To analyze the usefulness of diffusion magnetic resonance (MR) sequences before and after prostatic artery embolization (PAE) in patients with benign prostatic hyperplasia (BPH).
Material and methodsWe analyzed MR studies done before (7–10 days) and after (30 days) PAE in 19 patients with BPH treated with PAE between June 2012 and December 2013. We used 1.5Tesla scanners with body surface coils. In pre-PAE MR studies, we recorded mean b40 values and minimum (min) and maximum (max) apparent diffusion coefficient (ADC) values. In post-PAE MR studies, we recorded b40, b400, and b1000 values and min, mean, and max ADC values. We compared diffusion behavior/ADC before and after PAE and areas without ischemia. We correlated these with decreased prostatic volume (PV).
ResultsWe identified ischemia with contrast in 8 (42.1%) patients. No significant difference was found in mean b40 (p=0.1650) or in the b40 ratio (p=0.8868) between patients with ischemia and those without before PAE. Min b40, b40 ratio, and min ADC values differed significantly between ischemic areas and nonischemic areas within patients [p=0.048 (b40 min and ratio) and p=0.002 (min ADC)]. No significant correlation was found between the percentage decrease in PV and mean b40 (p=0.8490) or b40 ratio (p=0.8573).
ConclusionPost-PAE ischemia generates objective changes in diffusion and ADC values that enable ischemic sectors to be differentiated from nonischemic sectors. Future studies should analyze whether it is possible to subjectively differentiate between these areas through the visualization of nonischemic sectors and the feasibility of replacing them with contrast to detect ischemia.
Analizar la utilidad de la difusión en resonancia magnética (RM) antes y después de la embolización de arterias prostáticas (EAP) en pacientes con hiperplasia prostática benigna (HPB).
Material y métodoSe analizaron RM pre-EAP (7–10 días) y post-EAP (30 días) en 19 pacientes con HPB tratados con EAP entre junio de 2012 y diciembre de 2013. Se utilizaron equipos de 1,5 Tesla y bobina corporal de superficie. En RM pre-EAP se registraron valores b40 media, coeficiente de difusión aparente (CDA) mínimo (mín) y máximo (máx). En RM post-EAP se determinaron b40, b400, b1000 y CDA mín, media y máx. Se comparó el comportamiento en difusión/CDA antes y después del procedimiento y en áreas sin isquemia. Se correlacionó con la disminución de volumen prostático (VP).
ResultadosSe identificó isquemia con contraste en 8 pacientes (42,1%). Al comparar pacientes con isquemia vs. sin isquemia, la diferencia en b40 media (p=0,1650) y b40 cociente (p=0,8868) pre-EAP no fue significativa. Encontramos diferencia significativa entre valores b40 mín, b40 cociente y CDA mín de áreas isquémicas y no isquémicas del mismo paciente (p=0,048 [b40 mín y cociente] y 0,002 para CDA mín). No se encontró una correlación significativa para b40 media (p=0,8490) y b40 cociente (p=0,8573) al compararla con el porcentaje de reducción de VP.
ConclusiónLa isquemia post-EAP genera cambios objetivos en difusión y CDA que permitirían diferenciarla de sectores no isquémicos. Futuros trabajos deberán analizar si es posible una diferenciación subjetiva mediante visualización de sectores no isquémicos y la factibilidad de reemplazar las secuencias con contraste para detectar isquemia.
