To describe the magnetic resonance imaging (MRI) findings for the most common inflammatory and immune-mediated diseases that involve the brainstem.
ConclusionInflammatory lesions involving the brainstem are associated with a wide range of autoimmune, infectious, and paraneoplastic syndromes, making the differential diagnosis complex. Being familiar with these entities, their clinical characteristics, and their manifestations on MRI, especially the number of lesions, their shape and extension, and their appearance in different sequences, is useful for orienting the radiological diagnosis.
Describir los hallazgos en Resonancia Magnética (RM) de las principales enfermedades inflamatorias e inmunomediadas que afectan al troncoencéfalo.
ConclusiónEl diagnóstico diferencial de las lesiones inflamatorias localizadas en el troncoencéfalo es complicado debido al amplio espectro de enfermedades autoinmunes, infecciosas y síndromes paraneoplásicos que pueden causarlas. Conocer estas entidades, sus características clínicas y manifestaciones en RM, sobre todo en cuanto a número, morfología, extensión y apariencia en las diferentes secuencias, es útil a la hora de orientar el diagnóstico radiológico.
Inflammatory lesions of the brainstem develop as part of many diseases, mainly those of immune-mediated or autoimmune aetiology. These lesions share a common appearance on magnetic resonance imaging (MRI): they are hyperintense on T2-weighted sequences and have patchy contrast enhancement on gadolinium-enhanced T1-weighted sequences. In some cases, they show diffusion restriction.
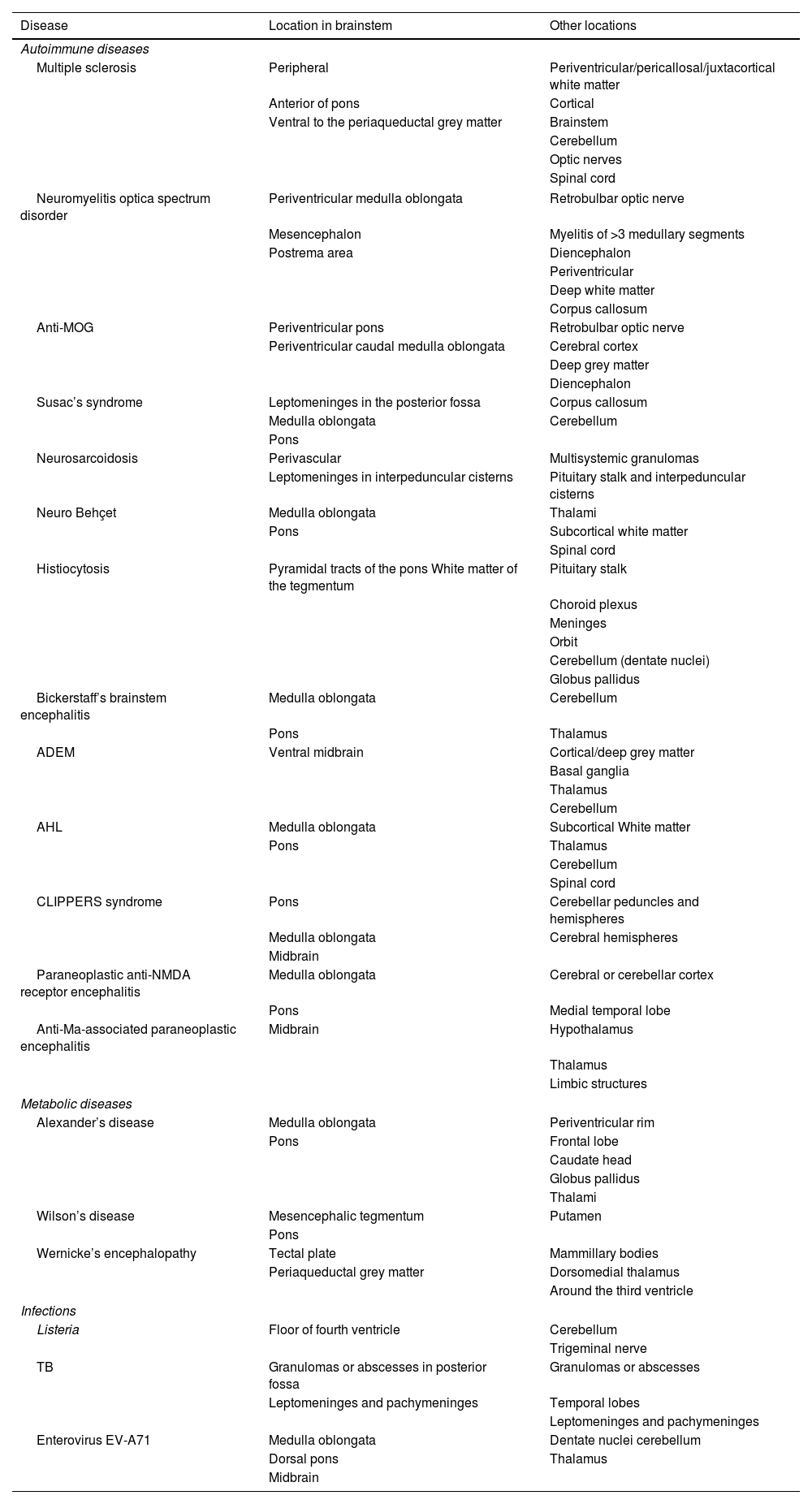
The characteristics that enable a diagnosis to be made are the morphology, number and distribution of lesions, in addition to their location within the brainstem and the presence of other associated lesions, both in the central nervous system (CNS) and in other organs (Table 1). Clinical, epidemiological and analytical findings are useful when establishing the diagnosis.
Overview of location of inflammatory lesions of each entity.
| Disease | Location in brainstem | Other locations |
|---|---|---|
| Autoimmune diseases | ||
| Multiple sclerosis | Peripheral | Periventricular/pericallosal/juxtacortical white matter |
| Anterior of pons | Cortical | |
| Ventral to the periaqueductal grey matter | Brainstem | |
| Cerebellum | ||
| Optic nerves | ||
| Spinal cord | ||
| Neuromyelitis optica spectrum disorder | Periventricular medulla oblongata | Retrobulbar optic nerve |
| Mesencephalon | Myelitis of >3 medullary segments | |
| Postrema area | Diencephalon | |
| Periventricular | ||
| Deep white matter | ||
| Corpus callosum | ||
| Anti-MOG | Periventricular pons | Retrobulbar optic nerve |
| Periventricular caudal medulla oblongata | Cerebral cortex | |
| Deep grey matter | ||
| Diencephalon | ||
| Susac’s syndrome | Leptomeninges in the posterior fossa | Corpus callosum |
| Medulla oblongata | Cerebellum | |
| Pons | ||
| Neurosarcoidosis | Perivascular | Multisystemic granulomas |
| Leptomeninges in interpeduncular cisterns | Pituitary stalk and interpeduncular cisterns | |
| Neuro Behçet | Medulla oblongata | Thalami |
| Pons | Subcortical white matter | |
| Spinal cord | ||
| Histiocytosis | Pyramidal tracts of the pons White matter of the tegmentum | Pituitary stalk |
| Choroid plexus | ||
| Meninges | ||
| Orbit | ||
| Cerebellum (dentate nuclei) | ||
| Globus pallidus | ||
| Bickerstaff’s brainstem encephalitis | Medulla oblongata | Cerebellum |
| Pons | Thalamus | |
| ADEM | Ventral midbrain | Cortical/deep grey matter |
| Basal ganglia | ||
| Thalamus | ||
| Cerebellum | ||
| AHL | Medulla oblongata | Subcortical White matter |
| Pons | Thalamus | |
| Cerebellum | ||
| Spinal cord | ||
| CLIPPERS syndrome | Pons | Cerebellar peduncles and hemispheres |
| Medulla oblongata | Cerebral hemispheres | |
| Midbrain | ||
| Paraneoplastic anti-NMDA receptor encephalitis | Medulla oblongata | Cerebral or cerebellar cortex |
| Pons | Medial temporal lobe | |
| Anti-Ma-associated paraneoplastic encephalitis | Midbrain | Hypothalamus |
| Thalamus | ||
| Limbic structures | ||
| Metabolic diseases | ||
| Alexander’s disease | Medulla oblongata | Periventricular rim |
| Pons | Frontal lobe | |
| Caudate head | ||
| Globus pallidus | ||
| Thalami | ||
| Wilson’s disease | Mesencephalic tegmentum | Putamen |
| Pons | ||
| Wernicke’s encephalopathy | Tectal plate | Mammillary bodies |
| Periaqueductal grey matter | Dorsomedial thalamus | |
| Around the third ventricle | ||
| Infections | ||
| Listeria | Floor of fourth ventricle | Cerebellum |
| Trigeminal nerve | ||
| TB | Granulomas or abscesses in posterior fossa | Granulomas or abscesses |
| Leptomeninges and pachymeninges | Temporal lobes | |
| Leptomeninges and pachymeninges | ||
| Enterovirus EV-A71 | Medulla oblongata | Dentate nuclei cerebellum |
| Dorsal pons | Thalamus | |
| Midbrain | ||
Anti-MOG: demyelination caused by anti-myelin oligodendrocyte glycoprotein antibodies; ADEM: acute disseminated encephalomyelitis; AHL: acute haemorrhagic leukoencephalitis; TB: tuberculosis.
The aim of this article is to provide an overview of entities that can cause inflammatory lesions of the brainstem, dividing them into three groups: autoimmune, infectious and metabolic.
Auto-immune diseasesMultiple sclerosisMultiple sclerosis (MS) is an acquired inflammatory disease of the CNS that begins between adolescence and the sixth decade, with a higher incidence among women and in countries far from the equator.
