In order to perform chest dose optimisation studies, the imaging phantom should be adequate for image quality evaluation. Since high-end phantoms are cost prohibitive, there is a need for a low-cost construction method with fairly available tissue substitutes.
Materials and methodsTheoretical calculations of radiological characteristics were performed for each of lung, cortical bone and soft tissues in order to choose appropriate substitute, then, cork, P.V.C. (Polyvinyl chloride) and water were chosen, respectively. Validation included, firstly, measuring CT Hounsfield Units (HU) of a real patient’s tissues then compared against their corresponding anatomies in the constructed phantom. Secondly, Signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) values were acquired in this study to evaluate the quality of images generated from the constructed phantom, then, compare their trends with a valid phantom under different exposure parameters (kVp and mAs).
ResultsFrom theoretical calculations, the percentage differences showed high accuracy of tissue substitutes when simulating real patient tissues; P.V.C. was ≥5.78%, cork was ≥4.46% and water ≥5%. The percentage difference (CT HU) between lung and cortical bone and their equivalent tissue substitutes were 10.44% and 0.53%–3.17%, respectively. Strong positive correlations were found for SNR when changing both kVp (0.79) and mAs (0.65). While the correlation strength of CNR values were found to be moderate when changing both kVp (0.58) and mAs (0.53).
ConclusionsOur low-cost phantom approved through CT HU that their materials replicate the radiological characteristics of real one-year-old child while SNR and SNR correlations confirmed its applicability in imaging and optimisation studies.
Para llevar a cabo estudios de optimización de dosis, el fantoma de imagenología debe ser adecuado para evaluar la calidad de la imagen. El coste de los fantomas de gama alta suele ser prohibitivo, por lo que es necesario hallar un método de construcción asequible que emplee sustitutos tisulares que sean razonablemente fáciles de obtener.
Materiales y métodosSe realizaron cálculos teóricos de las características radiológicas de cada uno de los pulmones, el hueso cortical y los tejidos blandos con el fin de elegir el sustituto adecuado; para ello se eligieron el corcho, el PVC (cloruro de polivinilo) y el agua, respectivamente. La validación consistió, en primer lugar, en la medición de las unidades Hounsfield (UH) de tomografía computarizada (TC) de los tejidos de un paciente real y su posterior comparación con las anatomías correspondientes en el fantoma construido. En segundo lugar, se obtuvieron los valores de relación señal/ruido (S/R) y de relación contraste/ruido (C/R) para evaluar la calidad de las imágenes generadas a partir del fantoma construido y comparar sus tendencias con un fantoma válido utilizando diferentes parámetros de exposición (valores kVp y mAs).
ResultadosPartiendo de los cálculos teóricos, las diferencias porcentuales exhibieron una precisión elevada en los sustitutos tisulares al simular los tejidos de un paciente real; con PVC fue de ≥5,78%, con corcho ≥4,46% y con agua ≥5%. La diferencia porcentual (UH de TC) entre el pulmón y el hueso cortical y sus sustitutos tisulares equivalentes fue del 10,44% y del 0,53% al 3,17%, respectivamente. Se encontraron fuertes correlaciones positivas para la S/R al variar tanto los valores de kVp (0,79) como de mAs (0,65). Por el contrario, se halló que la fuerza de correlación de los valores de la C/R era moderada al cambiar los valores kVp (0,58) y mAs (0,53).
ConclusionesSe ha constatado mediante las UH de TC que los materiales de nuestro fantoma asequible son una réplica de las características radiológicas de un paciente pediátrico de un año de edad, mientras que las correlaciones de la S/R y la C/R confirmaron su aplicabilidad en estudios mediante imágenes y de optimización.
Anthropomorphic imaging phantoms are considered to be the superior choice for any study in the diagnostic radiology because they provide a good approximation of patient anatomy and have excellent radiological fidelity.1 There are paediatric dosimetry phantoms (ATOM dosimetry phantoms) which simulate three paediatric ages: newborn, one and five years old.2 However, these phantoms have many limitations: they have been designed for dosimetry only and they may not be suitable for image quality assessments as they do not provide a sufficient level of realism when representing the human anatomy, especially for the chest region.3 In addition, these dosimetry phantoms are produced as a series of axial sections and generate shadows on the resultant X-ray images from the gaps between the phantom slices. Furthermore, ATOM dosimetry phantoms have been constructed in a way which does not allow the researcher to include any lesions inside the lung and this was one of the reasons to exclude this phantom from this work.
Commercially, there is a lack of anthropomorphic paediatric phantoms and to the authors’ knowledge there are only two phantoms available to simulate the paediatric chest. One, Lungman by Kyoto Kagaku4 (LUNGBOY PH-1C Multipurpose Pediatric Chest Phantom; Kyoto Kagaku Company Ltd, Japan), designed for a five-year-old child, two, the Gammex RMI© 610.5 However, in terms of the first one, the cost of this phantom is high and not available locally. Regarding the second one, it has been designed to simulate the neonatal chest phantom. The peadiatric age are divided into groups as follow; 0, 1, 5 10 and 15 year-old. In order to perform dose optimisation, there must be x-ray phantoms for each of the aforementioned age groups.2 As a results, there is limited availability of x-ray imaging phantoms within peadiatric age range in addition to the cost. One more reason is that there is a plan to include lesions inside the lung which will rule a conspicuity analysis of lesion visibility in paediatric chest radiography. ATOM dosimetry phantoms, on the other hand, have limitations in image quality evaluations due to the axial slices along their trunks that appears as black lines, which would disturb the appearance of chest anatomies including any lesion inserted in the phantom.6 In addition, those phantoms do not allow any insertion of a lesion within the lungs in order to conduct conspicuity analysis for lesions.
The process of constructing an X-ray imaging phantom requires careful consideration important criteria. Firstly, the materials selection (radiological substitutes) should provide a close response to radiation exposure, at imaging energy range, as if imaging real patient. Secondly, is the formability of tissue substitutes to shape internal and external human body parts.2,7 lastly, the coverage of peadiatric sizes is important in phantom construction, as the majority of the commercial phantoms are about the age of neonates or adults while there is urgent need for Phantoms representing the ages from 1 to 15 year-old.8
Many publications tried to use different types of phantoms to simulate the paediatric chest. These types of phantoms are limited because they do not represent the real patient anatomically nor radiologically and this means that anatomical shape and noise is not considered in the image quality assessments.9–13
The simulation of chest anatomies requires that their tissue equivalent substitutes should match radiological characteristics of human anatomies in addition to their availability and low-cost. Studies used low-cost materials for construction of pelvis imaging phantoms such as Plater of Paris for bone simulation and Poly methyl methacrylate (PMMA) for soft tissue,8 However, this method poses a challenge regarding insertion of lungs and lesion inside PMMA along the slices included in this construction method. Another study used ager gel to simulate soft tissue in order to construct a neonatal anthropomorphic imaging phantom.14 However, the agar gel can be inaccurate because the elemental composition for the agar gel is considered uncertain, also, it is unstable over relatively long periods.15 Other construction methods utilized resin-based materials within phantom construction,16–19 despite the high accuracy, the process of construction remain complicated and the cost of such materials is of considerable cost.
