Endometriosis is common in women of reproductive age; it can cause pelvic pain and infertility. It is important to diagnose endometriosis and to thoroughly evaluate its extension, especially when surgical treatment is being considered. Magnetic resonance imaging (MRI) with careful examination technique and interpretation enables more accurate and complete diagnosis and staging than ultrasonography, especially in cases of deep pelvic endometriosis. Furthermore, MRI can identify implants in sites that can be difficult to access in endoscopic or laparoscopic explorations.
In this article, we describe the appropriate MRI protocol for the study of pelvic endometriosis and the MRI signs of pelvic organ involvement. It is necessary to know the subtle findings and to look for them so we can ensure that they are not overlooked. We describe clinical grading systems for endometriosis and review the diagnostic efficacy of MRI in comparison with other imaging techniques and surgery.
La endometriosis es una afección frecuente en mujeres en edad reproductiva, que puede causar dolor pelviano e infertilidad. Es importante el diagnóstico correcto y evaluar de forma completa su extensión, especialmente cuando se plantea un tratamiento quirúrgico. La resonancia magnética (RM), con técnica de exploración e interpretación radiológica cuidadas, permite un diagnóstico y estadificación más precisos y completos que la ecografía, especialmente en la endometriosis pelviana profunda. Además, en RM se pueden identificar implantes en localizaciones de difícil acceso para exploraciones endoscópicas o laparoscópicas.
En este artículo describimos el protocolo de RM adecuado para estudiar la endometriosis pelviana, detallando la semiología en RM de la afectación en los órganos de la pelvis. Es necesario conocer y buscar hallazgos sutiles para evitar que pasen desapercibidos. Se describen sistemas de gradación clínica de la endometriosis y se revisa la eficacia diagnóstica de la RM comparada con otras técnicas de imagen y con la cirugía.
Endometriosis is defined as the presence of glandular and endometrial stromal tissue outside the uterine cavity. It is a common disease. There can be endometriotic foci in up to 10 per cent of women of reproductive age who many times remain asymptomatic. It is one of the leading causes of infertility and pelvic pain and, although the symptoms are highly variable, they are usually associated with the location of the implants.1,2
The presence of ectopic endometrial tissue is usually considered the effect of metastasic implants that reach the peritoneal cavity due to retrograde menstruation and that may also spread along the vascular route or in surgical scars. Other authors support the metaplastic theory–a differentiation of peritoneal cells toward the endometrial tissue.2
Even though endometriotic foci may be diagnosed in any organs, the pelvic location is by far the most common of all. There are three (3) different types of affectation due to endometriosis in the pelvis: ovarian endometriomas, endometriotic implants in the peritoneal surface, and deep pelvic endometriosis.1 The affectation of at least 5mm underneath the peritoneal surface is defined as deep. The deep implants are usually associated with fibrous or smooth muscle proliferation and commonly associated pelvic pain and reproductive difficulties.3
The clinical symptomatology is highly variable and physical examination is not very reliable, this is why the diagnosis of endometriosis is not easy and is usually delayed 7–10 years since symptom onset.4 Ovarian endometriotic cysts are usually diagnosed through an ultrasound, although the magnetic resonance imaging (MRI) may be useful in some cases, since it allows a more specific diagnosis of endometrioma and better differentiates blood clots from blood debris in solid areas that are more common of neoplastic cysts.5,6 Also, the MRI has a higher sensitivity than the ultrasound for the detection of deep endometriotic implants in some locations of the pelvis, that sometimes are harder to diagnose through laparoscopy due to the presence of adhesions and their subperitoneal location.7 The definitive diagnosis of endometriosis is based on the histological study of implants.8
The management of endometriosis is initially medical, while surgery is indicated in cases that do not respond well, or in the presence of complications. Surgery should be conservative, especially to preserve fertility, yet a complete resection of the implants should be attempted, in particular with deep implants. That is why knowing the exact location and spread of the disease is important before the surgery.3
This paper discusses the MRI protocol for the study of pelvic endometriosis, and details the MRI semiology of the affectation of pelvic organs. The clinical grading scales of endometriosis are described and the diagnostic effectiveness of MRI is reviewed.
Technique of pelvic magnetic resonance imaging for the study of endometriosisThe exploratory method of MRIs for the assessment of pelvic endometriosis is a study similar to the study of other gynecological conditions, although for an adequate detection of pelvic implants, it is important to take care of details for the sake of study technique and image interpretation.9
One high field magnet and anterior and posterior multi-element anthenas are recommended. 3T machines can give us higher spatial resolution than 1.5T machines, but this also comes with more MRI artifacts. The study can be performed any day of the menstrual cycle, and knowing the date of the last period may help us interpret some MRI findings.10
The application of gel into the vagina, and gel or water into the rectum may facilitate the detection of implants in the wall of these organs (Fig. 1).11 For the same reason, it is better if the bladder shows moderate repletion that may be achieved not urinating 1h before the study. If the bladder is too filled it may include movement artifacts and even modify the anatomy of the pelvis.
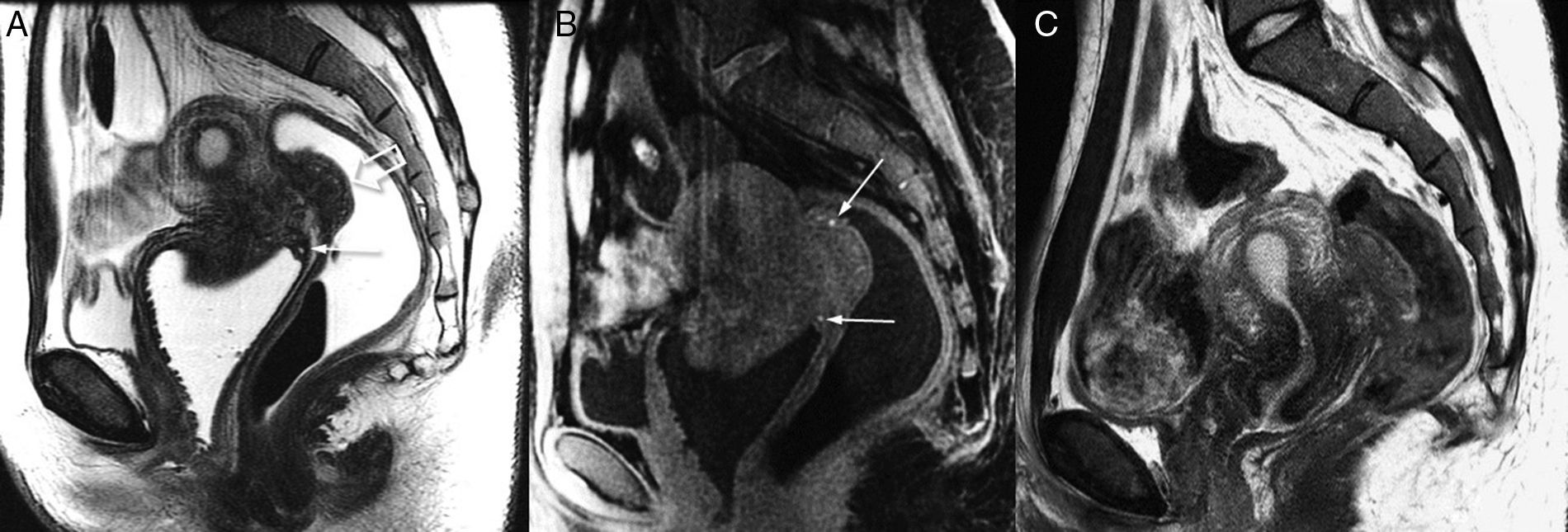
Deep endometriosis of rectum wall and fornix vaginal. Utility of applying gel into rectum and vagina (3T). T2-weighted sagittal image (A) showing hypointense wall thickening of vaginal posterior recess with small hyperintense glandular foci (arrow). The significant focal thickening of rectal wall (hollow arrow) is indicative of deep affectation, although it does not reach the rectal lumen: in the posterior endoscopy, it was described as extrinsic compression. In the T1-weighted image with fat suppression (B) small hemorrhagic hyperintense foci may be seen (arrows) both in the rectal injury and the vaginal wall. The endometriotic affectation of rectum and vagina is in continuity with the implant in the lowest part of the pouch of Douglas. As a correlation (C) one T2-weighted sagittal image of the MRI study of this same patient acquired weeks before is shown; such image contributed to the diagnosis of pelvic endometriosis (unsuspected clinically), yet the assessment of the spread of affectation is poorer due to the lack of vaginal, rectal, and urinary bladder distension.