Benign prostatic hyperplasia (BPH) has a high prevalence rate in men older than 50 years; it is usually associated with lower urinary tract symptoms, in particular intermittence, decrease of urinary flow, nocturia, the sensation of incomplete vesical void, increase in the frequency and urge to urinate.1,2
A population prospective study established that a 60-year-old man with a BPH diagnosis has a 23% chance of suffering acute urinary retention if he survives 20 years. It also showed that urinary retention risk increases with age.3
Based on the seriousness of the symptoms and clinical evolution, there are several options for managing this pathology, ranging from observation and medication to surgical intervention. The medical treatment and surgical procedures used so far have allowed us to diminish the symptoms, but they have also communicated co-morbidities and complications4; transurethral resection or open adenectomy are considered the therapeutic choice for those patients who are refractory to pharmacological treatment or for those with urinary obstruction.5,6
With the goal of reducing complexity, duration and risk of complications associated with the traditional treatments, new, less invasive therapeutic options have been developed, such as thermotherapy or ablation with a transurethral needle, Holmium laser resection and photoselective vaporization; these alternatives have not yet shown greater efficiency than traditional treatments to control the symptomatology of BPH patients. In addition, they are not widely available and usually have a high cost.7
It has been recently suggested that prostatic artery embolization (PAE) for the treatment of BPH in men can be as effective as uterine embolization for the treatment of fibroma in women. The control and follow-up of post-embolization with images in both procedures is essential to document the presence of post-treatment ischemia.6,8,9
Such control can be performed through ultrasounds (US) or magnetic resonance images (MRI). The latter has been considered for prostate evaluation, and its main advantages include the safe differentiation between the transition region and the peripheral region, the accurate determination of glandular volume and an easy detection of glandular devascularization.10
The use of MRI in the assessment before and after the procedure is an extension of what was learned in the treatment of uterine myomas.11 While T1-weighted TSE sequences, with and without IV contrast show us devascularized sectors and changes secondary to the devascularization or necrosis obtained by embolization, the diffusion/apparent diffusion coefficient (ADC) sequences have proven to be useful in the assessment of volume reduction12 and in the detection of post-embolization ischemia of uterine myomas.13 There are not any publications today about the usefulness of these sequences in pre- and post-PAE evaluation.
The goal of the present article is to analyze the usefulness of diffusion/ADC sequences in the pre- and post-embolization assessment of the prostate gland.
Material and methodPopulationThis study was conducted in the setting of a research protocol approved by the research protocol committee in our institution. All patients included signed their prior informed consent, the pre- and post-PAE MRI were performed together with the clinical examination and the laboratory tests in the time and manner established in the aforementioned protocol.
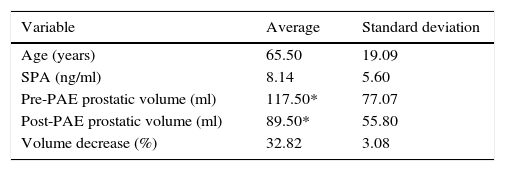
A retrospective analysis was carried out of the pre- and post-PAE MRI of 19 patients diagnosed with BPH refractory to medical treatment, the patients were over 50 years of age, with a prostate volume (PV) greater than or equal to 50ml who had undergone PAE between June 2012 and December 2013. We excluded patients diagnosed with diabetes mellitus, other urological pathologies associated with BPH, stroke in the last 6 months, history of pelvic radiotherapy, contraindications to undergo angiography and patients whose MRIs were obtained outside the institution (Table 1).
Variables obtained from electronic medical records.
| Variable | Average | Standard deviation |
|---|---|---|
| Age (years) | 65.50 | 19.09 |
| SPA (ng/ml) | 8.14 | 5.60 |
| Pre-PAE prostatic volume (ml) | 117.50* | 77.07 |
| Post-PAE prostatic volume (ml) | 89.50* | 55.80 |
| Volume decrease (%) | 32.82 | 3.08 |
SPA: specific prostatic antigen; PAE: prostatic artery embolization.
Prostatic embolization was performed outpatiently in all cases. An angiographic machine with digital subtraction was used (Artis Zeego®; Siemens Medical Solutions, Erlangen, Germany), in a hybrid OR of the hospital Angiography and Endovascular Therapy Unit.
A vesical tube was inserted in all patients and they were administered a 400mg dose of IV ciprofloxacin prior to the intervention. The procedure was performed through percutaneous puncture and catheterization of the right common femoral artery with local anesthesia. A panoramic aortography was performed followed by selective angiographies of both hypogastric arteries for the identification of the vascular anatomy of the pelvis paying special attention to the origin of prostate arteries.
Once they had been recognized, the prostate arteries were catheterized selectively using a microcatheter (Pro Great® 2,7, Terumo, USA, or Maestro® 2.4, Merit Medical, South Jordan, USA) placed co-axially through a 5 Fr angiographic catheter. At the discretion of the operator in uncertain cases angiotomographic images were obtained using cone beam computed tomography (Cone Beam CT).