According to the above-mentioned, there is a high cost and a limited availability for paediatric imaging phantoms, specially the chest area, over the age range (0–15) in addition to the ability to use them to study lesion detection in lungs. Accordingly, the aim of this study is to design and validate paediatric chest phantom for dose optimisation and lesion detection studies.
Materials and methodsPhantom design methodA paediatric chest phantom has been constructed to serve the purpose of this research and will facilitate evaluation of the factors that affect dose, lesion detection and image quality optimisation. An essential requirement for the phantom is that it provides an acceptable level of simulation of the chest region in a paediatric patient. The first stage (Anatomical equivalence) in the phantom construction was to determine the morphology and the physical dimensions of the phantom. The second stage (Radiological equivalence) was to find suitable radiological tissue equivalent substitutes for the bone, lung and soft tissue to be, then, formulated according to the organs’ shapes that were determined in first stage.
Anatomical equivalenceA unique methodology has been used to design and construct the phantom for use in optimisation studies with anatomical shape and dimension to be close to real patient anatomies. The proposed method will also allow the required range of phantoms to be constructed for different ages and dimensions.
The first stage in the phantom construction process was to determine the morphology and the physical dimensions of the phantom, these included the overall height, width and depth as well as the sizes of the internal anatomical structures. Therefore, measurements were taken from a standard size of one-year-old ATOM dosimetry phantom using CT scan data (120 kVp and a 1mm reconstructed slice thickness). Then, using the available CT data, dimensional measurements were undertaken using the RadiAnt DICOM Viewer. The rationale for selecting the ATOM dosimetry phantom was to measure the morphology and the physical dimensions for the phantom was to avoid the size variability of clinical CT data for the same age as well as the reliance on the clinical CT data for one patient which could not give the standard size for the specific age. Generally, the ATOM dosimetry phantom represents the average of the ages for the group.
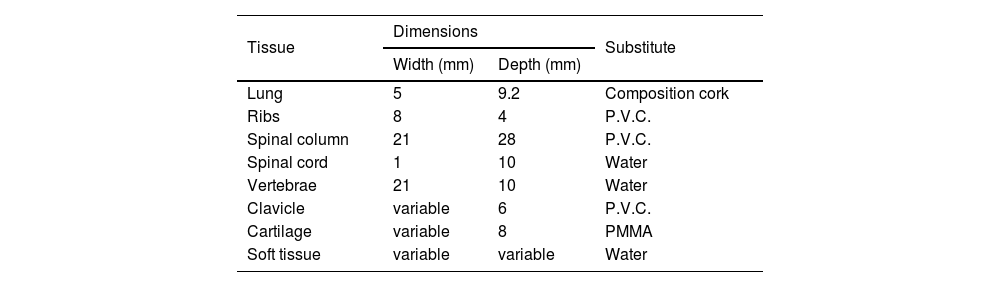
Measurements that have been included were those of the lung, spine, ribs, sternum and clavicle, where all of them are shown in Figs. 1–6 in the Appendix. In terms of the lung, the depth and the width have been determined from sagittal and coronal reconstructions as shown in Figs. 1 and 2, in the Appendix, respectively. Their corresponding values were equal to 9.2 and 5.0cm, respectively. Using 3D image processing the overall 3D shape of the lung was estimated as shown in Fig. 3 in the Appendix. The size and shape changes of the lung from the anterior to posterior surfaces as well as the differences between right and left lung were taken into consideration.
With reference to the spinal column, both the spinal cord and the vertebra were taken into consideration and the depth of the spine, spinal cord, the vertebra together with the length of the vertebra were calculated from the sagittal reformats and their values were equal to 28, 10, 10 and 2mm, respectively (as illustrated in Fig. 4a in the Appendix). The width of the spine was determined from the coronal reformats (as shown in Fig. 4b in the Appendix) and construction was further simplified by calculating the average width values across all the visible spinal segments, 21mm.
With respect to the sternum, this was formed from six segments all different in their individual depths and sizes. The depth of the sternum was measured from the sagittal reconstructions and the width was measured from a 3D bone volume rendering as shown in Figs. 4a and 5, in the Appendix, respectively.
The thickness of the ribs was determined using axial CT slices and in general they are equal to around 4mm, as shown in Fig. 6a in the Appendix, and their width was measured again using a 3D bone volume rendered image and in general they are all equal to around 8mm, as shown in Fig. 5 in the Appendix. Finally, the clavicle dimensions ware obtained from axial slices view shown in Fig. 6b in the Appendix.