According to the McDonald criteria, diagnosis is possible by MRI if dissemination in time and space can be demonstrated for the lesions.1 The lesions are ovoid, circumscribed and hyperintense on T2. They are perivenular, with a hypointense central line or dot (central vein sign) visible on the T2* sequence, which can help differentiate them from other inflammatory diseases.2 Chronic lesions appear as hypointense black holes on T1, while acute lesions reflect gadolinium uptake and may restrict diffusion.1
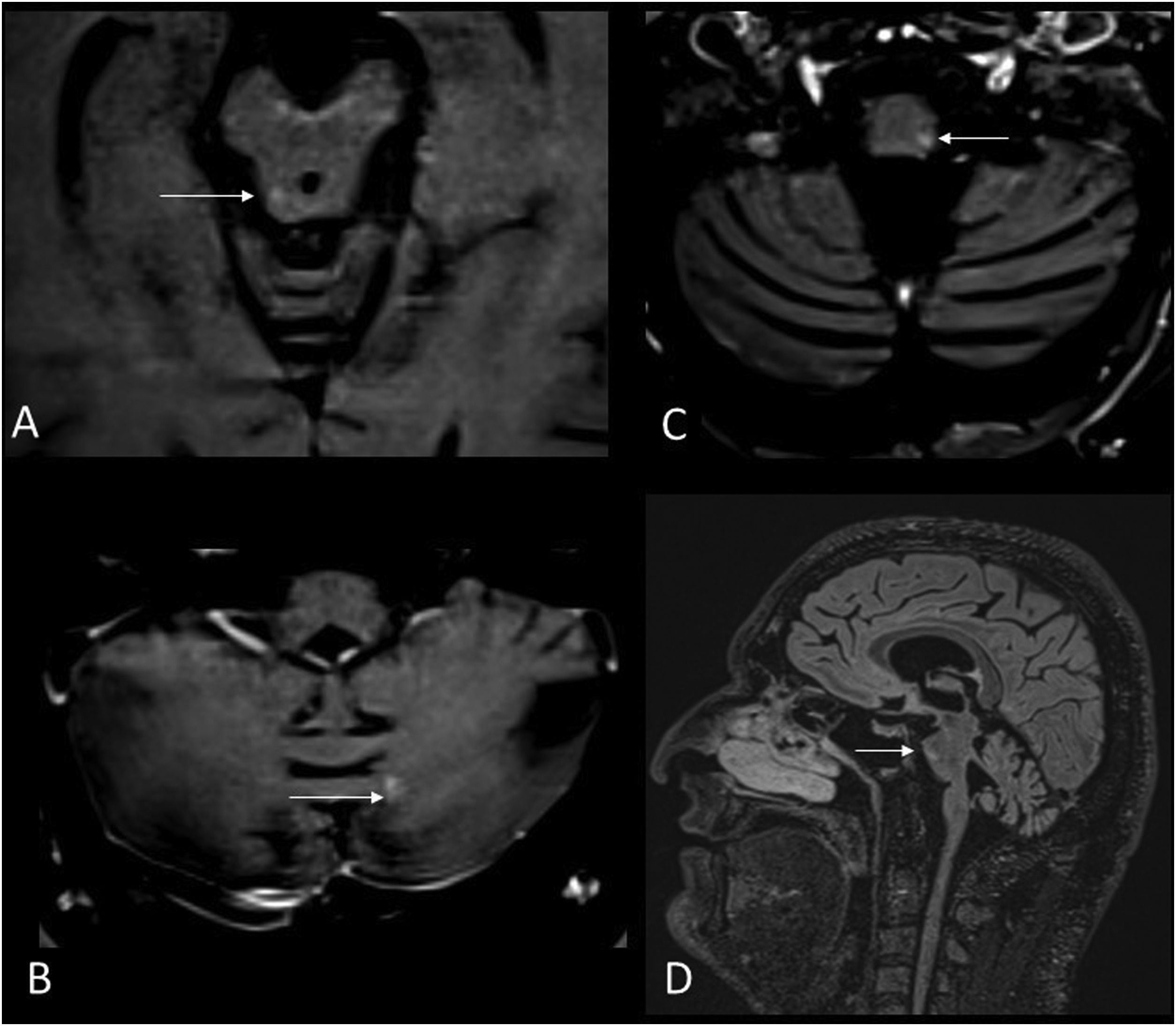
Typical locations are the periventricular, pericallosal, cortical and juxtacortical white matter, the brainstem and cerebellum, the optic nerves and the spinal cord. Brainstem lesions are peripheral, and are more often found in the pons than in the medulla oblongata. Typical locations include the anterior part of the pons, the trigeminal input zone, or ventral to the periaqueductal grey matter (Fig. 1).
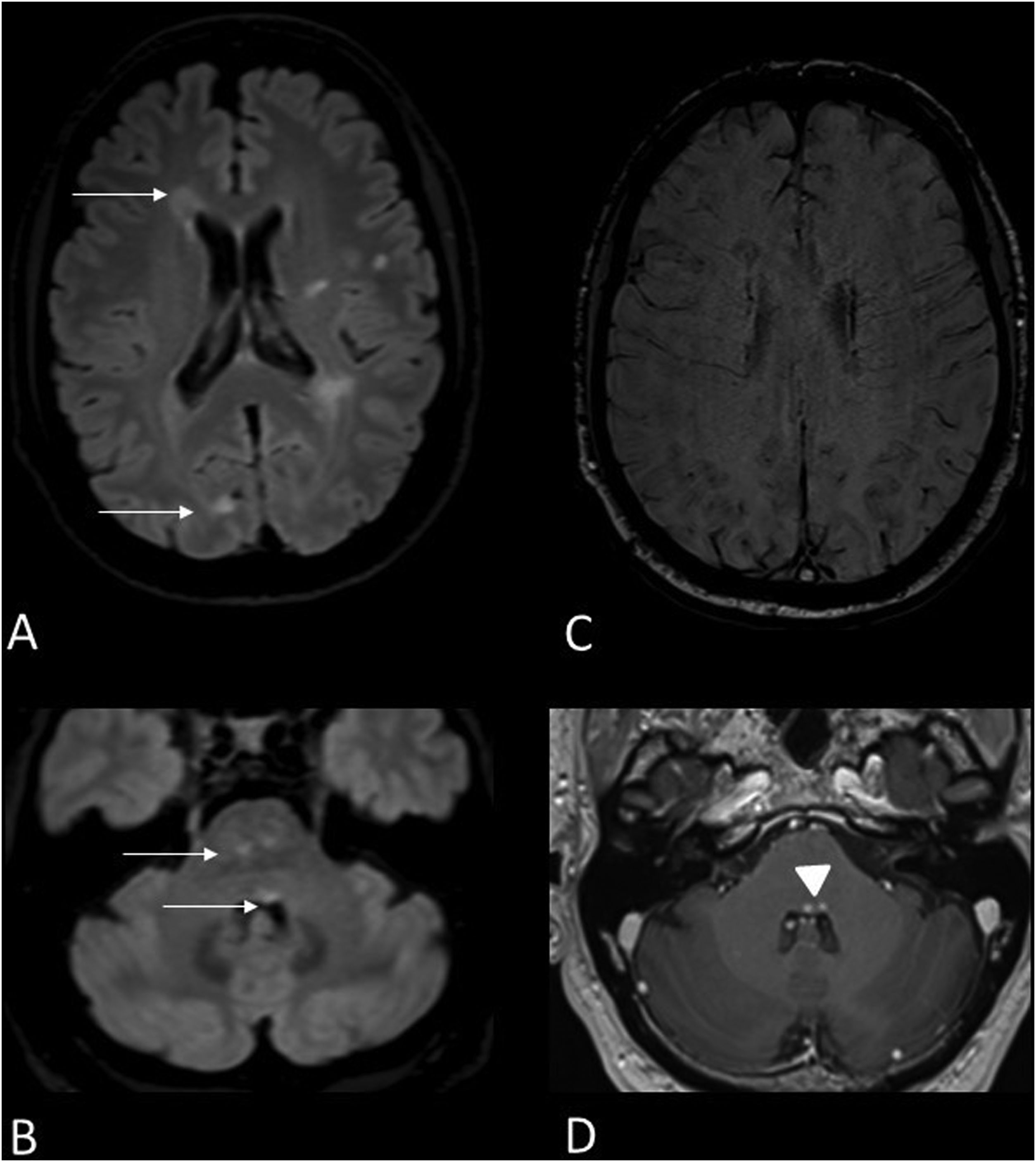
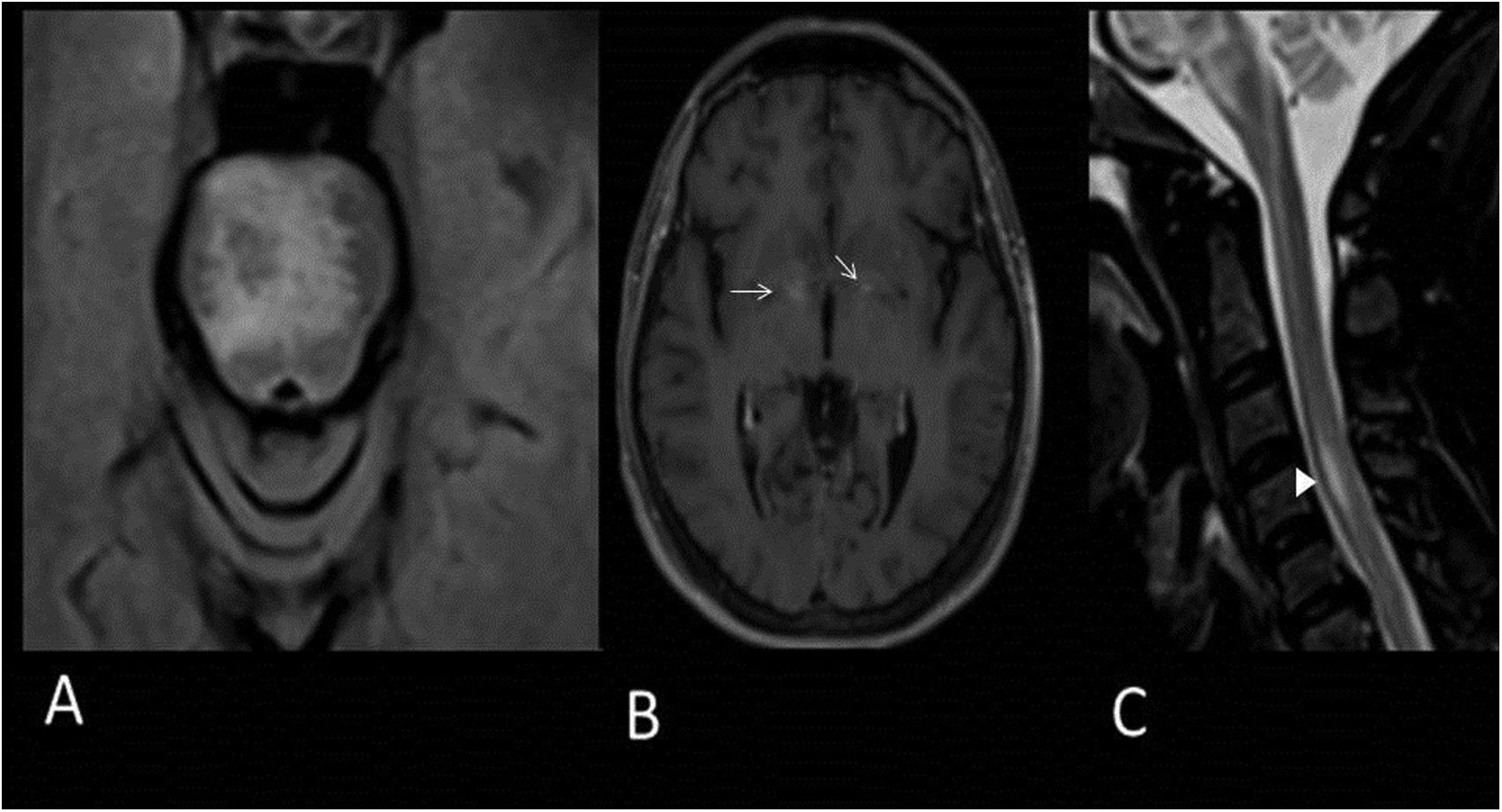
50-year-old male with MS presenting with internuclear ophthalmoplegia. MRI images of brain: Axial T2-FLAIR (A,B), magnetic susceptibility-weighted (C) and gadolinium-enhanced T1 (D). Hyperintense demyelinating lesions on T2-FLAIR sequence located in the periventricular white matter, juxtacortical in the right occipital lobe (A), central in the midbrain and on the floor of the fourth ventricle (B) (arrows). A central vein sign reveals a right frontal periventricular lesion (C). The periventricular lesions of the fourth ventricle also show contrast enhancement (D) (arrowhead).