With the microcatheter placed in the right position, a free-flow embolization was performed with trisacryl microspheres (Embosphere®, Biosphere Medical, South Jordan, USA), until the prostate arterial flow was occluded; 100 micron (μ) embospheres were used in eight patients, 200μ embospheres in one patient and 300μ embospheres in eight; two patients were embolized with 200μ PVA particles (Contour; Boston Scientific/Target Vascular, Fremont, California) After performing a post-embolization angiographic control, the angiographic catheter was removed and manual compression was exerted in the place of the puncture.
The patients were discharged 6h later, without the vesical tubes and medicated with oral antibiotics (ciprofloxacin 1g/day) for eight days and non-steroid anti-inflammatory drugs on demand. They were all contacted by telephone during the first week and the symptoms or complications were recorded and categorized following the classification proposed by the American Society of Interventional Radiology.6
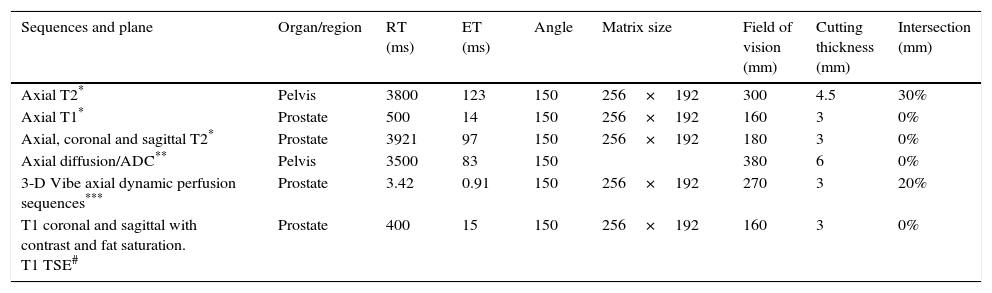
Magnetic resonance prostate protocol before and afterThe MRI prior to the embolization was performed between 7 and 10 days before the procedure, while the post-embolization control was carried out 30 days after the embolization. The studies were performed with 1.5Tesla machines (Magnetom Avanto® or Essenza®; Siemens Medical Solutions, Erlangen, Germany) using a body phased array surface coil. The posterior surface of the prostate was taken as a reference for the orientation of the surface sequences. Transversal images were oriented perpendicular to the posterior surface of the prostate (from the base to the apex) and coronal images parallel to the above-mentioned plane (from the symphysis to the rectum).
The protocol included sequences without contrast: transversal T1 Turbo Spin Eco (TSE) and T2 TSE in the transversal, sagittal and coronal planes; diffusion/ADC sequences with values of b: 40, 400 and 1000s/mm2.
After the administration of IV gadolinium (gadoterate meglumine at a dose of 0.1mmol/kg with a flow of 3ml/s, followed by 20ml of saline solution at a similar flow) the sequences were acquired with a dynamic technique (flash 3D Vibe) oriented on the transversal plane, using consecutive acquisitions every 20s, for 5min, and finally late acquisition after 7min with TSE T1 sequences on the transversal, coronal and sagittal planes (Table 2).
Parameters of magnetic resonance sequences.