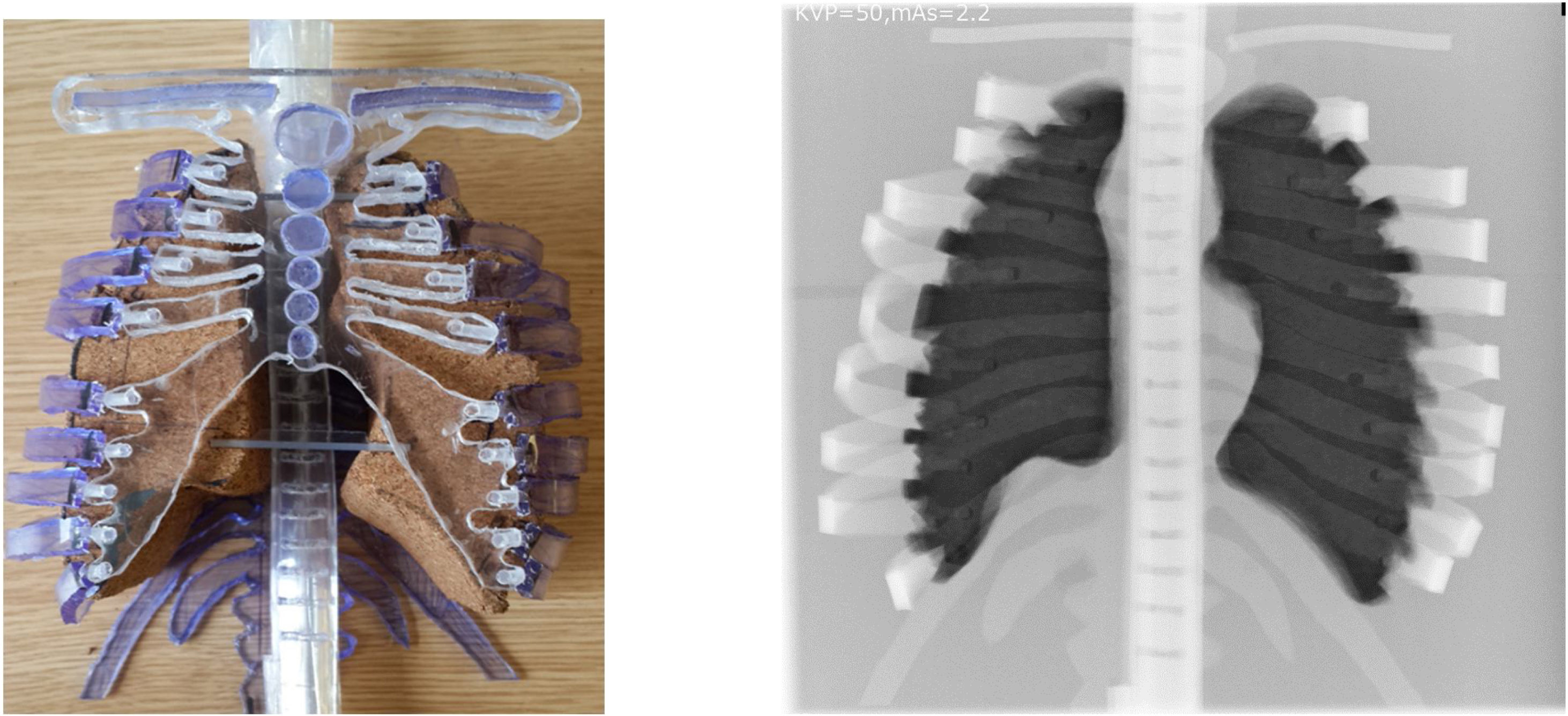
The phantom was purposefully designed in way that allows all the four parts of the phantom (lung, spine, rib cage and cartilage) to be removed and the lung was constructed in way which allowed lesions to be inserted. Also, the lung has been painted by waterproof spray to ensure that it does not absorb any water (soft tissue equivalent substitute) from the environment. The overall measurement values of the internal anatomical structure and their equivalent substitute are listed in Table 1. The design of the phantom and its resultant radiograph are shown in Fig. 1a and b, respectively, and it represents the first prototype phantom to simulate the chest region for one year age child. Although this phantom was constructed in a way to take into account the anatomical and radiological issues some limitations can be seen in this prototype. One, the geometry of the clavicle does not reflect the same shape for that of the real patient; two, lung edges are very sharp and not curved as would be for a real lung; three, phantom handling is not comfortable (because all of the phantom parts are removable), especially for the phantoms with five and ten ages because the large size of the lung and the other parts may lead to some difficulties with phantom control.
Dimensions and equivalent substitutes of the internal anatomical structures for the phantom.
| Tissue | Dimensions | Substitute | |
|---|---|---|---|
| Width (mm) | Depth (mm) | ||
| Lung | 5 | 9.2 | Composition cork |
| Ribs | 8 | 4 | P.V.C. |
| Spinal column | 21 | 28 | P.V.C. |
| Spinal cord | 1 | 10 | Water |
| Vertebrae | 21 | 10 | Water |
| Clavicle | variable | 6 | P.V.C. |
| Cartilage | variable | 8 | PMMA |
| Soft tissue | variable | variable | Water |
In the second stage, the tissue substitute materials that were used in this work were selected to serve four aims: one; similar attenuation coefficients to that of real patient tissue, two; to provide easier and reproducible options for phantom construction, three; materials that were available commercially and relatively economically, and four; they can be easily formulated according to the shape of the organs within the chest.
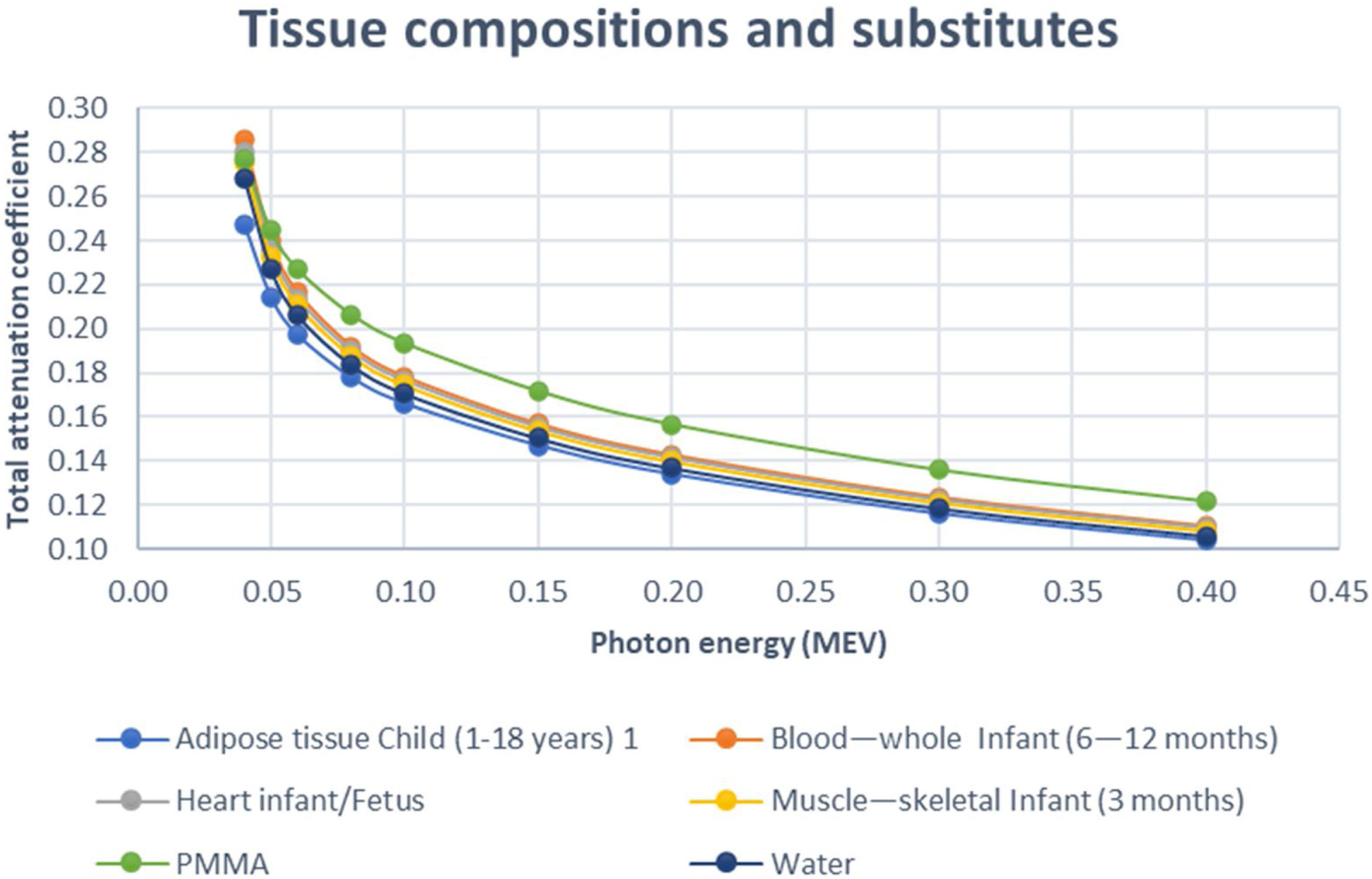
In this study, the total attenuation coefficient of each of the neonate body tissues; soft tissues (including: adipose tissue, blood—whole, heart and muscle—skeletal), cortical bone and lung along with their tissue substitutes have been calculated using the XCOM software.20 This program is accessed through NIST (National Institute of Standards and Technology) web database which enable calculating photon cross-sections for photoelectric absorption, scattering, pair production, in addition to the total attenuation coefficient. These calculations are applicable for any element, mixture or compound with atomic number Z≤100, covering energy range; 1keV–100GeV.20 Furthermore, the elemental composition for the tissue substitute materials that is used in this software was obtained from White et al.,21 and by comparison, these data have been reported for the human body.22 There are similarities found in body composition of soft tissues, cortical bone and lungs between the following ages; fetus, neonate, infant and paediatric,23 then the total attenuation coefficients of each tissue type of these ages are expected to be close.