The presence of oligoclonal bands in the cerebrospinal fluid (CSF) supports the diagnosis.1 If pleocytosis is present, it is mononuclear and less than 50 cells/μl.
Neuromyelitis optica spectrum disorderNeuromyelitis optica spectrum disorder is an autoimmune demyelinating astrocytopathy of the CNS. Anti-quaporin 4 IgG (AQP4-IgG) antibodies are characteristic.3 The average age at symptom onset is 39, although it can occur in children and the elderly, and is nine times more frequent in women.4
Optic neuritis manifests as a thickening and hyperintensity of a long segment of the retrobulbar optic nerve; it may be unilateral or bilateral. It also presents as longitudinally extensive myelitis, involving ≥ 3 medullary segments.3 Encephalic lesions occur in periependymal regions, deep white matter, in the pericallosal region, in the cerebellum, in the diencephalon, and in the brainstem, more specifically the medulla oblongata around the fourth ventricle.3 Brainstem lesions are linear and hyperintense on T2 and may merge with cervical lesions.5
Diencephalic lesions are located around the third ventricle and in the mesencephalon and usually have patchy contrast uptake (Fig. 2).
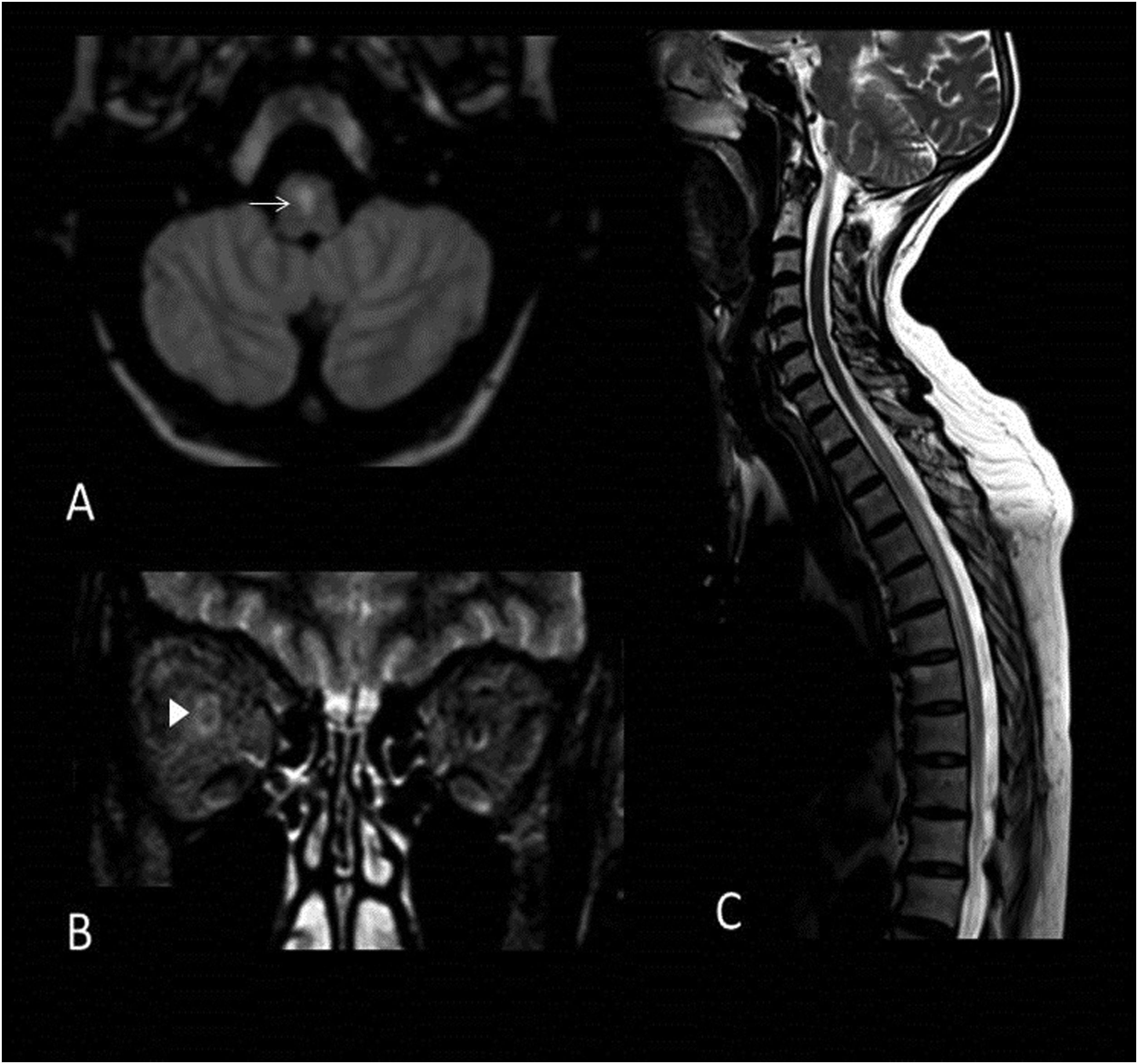
49-year-old woman with suspected myelitis, decreased visual acuity of the right eye and AQP4-IgG antibodies. MRI images of brain and cervicodorsal spine: Axial T2-FLAIR (A), coronal MRI (B) and sagittal T2 (C). Hyperintense lesion on T2-weighted sequences in central location of the medulla oblongata (arrow) (A). Extensive right retrobulbar optic neuritis (arrowhead) (B). Dorsal medullary lesion longitudinally extensive and hyperintense on T2 sequence that extends between levels D1 and D7 (C).
Up to 42% of patients with neuromyelitis optica spectrum disorder who are seronegative for AQP4-IgG have anti-MOG antibodies.6 This principally affects children and young adults with bilateral optic neuritis or a simultaneous presentation of myelitis and monophasic/chronic relapsing optic neuritis.
Lesions are found in the cortex and deep grey matter, subcortical and deep white matter, retrobulbar optic nerve and diencephalon, as well as in the periventricular white matter of the fourth ventricle, in the pons and in the caudal medulla oblongata. The lesions are hyperintense, large and oedematous on T2 sequences (Fig. 3).
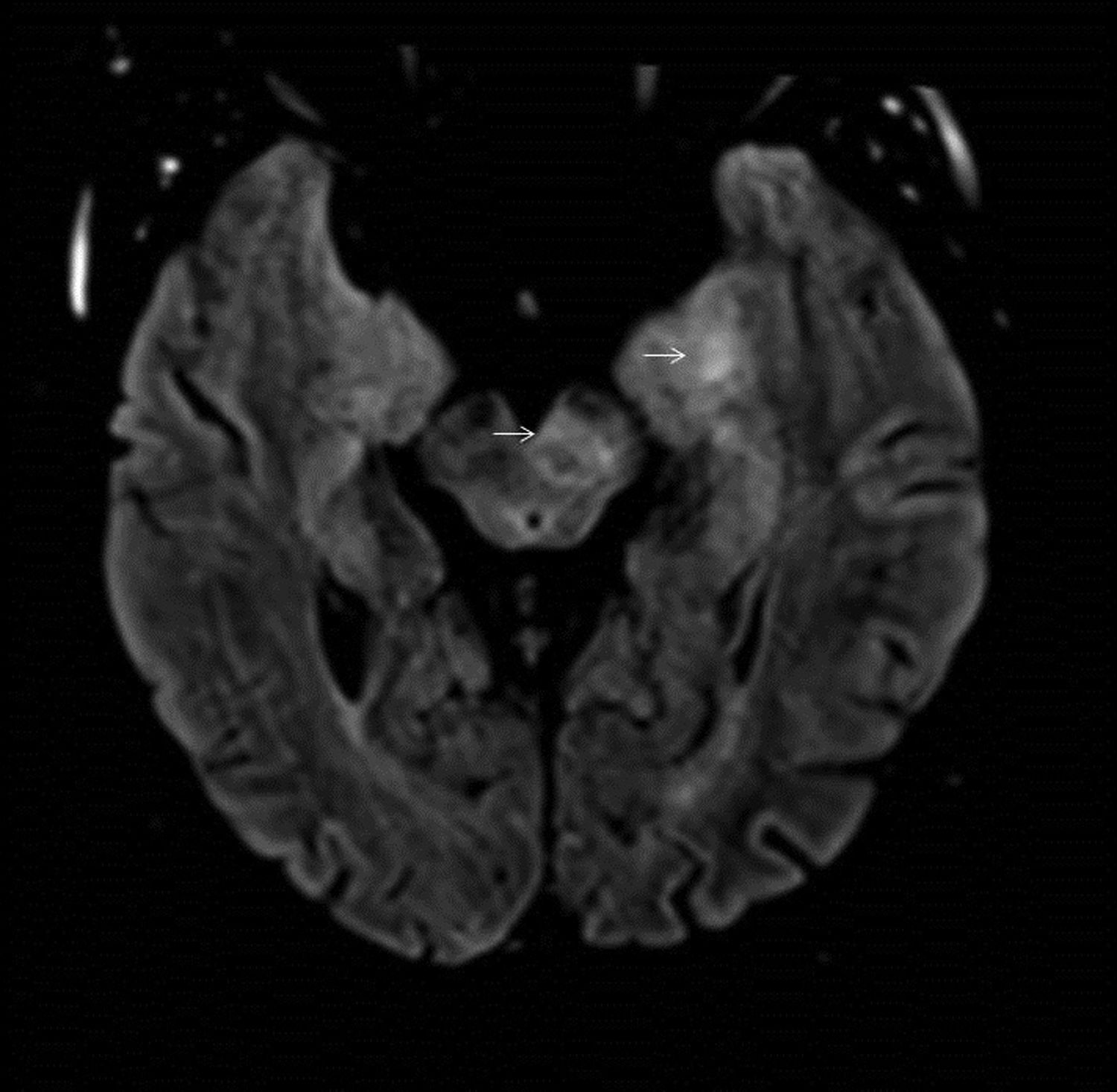
33-year-old male with suspected autoimmune encephalitis and the presence of anti-MOG antibodies. Images of MRI of brain: Axial T2-FLAIR (A,C,D) and T1 black-blood with gadolinium (B). Cortical hyperintensity in left parietal and temporal lobes (A) with areas of patchy enhancement after contrast administration (B). Extensive hyperintense inflammatory lesion on T2-weighted sequences located on the left side of the midbrain (arrow) (C) extending caudally to the ipsilateral pons and the periventricular white matter of the fourth ventricle (arrowhead) (D).