To reduce artifacts due to intestinal peristalsis, fasting for 4–6h before the study is recommended, and if artifacts persist, they may also be reduced through the injection of anti-peristaltic drugs like glucagon or butilescopolamine. A moderate compression on the pelvis, spatial saturation bands, while changing the sentence and frequency coding may also help reduce the occurrence of artifacts. Very fast T2-weighted sequences (SSTSE or SSFSE) are another option to take into consideration if there is still movement and especially if the patient is uncomfortable or suffers from claustrophobia, and in some works they have showed good diagnostic efficiency,12 although they should not replace the high-resolution T2-weighted sequences that will be discussed now.
MRI studies are based on T2-weighted (fast or turbo SE) sequences without fat suppression, acquired in three (3) orthogonal planes, though they may be oblique based on the position of the uterus or other pelvic organs. The acquisition of one TSE 3D sequence and reconstruction in differente planes is another alternative if we want to reduce the study time,13 although the T2-weighted contrast agent is a little different and may show even more artifacts (Fig. 2). Also it is essential to acquire T1-weighted images with and without selective fat suppression in order to detect and characterize the hemorrhagic foci (Fig. 1).9
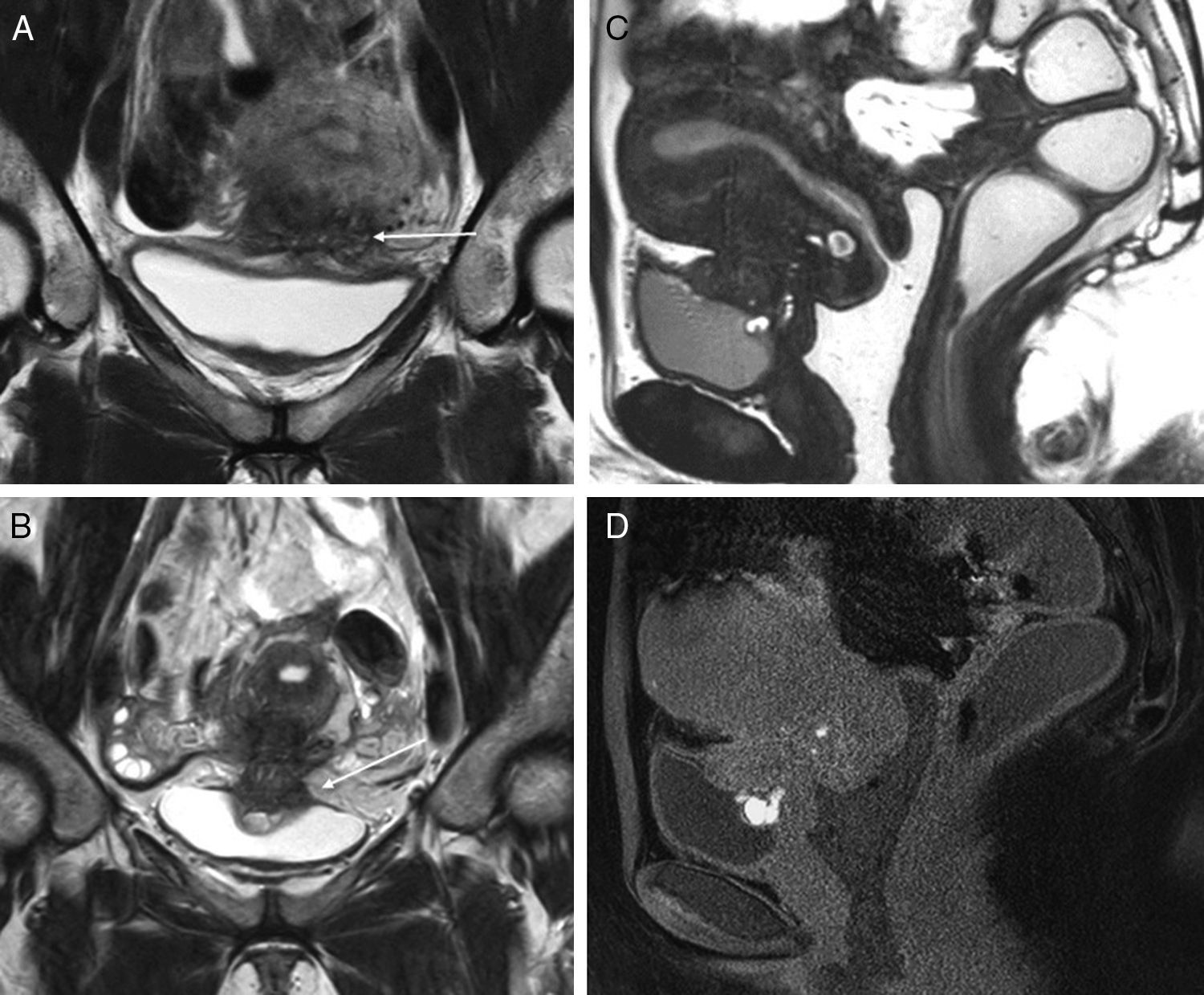
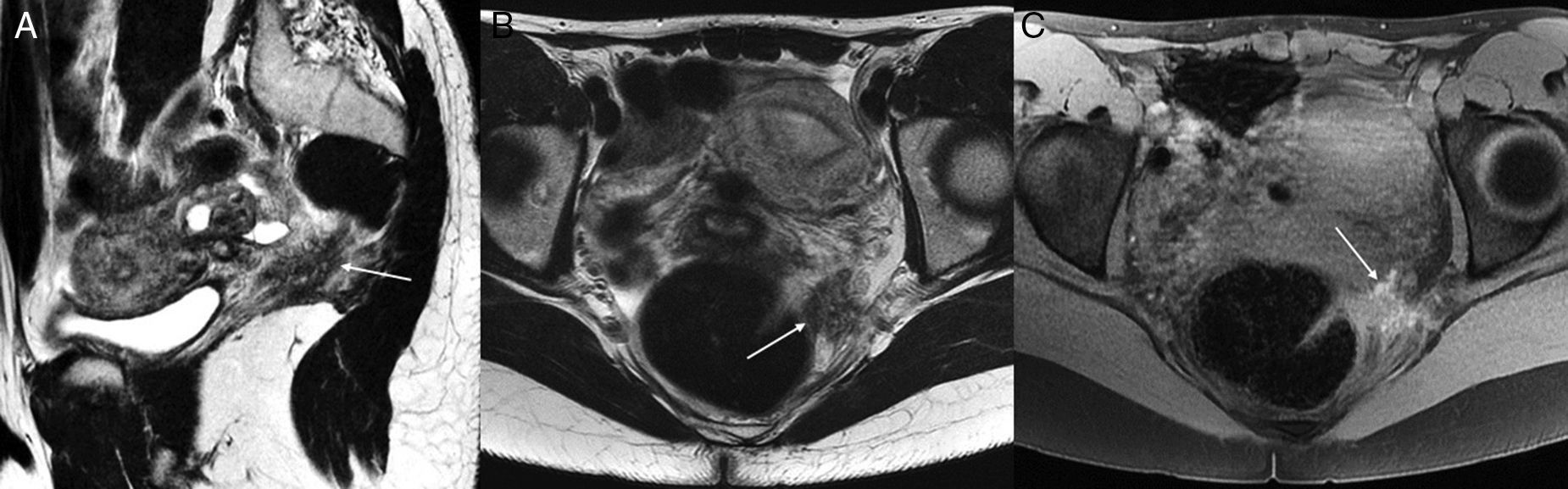
Implants in the anterior compartment of the pelvis (3T). The endometriotic implants affecting the urinary bladder wall are usually consistent with peritoneal implants in the anterior compartment that later have a profound infiltration. A shows one coronal TSE T2-weighted image of an implant in the vesicouterine recess (arrow), with infiltration of the uterine anterior side and subperitoneal fat in the region of the vesical dome, but no affectation of the whole thickness of the bladder wall. Images B and D show another case with affectation in the same area, but with more profound infiltration of the myometrium and the vesical wall, with clear signs of detrusor muscle affectation (thickening and glandular and hemorrhagic foci very close to the lumen of urinary bladder). No affectation of the posterior compartment of the pelvis. Image C shows one 3D TSE acquisition with great spatial resolution (cutting thickness 1.6mm) and contrast between different soft tissues is slightly different to the 2D TSE-weighted sequences (images A and B). D is a gradient-echo T1-weighted image with fat suppression showing hemorrhagic hyperintense foci.