Three different types of tissue equivalent substitutes namely; P.V.C., cork and water were used in this work in order to simulate the bone, lung and soft tissue, respectively. The aforementioned materials are of high availability and low cost but most importantly are of high simulation properties regarding the radiological characteristics of human tissues. Starting with the P.V.C. which has being regarded as a tissue substitute that can be used to simulate human bone.21 The cork on the other hand is considered to be one of the best substitutes for the human lung because its physical properties are similar to that of the human lung.24 It is also reported by the ICRU 44 to be potential tissue substitute for human lungs. Finally, water has been repeatedly reported to be successful substitute for soft tissues.21
Phantom validation methodIn addition to the theoretical calculation of the linear attenuation coefficient (considered as theoretical validation) as described in the above section “Radiological equivalence”, the study contained two different methods to validate the constructed phantom: one, by measuring the Hounsfield unit (HU), using CT scanning, for different regions of interest (ROI); two, comparing the response (correlation) of the phantom with a reference phantom (Gammex RMI© 610) using the physical measurements signal to noise ratio (SNR) and contrast to noise ratio (CNR) on a digital radiograph over changing different radiographic parameters (kVp and mAs).
CT Hounsfield unit validationDifferent materials have different X-ray attenuation characteristics that represented by CT HU numbers, which varies according to the density of the patient tissue or its equivalent substitute. Measuring HU values from CT scans considered to be reliable method in identifying and comparing the composition and density of tissues along with their radiological equivalent materials.25,26 Therefore, measuring CT HU was considered in the experimental validation of the phantom, where the measurement were acquired from a real patient’s Lung, spinal column, ribs then compared against their corresponding anatomies in the constructed phantom.
Pixel value validationThe value each pixel within digital x-ray image is dependent on the amount X-rays attenuation after they pass through the corresponding body part.27 The information included in a digital radiograph is determined by number of pixels it contains.28 SNR is considered as a common approach used to estimate the overall visibility information appears on the radiograph.29 SNR is a physical measure of image quality and is extensively used in optimisation studies.30 The physical image quality measures (SNR and CNR) have been used in comparing image quality trend and validating phantom images.8 Thus, SNR and CNR values were acquired in this study to evaluate the quality of images generated from the constructed phantom, then, compare their trends with a valid phantom (Gammex RMI© 610) under different exposure parameters (kVp and mAs).