It is important to note the following points regarding brainstem lesions and the main immune-mediated diseases:
- □
In MS they are peripheral and mostly appear in the pons.
- □
In neuromyelitis optica they are periventricular and appear mainly in the medulla oblongata.
- □
In demyelination caused by anti-MOG antibodies, they are periventricular, large and oedematous.
Susac’s syndrome is a type of arteriolar endotheliopathy that causes the clinical triad of encephalopathy, central retinal artery occlusion and low-frequency sensory hypoacusis.7 It most commonly affects women aged between 20 and 40 who, in cases involving encephalopathy, often suffer from memory and behavioural disturbances, confusion, ataxia, dysarthria, paranoid psychosis, headache and occasional mutism.8 CSF levels reveal protein elevation (>1.5 g/l), mild pleocytosis and no oligoclonal bands.8
Hyperintense multifocal lesions of 3–7 mm are seen on MRI using the T2-weighted sequence. They invariably affect the corpus callosum. They are described as “snowball-like” when round and centrally located in the corpus callosum, and “icicle-like” when wedge-shaped and extending from the roof of the corpus callosum without making contact with its base.9
In the acute phase, enhancing lesions and diffusion-restricting microinfarcts appear. In fulminant cases, nodular leptomeningeal uptake appears in the posterior fossa.9,10
Thirty percent of patients have punctate lesions in the cerebellar hemispheres and brainstem10 similar to those associated with CLIPPERS, but with a lower uptake of gadolinium.
NeurosarcoidosisSarcoidosis is a systemic granulomatous disease that rarely affects the CNS. When it does, it causes clinical brainstem and cerebellar syndromes.11 The appearance of granulomatous lesions in other systems on CT scans is suggestive of the diagnosis.
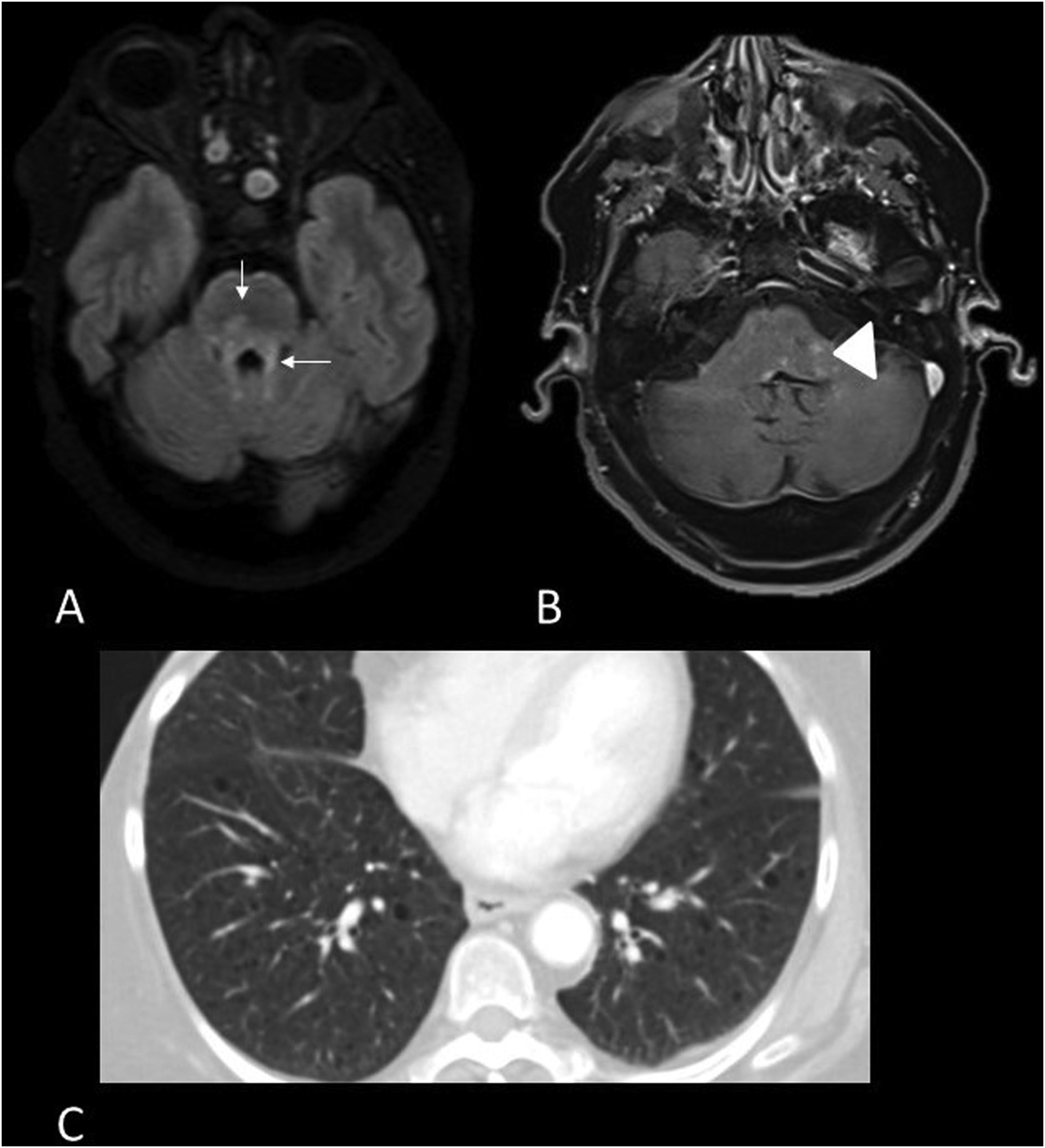
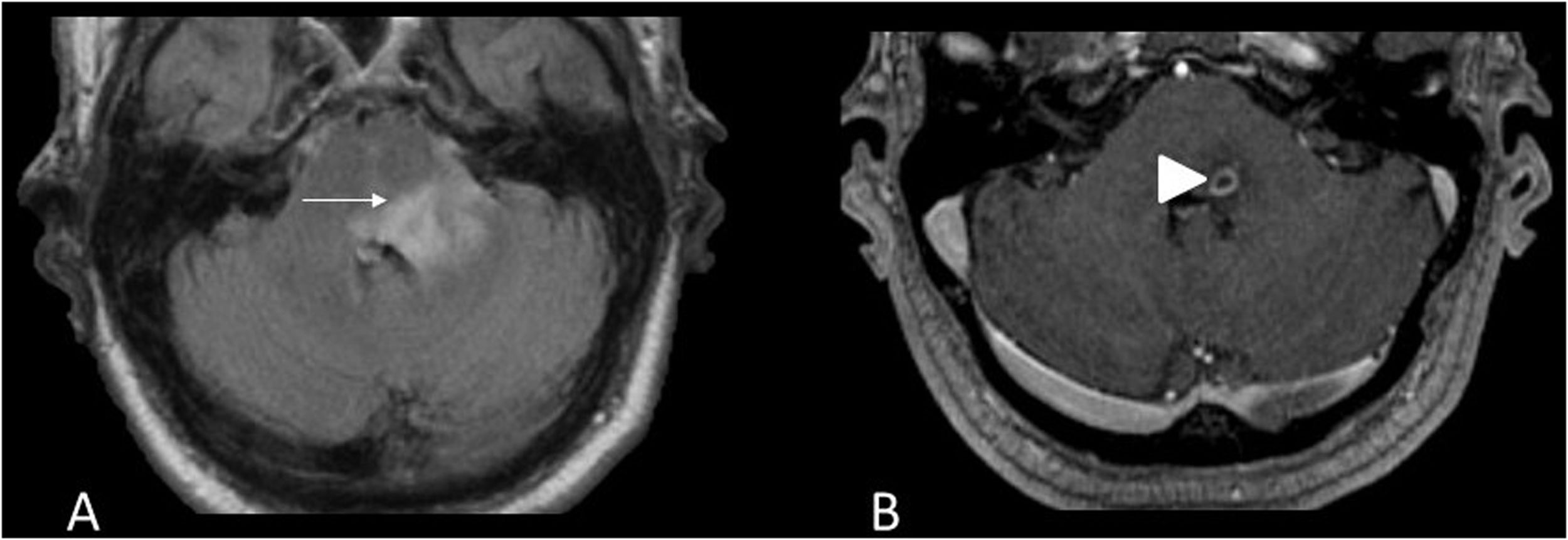
Imaging shows nodular, diffuse or perivascular leptomeningeal contrast enhancement, mainly in the pituitary stalk and interpeduncular cisterns.12 The brainstem lesions are hyperintense on T2, with no specific morphology. Granulomas are also present and form true sarcoid masses, especially in the posterior fossa that are hypointense on T2 and have discontinuous contrast uptake (Fig. 4). Due to meningeal inflammation and masses that may obstruct CSF drainage, and hydrocephalus may be present.