Recent studies suggest that susceptibility weighted imaging (SWI) may have a higher sensitivity than T1-weighted images for the detection of hemorrhagic foci in pelvic endometriosis.14,15 However, pelvic SWIs have too many artifacts and they are not sequences of common clinical use, which is why the outcomes should be interpreted with caution and always in correlation with other sequences.
Although some authors use IV contrast for the assessment of endometriosis,16 we do not use gadolinium contrasts on a routine basis. Deep endometriotic implants have an important fibrous component, which this is why they capture the late stages of contrast, but other inflammatory or tumor injuries and anatomical structures like pelvic veins do exactly the same, and this is why post-contrast sequences do not add anything new to diagnosis.17 On the other hand, IV contrast is useful for the assessment of complex adnexal injuries as long as the T2 and T1-weighted images with and without fat suppression do not show any specific characteristics and on suspicion of malignant neoplasms.18
We conducted our MRI studies using 1.5 and 3T machines upon availability. The study is based on T2-weighted 2D images in three (3) planes (without fat suppression, 3–4mm-cut thickness) and T1-weighted 3D images with and without fat suppression. Gel is applied into the vagina and rectum on suspicion of implants in the compartment located posterior to the pelvis. In the presence of movement artifacts, we added fast SSTSE sequences, or injected anti-peristaltic drugs. We did not think that the SWI sequences were useful due to the low quality of the images obtained.
In our usual protocol we also acquire a series of diffusion-weighted imaging (DWI). It is a fast sequence that helps in the detection of unsuspected injuries (neoplastic, inflammatory, adenopathies), although its utility for the differential diagnosis of endometriosis is limited since endometriotic imlants may show diffusion restriction similar to other tumor or inflammatory injuries of the pelvis.9