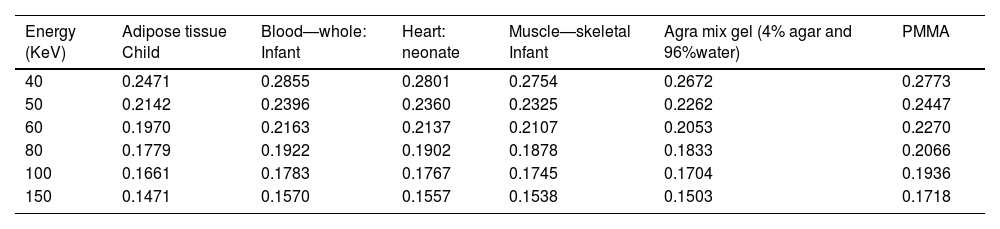
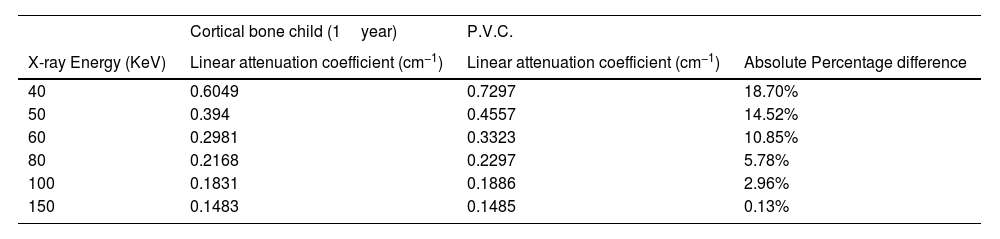
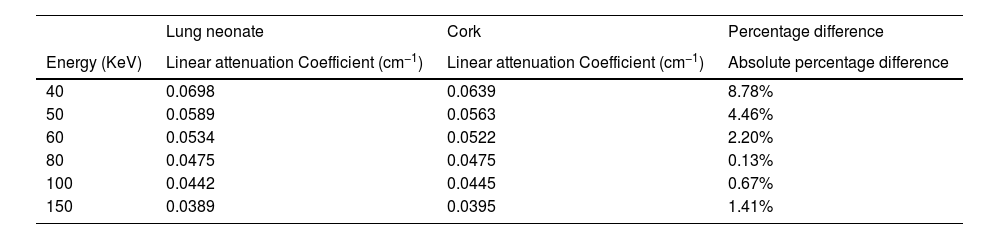
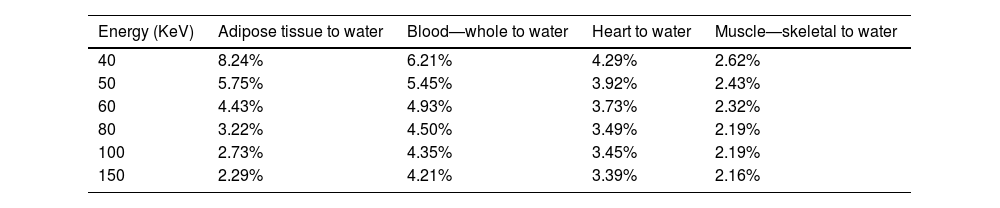
ResultsRadiological characteristics of bone, lung and soft tissue substitutesThe values of linear attenuation coefficient of all soft tissues and their substitutes are listed in Table 2). It can be seen from Table 3 below that Percentage difference between linear attenuation coefficient of 1-year-old cortical bone child and its tissue substitute (P.V.C.) ranged from 0.13% to 18.6%. Giving the most energy used during the chest imaging of one-year old child (≥80kVp), then the effective percentage difference would be from 5.78% then it becomes less as energy increases, see Table 3. While for the lung tissue substitute (cork), the percentage difference from real lung tissue varied from 0.13% to 4.46% over the range 50–150keV (except for 8.78% at 40keV which is not within the energy range commonly used during chest imaging), see Table 4. Lastly, Water has shown to be the adequate choice for this application because its linear attenuation coefficient is very similar to that of the neonate soft tissues (adipose tissue, blood—whole, heart and muscle—skeletal) as shown in Table 5. Where all the percentage differences were ≥5% at energy range 60–150 over all types of soft tissues. Except for the lower energies, the percentage differences were higher than 5% for the adipose tissue and blood. To sum up all, these percentage differences showed high accuracy in simulation of real patient tissues in term of radiological characteristics. As a results, it is possible for paediatric phantoms to use water to simulate soft tissues (adipose tissue, blood—whole, heart and muscle—skeletal), cork to simulate lungs and P.V.C. to simulate cortical bone.
Linear attenuation coefficient (cm−1) of different soft tissues within chest area and their equivalent substitutes over diagnostic x-ray energy range (40–150 KeV).
| Energy (KeV) | Adipose tissue Child | Blood—whole: Infant | Heart: neonate | Muscle—skeletal Infant | Agra mix gel (4% agar and 96%water) | PMMA |
|---|---|---|---|---|---|---|
| 40 | 0.2471 | 0.2855 | 0.2801 | 0.2754 | 0.2672 | 0.2773 |
| 50 | 0.2142 | 0.2396 | 0.2360 | 0.2325 | 0.2262 | 0.2447 |
| 60 | 0.1970 | 0.2163 | 0.2137 | 0.2107 | 0.2053 | 0.2270 |
| 80 | 0.1779 | 0.1922 | 0.1902 | 0.1878 | 0.1833 | 0.2066 |
| 100 | 0.1661 | 0.1783 | 0.1767 | 0.1745 | 0.1704 | 0.1936 |
| 150 | 0.1471 | 0.1570 | 0.1557 | 0.1538 | 0.1503 | 0.1718 |
Absolute percentage difference between linear attenuation coefficient of 1-year-old Cortical bone child and P.V.C.
| Cortical bone child (1year) | P.V.C. | ||
|---|---|---|---|
| X-ray Energy (KeV) | Linear attenuation coefficient (cm−1) | Linear attenuation coefficient (cm−1) | Absolute Percentage difference |
| 40 | 0.6049 | 0.7297 | 18.70% |
| 50 | 0.394 | 0.4557 | 14.52% |
| 60 | 0.2981 | 0.3323 | 10.85% |
| 80 | 0.2168 | 0.2297 | 5.78% |
| 100 | 0.1831 | 0.1886 | 2.96% |
| 150 | 0.1483 | 0.1485 | 0.13% |
Absolute percentage difference between linear attenuation coefficient of Lung neonate and Cork.
| Lung neonate | Cork | Percentage difference | |
|---|---|---|---|
| Energy (KeV) | Linear attenuation Coefficient (cm−1) | Linear attenuation Coefficient (cm−1) | Absolute percentage difference |
| 40 | 0.0698 | 0.0639 | 8.78% |
| 50 | 0.0589 | 0.0563 | 4.46% |
| 60 | 0.0534 | 0.0522 | 2.20% |
| 80 | 0.0475 | 0.0475 | 0.13% |
| 100 | 0.0442 | 0.0445 | 0.67% |
| 150 | 0.0389 | 0.0395 | 1.41% |
Absolute percentage difference between Linear attenuation coefficient of soft tissues and water over diagnostic x-ray energy range (40–150 KeV).