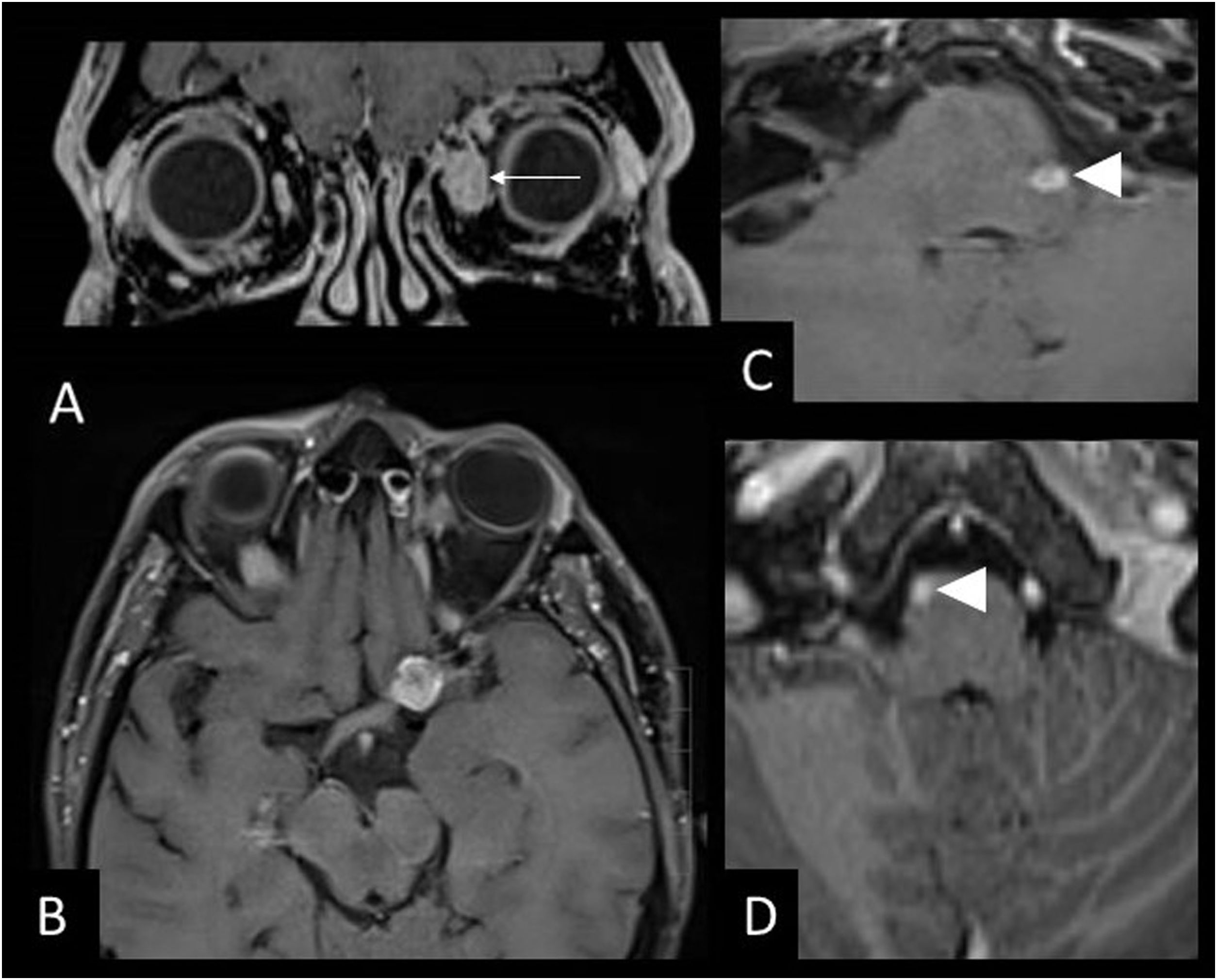
53-year-old male with sarcoidosis, left eye pain and headache. MRI images of the brain: Coronal T1 with gadolinium (A), axial T1 black-blood with gadolinium (B,C) and axial T1 with gadolinium (D). Sarcoid masses are hyperenhancing in the belly of the left medial rectus muscle (arrow) (A) and in the left supraclinoid leptomeninges (B) and bilateral anterior infratentorial masses in the pons (C,D) (arrowheads).
Neuro-Behçet’s disease is a multisystemic vasculitis affecting small vessels.11 The brainstem symptoms may coexist with hemispheric or spinal cord symptoms. It is often associated with oral and genital ulcers, skin changes and fever.
The lesions are oedematous, hyperintense on T2, with microhaemorrhages only visible on susceptibility weighted imaging, and acute contrast-enhancing lesions. The brainstem is the most commonly affected region. Other common locations are the basal ganglia, thalami and, less frequently, the subcortical white matter and the spinal cord (Fig. 5).
42-year-old male with Behçet's disease who is beginning to show cerebellar signs and marked pyramidalism. MRI images of the brain: Axial T1-weighted with gadolinium (A,B,C) and sagittal T2-FLAIR (D). Small hyperintense lesions exhibiting contrast enhancement in the midbrain (A), left cerebellar hemisphere (B) and medulla oblongata (C,D) (arrows) and infratentorial atrophy.
Venous thrombosis and optic neuritis are frequently associated. Black holes and infratentorial atrophy develop in chronic disease.13
HistiocytosisLangerhans cell histiocytosis usually affects children11 while Erdheim-Chester disease is a rare form of histiocytosis that affects adults.14 Symptoms include bone pain and skin and visceral changes. Neurological symptoms include diabetes insipidus, cognitive impairment, dysarthria, dysphagia and gait disturbances.11
Space-occupying lesions with intense contrast enhancement appear in the pituitary stalk, choroid plexus, meninges, brainstem and orbit. There are also lesions that appear hyperintense and symmetrical on T2, progressively appearing in the cerebellum, globus pallidus and pons.
Brainstem lesions are less common and usually occur in the pyramidal tracts of the pons and in the white matter of the tegmentum associated with lesions in the dentate nuclei. The lesions are hyperintense on T2 and have a mottled appearance, extending to the cerebellar peduncles.15 Acute lesions may retain contrast for weeks (Fig. 6).
67-year-old woman with Langerhans cell histiocytosis and subacute cerebellar involvement. MRI images of brain: Axial T2-FLAIR (A) and axial gadolinium T1 (B) and thoracic CT (C). Hyperintense patchy lesions on T2-FLAIR sequence in pontine pyramidal tracts extending into the cerebellar peduncles (arrows) (A) and showing areas of contrast enhancement (arrowhead) (B). Small bilateral pulmonary cysts (C).
Bickerstaff’s brainstem encephalitis is a post-infectious encephalitis, a variant of Guillain-Barré syndrome and Miller Fisher syndrome, with anti-GQ1b antibodies in the CSF.16 Patients have an altered level of consciousness, ataxia, ophthalmoplegia and signs of upper motor neuron injury, which appear after a viral infection.17
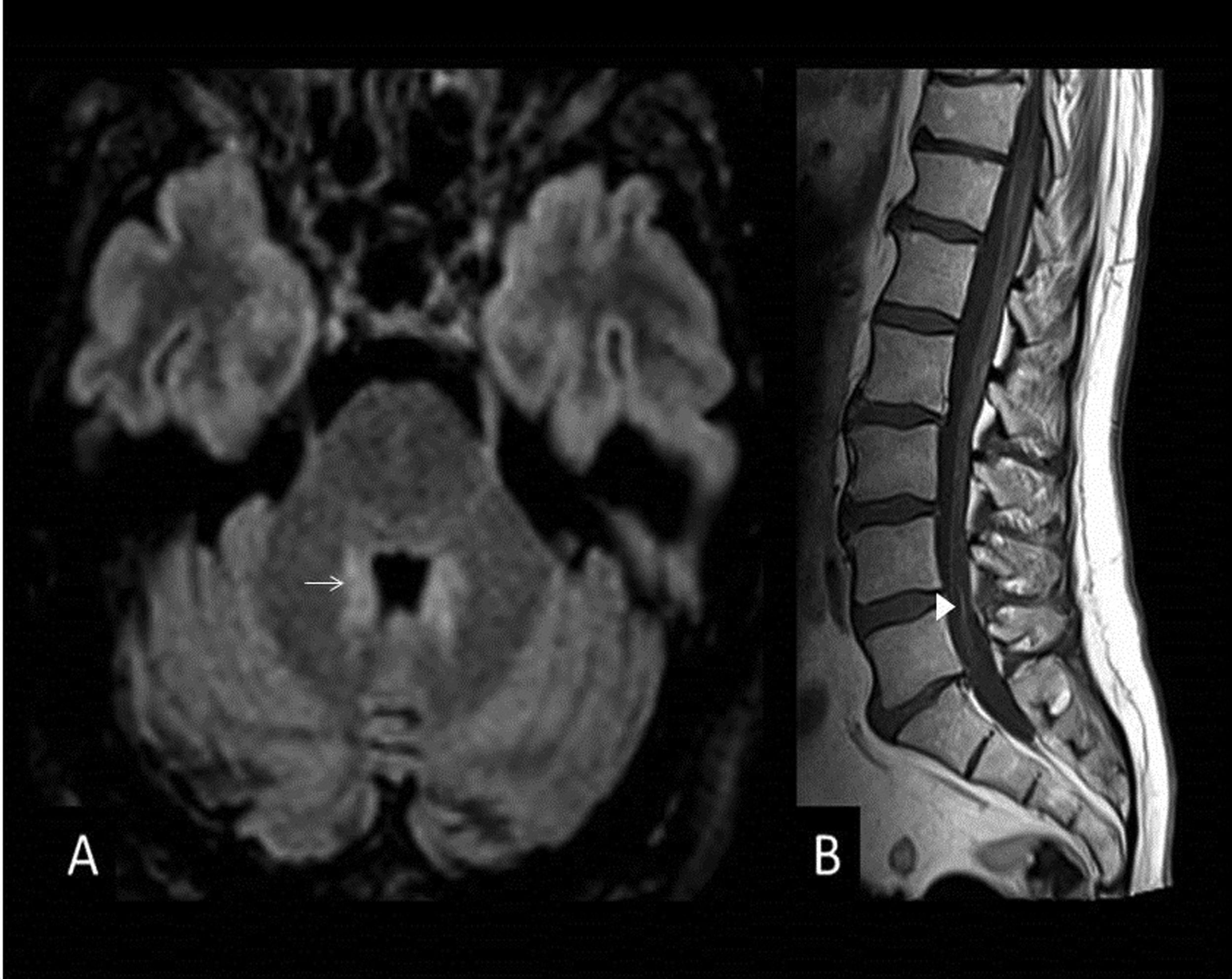
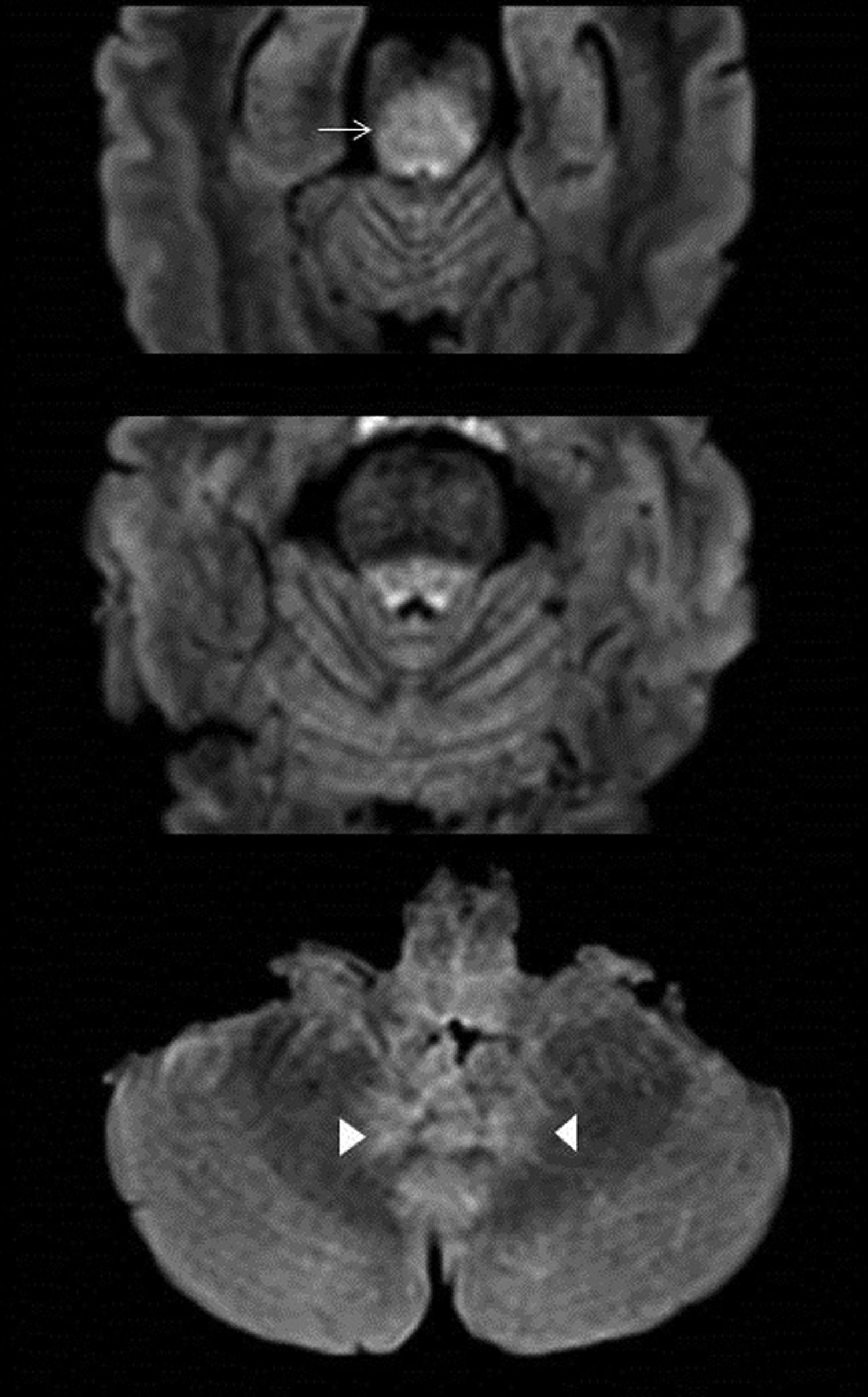
No lesions are observed on MRI in up to 70% of cases; when they are seen, they show little or no enhancement on contrast-enhanced sequences, sometimes restrict diffusion and are hyperintense on T2. Lesions are located in the pons, medulla oblongata, cerebellum, and thalamus, and most disappear along with the clinical presentation17 (Fig. 7).
66-year-old male with pain in the posterior side of the right lower limb, an inability to urinate and a fever. MRI images of brain: Axial T2-FLAIR (A). Bilateral hyperintense lesions on FLAIR surrounding the fourth ventricle without mass effect (arrow) (A). Sagittal T1-weighted image with gadolinium of the lumbar spine (B). There is thickening and discrete enhancement of the cauda equina (arrowhead). Findings consistent with Bickerstaff's brainstem encephalitis.
