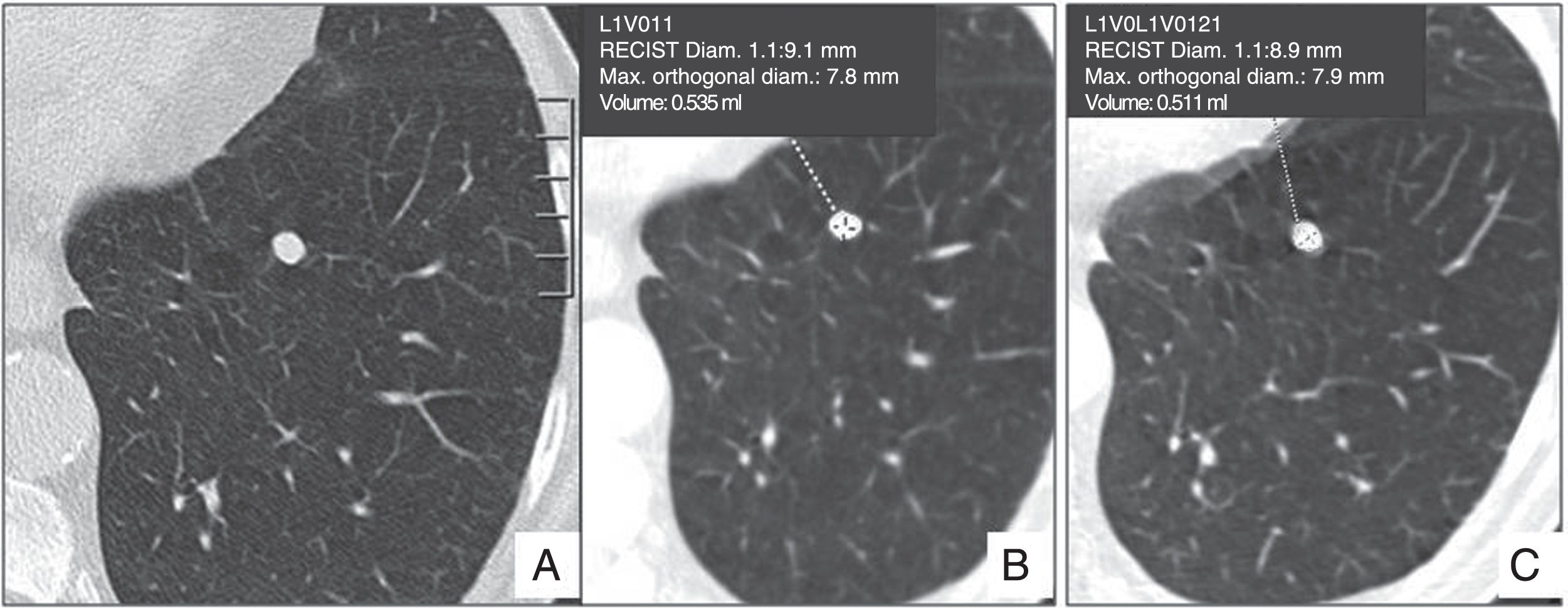
Nodule measuring over 8mm. The risk of malignancy is around 3%, depending on the morphology and location. The following options are available: follow-up CT at three months, positron emission tomography-computed tomography (PET-CT) or biopsy, based on preferences, individual risk, morphology, nodule location, comorbidities, etc. (Fig. 9).
73-year-old male, smoker. (A and B) Emphysema in right lower lobe and solid nodule measuring 8mm, volume 535mm3, with smooth margins. This is a high-risk patient, but the morphology and location of the nodule are not suspicious for malignancy, so a computed tomography follow-up at 3 months is recommended (C), that shows no changes in growth. Periodic follow-ups should be performed for 2 years, until stability is confirmed.
Nonsolid nodule measuring less than 6mm. Follow-up is not recommended. In certain cases with a nodule measuring close to 6mm with a suspicious morphology or with some other risk factor, follow-up CT at two and four years can be considered.
Nonsolid nodule equal to or greater than 6mm. A preliminary follow-up is recommended at 6–12 months, and then every two years for up to five years (Fig. 10).