| Energy (KeV) | Adipose tissue to water | Blood—whole to water | Heart to water | Muscle—skeletal to water |
|---|---|---|---|---|
| 40 | 8.24% | 6.21% | 4.29% | 2.62% |
| 50 | 5.75% | 5.45% | 3.92% | 2.43% |
| 60 | 4.43% | 4.93% | 3.73% | 2.32% |
| 80 | 3.22% | 4.50% | 3.49% | 2.19% |
| 100 | 2.73% | 4.35% | 3.45% | 2.19% |
| 150 | 2.29% | 4.21% | 3.39% | 2.16% |
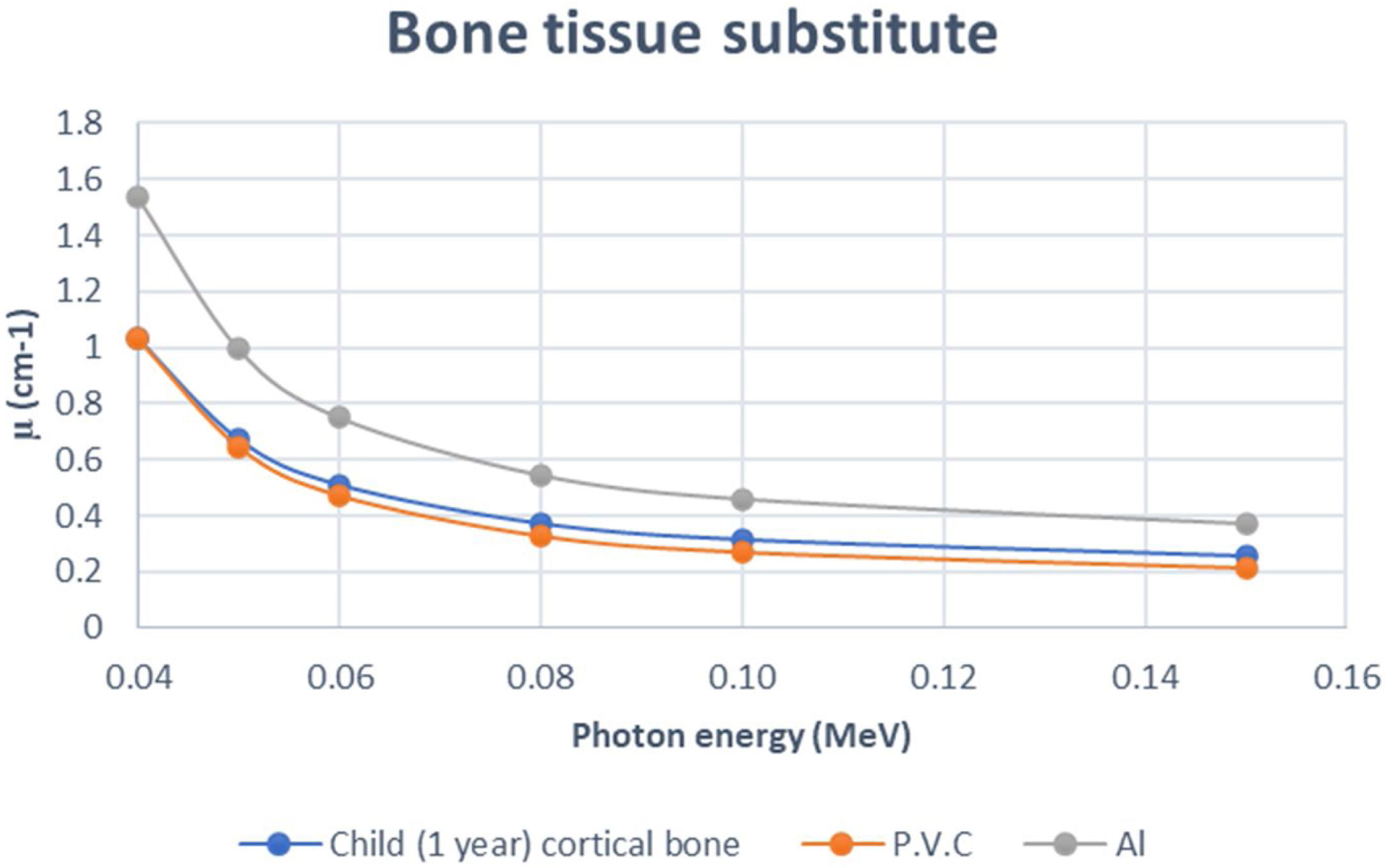
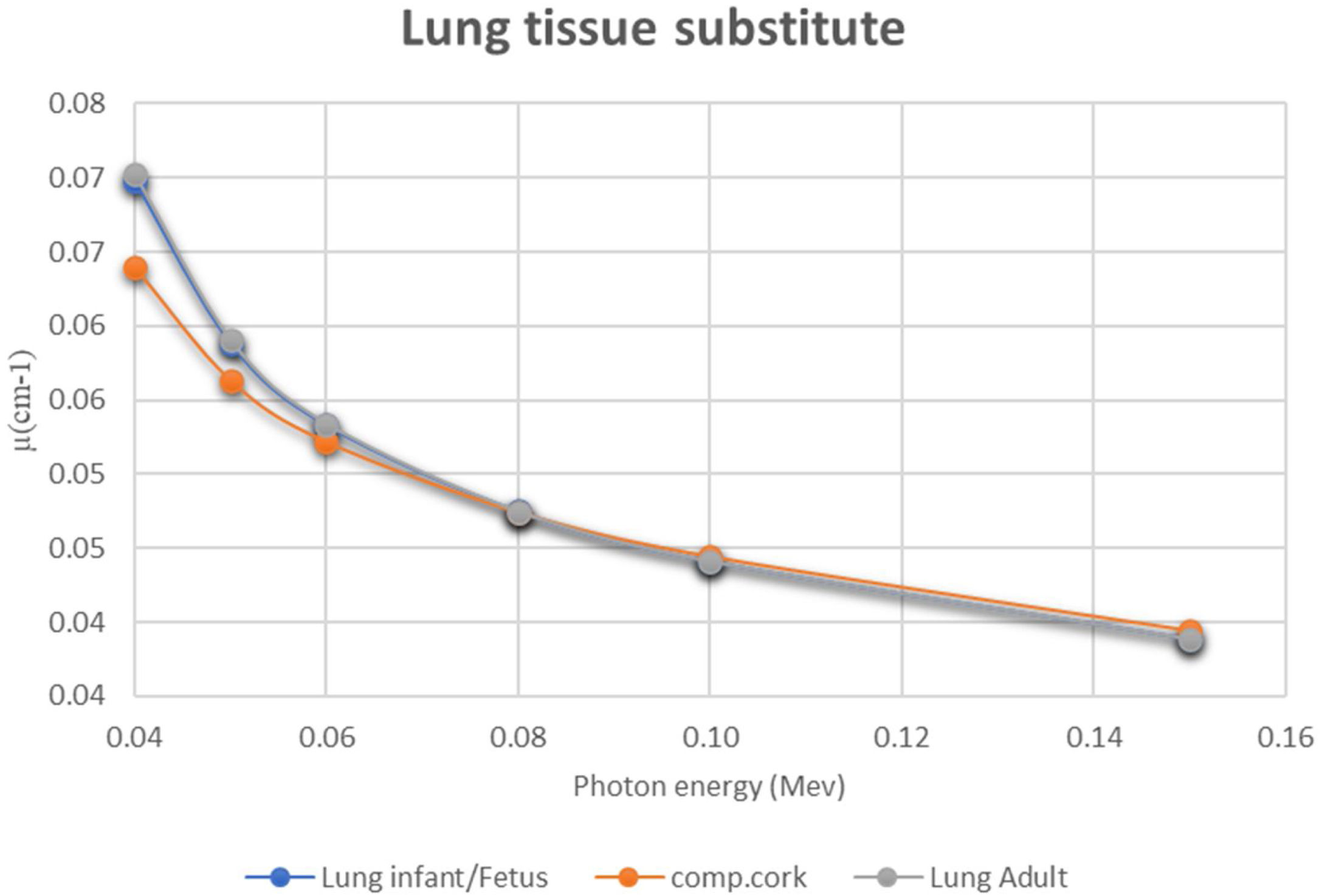
In addition to the simulation accuracy in radiological characteristics, x-ray interactions of one-year old cortical bone against the P.V.C., showed a coherent behavior when increasing the x-ray energy, see Fig. 2. Comparing to Aluminum (Al), P.V.C. showed closer linear attenuation coefficient values to the cortical bone of one year child (see Fig. 2). The same coherent patterns were seen for cork against lung neonate (see Fig. 3) and water against different neonate soft tissues; adipose tissue, blood—whole, heart and muscle—skeletal (see Fig. 4) when increasing the x-ray energy. Also, it is noticeable the similarity in the radiational characteristics of real human lungs.