Acute disseminated encephalomyelitis (ADEM) is a demyelinating inflammatory disease that presents with encephalopathy and multifocal neurological deficits following infection or vaccination.18 It is typical of children and follows a rapidly progressive monophasic course.19
MRI shows extensive, ill-defined and asymmetric reversible lesions affecting the cortical and deep grey matter, basal ganglia, thalamus, cerebellum and brainstem.10,19 Lesions are commonly found in the posterior fossa, but when compared to MS they are larger, less well defined and often located in the ventral midbrain.10
Acute haemorrhagic leukoencephalitis or Weston Hurst diseaseAcute haemorrhagic leukoencephalitis (AHL) or Weston Hurst disease is the most severe form of ADEM. It is a rare sporadic demyelinating disease that affects young people and causes fulminant haemorrhagic inflammation of the white matter.
Neurological manifestations are preceded by upper respiratory tract infections, which may be accompanied by high fever, CSF neutrophilic pleocytosis and leukocytosis.
Inflammatory lesions are bilateral, asymmetrical and may occur in the subcortical white matter, thalamus, cerebellum, brainstem and spinal cord.20 Large, swollen demyelinating lesions also appear that are hyperintense on T2, with less oedema than would be expected for their size. These lesions may have patchy contrast uptake. In addition, haemorrhagic foci are characteristically visible on susceptibility-weighted sequences.20 (Fig. 8).
54-year-old woman with AHL who presented with rapidly progressive decrease in strength in her left extremities with a subsequent decrease in level of consciousness. MRI images of brain: sagittal T1 with gadolinium (A) and SWI (B). Tumefactive lesion involving the pons with contrast enhancement (A) and punctate haemorrhagic foci (arrow) (B).
Systemic lupus erythematosus is an autoimmune disease that can affect the CNS,11 manifesting as supratentorial cerebritis, aseptic meningitis, stroke, demyelination, transverse myelitis or brainstem encephalitis.21 Acute lesions are hyperintense on T2, hypointense on T1, with possible punctate or peripheral contrast uptake22 (Fig. 9). These lesions correspond to areas of oedema, inflammatory infiltrates and demyelination.
41-year-old woman with SLE presenting with 12 h of dizziness and binocular diplopia. MRI images of brain: Axial T2-FLAIR (A), axial T1 with gadolinium (B) and sagittal T2 (C). Hyperintense lesions on T2 in the pons (A) and in the diencephalic region with punctate contrast enhancement (arrows) (B). Hyperintense lesion on T2 in the anterior portion of the C3 and C4 medullary segments (arrowhead) (C).
CLIPPERS syndrome is an immune-mediated inflammatory brainstem and cerebellar disease23 whose diagnosis is confirmed by biopsy. The lesions and clinical symptoms resolve with corticosteroid treatment.10
On T2-weighted MRI, hyperintense lesions are observed with homogeneous or curvilinear punctate (<3 mm) gadolinium uptake in the pons. The lesions do not restrict diffusion and do not usually exert a mass effect.24
As the density of lesions in the pons decreases, others appear in the cerebellar peduncles, medulla oblongata, midbrain, cerebral hemispheres and cerebellar hemispheres.23
Pontocerebellar atrophy also occurs, leading to permanent disabilities, even after clinical and radiological signs have improved.25
Paraneoplastic encephalitisIn the context of various paraneoplastic syndromes, antibodies that cause cerebral inflammation may develop against synaptic receptors, cell surface proteins or intracellular antigens. The most frequently associated neoplasm is microcytic lung carcinoma.11
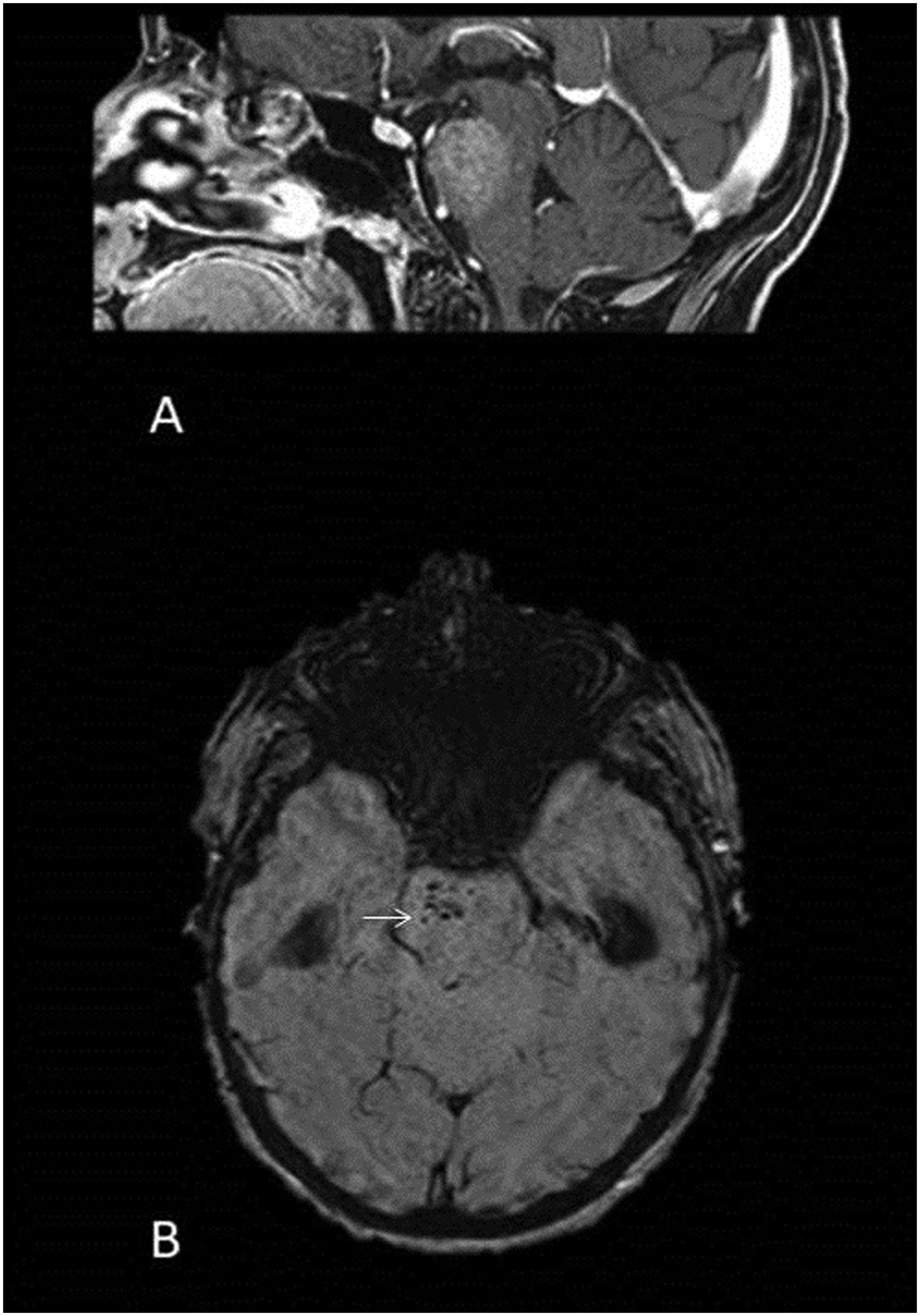
The most common form is limbic encephalitis with bilateral hyperintensity of the temporal lobes on T2 (Fig. 10). However, they occasionally exhibit antineuronal autoantibodies that are associated with specific patterns on MRI.