MRI semiology of pelvic edometriosisOf the three (3) types of pelvic endometriosis that exist, the most difficult one to diagnose in an MRI are implants in the peritoneal surface that in the laparoscopy may be of millimetric sizes.19 In the MRI they can only be properly diagnosed when they show hemorrhagic contents like hyperintese foci in the T1-weighted with fat suppression.
Peritoneal implants may cause adhesions between the pelvic organs and the intestinal loops. In the pelvic MRI in women with endometriosis it is a common finding that ovaries are located in a lower position, centered in the pelvis, while the uterus remains in retroflexion. Also we may find hypointense bands among organs (Fig. 3), “peaks” of retraction in the bowel wall or loculations in the peritoneal fluid. In the transvaginal ultrasound, the diagnosis is even harder, even though in expert hands, adhesions when moving the pelvic organs may be detected.20
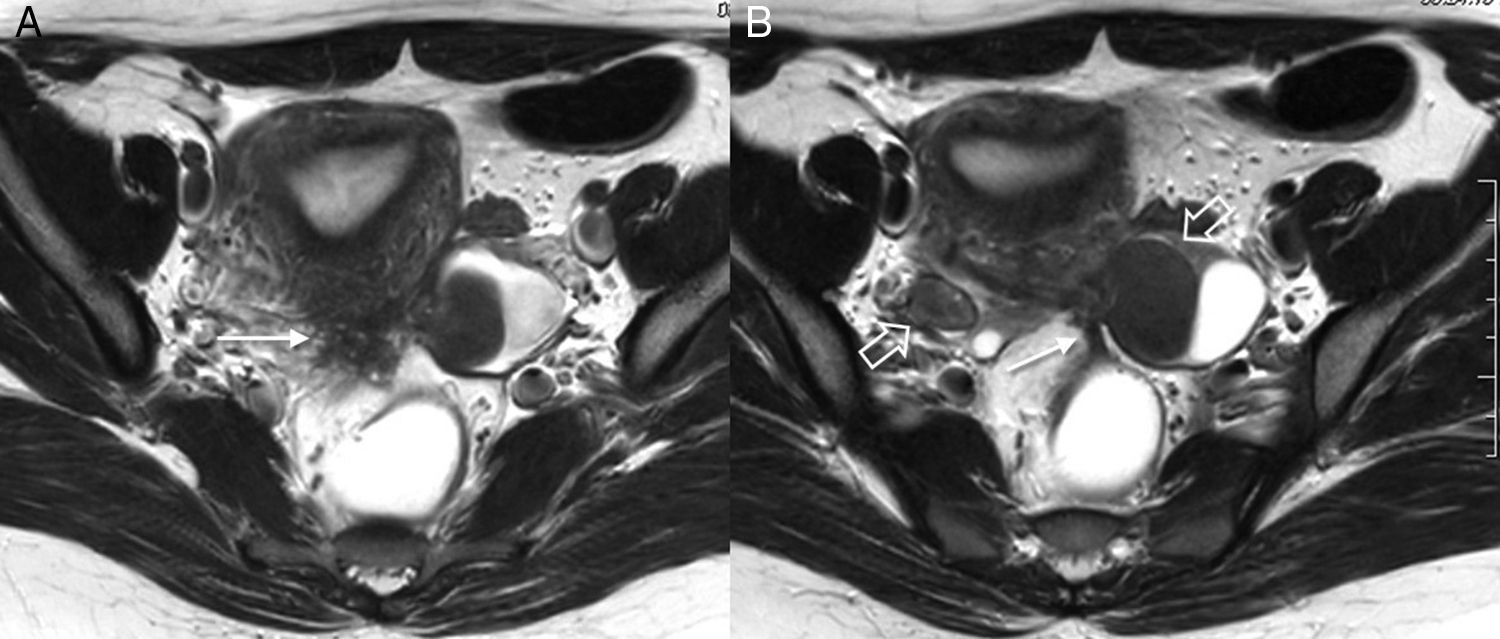
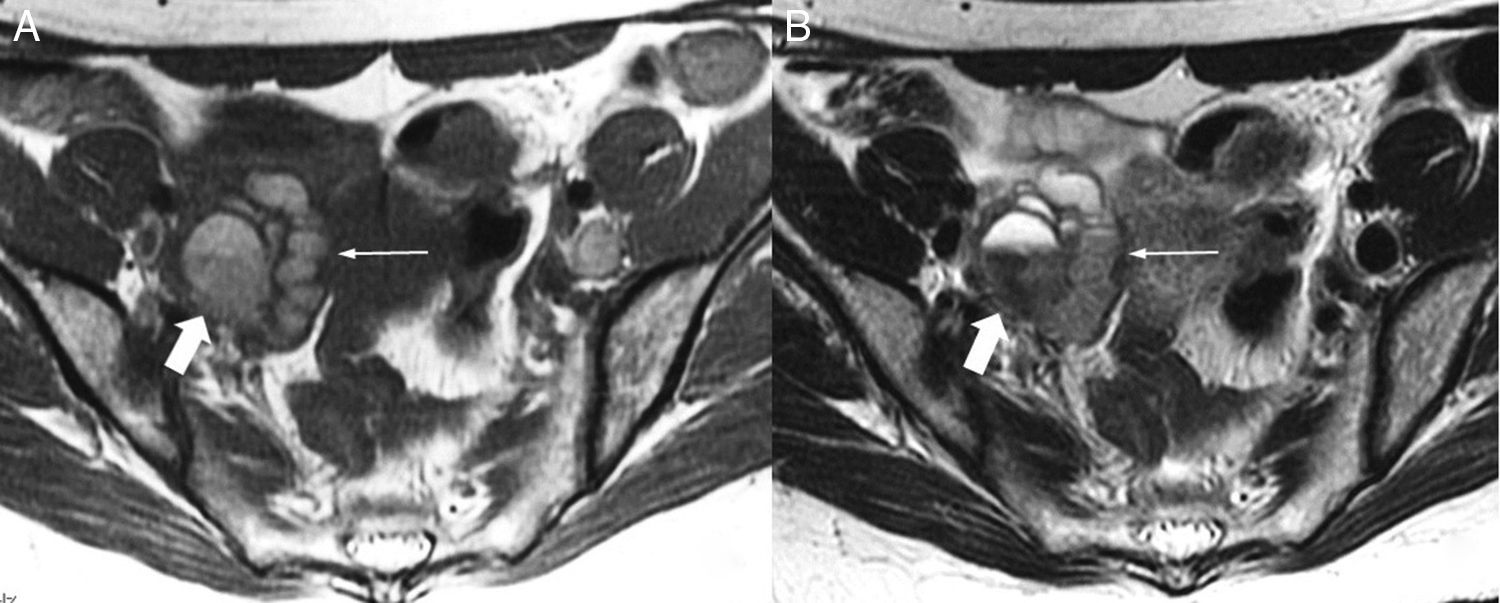
Implant in the posterior uterine side with adhesions to ovaries and rectus bridle. Axial TSE T2-weighted images (3T). Endometriotic implant identified in the posterior side of the uterus in the uterine torus region (arrow in image A). Both ovaries (hollow arrows in image B) are located behind the uterus with adhesions between the uterus and the ovaries and endometrioma in the left ovary. Also, in this case we can see one fibrous band due to adhesions between the anterior side of the rectum and the uterine implant (long arrow in B).
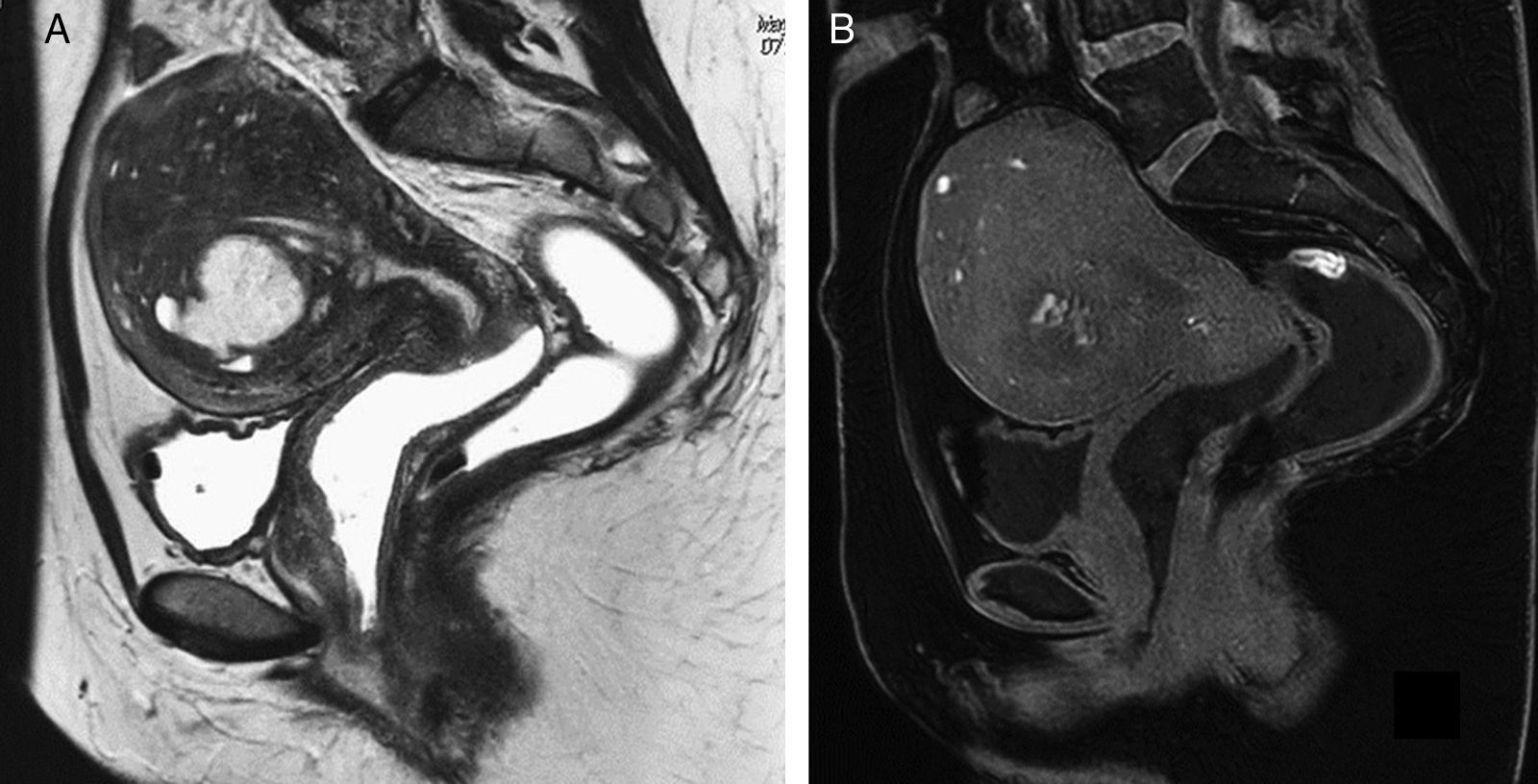
The ovary is the most common location of endometriosis and the most common imaging findings are endometriomas or endometriotic cysts. These cysts are due to cyclic bleeding of the endometrial tissue and their content is consistent with products of blood decay in different evolutionary stages. Endometriomas may be diagnosed through an ultrasound,5 but the MRI semiology is more specific: usually hyperintese cysts in the T1-weighted images