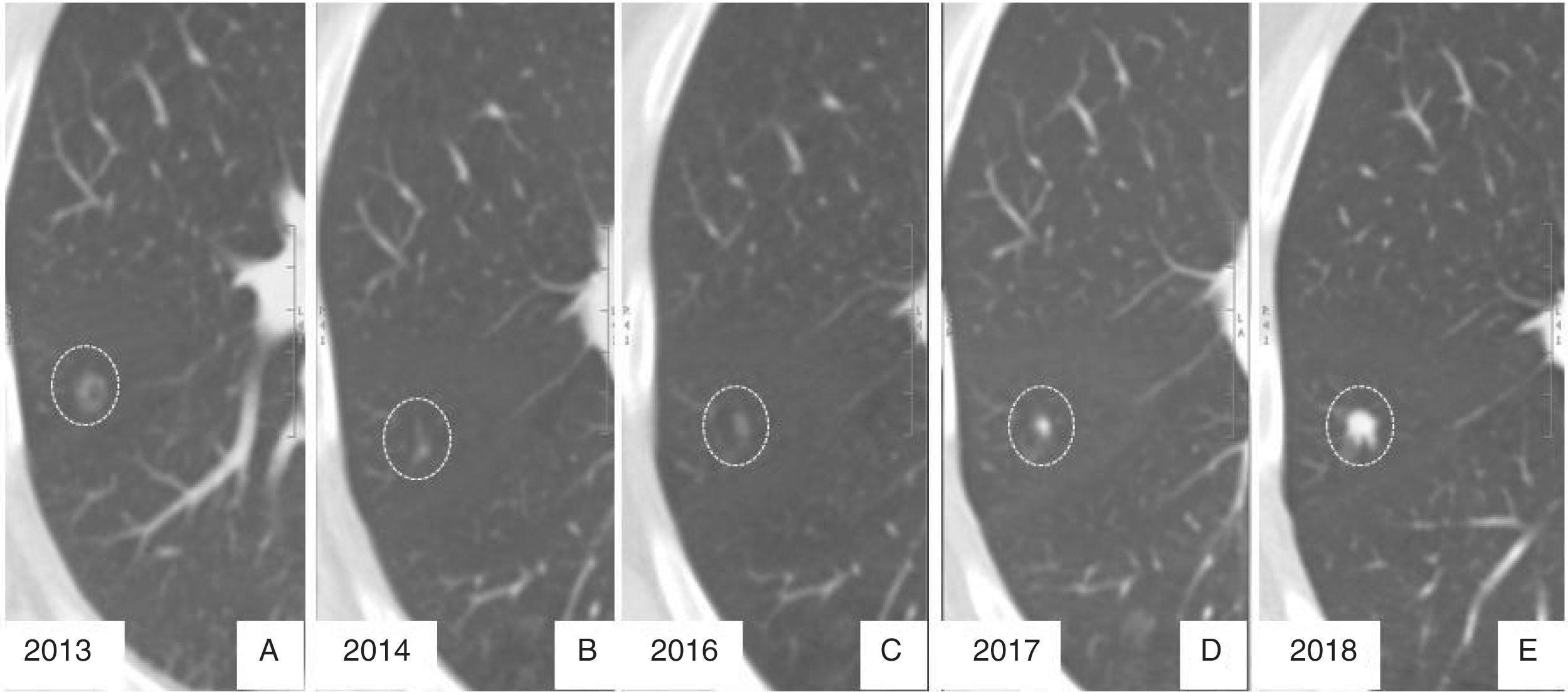
65-year-old male, smoker of 30 pack-years. (A) Nonsolid nodule in left upper lobe. (B and C) At the 12 and 24 month follow-ups, respectively, the nodule shows slow, progressive growth, in addition to the appearance of a bronchogram inside it. Surgical resection is indicated, and the pathological diagnosis is non-invasive adenocarcinoma.
Nonsolid nodules are slow-growing lesions in which earlier follow-up does not affect the final outcome. Follow-up should be performed at six months when there are findings suggestive of malignancy, particularly size greater than 10mm and small bubbles inside, or if a longer interval will cause anxiety in the patient, since these nodules will sometimes disappear.