57-year-old male with diplopia and gait instability who presented with a primary pulmonary neoplasm in the staging study. T2-FLAIR axial brain MRI image. Bilateral hyperintense areas of left predominance in the medial region of the temporal lobe and on the left side of the midbrain (arrows).
In encephalitis secondary to anti-NMDA receptor antibodies, MRI of the brain may be normal, but if alterations are present they usually appear in the cerebral cortex or cerebellum, medial temporal lobe and occasionally in the brainstem.26
In anti-Ma-associated encephalitis, hyperintense lesions that rarely uptake contrast appear in the midbrain, hypothalamus, thalamus and limbic structures.
Metabolic alterationsAlexander diseaseThis rare and fatal disease is also known as fibrinoid leukodystrophy. It is usually diagnosed in childhood, although there are childhood, juvenile and adult variants.
The adult form may be indistinguishable from MS on imaging, even with oligoclonal bands in the CSF. In such cases, detection of GFAP mutation is useful.27 The disease starts in the frontal region and progressively spreads to posterior areas in the following order: caudate head, globus pallidus, thalami and brainstem. In early stages subcortical U-fibres are damaged and in late stages there is cystic contrast-enhancing leukomalacia.28
The lesions, inflammatory in appearance, are symmetrical and bilateral in the frontal white matter (Fig. 11). There is a characteristic hyperintense periventricular rim28 and obstructive hydrocephalus may be observed.
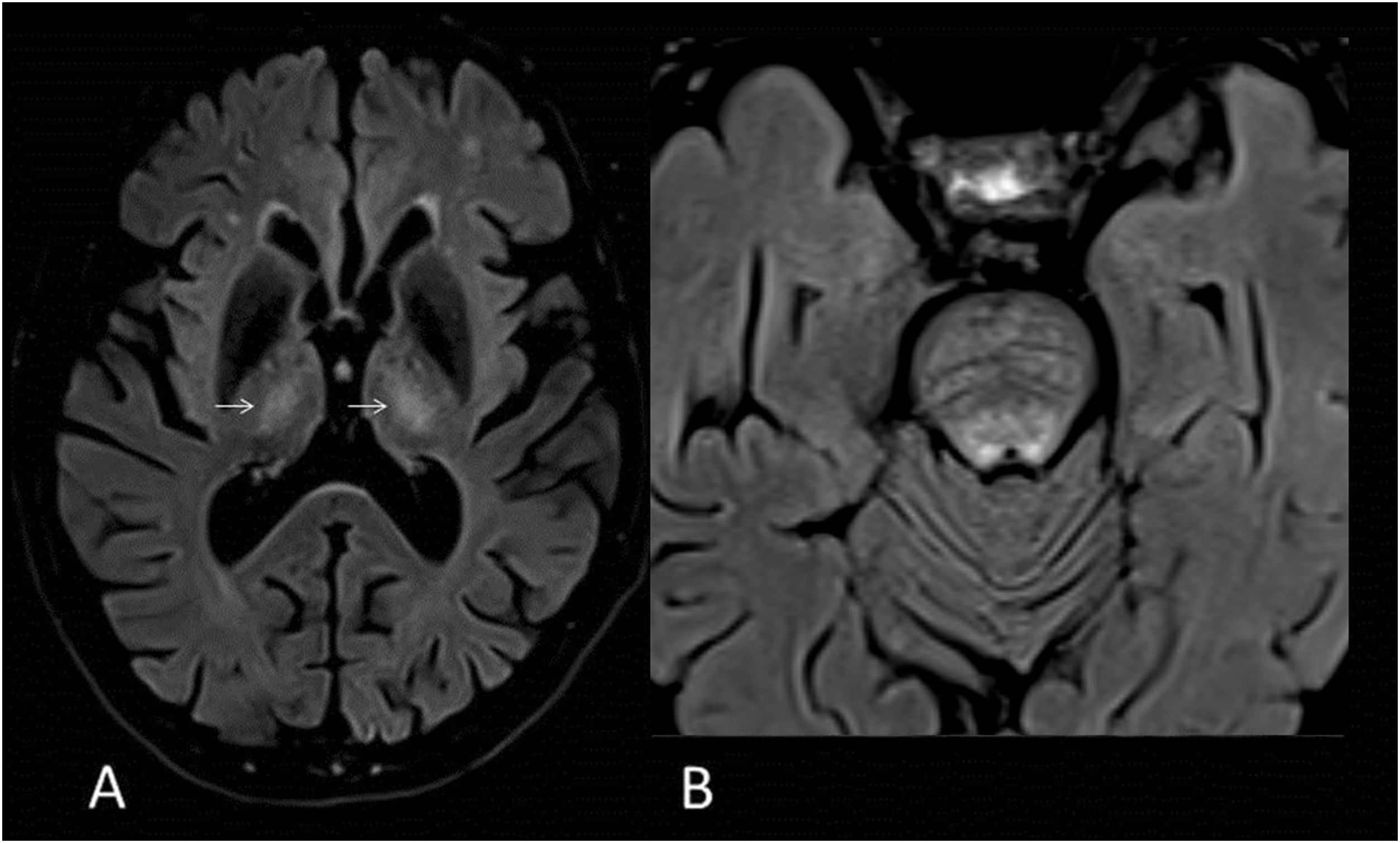
35-year-old male with bilateral neuroretinitis since childhood, weakness and pyramidalism of the lower extremities secondary to Alexander's disease. MRI images of brain: T2-FLAIR sagittal (A), diffusion (B), axial T1 with gadolinium (C) and axial T2-FLAIR (D). Pontomedullary lesion (arrow) hyperintense on FLAIR (A) with diffusion restriction (B) and areas of contrast enhancement (C). Hyperintense periventricular rim on FLAIR (D).
Wilson’s disease is a rare autosomal recessive disorder resulting from abnormal copper metabolism.11 Symptoms appear in adulthood and include neurological manifestations such as dystonia, dysarthria, hand tremors, pseudoparkinsonism and cerebellar symptoms.
The most common finding is putaminal hyperintensity on T2. Hyperintense areas may also appear on T2 in the mesencephalic tegmentum and in the pons, giving rise to the double panda sign. In patients with neurological symptoms, the affected areas are hypointense on T1. Diffusion restriction may be observed in early stages29 (Fig. 12).
Wernicke’s encephalopathyWernicke’s encephalopathy is a disease suffered by alcoholic patients caused by a deficiency of thiamine.11
The lesions exhibit typical inflammatory features on imaging and appear in the mammillary bodies, the dorsomedial thalamus, the tectal plate, the periaqueductal grey matter and around the third ventricle.30
Infectious aetiologyBrainstem inflammation can also be caused by infections. It is crucial to rule these out before initiating immunotherapy, as it could lead to further progression of the infection.