with fat suppression, and shading in T2-weighted images. It is interesting to mention here that the loss of signal in the T2-weighted images is a very specific sign of endometrioma whether intense, subtle, in layers or with “debris” kind of content (Fig. 4). The identification of multiple cyst ovaries or hyperintense foci in the T1-weighted images, even without loss of signal in the T2-weighted images, is also suggestive of endometriosis.21,22
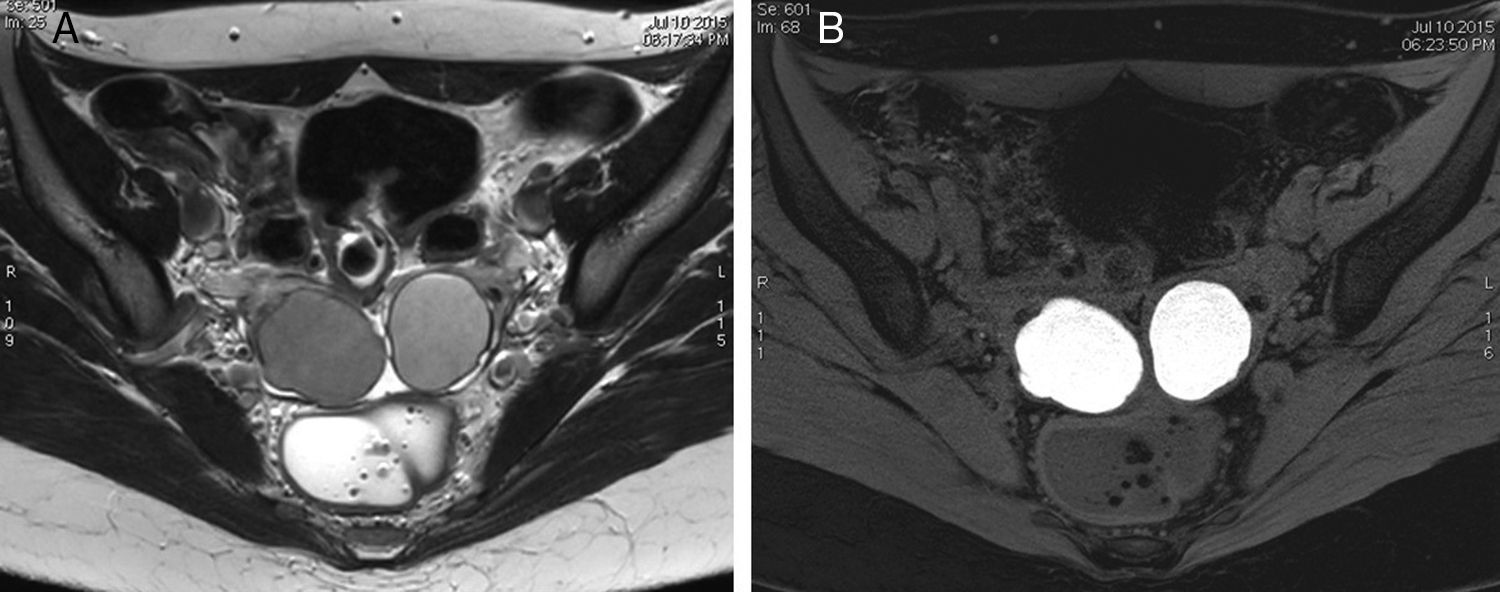
Bilateral ovarian endometriosis (3T). (A) TSE T2-weighted image. (B) Gradient-echo T1-weighted image with fat suppression. Both ovaries are located in a more central and posterior position than usual and in both ovaries we can see cysts with typical signs of endometriomas: they are hyperintense in the T1-weighted image with fat suppression (B), while in the T2-weighted (A) they show slight shading and a lower than water signal; in this case no fluid-fluid levels can be identified. These characteristics are typical of endometriomas.
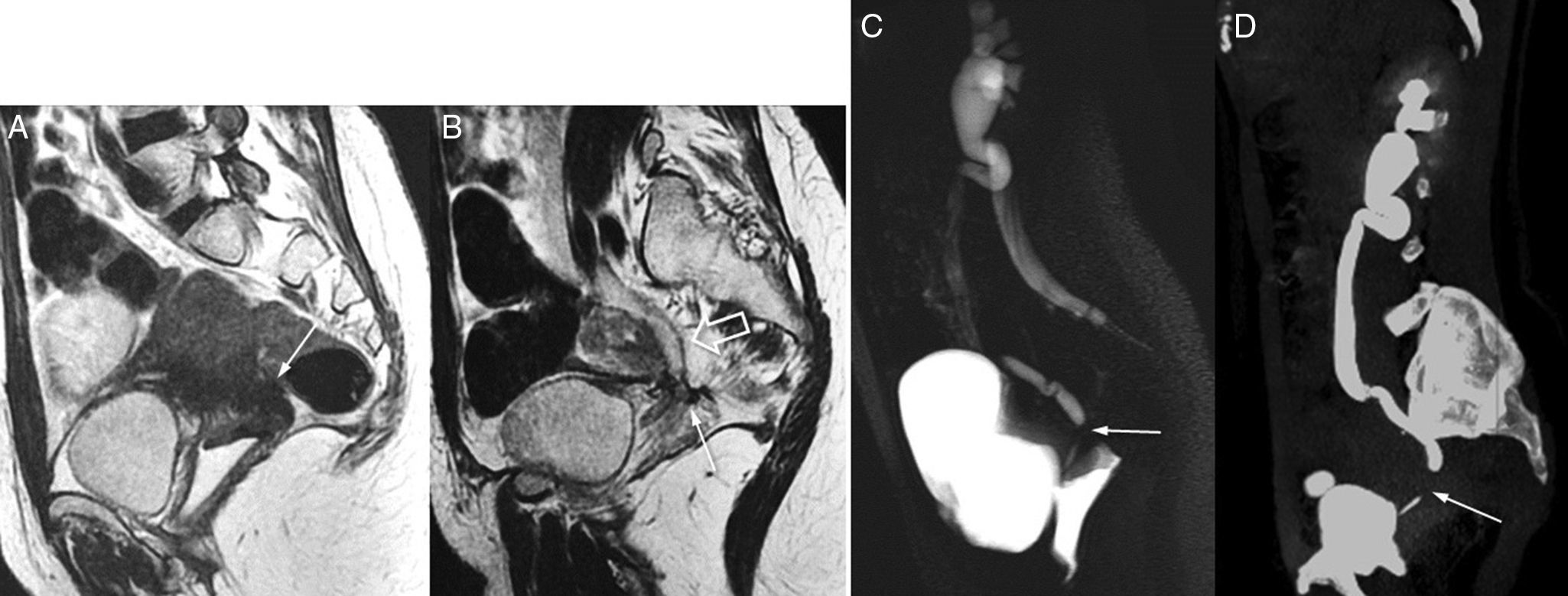
Another almost pathognomonic finding of endometriosis is hematosalpinx.22 One Fallopian tube dilated due to hyperintense content in the T1-weighted images is diagnostic of hematosalpinx. If the content shows low signal in the T2-weighted images, it may simulate one endometrioma but it is usually easy to differentiate it as long as we can identify its inner serpiginous morphology or incomplete septa (Fig. 5).
Hematosalpinx (1.5T). The identification of Fallopian tube dilation due to blood content is diagnostic of endometriosis. The diagnosis of hematosalpinx is easy when we identify one serpiginous tubular structure or with incomplete septa, with hyperintense signal in the T1-weighted image (image A, long arrow) and hypointense signal or shading in the T2-weighted image (image B). The infundibular region of the tube may be too dilated due to blood content, and simulate one ovarian cyst, which is why it can be hard to distinguish it from one endometrioma (thick arrow).