Solitary part-solid pulmonary noduleNodule measuring less than 6mm. Follow-up is not recommended. In nodules of this size, the presence of a solid component is very difficult to establish reliably, so they are managed in the same way as nonsolid nodules.
Nodule equal to or greater than 6mm. This can be secondary to an infection, and will resolve in the short term; therefore, a preliminary follow-up must always be performed after three to six months to confirm persistence39 (Fig. 11). If the nodule persists, it should be managed according to the size of the solid component.
If the solid component measures less than 6mm. There is a high probability of adenocarcinoma, but most are in situ or minimally invasive adenocarcinomas. Follow-up CT scan is recommended every year for five years.
If the solid component is equal to or greater than 6mm. The probability of invasion and metastasis increases with the size of the solid component. A solid component measuring more than 5mm is associated with a high probability of local invasion, and this parameter is included in the new T staging of lung adenocarcinoma.40 In nodules with a suspicious morphology, such as lobulated margins, with a solid component larger than 8mm, cystic component, or growth of the solid component, surgical resection, biopsy or PET-CT should be considered. However, it must be borne in mind that the yield of PET-CT and biopsy to differentiate benignity from malignancy in these types of nodules is low, so more radical options, such as surgery9, can be considered (Fig. 12).
A 57-year-old man in annual follow-up due to a nonsolid lesion in the anterior segment of the left upper lobe (A). (B and C) Growth is dubious in the 16- and 28-month follow-up scans. (D) In the 3-year follow-up, solid nodules (arrows) appear, so surgical resection is indicated, which gives a diagnosis of non-invasive papillary adenocarcinoma.
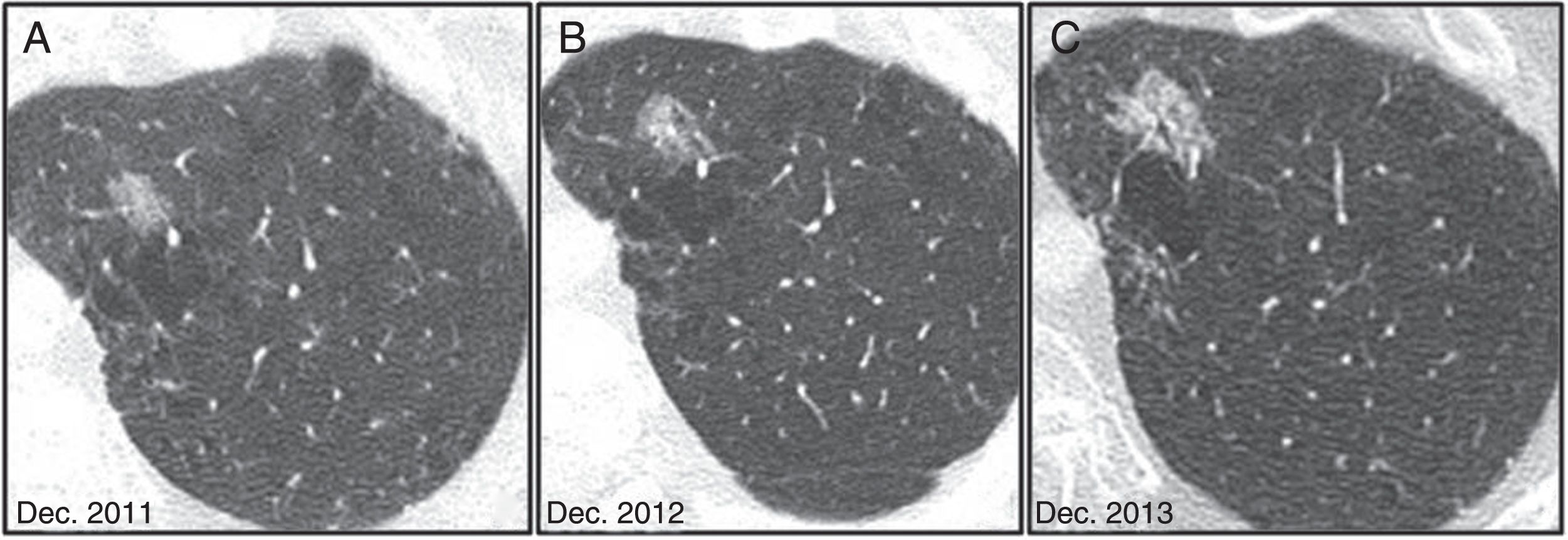
It is important to bear in mind that some malignant nodules will initially shrink in size as they increase in density. This is most frequently observed in subsolid nodules, and should not mislead the clinician into considering the nodule is benign9 (Fig. 13).