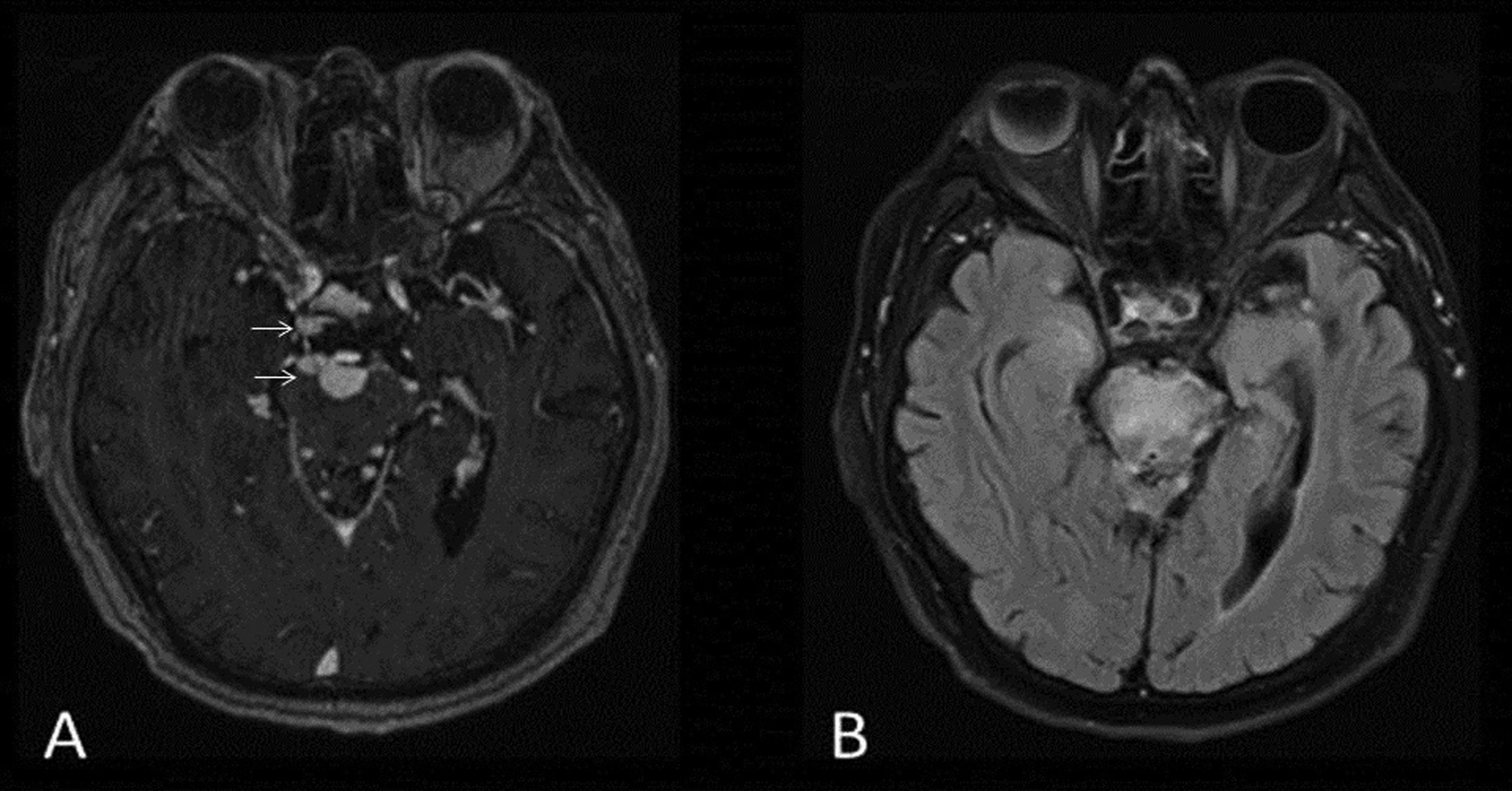
ListeriaListeria monocytogenes affects the CNS and has a mortality rate of 32% and permanent neurological sequelae affecting 55% of patients. It occurs in both immunocompromised and immunocompetent patients and can occur asymptomatically or be accompanied by neurological symptoms, especially in middle-aged men.11,31 It causes inflammatory lesions on the floor of the fourth ventricle and in the cerebellum. These lesions appear on T2 as hyperintense brainstem microabscesses with ring enhancement following contrast administration and centrally restricted diffusion, as well as gadolinium-enhancing trigeminal neuritis32 (Fig. 13).
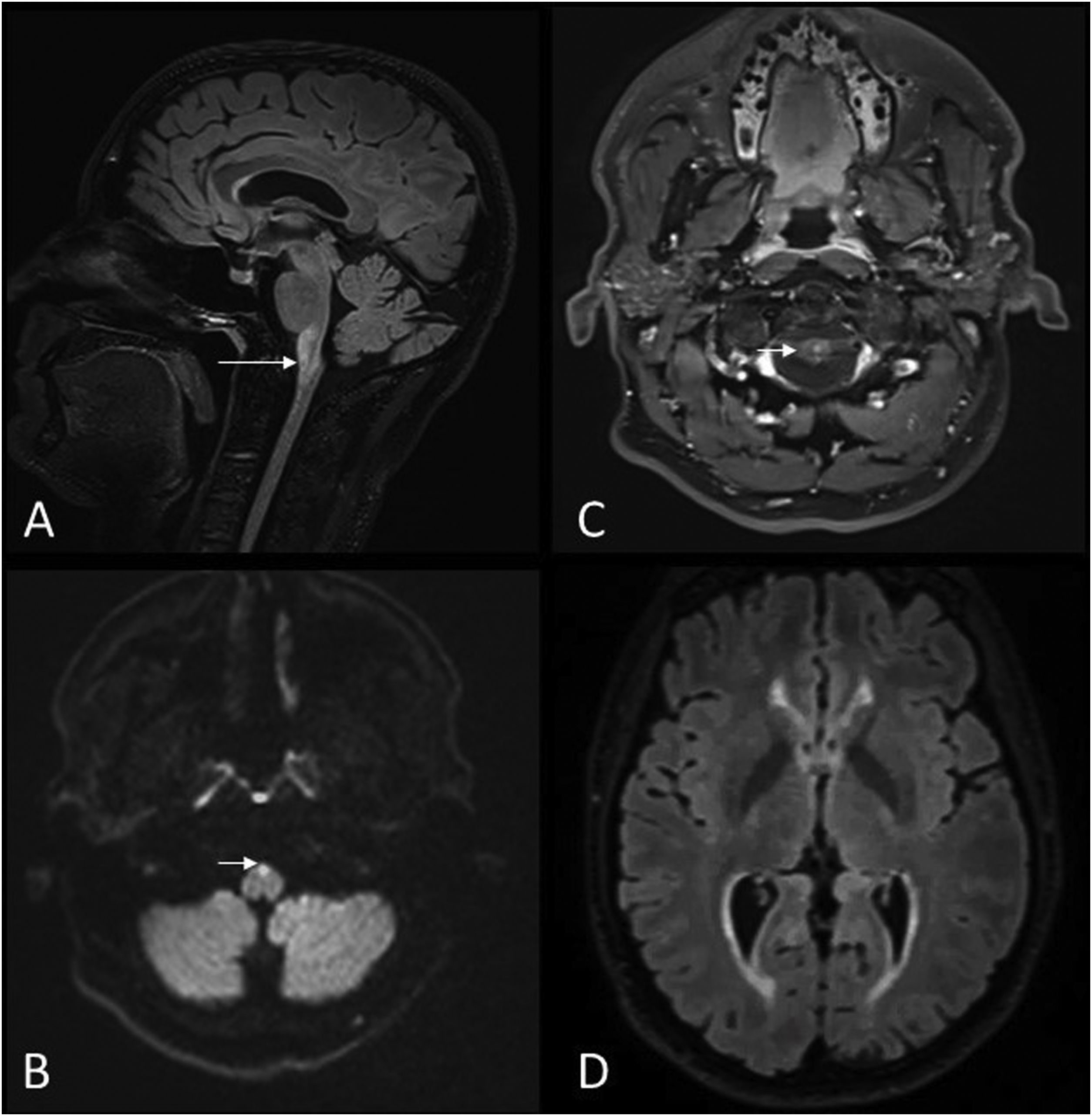
49-year-old male with suspected rhomboencephalitis and lumbar puncture positive for Listeria. MRI images of brain: axial T2-FLAIR (A) and axial T1 with gadolinium (B). Abscess with contrast enhancement in ring pattern (arrowhead) (B) within a hyperintense area on FLAIR in the dorsal pons extending to the left superior cerebellar peduncle (arrow) (A).
Enterovirus is the main infectious agent of brainstem encephalitis after Listeria. The most common subtype is EV-A7, which causes hand, foot and mouth disease, acute gastroenteritis and upper respiratory tract infections. In 25% of cases, particularly in children and adolescents, it causes neurological symptoms.33
The location of EV-A71 subtype lesions is characteristic: dorsal pons, medulla oblongata, followed by dentate nuclei, cerebellum, midbrain and thalamus, with no other associated supratentorial lesions34 (Fig. 14).
61-year-old woman with acute febrile syndrome in the context of viral meningitis caused by enterovirus, presenting with fever, speech impairment, inability to walk and intention tremor. Axial T2-FLAIR images of brain MRI. Hyperintense areas on the right side of the dorsal pons (arrow), and in bilateral cerebellar dentate nuclei (arrowheads) and medulla oblongata.
CNS involvement in tuberculosis is rare. It usually occurs with high morbidity and mortality in immunocompromised patients, especially when it is associated with HIV.11,35
Tuberculosis can cause meningitis, hydrocephalus, cerebritis, tuberculomas, abscesses and spinal tuberculosis. On MRI, brainstem encephalitis is associated with leptomeningeal gadolinium uptake in the interpeduncular cisterns. The CSF appears cloudy, is hyperintense on FLAIR and takes up contrast.35
Non-caseating tuberculomas have homogeneous contrast uptake while non-caseating tuberculomas enhance in a ring pattern with a hypointense centre on T2-weighted images35,36 (Fig. 15).
It should be noted that Listeria monocytogenes and enteroviruses are the most frequent infectious agents of brainstem encephalitis.
- □
Listeria generates periventricular inflammatory lesions, microabscesses and trigeminal neuritis.
- □
Enterovirus EV-A7 causes lesions in the pons, medulla oblongata, dentate nuclei, midbrain and thalami.
56-year-old male with disorientation, decreased consciousness and suspected tuberculous meningitis. MRI images of brain: axial T1 with gadolinium (A) and axial T2-FLAIR (B). Multiple contrast-enhancing nodules in the subarachnoid space located in the interpeduncular cisterns, corresponding to non-caseating tuberculomas (arrows) (A). Extensive hyperintense oedema in the pons (B).
A wide spectrum of diseases cause brainstem inflammation. An understanding of their clinical and epidemiological characteristics as well as their presentation in terms of number, distribution, morphology and behaviour on different MRI sequences facilitates diagnosis. Early diagnosis is important as it permits infection to be excluded, treatment to be established and specific tests to be performed in a timely manner.
FundingThis research has not received funding support from public sector agencies, the business sector or any non-profit organisations.
Authorship- 1.
Research coordinators: CGdAS, JJGM.
- 2.
Study concept: CGdAS, JJGM, IAM, LAM.
- 3.
Study design: CGdAS, JJGM.
- 4.
Data collection: N/A.
- 5.
Data analysis and interpretation: N/A.
- 6.
Statistical processing: N/A.
- 7.
Bibliographic search: CGdAS.
- 8.
Drafting of article: CGdAS.
- 9.
Critical review for important intellectual content: CGdAS, JJGM, IAM, LAM, RFP.
- 10.
Approval of final version: CGdAS, JJGM, IAM, LAM, RFP.
The authors declare that they have no conflicts of interest.