Deep implants may be useful in MRIs since the fibrous component is predominant (hypointense in the T2-weighted images, and intermediate in the T1-weighted images) with few or no glandular or hemorrhagic foci. Although deep endometriosis may be present in all locations of the pelvis, the most common affectation is retrouterine (v. Fig. 3) located in the posterior compartment of the pelvis with an obliterated pouch of Douglas at the bottom.16,23
Here we should pay special attention to any hypointense tissue invasions, thickenings or retractions in the most common locations: uterine posterior side, retrocervical area, uterosacral ligaments, vaginal vault (posterior formix), rectovaginal septum, and the rectosigmoid anterior side. The presence of hemorrhagic foci facilitates and secures diagnosis, but any hypointense, nodular o in plaque thickenings of any of these structures that in the T2-weighted images should be suspicious.23 Affectation due to endometriosis spreads by continuity in these structures that are posterior to the pelvis (v. Fig. 1), but we should also pay special attention to any possible isolated endometriotic foci in these organs.
When implant surgery in the sigma rectum pouch is being planned it is important to determine if there is a profound affectation of the bowel wall since this usually conditions resection and colorectal anastomosis. Sometimes it is not easy to determine through an MRI if one rectal endometriotic implant is superficial or deeply infiltrated, but in the presence of deformity and thickening of the rectal or sigmoid “C” or “mushroom cap” wall, it usually implies affectation of the muscular wall.24
With much less frequency (<10 per cent) endometriotic implants affect other parts of the proximal intestinal tract different from the sigma rectum pouch: small bowel loops, caecum, or appendix. The diagnosis of these injuries is hard to achieved through an MRI of the pelvis and on clinical suspicion of small bowel affectation, a study through magnetic resonance enterography (MRE) (Fig. 6),25 or computed tomography (CT) scan may be indicated.
Intestinal endometriosis. Endometriotic implants located in small bowel loops may be identified through MRI studies of the pelvis, but on clinical suspicion of small bowel affectation it is better to perform one MR-enterography since the fluid-induced distension of the loops facilitates the detection of the implants in the bowel wall. This patient presented to the emergency room with clinical manifestations of intestinal obstruction, and the portal phase CT scan with IV iodinated contrast and without oral contrast (A) showed one incomplete obstruction due to one injury of extrinsic appearance in an ileal loop of the pelvis (arrow) with adhesions between loops. Management was conservative. One week later one MR-enterography was performed (B and C, axiales SSTSE, 3T-weighted images) that identified two (2) endometriosis implants with small bowel affectation, one in the pelvis and the other more cranial (arrows).
The affectation of the uterine body associated with endometriosis may be due to peritoneal implants (in the serosa of the posterior or anterior side) that later infiltrate profoundly while affecting the endometrium and usually associating adhesions (v. Fig. 2).
Histologically, in the adenomyosis there is also presence of endometrial tissue in the myometrium, but in this case it is due to spread from the endometrial cavity, which is why adenomyosis is not included in the concept of deep endometriosis.
In the MRI, adenomyosis looks like a thickening of the myometrial junction (highly suggestive of adenomyosis when thickness >12mm–more common in the posterior side) and in some cases glandular foci (hyperintense in the T2-weighted images) and/or hemorrhagic foci (hyperintense in the T1-weighted images) may be seen that will allow a more specific diagnosis (Fig. 7).22,26,27 Affectation of the internal myometrium may be diffuse but also nodular, and we may call adenomyomas to the myometrial endometriotic nodules that, sometimes, may be difficult to distinguish from myomas in the MRI. This has one histological explanation, since the presence of glandular and endometrial stromal tissue in the myometrium conditions smooth muscle hypertrophy and hyperplasia.
Extensive uterine adenomiosis (3T-weighted image). Image A (sagittal TSE T2-weighted image) shows significant hypointense thickening of the myometrial junction and inside this junction we can see hyperintense foci consistent with glandular areas. In the T1-weighted image with fat suppresion (B) some of these foci are hyperintense, indicative of hemorrhagic content, which allows us to achieve a more specific diagnosis.
When there is affectation due to round or broad ligament endometriosis, these ligaments will be thickened and lead to an anomalous position of the uterus.28
Less common is the endometriosis of the anterior pelvic compartment where it may affect the anterior side of the uterus, the vesicouterine peritoneal recess (leading to adhesions), the urinary bladder and the vesicovaginal septum.
Affectation due to endometriosis in the urinary bladder may be located in the vesical dome and initiate in the serosa (due to one peritoneal implant), and from there profoundly infilatrate the wall.29 When there is affectation of the detrusor muscle, the urinary symptomatology is something common (dysuria, pollakiuria, and more commonly hematuria). Most of the times the cytoscopic exploration does not help achieve diagnosis because the vesical urothelium is usually normal, which is why the radiological diagnosis is that important. In the MRI it usually looks like a nodular thickening of the bladder, normally in its dome or in the vesicovaginal septum. If the bladder is moderately distended, it may be possible to assess accurately any muscle layer affectation (Fig. 2), but it is still hard to know for sure whether the implant reaches the lumen. Glandular and hemorrhagic foci are common findings.29,30
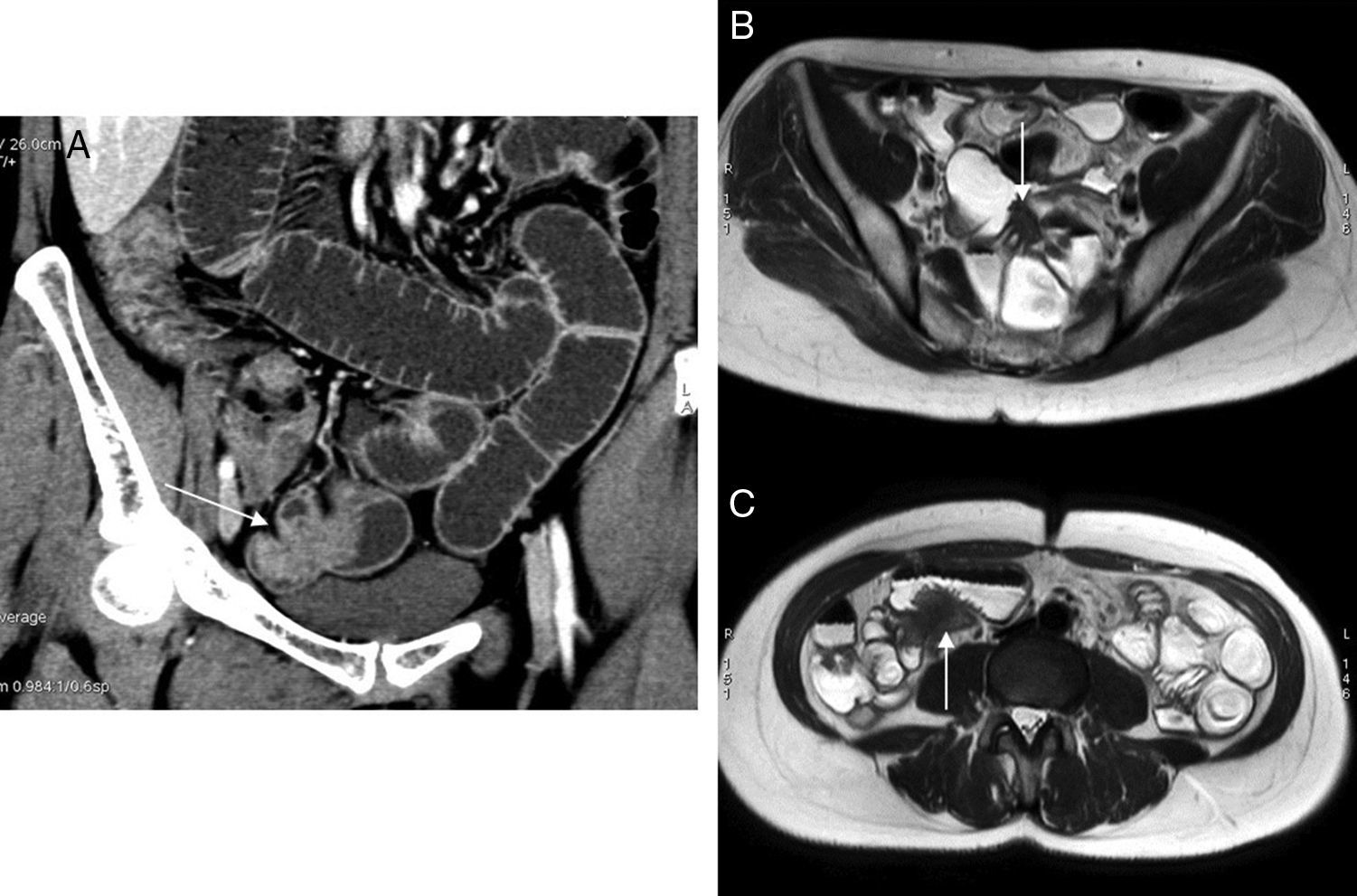
Although it is more rare, the diagnosis of a possible ureteral affectation is also very important, since it may lead to obstructive uropathies and renal function impairment. Ureter affectation is usually extrinsic due to one deep endometriotic implant that contacts or surrounds the ureter, most of the times in its pelvic trajectory.30,31 This is why when performing an MRI of a female pelvis with endometriosis we should always assess the trajectory of ureters looking for possible dilations, changes of caliber, or contact with a deep endometriotic implant that may be small and without hemorrhagic foci (Fig. 8). On suspicion of ureteral affectation, the MRI of the pelvis should be completed with a look at the patient's kidneys to rule out hydronephrosis.