| Sequences and plane | Organ/region | RT (ms) | ET (ms) | Angle | Matrix size | Field of vision (mm) | Cutting thickness (mm) | Intersection (mm) |
|---|---|---|---|---|---|---|---|---|
| Axial T2* | Pelvis | 3800 | 123 | 150 | 256×192 | 300 | 4.5 | 30% |
| Axial T1* | Prostate | 500 | 14 | 150 | 256×192 | 160 | 3 | 0% |
| Axial, coronal and sagittal T2* | Prostate | 3921 | 97 | 150 | 256×192 | 180 | 3 | 0% |
| Axial diffusion/ADC** | Pelvis | 3500 | 83 | 150 | 380 | 6 | 0% | |
| 3-D Vibe axial dynamic perfusion sequences*** | Prostate | 3.42 | 0.91 | 150 | 256×192 | 270 | 3 | 20% |
| T1 coronal and sagittal with contrast and fat saturation. T1 TSE# | Prostate | 400 | 15 | 150 | 256×192 | 160 | 3 | 0% |
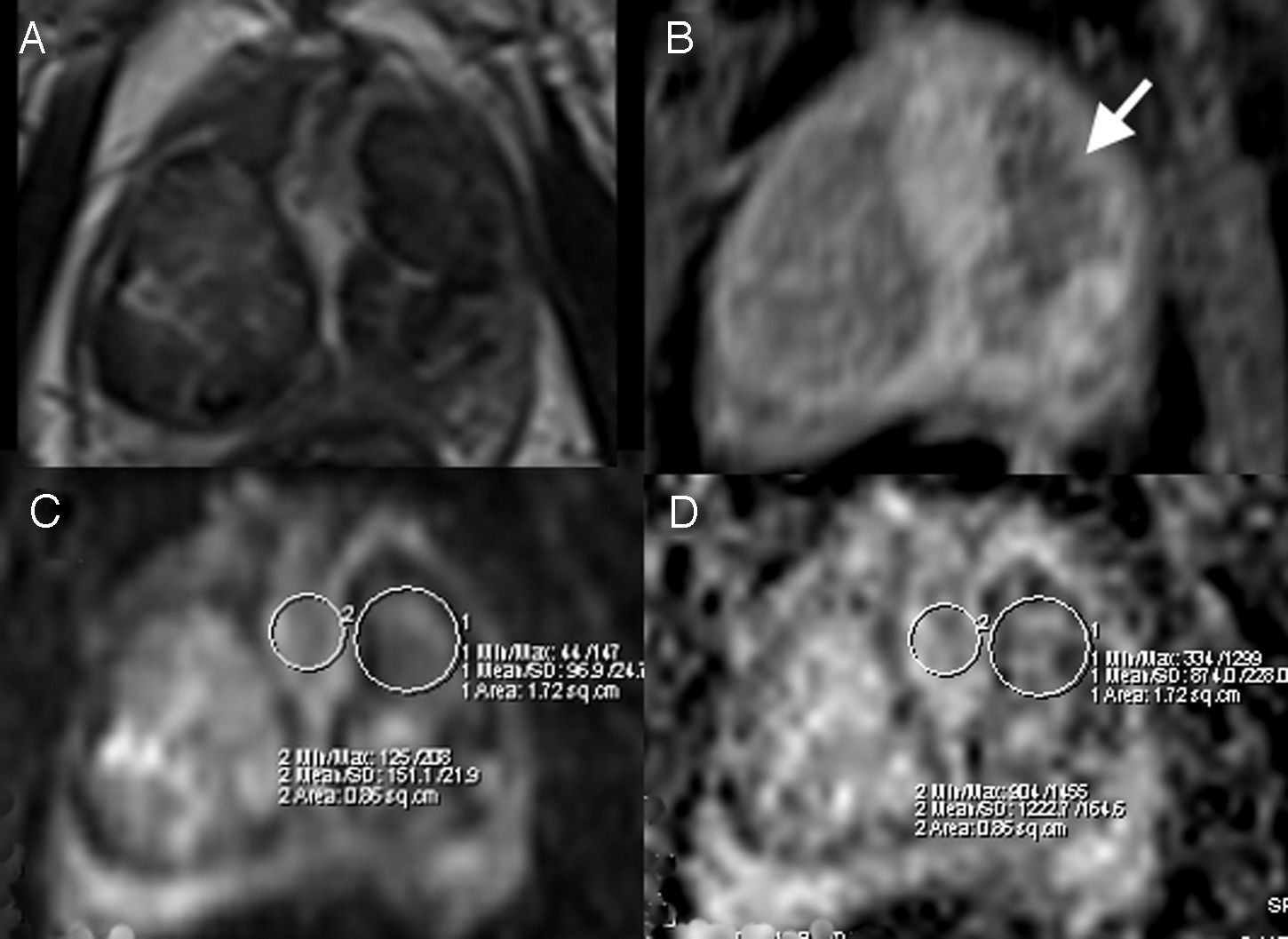
All patients underwent a prostate MRI before and after the embolization. All the studies were reviewed by a specialist in image diagnosis with 8 years of experience in prostatic images. Taking as a reference the location of the ischemic areas detected in T1 sequences with post-embolization contrast, the performance of such areas was analyzed in diffusion/ADC before and after the procedure and they were compared with areas without ischemia within the same gland and their relation with the reduction of the prostatic volume between both MRIs was studied (Fig. 1). The following variables were recorded in the diffusion/ADC sequences of the MRI performed before and after the PAE:
- •
In the pre-PAE MRIs b40 median values were analyzed, minimum (min) and maximum (max) ADC. The measurements were made using as reference cut-off value of the glandular middle third in TSE T2 axial sequences where it was possible to identify as a reference the focal areas with the greatest signal intensity, interpreted as hyperplasia sectors of epithelial predominance.14 In addition, the signal intensity of the gluteal muscle was determined (b musc.) to minimize the variability between the diffusion of the two machines used through the calculation of b40 quotient (b40 mean/b muscle).