In this study, two different methods were used to validate the recently constructed phantom: one, by measuring the Hounsfield unit (HU), using CT scanning, for different ROIs; two, comparing the response (correlation) of the phantom with a reference phantom (Gammex RMI© 610) using the physical measurements (SNR and CNR) on a digital radiograph over changing different radiographic parameters (kVp and mAs).
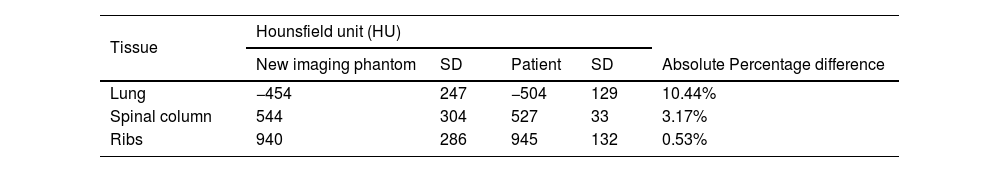
CT Hounsfield unit validationIn terms of HU values were measured in three different ROIs (spinal column, ribs and lung) for both the new constructed chest phantom and the one-year aged patient then the results were compared with each other as shown in Table 6. Both the imaging phantom and the patient were subjected to a helical CT scan using 80kVp and a 0.6mm reconstructed slice thickness. The CT number (HU) was measured for both; the cork (−454 HU) and the patient lung (−504 HU), they were of close values (percentage difference; 10.44%) this, also, ensure that the commercial cork used in this phantom is matched with the theoretical calculations, which were used to find tissue equivalent substitutes. The percentage difference in HU values between the P.V.C. and the one-year-old cortical bone was 3.17% for spinal column and 0.53% for ribs. The is no need to measure the water (soft tissue) as the CT HU are already calibrated using water and the calculations of linear attenuation (μ) should be adequate as no impurity is expected from using water.
The results of the CT HU values listed in Table 6 show that there is an excellent agreement between the HU of different ROIs for the real patient compared with that for the phantom.
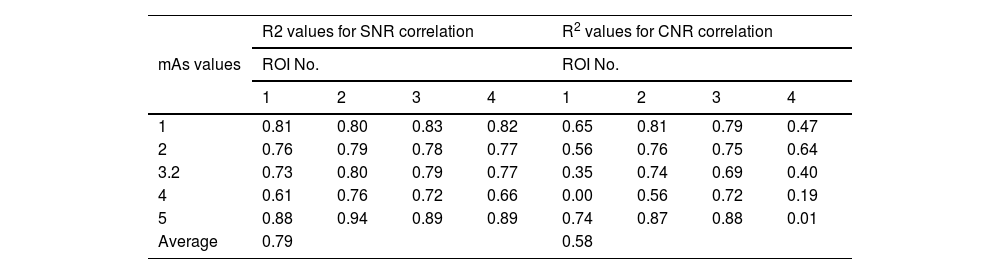
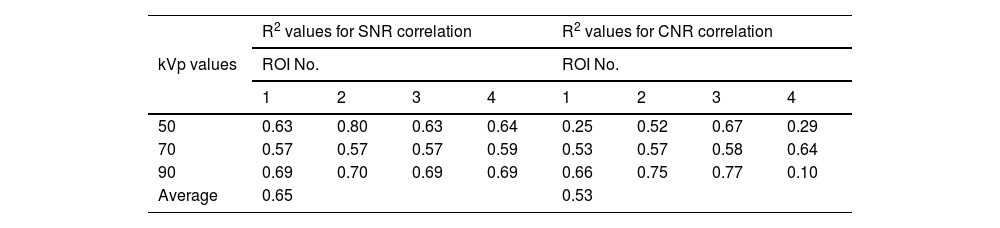
Pixel value validationThe coefficient of determination (R2) values has been used to examine the correlation degree between the trends of SNR and CNR values for the constructed phantom compared with that for the Gammex RMI© 610 phantom and their values. Regarding the trend of SNR and CNR values when changing kVp, it can be seen from Table 7 that when changing kVp values, the average correlation for SNR and CNR is 0.79 and 0.58, respectively. While when changing mAs values (Table 8), the average correlation for SNR and CNR is 0.65 and 0.53, respectively. The strength of aforementioned correlations were found to be strong positive for SNR values when changing both kVp and mAs, see Tables 7 and 8. While the correlation strength of CNR values were found to be moderate when changing both kVp and mAs (Tables 7 and 8).