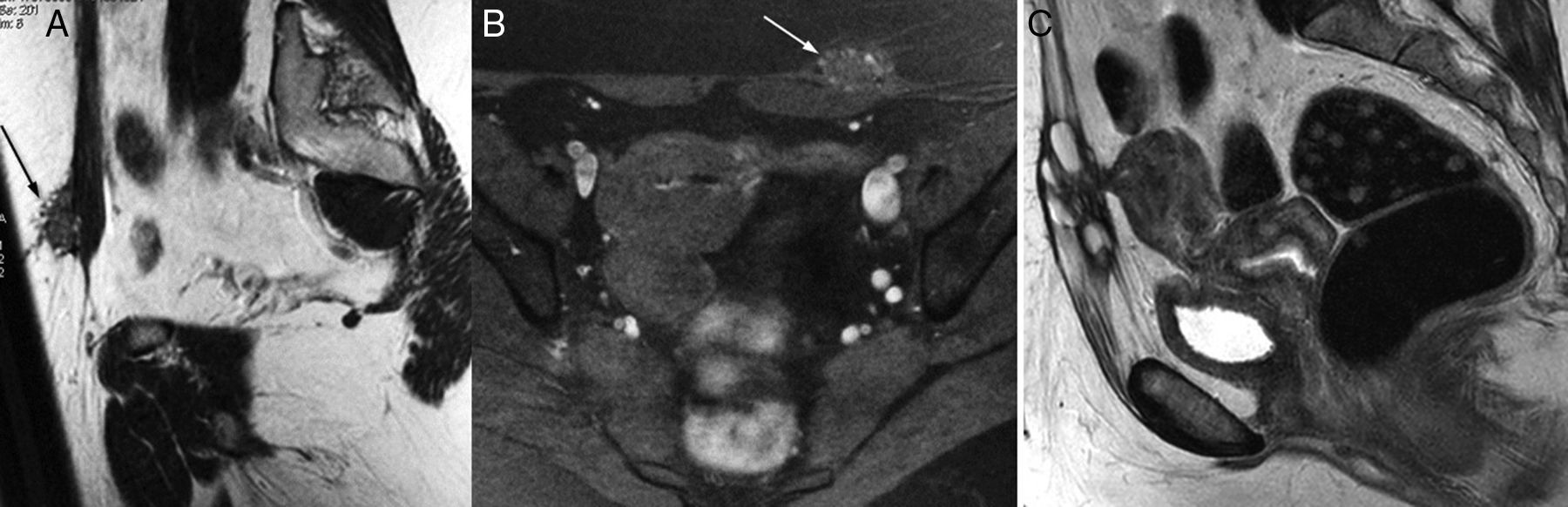
Ureteral affectation due to endometriosis. Left obstructive uropathy. (A and B) FSE T2-weighted images (1.5T) showing one endometriotic implant in the pouch of Douglas region and uterine torus (arrow in A) running across the left uterosacral ligament (arrow in B) and surrounding the pelvic ureter that is proximally dilated (hollow arrow). (C) In these cases it is very useful to complement the MRI study with thick cut, large field SSFSE T2-weighted images (MRI urography without IV contrast, acquisition time: 1–2s per cut). In this sagittal-oblique cut we can identify clear signs of obstructive uropathy, with pielocalicial system and proximal ureteral dilation, 1cm-long pelvic ureter stenosis (arrow) and non-dilated normal final section of the ureter. (D) Excretory phase CT urography CT; sagittal-oblique MIP reconstruction. Good correlation shown between the CT urography findings and the MRI findings without contrast.
As we already mentioned, the affectation of the vagina due to endometriosis usually occurs in its vault due to peritoneal implants in the posterior compartment that progress in depth. On suspicion of vaginal affectation it is recommended to re-apply gel into the vagina in order to distend the recesses and make a better assessment of its wall. The affection of the fornix posterior is more common and it may extend toward the rectovaginal septum. Isolated endometriotic implants in the anterior vagina wall or the rectovaginal septum are less common.23
Other common locations of pelvic endometriosis are trajectories or surgical wounds and, in particular, previous c-section scars. If the patient is in discomfort or shows nodular thickening in the area of the surgical scar, we should suspect presence of endometriotic implant, and although the ultrasound may be a good idea, the MRI allows us to achieve a more specific diagnosis since it usually detects small hyperintense foci in the T1-weighted images, which in turn allows us to distinguish endometriotic implants from hypertrophic scars or desmoid tumors (Fig. 9).32,33
Abdominal wall endometriosis (3T-weighted images). Endometriotic implants in surgical scars, especially after a c-section, are common. There are not always in midline location. One predominantly hypointense 2-cm nodule located in front of the left anterior rectus muscle fascia is shown with hyperintese areas in the T2-weighted images (A) and hyperintense areas in the T1-weighted images with fat suppression (B) consistent with glandular and hemorrhagic foci. It is characteristic of endometriosis and the location is consistent with the most lateral part of a Pfannestiel incision. (C) Sagittal TSE T2-weighted image of another patient showing one large endometriotic implant in a previous c-section scar of anterior midline location, with adhesion and uterine fundus retraction.
We can also find endometriotic implants in other pelvic scars like laparoscopy entry sites or episiotomies, but these are less common.32
There are other uncommon locations of deep pelvic endometriosis that, although rare, are interesting to know because they may lead to very peculiar symptomatology: extraperitoneal implants in the trajectory of the sciatic nerve (neuropathic pain of cyclical nature) (Fig. 10), or in the perianal region (perianal discomfort and rectal mass).34
Extraperitoneal deep pelvic endometriosis. Twenty-four year old-female with left sciatic radiating pain consistent with menstrual periods. One deep endometriotic implant may be seen (arrows) running across the left uterosacral ligament and contacting the mesorectal fascia and the levator ani muscle. It is also adjacent to the trajectory of the left sciatic nerve before leaving the pelvis. (A and B) Sagittal and coronal FSE T2-weighted images. (C) Axial T1-weighted image with fat suppression; 1.5T.