- •
In the post-PAE MRIs the minimum, mean and maximum values of b40, b400 and b1000 were determined, as well as the minimum, mean and maximum ADC of the ischemic areas; also the same values were recorded in non-ischemic sectors of the same image. The presence or absence of ischemia was established taking as a reference the performance with contrast on the same cut plane in TSE T1 axial sequences with fat saturation. The values b40 mean, b40 quotient, minimum and maximum ADC of the pre-PAE MRI were correlated with the presence of ischemia in the post-PAE MRI.
Patient #3. Post-PAE magnetic resonance images. Transversal cuts. (A) T2 TSE. (B) T1 TSE with contrast and fat saturation. (C) b40 diffusion. (D) ADC. Ischemic area (white arrow) in B; in C and D the measurement of the region of interest (ROI) in ischemic (1) and non-ischemic (2) areas is shown in the same patient with minimum, mean, and maximum determination of b value and ADC.
PV was determined before and after embolization through planimetry using all the images in TSE T2 sequences on the transversal plane, in which it was possible to identify the gland from the glandular base to the apex. The contour was traced on each transversal image following the prostatic capsule and volumetry was performed based on the sum of areas using the software provided at the Vitrea® Computer 3-D Reconstruction Workstation. The PV reduction between both MRIs was calculated.
Statistical analysisThe continuous variables were presented as median and standard deviation, and the categorical variables as frequency measurements. The chi-squared and the Fisher tests were used for the categorical variables as appropriate, and the Mann–Whitney, Wilcoxon Tests or T-test for the continuous variables based on their distribution. The continuous variables were compared to one another using the statistic regression test. One p<0.05 value was taken as significant. The tests were performed using the statistic software MedCalc for Windows, version 12.5 (MedCalc Software, Ostend, Belgium).
ResultsNineteen patients with a mean age of 65.5 years (ranging between 52 and 86 years) were studied. In eight of them (42.1%) it was possible to identify ischemic areas in T1-weighted TSE sequences with contrast. The median values and standard deviation of the specific prostatic antigen (SPA), pre- and post-PAE PV and the percentage of volume reduction can be seen in Table 1.
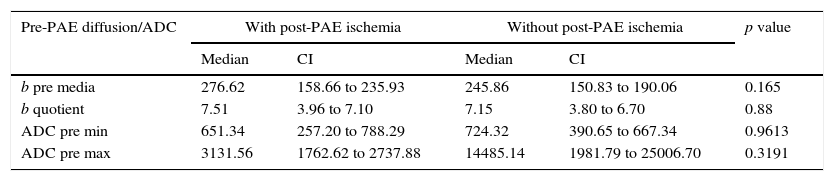
The pre-PAE mean b40 and quotient b40 values in patients with ischemia in the post-PAE MRIs did not show significant differences from the comparative study with those from patients without ischemia with p=0.1650 values for mean b and p=0.8868 for quotient b, with confidence intervals (CI) with wide overlapping. Similar results can be seen after analyzing the min and max ADC values with relation to the presence or absence of ischemia (Table 3).