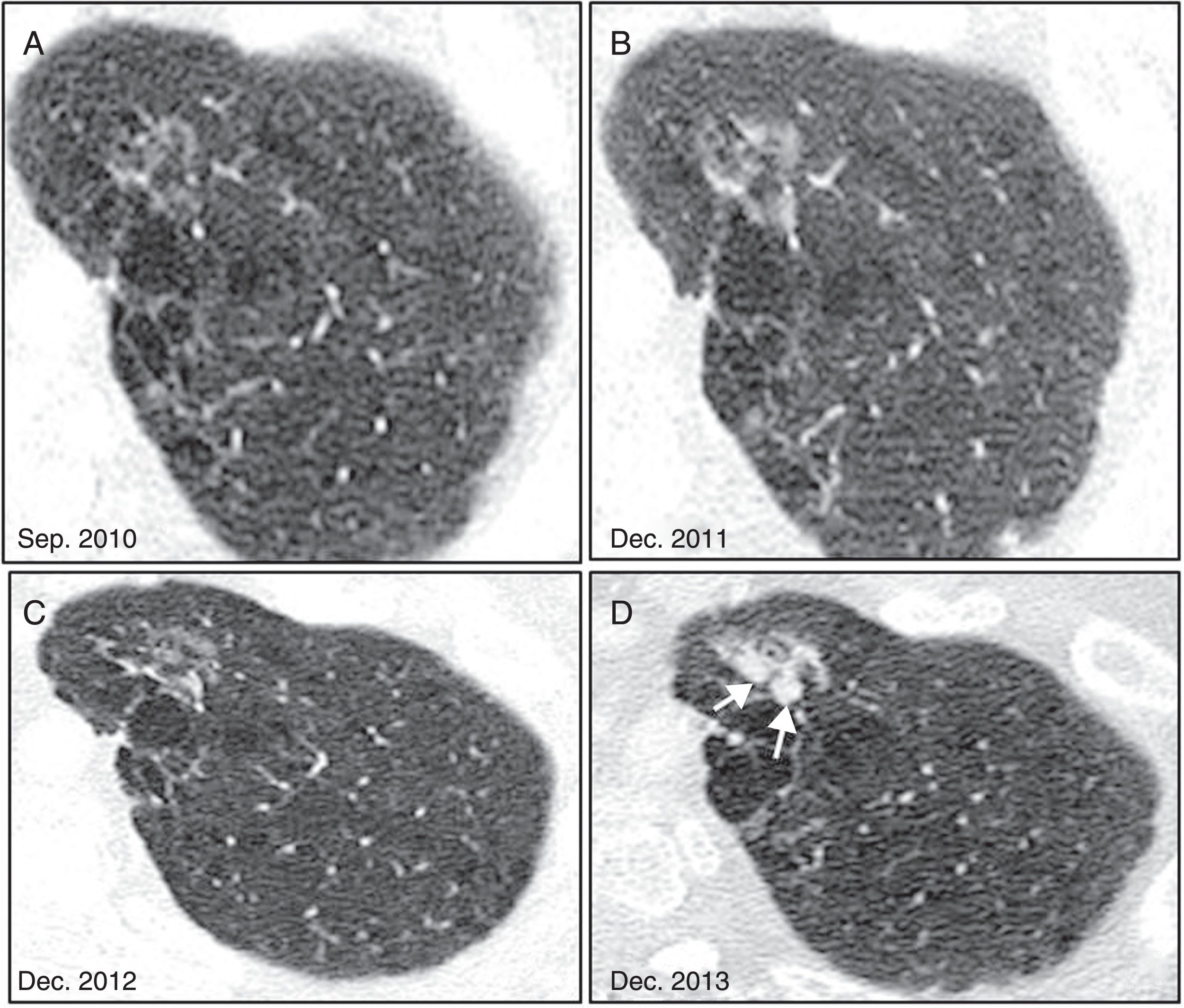
Radiological evolution of invasive adenocarcinoma. (A) Nonsolid nodule in right upper lobe, detected incidentally in 2013. (B) In the 12-month follow-up, the nodule appears to shrink. (C) In a new CT scan performed at 3 years, growth is dubious, so follow-ups are performed at 4 and 5 years (D and E), which confirm clear growth pattern with an increase in density.