Several severity scales have been described in an attempt to measure how serious the affectation due to pelvic endometriosis really is.35 The most widely used in gynecology is the scale system described and reviewed by the American Society of Fertility for Reproductive Medicine (r-ASRM or r-AFS) that assigns a particular score to each and everyone of the injuries diagnosed based on their location, size, and affectation depth. By adding all these scores, endometriosis is categorized into four (4) different stages from minor to major condition, yet this categorization will not provide us with information on the location of the disease, and the correlation with clinical symptomatology is poor. Recently, German authors have come up with a different reviewed scale (r-ENZIAN score) that includes the exact pelvis location of deep endometriotic implants which makes this scale more suitable for surgical planning purposes; however, it is a more complex categorization and not as widely accepted. Even though this scale is based on surgical findings, it may be useful for staging purposes when performing MRIs.36
Differential diagnosis of endometriotic injuries in magnetic resonance imaging of the pelvisThe diagnosis of pelvic endometriosis through an MRI is usually very specific. Ovarian endometriomas are different from other cystic injuries in the low signal they show in T2-weighted images and in the high signal they show in T1-weighted images with selective fat suppression (here we should not use STIR, it can also annul the signal of methemoglobin). Unlike endometriomas, sebaceuous cysts (dermoid) reduce their signal with the specific fat saturation.21,22 In the presence of one ovarian cyst that is hyperintense in the T1-weighted images, the low signal shown in the T2-weighted sequences is specific of endometrioma compared to one hemorrhagic cyst, while the presence of multiple hemorrhagic cysts (even without shading in T2-weighted sequences) is highly suggestive of ovarian endometriosis.22
At times we may think of performing differential diagnosis with one cystic ovarian neoplasm, especially when content or septa in the cysts may be identified. In this case it is useful to inject IV contrast in order to distinguish the uptaking solid nodules of blood clots in the cyst.16,18 Conducting basal image subtraction with contrast facilitates the detection of uptake in all those possible solid nodules within the cyst that show high signal in the T1-weighted images. The overall incidence of ovarian tumors in women with endometriosis is low due to the age of onset of this condition. However, there are series that discuss higher incidences of ovarian carcinomas in patients with endometriosis (1.5–2×). Clear cell carcinoma and endometroid cancer are the most common subtypes, and their prognosis is better than that of other ovarian malignant neoplasms. The decidual changes in the ectopic endometrial tissue during pregnancy may simulate malignant transformation of the endometrioma,22 although the higher signal shown in the T2-weighted images and the lower thickness from the solid area may help while performing differential diagnosis.37
Deep endometriotic implants in other locations (rectum, bladder, vagina) may simulate primary tumors of these organs, yet we can achieve diagnosis thanks to the presence of hemorrhagic foci and the coincidence of adnexal endometriotic signs or in other areas of the pelvis, although there may be deep endometriosis without endometriomas in the ovaries.
The other way round is also possible since infiltrating malignant neoplasms in the pelvis may simulate or coexist with deep endometriosis. Due to their frequency and location we should pay special attention to differential diagnosis with parametrial infiltration following cervix tumors. T2-weighted images and DWIs of the uterine neck usually help achieve diagnosis.18
Diagnostic performance of magnetic resonance imaging in pelvic endometriosisAccording to the different papers published in the specialized medical literature, the diagnostic performance of MRIs in the study of pelvic endometriosis is varied, since there are significant differences among the studies published when it comes to MRI technique used, type of endometriotic injuries studied, and reference pattern used.
Medeiros et al. recently published one meta-analysis on the diagnostic efficiency of pelvic MRIs in the diagnosis of deep endometriosis.38 Such meta-analysis reviews 20 studies published comparing the findings from high field MRIs (1.0–3.0T) to surgical findings (laparoscopic or open) and histology as a reference pattern. In sum, the MRI studies showed 83 per cent sensitivity and 90 per cent specificity in the diagnosis of injuries due to endometriosis. The location that showed the highest sensitivity was the pouch of Douglas (sensitivity=89 per cent, specificity=94 per cent), while in the anterior compartment, the MRI sensitivity was lower (bladder sensitivity=64 per cent, specificity=98 per cent). This may be due the fact that since the posterior compartment is the most common location of deep endometriosis, the MRI is more precise though it is also true that, sometimes, in these MRI studies we do not pay as much attention to the bladder (right degree of depletion, detailed review of images).
There are very few studies published where the diagnostic effectiveness of the MRI and the transvaginal ultrasound in pelvic endometriosis is compared directly. In Grasso's series,39 the transvaginal ultrasound showed similar effectiveness to the MRI in certain pelvic locations (ovaries, uterus, vagina), yet the MRI better detected the implants in the sigma rectum pouch, the bladder or extraperitoneal affectation. In studies published in gynecological journals, Bazot40 finds that both transvaginal and transrectal ultrasounds had similar effectiveness to the MRI for the diagnosis of bowel implants, but that the MRI was significantly better for vaginal and uterosacral ligament affectations. In Gauche Cazalis’ comparative study,41 the transvaginal ultrasound was the best modality for the diagnosis of endometriomas, the transrectal ultrasound was the best modality for rectosigmoid and rectovaginal septum affectation, while the MRI was the most effective modality for the affectation of uterine torus, uterosacral ligaments, and bladder. In Saccardi's study,42 the MRI (using a 1.0T machine) was far better than transvaginal ultrasound for the diagnosis of posterior deep endometriosis, but with the use of a system that keeps the vagina distended while induced by serum (sonovaginography) they achieved similar results to those of the MRI. However, in the study by Abrao's Brazilian team,43 the MRI performed in an external center was inferior to transvaginal ultrasound and only slightly better than clinical exploration. In general, most authors agree that the initial imaging exploration should be the ultrasound, that the MRI is a good additional exploratory tool on suspicion of deep pelvic endometriosis, and that the results coming from the ultrasound are not conclusive or when planning surgical treatment.
ConclusionMRIs of the pelvis are more widely used in the assessment of women with endometriosis.
The initial imaging study on suspicion of endometriosis should be a detailed ultrasound exploration. MRI studies are beneficial for the characterization of some adnexal injuries detected in the ultrasound and also for the diagnosis and complete staging of affectation due to pelvic endometriosis. Deep endometriotic implants are usually the most symptomatic of all and their clinical and ultrasound diagnosis may not be easy.
When the MRI is used for presurgical planning it is important to diagnose and locate all endometriotic implants (deep ones in particular) so that the surgical treatment may be effective with a complete resection of the implants, and conservative.
This is why an adequate study protocol for MRIs is necessary; also it is also important to know the MRI semiology of the different forms of pelvic endometriosis that exist. One detailed review of the images for a good diagnosis of deep endometriotic injuries is necessary since, most of the times, they are very subtle in the MRI because the fibrotic component is usually predominant. Lastly, the radiological findings should be adequately communicated to gynecologists in plain language.
Ethical disclosuresProtection of people and animalsThe authors declare that no experiments with human beings or animals have been performed while conducting this investigation.
Confidentiality of dataThe authors confirm that in this article there are no data from patients.
Right to privacy and informed consentThe authors confirm that in this article there are no data from patients.
Authors- 1.
Manager of the integrity of the study: RM.
- 2.
Study idea: RM and JB.
- 3.
Study design: RM and JB.
- 4.
Data mining: RM and JB.
- 5.
Data analysis and interpretation: RM and JB.
- 6.
Statistical analysis: NA.
- 7.
Reference: RM and JB.
- 8.
Writing: RM.
- 9.
Critical review of the manuscript with intellectually relevant remarks: JB.
- 10.
Approval of final version: RM and JB.
The authors declare no conflict of interests associated with this article whatsoever.
Please cite this article as: Méndez Fernández R, Barrera Ortega J. Resonancia magnética de la endometriosis pelviana. Radiología. 2017;59:286–296.