Variable correlation in pre-PAE diffusion/ADC with presence of post-PAE ischemia.
| Pre-PAE diffusion/ADC | With post-PAE ischemia | Without post-PAE ischemia | p value | ||
|---|---|---|---|---|---|
| Median | CI | Median | CI | ||
| b pre media | 276.62 | 158.66 to 235.93 | 245.86 | 150.83 to 190.06 | 0.165 |
| b quotient | 7.51 | 3.96 to 7.10 | 7.15 | 3.80 to 6.70 | 0.88 |
| ADC pre min | 651.34 | 257.20 to 788.29 | 724.32 | 390.65 to 667.34 | 0.9613 |
| ADC pre max | 3131.56 | 1762.62 to 2737.88 | 14485.14 | 1981.79 to 25006.70 | 0.3191 |
ADC: apparent diffusion coefficient; PAE: prostatic artery embolization; CI: confidence interval; max: maximum; min: minimum.
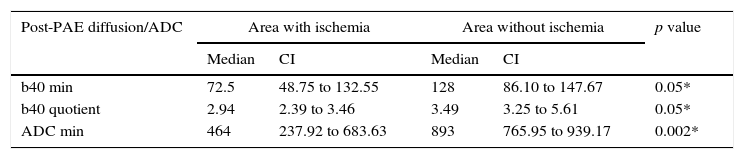
When comparing the min b40, quotient b40 and min ADC values between the ischemic and non-ischemic areas in the same patient, it is possible to see a significant difference with p=0.048 values for min b40 and quotient and 0.002 for min ADC; the confidence intervals and median are summarized in Table 4.
Variable correlation in post-PAE diffusion/ADC in-between areas with or without ischemia.
| Post-PAE diffusion/ADC | Area with ischemia | Area without ischemia | p value | ||
|---|---|---|---|---|---|
| Median | CI | Median | CI | ||
| b40 min | 72.5 | 48.75 to 132.55 | 128 | 86.10 to 147.67 | 0.05* |
| b40 quotient | 2.94 | 2.39 to 3.46 | 3.49 | 3.25 to 5.61 | 0.05* |
| ADC min | 464 | 237.92 to 683.63 | 893 | 765.95 to 939.17 | 0.002* |
ADC: apparent diffusion coefficient; PAE: prostatic artery embolization; CI: confidence interval; max: maximum; min: minimum.
When we conducted a regression analysis between the mean b40 and quotient b40 values with the percentage of volume reduction, we cannot find any significant correlations with p=0.8490 and 0.8573 values, respectively.
DiscussionIn the embolization of uterine fibromas, the infarction/ischemic areas have showed transitory increase in signal intensity (SI) in b values and persistent decrease in ADC15; in our group we were unable to show similar changes, which can be related with the short follow-up period of our population; nevertheless, the significant differences that we found between ischemic and non-ischemic areas for min b40, quotient b40 and ADC values could represent an objective tool for the identification of post-PAE ischemia in diffusion/ADC without having to use sequences with contrast for its detection, with the corresponding simplification of the MRI protocol and less time for the study to be completed.
In our group of patients, all the infarction areas detected in T1 sequences with contrast correlated with evident changes in the diffusion/ADC sequences in post-embolization MRIs, however. We have not found findings similar to the ones described in the diffusion/ADC sequences for the embolization of uterine myomas.15
When comparing min b40, quotient b40 and min ADC values between the ischemic and non-ischemic areas of the same patient, significant differences are observed. These objective changes added to the visual subjective identification of the infarction areas in eight patients would allow us to hypothesize that such ischemic areas could be spotted through diffusion/ADC sequences without using T1 sequences with gadolinium as part of the study protocol in post-embolization MRIs.
The physiopathological reasons behind these findings should be the object of study in the future. A possible hypothesis behind the changes observed in diffusion and ADC could be that a hemorrhagic component is observed in several areas of post-PAE necrosis, considering that both blood and its components have an impact on such sequences as it has been published on the nervous tissue16 though it is not possible to make an exact correlation due to the differences existing between both tissues and because we did not have an anatomopathologic correlation in the present study.