The correlation values (R2) between the SNR and CNR for the phantom compared with that for Gammex RMI© 610 phantom for different ROI during changing kVp values (from 50 to 110 by 5 increment).
| mAs values | R2 values for SNR correlation | R2 values for CNR correlation | ||||||
|---|---|---|---|---|---|---|---|---|
| ROI No. | ROI No. | |||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| 1 | 0.81 | 0.80 | 0.83 | 0.82 | 0.65 | 0.81 | 0.79 | 0.47 |
| 2 | 0.76 | 0.79 | 0.78 | 0.77 | 0.56 | 0.76 | 0.75 | 0.64 |
| 3.2 | 0.73 | 0.80 | 0.79 | 0.77 | 0.35 | 0.74 | 0.69 | 0.40 |
| 4 | 0.61 | 0.76 | 0.72 | 0.66 | 0.00 | 0.56 | 0.72 | 0.19 |
| 5 | 0.88 | 0.94 | 0.89 | 0.89 | 0.74 | 0.87 | 0.88 | 0.01 |
| Average | 0.79 | 0.58 | ||||||
The correlation values (R2) between the SNR and CNR for the phantom compared with that for Gammex RMI© 610 phantom for different ROI during changing mAs values (from 1 to 5 by 1 increment).
| kVp values | R2 values for SNR correlation | R2 values for CNR correlation | ||||||
|---|---|---|---|---|---|---|---|---|
| ROI No. | ROI No. | |||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| 50 | 0.63 | 0.80 | 0.63 | 0.64 | 0.25 | 0.52 | 0.67 | 0.29 |
| 70 | 0.57 | 0.57 | 0.57 | 0.59 | 0.53 | 0.57 | 0.58 | 0.64 |
| 90 | 0.69 | 0.70 | 0.69 | 0.69 | 0.66 | 0.75 | 0.77 | 0.10 |
| Average | 0.65 | 0.53 | ||||||
Regarding results of the tissues radiological characteristics (bone, Lung and soft tissue), it appears that there are considerable agreement 1-year-old cortical bone child and its tissue substitute (P.V.C.) (Table 3), this agreement (percentage difference) reflects the simulation ability of P.V.C. as theoretical radiological equivalent substitute. Also, the agreement between lung neonate and Cork (Table 4) approved high ability of cork material to be radiological substitute for neonate lung. Finally, the percentage agreement in radiological simulation between water and all the soft tissues (Table 5) showed that water can be reliable substitute for soft tissues in imaging phantom despite the low solidity of the phantom as a result of using water. This issue can be controlled by using a solid vessel made from PMMA, which is a soft tissue substitute as well, and thus, would not affect the x-ray image of the phantom.
Phantom validation methodCT Hounsfield unit validationThe CT (HU) quantities (Table 6) indicate a high agreement between the constructed phantom materials and the real patient tissues. These levels of CT HU agreement are related to the X-ray attenuation coefficient and, thus, refers to high experimental validity of materials used in the phantom.31 Percentage differences values from Table 6, between spinal column and ribs from the patient and tissues their substitutes (P.V.C.), were recorded at >5%. Therefore, P.V.C. regarding the tissue classification system,21,32 is shown to be a class A substitute and listed within an tolerable error rang of 5%.21,32,33 While when comparing the neonate lung and cork, the percentage difference was 10.44%, which is considered as class B substitute.32 Such accuracy can be justified due to the nature of measuring lung which does not has block of tissues to measure but instead has tissue with air gabs, then, the measuring of HU would give more variations.
Pixel value validationThe high correlation found between the two phantoms regarding the physical measures of image quality (Tables 7 and 8), where moderate and strong correlations were found. After considering size difference between the 0-year-old phantom (Gammex RMI© 610) and the one-year-old phantom (the constructed phantom). Since SNR is a reliable measure of physical image quality and is widely used in optimisation researches,30 such correlation strength can be evidence for phantom validity as well as evidence for the high level of structural and material simulation of the phantom because of the similarity in responses when changing exposure factors (kVp and mAs). Hence, the production of the subsequent age representative X-ray images is achievable using the constructed phantom.
ConclusionA number of validation approaches were undertaken to investigate the capability of the constructed phantom in generating reliable radiographs for optimisation studies. Studying the radiological interactions of different tissues substitutes showed relatively low percentage differences, which lead to a conclusion that the most suitable material would be P.V.C., cork and water as substitute for cortical bone, lung and soft tissues, respectively. Regarding the CT validation method, the result of percentage differences showed high correlations this means that the materials used in construction of the phantom would replicate the radiological characteristics of a human one-year-old chest. On the other hand, pixel value validation demonstrated a moderate and strong correlation between the trend in physical image quality of a commercial valid phantom and our phantom images, at different exposure factors. The results from the experiments lead to evidence that the constructed one-year-old chest phantom is valid for optimisation studies and general radiography. In terms of availability, the radiological equivalent materials that used within this chest phantom are widely accessible with a relatively low cost, this makes manufacturing process quite simple. Also, CT human data are widely available and require no simple knowledge to operate. In summarizing conclusions, the phantom is valid for optimisation studies and can be formed from low-cost and available materials with fairly simplicity in construction. It is expected for these materials and construction method to have worldwide utility in places that lack financial support and access to high-end commercial anthropomorphic phantoms.
However, this work has some limitations related to the process of phantom manufacturing, in general, for example; several tissue substitutes are classified for industrial confidentiality purposes and are not, in general, available.34,26 Another example is the manufacturing process during the preparation and geometrical formation of tissue substitutes materials.32 Therefore, the inclusion of lung nodules in their real anatomical shape together with the rest of lung tissue is difficult and would necessitate using other technologies such as 3D printing, which still in developing process.35,36 However, this limitation can have slight effect on the phantom when used in optimising and lesion visibility studies because there are lesion equivalent balls that can be inserted inside the cork to represents the visibility of a lesion or a nodule (small lesion ball). on the other hand, specific limitations in the constructed phantom are existed despite it was constructed in a way to take into account the anatomical and radiological accuracy. One, the geometry of the clavicle does not reflect exactly the shape of real patient. Two, phantom handling is not simplified because all of the phantom parts are removable and need to be placed in the specific anatomical area. Three, it would be useful to specifically simulate the material destinations of the thorax such as bony components (including: vertebrae, costal arches, sternum and clavicles) and soft tissues within the both lungs). However, their relatively close radiational characteristics lead to neglect their effect on image quality when undertaking dose optimization studies. In Future, significant improvements on the phantom can be achieve by using some advance machines such as the 3D cutting machines and the above limitations can be tackled. One last point to declare is that, using the construction method in this work, other studies are needed to construct phantoms for other paediatric ages; 5, 10 and 15 year-old.
FundingThere was no source of funding.
Conflict of interestThere is no conflict of interest statement to be declare for this study.
The author gratefully thanks the support from: AL-Zahraa University for Women, Karbala, Iraq.