Nodule measuring less than 6mm. These are most frequently caused by granulomas secondary to infection or benign intrapulmonary ganglia. They do not require follow-up in low-risk patients, and in high-risk patients 12-month follow-up can be considered.
When there is at least one nodule greater than or equal to 6mm. Follow-up at 3–6 months is recommended, and a further scan at 18–24 months can be considered, depending on the estimated risk.
In these cases, the most probable aetiology is metastasis, especially when located in the lower lobes and in the periphery of the lung, or when size varies greatly. The growth of metastases is usually noticeable within three months.
The nodule with the most suspicious morphology, although not necessarily the largest, is called the dominant nodule, in other words, the most suspicious for malignancy. This nodule is taken as a reference, and management guidelines for solitary pulmonary nodules should be followed, although the growth of the remaining nodules must also be monitored.
Multiple subsolid nodulesNodule measuring less than 6mm. Infection is the most likely cause, so a preliminary follow-up at three to six months is recommended to confirm persistence. If the nodules persist, a follow-up scan should be performed at two and four years to confirm stability, since there is a possibility that they may be secondary to multiple atypical adenomatous hyperplasia and/or adenocarcinomas in situ.
Nodules greater than or equal to 6mm. When there is at least one nodule greater than or equal to 6mm, follow-up should be based on the most suspicious lesion. Follow-up CT should be performed at three to six months to rule out the possibility of the nodules being infection-related, and, if they persist, multiple primary adenocarcinomas should be considered. The presence of more than one suspicious nodule increases the likelihood of malignancy with respect to a solitary nodule.
Guidelines for the management of nodules detected in studies other than chest CTNodules can be detected incidentally in neck, abdomen or cardiac CT scans:
- 1.
Nodule measuring less than 6mm. Do nothing.
- 2.
Nodule measuring 6-8mm. Follow-up chest CT within 3–12 months, depending on the morphology and clinical risk.
- 3.
Nodules greater than 8mm or with high suspicion of malignancy. Complete the study with chest CT.
Partial CT studies limited only to the nodule are not recommended for follow-up.
In conclusion, lung nodules detected incidentally should be studied with an appropriate CT technique, correctly measured and classified as solid, nonsolid and part-solid. Before deciding how to manage a nodule, it is important to classify the patient as low-, intermediate- or high-risk before following the recommended management guidelines. Nodules with calcium deposits, fat, or with a morphology typical of perifissural or intrapulmonary ganglia are considered benign and do not require follow up. Periodic follow-ups should be performed to confirm stability for two years in the case of solid nodules and for five years in the case of subsolid nodules.
Authorship- 1.
Responsible for the integrity of the study: CTL.
- 2.
Study conception: CTL.
- 3.
Study design: CTL, CDSG.
- 4.
Data collection: CTL, CDSG, ASV.
- 5.
Data analysis and interpretation: CTL, CDSG, EUP, CJB.
- 6.
Statistical processing: not applicable.
- 7.
Literature search: CTL, CDSG, EUP, CJB, ASV.
- 8.
Drafting of the article: CTL.
- 9.
Critical review of the manuscript with intellectually significant contributions: CTL, CDSG, EUP, CJB.
- 10.
Approval of final version: CTL, CDSG, EUP, CJB, ASV.
The authors declare that they have no conflicts of interest.
Please cite this article as: Trinidad López C, Delgado Sánchez-Gracián C, Utrera Pérez E, Jurado Basildo C, Sepúlveda Villegas CA. Nódulo pulmonar incidental: caracterización y manejo. Radiología. 2019. https://doi.org/10.1016/j.rx.2019.03.002