At present MRI is the image modality offering the greatest accuracy to evaluate the prostate and it is probably the most appropriate one for evaluation pre and post-PAE.8,17 The role of the different sequences in the identification of ischemia in post-PAE studies or which is the most appropriate protocol for pre- and post-embolization MRI has not been defined yet.
The infarction areas have been described as prostatic gland sectors without contrast enhancement in T18 TSE sequences; in our group we have found infarctions behaving like this in sequences with contrast in eight patients (42%).
Recent publications claim to have detected such areas in approximately 70–80% of cases.17,18 Such differences can be associated with the procedure technique and the different size of embolization material used when comparing both groups of patients and among the patients of our group.
One of the goals of PAE is to minimize PV. PV reduction rates between 20 and 26.5% at 30 days post-embolization have been reported, results that are similar to those found in our group; however, the direct relation between the reduction of PV and clinical improvement has not been proven yet.8,18 In our analysis we have not found any correlations between b and ADC values that would allow us to predict what prostates will undergo a major PV reduction which might be explained by the fact that only one T1 TSE gland cut with contrast was assessed for the identification of the most representative ischemic areas. Possibly, an analysis of several cuts or the entire prostatic volume will allow us to obtain results with greater predictive value about glandular volume reduction.
The lack of justification to perform biopsies or prostatectomies after the procedure did not allow us to correlate the findings in diffusion/ADC sequences with the histopathologic changes; this is why the reason for the changes in the images are only hypotheses based on comparisons with what has been published about the embolization of myomas. On the other hand, the short follow-up period (30 days) makes the comparison and parallelism difficult with the findings observed in the infarction areas with post-embolization MRIs of uterine myomas, since such studies have a larger observation period.13
These results show that the mean b40, quotient b40, min and max ADC values in pre-PAE MRI do not predict the presence of ischemia or major reduction of volume in post-embolization MRIs; nevertheless, min b40, quotient b40 and min ADC values between ischemic and non-ischemic areas in the same patient showed significant differences attributable to recordable objective changes which could allow us to differentiate ischemic areas from non-ischemic areas in diffusion/ADC sequences.
Like we mentioned, our analysis had several limitations; the small number of patients and the fact that the ischemic areas in T1 sequences with contrast were observed in eight of the 19 patients determine that the groups compared are too small which in turn makes the observed changes lose statistic significance. Also the different sizes of embolization material used throughout the protocol, which we attribute to the learning curve, could influence the expression of the infarction areas, which would in turn influence the visual identification of such areas and the b values in diffusion and ADC.
In sum the post-PAE prostatic ischemic areas generate changes in diffusion/ADC which would allow us to differentiate them from non-ischemic sectors shown in low SI b40 and ADC values.
Future studies with larger populations and longer follow-up periods should analyze if the findings in ischemic areas in diffusion/ADC allow us to do a safe objective visual differentiation from non-ischemic sectors and if it is possible to replace the current role of sequences with contrast in the detection of post-embolization prostatic ischemia.
Ethical responsibilitiesProtection of people and animalsThe authors declare that no experiments with human beings or animals have been performed while conducting this investigation.
Data confidentialityThe authors declare that in this article there are no data from patients.
Right to privacy and informed consentThe authors declare that in this article there are no data from patients.
Authors contribution- 1.
Manager of the integrity of the study: ES.
- 2.
Study idea: ES, JO, NK, RGM.
- 3.
Study design: ES, AK, JO.
- 4.
Data mining: ES, NK, NN.
- 5.
Data analysis and interpretation: JO, AK.
- 6.
Statistical analysis: AK.
- 7.
Reference search: ES.
- 8.
Writing: ES, JO, NK.
- 9.
Critical review of the manuscript with intellectually relevant remarks: ES, JO, AK, NN, RGM.
- 10.
Approval of final version: ES, JO, AK, NK, NN, RGM.
The authors declare no conflict of interests associated with this article whatsoever.
Please cite this article as: Serrano E, Ocantos J, Kohan A, Kisilevsky N, Napoli N, García-Mónaco R. Resonancia magnética de próstata: utilidad de la secuencia de difusión en la detección de isquemia postembolización en pacientes con hiperplasia prostática benigna. Radiología. 2016;58:129–135.